Abstract
Limitations of current viral-based gene therapies for malignant tumors include lack of cancer-specific targeting and insufficient tumor delivery. To ameliorate these problems and develop a truly effective adenovirus gene-based therapy for cancer, we constructed a conditionally replication competent adenovirus (CRCA) manifesting the unique properties of tumor-specific virus replication in combination with production of a cancer-selective cytotoxic cytokine, melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24), which embodies potent bystander antitumor activity. Cancer cell selective tropism was ensured by engineering the expression of the adenoviral E1A protein, necessary for viral replication, under the control of a minimal promoter region of progression elevated gene-3 (PEG-3), which functions selectively in diverse cancer cells with minimal activity in normal cells. In the E3 region of this CRCA, we introduced the mda-7/IL-24 gene, thereby mediating robust production of this cytokine as a function of adenovirus replication. Infection of this CRCA (designated Ad.PEG-E1A-mda-7) in normal mammary epithelial cells and breast cancer cells confirmed cancer cell selective adenoviral replication, mda-7/IL-24 expression, growth inhibition, and apoptosis induction. Injecting Ad.PEG-E1A-mda-7 into human breast cancer xenografts in athymic nude mice completely eradicated not only the primary tumor but also distant tumors (established on the opposite flank of the animal) thereby implementing a cure. This dual cancer-specific targeting strategy provides an effective approach for treating breast and other human neoplasms with the potential for eradicating both primary tumors and metastatic disease. Additionally, these studies support the potential use of mda-7/IL-24 in the therapy of malignant cancers.
Keywords: bystander antitumor activity, conditionally replication competent adenovirus, PEG-Prom, mda-7/IL-24, in vivo tumorigenesis
The ultimate goal of cancer therapy is a minimally or nontoxic treatment regimen that will ensure complete eradication of both primary and metastatic cancers, thereby producing a cure for this debilitating, life-shortening, and pervasive disease. At present, achieving this objective remains elusive despite improvements in therapies for specific neoplastic conditions. Although potentially effective in early stages of the disease, the standard treatment protocols of surgery, chemotherapy, and radiotherapy, alone or in various combinations, fall short of restraining the disease, which can ultimately progress to metastasis and a failure to respond to therapy (1). These harsh realities highlight the need for alternative modalities of treatment, either alone or in combination, to effectively manage this clinical condition. Newer approaches currently being pursued include gene therapy (targeting specific defects in cancer cells, replacing defective tumor suppressor genes, or promoting cancer-specific viral replication) and immunotherapy (2–5). Conventional treatment failures raise the relevant question of what would constitute an effective cancer therapy. Clearly, a course of treatment that evokes complete destruction of tumor cells with little or no injurious effects toward normal cells represents the definitive objective of cancer gene therapy. In the present work, we describe a dual gene therapy approach using a cancer cell-selective promoter and a cancer cell-specific apoptosis-inducing cytokine gene displaying profound activity toward primary and distant breast tumors, thereby providing a valuable therapeutic for localized and metastatic cancers.
Cancer gene therapy typically involves delivery of tumor suppressor, apoptosis-inducing, or suicide genes directly into tumor cells (2). Replication incompetent adenoviral (Ad) vectors are frequently used for this purpose because they promote high-level transgene expression (6). However, in most instances, engendering a discernible and significant antitumor response requires administering the Ad multiple times, which can trigger an immune response and viral clearance. In these contexts, conditionally replication competent adenoviruses (CRCA) are currently being evaluated because of their effectiveness in killing cancer cells by replication and thus requiring fewer administrations (7, 8). Of equal importance, although antiadenovirus neutralizing Abs significantly attenuate the activity of a replication-incompetent Ad, they have limited or no effect on the activity of a replication-competent Ad (9). This finding argues that administration of a replication-competent Ad in patients with preexisting Ad immunity would still prove efficacious.
The most important facet of using a CRCA is to ensure cancer cell-specific replication, and many unique strategies have been developed to achieve this objective. One approach currently being evaluated clinically uses ONYX-015, a replication-competent Ad that propagates preferentially, although not exclusively, in p53 mutant cells (10). A similar replication-competent Ad, ONYX-411, exploits defects in the Rb pathway, a recurrent alteration in tumor cells (11). The rationale for using such viruses is that because of intact p53 or Rb pathways, these Ads will not replicate in normal cells, while selectively reproducing and inducing cytolysis in tumor cells containing intrinsic defects in p53 or Rb pathways. A problem confounding this approach relates to the multitude of genetic and phenotypic changes occurring in primary tumors that are commonly exacerbated during tumor progression, thereby restricting the effectiveness of these types of viruses.
To circumvent the problem of tumor-cell specificity, we engineered a CRCA in which replication is driven by a minimal active region of the promoter of progression elevated gene-3 (PEG-3), which functions selectively in diverse cancer cells with limited activity in normal cells (12). PEG-3 was cloned as an up-regulated transcript from a transformation progression rodent cancer model, and attractively, the activity of its promoter (PEG-Prom) was found to be significantly and often markedly higher not only in rodent but also in human cancer cells of diverse origin when compared with normal cells (12–15). The cancer cell specificity of the PEG-Prom is governed by two transcription factors, AP-1 and PEA-3, which are overexpressed, either singly or in combination, in virtually all types of cancers (16–19). Using the PEG-Prom to drive GFP or luciferase by means of a replication-incompetent Ad confirmed prominent cancer cell-specific transgene expression in human prostate and breast cancer cells as well as in malignant glioma cells (12). These observations prompted us to investigate the use of the PEG-Prom to drive expression of the E1A gene, necessary for Ad replication, to create a cancer cell-specific CRCA.
Because cancer cells are genetically and phenotypically complex and frequently harbor multiple abnormalities, we reasoned that simply inducing Ad replication in a subset of tumor cells might not be adequate to ensure complete eradication of the disease, especially when compounded by the spread of neoplastic cells to multiple organs. Based on this consideration, we engineered melanoma differentiation associated gene-7 (mda-7/IL-24) to be simultaneously expressed from the E3 region of our CRCA. mda-7/IL-24 was cloned as a gene transcript upregulated during differentiation of melanoma cells, which possesses cancer cell-selective apoptosis inducing properties without harming normal cells in vitro, in vivo in animal models, and recently in a phase I clinical trial (20–29). Intriguingly, mda-7/IL-24 not only induces apoptosis but also has immune modulatory and antiangiogenic properties as well as potent antitumor bystander effects, making it an ideal candidate for cancer gene therapy (23, 25, 30–34). Owing to these essential qualities as a potential gene therapeutic for cancer, a replication-incompetent Ad expressing mda-7/IL-24 (Ad.mda-7) is currently being evaluated for clinical efficacy in phase II clinical trials (23, 25, 29).
The present studies focus on breast cancer, a common female cancer accounting for 32% of all cancers in women (35). It is estimated that in 2005, a total of 211,240 new cases of invasive breast cancer will be diagnosed in the United States, and the estimated death toll from all forms of breast cancer will be 40,410 (35). Because no consistently successful therapy exists for metastatic breast cancer, we evaluated the effect of Ad.PEG-E1A-mda-7 in normal and human breast cancer cells by using in vitro cell culture and in vivo in nude mouse tumor models. We reasoned that should cancer-cell-specific efficacy be apparent in this breast cancer model, our dual targeting strategy could be of immense benefit to a significant patient population suffering from primary and metastatic forms of this disease. This possibility has now been validated, indicating that in an experimental animal model complete eradication of both primary and distant breast cancers result after administration of Ad.PEG-E1A-mda-7. This work provides a solid foundation for developing a potentially effective virus-based therapy for patients with breast cancer, with clear relevance to additional neoplastic diseases.
Materials and Methods
Cell Lines, Culture Conditions, and Viability Assays. MCF-7, T47D, MDA-MB-157, and MDA-MB-231 breast cancer cells and human embryonic kidney (HEK)-293 cells were obtained from American Type Culture Collection and cultured as described in ref. 22. The 184A1 and 184AA2 cells were kindly provided by Martha Stampfer (Lawrence Berkeley National Laboratory, Berkeley, CA) and were cultured in mammary epithelial growth medium containing supplements (MEGM BulletKit, CC-3051; BioWhittaker) and transferrin (5 μg/ml) and isoproterenol (10 mM). Cell viability was determined by standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assays (26).
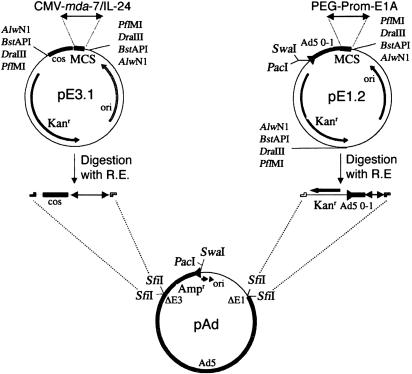
Construction of a CRCA. To construct the CRCA (Ad.PEG-E1A-mda-7), the AdenoQuick cloning system from OD260 (Boise, ID) was used (36). This system uses two shuttle vectors, pE1.2 and pE3.1, in which the transgene cassettes PEG-Prom driving E1A and the CMV promoter driving mda-7/IL-24 were inserted, respectively, before being transferred into a large Ad plasmid (Fig. 1). Ad amplification, purification, titration, and infection were performed as described in ref. 37. Similar strategies were used to generate Ad.CMV-E1A-mda-7. Ad.CMV-mda-7 and Ad.PEG-mda-7 were constructed as described in ref. 12.
Fig. 1.
Construction of Ad.PEG-E1A-mda-7. pE1.2 and pE3.1 are the shuttle vectors in which the PEG-3 gene promoter (PEG-Prom) driving the E1A gene (PEG-Prom-E1A) and the CMV promoter controlling the mda-7/IL-24 gene (CMV-mda-7/IL-24) were ligated, respectively, at the multiple cloning site (MCS). The promoter plus transgene cassettes was digested out by restriction enzyme (R.E.), e.g., AlwNI, BstAPI, DraIII, or PflMI and ligated into SfiI-digested Ad vector plasmid Ad
Annexin V-Binding Assay. Annexin V binding assays were performed as described in ref. 26.
Preparation of Whole-Cell Lysates and Western Blot Analyses. Preparation of whole-cell lysates and Western blot analyses was performed as described in ref. 24. The primary Abs used were anti-E1A (1:1,000; mouse monoclonal; Upstate Biotechnology, Lake Placid, NY), anti-mda-7 (1:2,000; rabbit polyclonal), anti-c-JUN (1:1,000; rabbit polyclonal; Santa Cruz Biotechnology), anti-PEA-3 (1:500; mouse monoclonal; Santa Cruz Biotechnology), and anti-EF1α (1:1,000; mouse monoclonal; Upstate Biotechnology).
Human Breast Cancer Xenograft in Athymic Nude Mice. T47D cells (2 × 106) were injected s.c. in 100 μl of PBS in both flanks of male athymic nude mice (NCRnu/nu; 4 wk old; ≈20 g of body weight) (22, 38). After establishment of visible tumors of ≈75 mm3, requiring ≈4- to 5-d, intratumoral injections of different Ads were given only to the tumors on the left flank at a dose of 1 × 108 plaque-forming units in 100 μl of PBS. No injection was given to the right-sided tumors. The injections were given three times a week for the first week and then twice a week for two more weeks for a total of seven injections. A minimum of five animals was used per experimental point. Tumor volume was measured twice weekly with a caliper and calculated by using the formula π/6 × larger diameter × (smaller diameter)2. At the end of the experiment, the animals were killed, and the tumors were removed and weighed.
Transient Transfection and Luciferase Assays. Transient transfection and luciferase assays were performed as described in ref. 12.
Statistical Analysis. Statistical analysis was performed by using ANOVA, followed by Fisher's protected least significant difference analysis. P < 0.05 was considered significant.
Results
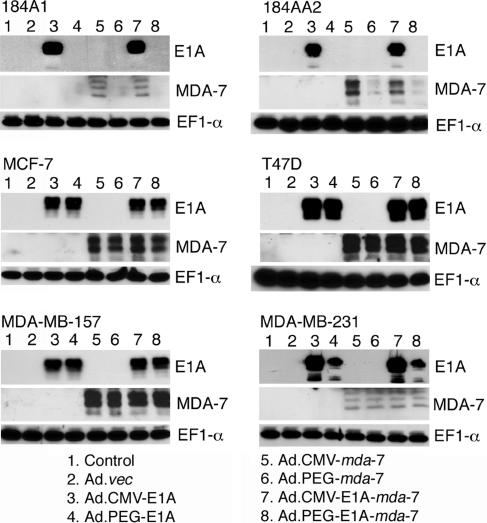
To test the dual cancer-specific targeting stratagem and to evaluate the relative effectiveness of Ad.PEG-E1A-mda-7, we created a series of additional Ads, including Ad.CMV-E1A-mda-7, in which viral replication is controlled by the CMV promoter and which also expresses mda-7/IL-24, and Ad.CMV-E1A and Ad.PEG-E1A, in which viral replication is controlled by the CMV promoter or the PEG-Prom, respectively. Additionally, we used Ad.CMV-mda-7 and Ad.PEG-mda-7, replication-incompetent Ad in which the CMV or the PEG promoter drives mda-7/IL-24 expression, respectively (12). A replication-incompetent empty Ad, Ad.vec was used as a control. Experiments were performed in four breast cancer cell lines, MCF-7 (wt p53), T47D (mut p53), MDA-MB-157 (p53 null), and MDA-MB-231 (mut p53), and two normal immortal mammary epithelial cell lines, 184A1 and 184AA2. The functionality of these constructs was ascertained after Ad infection by monitoring protein levels of MDA-7/IL-24 and E1A, a marker for Ad replication, by Western blot analysis after appropriate viral infection (Fig. 2). Western blot analysis detects multiple E1A gene products ranging from 36 to 50 kDa and multiple glycosylated forms of MDA-7/IL-24 protein ranging from 21 to 28 kDa.
Fig. 2.
PEG-Prom promotes Ad replication and transgene expression selectively in breast cancer cells. The indicated cells were uninfected (control) or infected with the indicated Ad (as described at the bottom of the figure) at a multiplicity of infection of 100 plaque-forming units/cell for 48 h. The expressions of E1A, MDA-7/IL-24, and EF1-α proteins were analyzed by Western blot
Infection of 184A1 or 184AA2 cells with Ad.CMV-E1A or Ad.CMV-E1A-mda-7, but not Ad.PEG-E1A or Ad.PEG-E1A-mda-7, resulted in production of E1A proteins; whereas in breast carcinoma cells, infection with all four replication-competent Ad generated E1A proteins (Fig. 2). No E1A proteins were detected in any cell line after infection with replication-incompetent Ads. In 184A1 and 184AA2 cells, infection with Ad.CMV-E1A-mda-7 and Ad.CMV-mda-7 resulted in MDA-7/IL-24 production, whereas infection with Ad.PEG-mda-7 or Ad.PEG-E1A-mda-7 resulted in barely detectable (184A1) or very low (184AA2) levels of MDA-7/IL-24 production (Fig. 2). In breast cancer cells, infection with Ad.CMV-mda-7, Ad.PEG-mda-7, Ad.CMV-E1A-mda-7, or Ad.PEG-E1A-mda-7 generated significant MDA-7/IL-24 production. No MDA-7/IL-24 protein production could be detected in control uninfected cells or after infection with Ad.vec, Ad.CMV-E1A, or Ad.PEG-E1A. In 184A1 and MDA-MB-231 cells, MDA-7/IL-24 protein levels were reduced in comparison with the other cell types studied after infection with Ad.CMV-mda-7 and Ad.CMV-E1A-mda-7. Because a similar reduction in MDA-7/IL-24 protein levels was evident in MDA-MB-231 cells infected with PEG-Prom driven constructs, this reduced expression could reflect a difference in MDA-7/IL-24 transgene expression in these cell types. In total, these findings document that the PEG-Prom facilitates cancer cell-selective replication of Ad and mda-7/IL-24 expression.
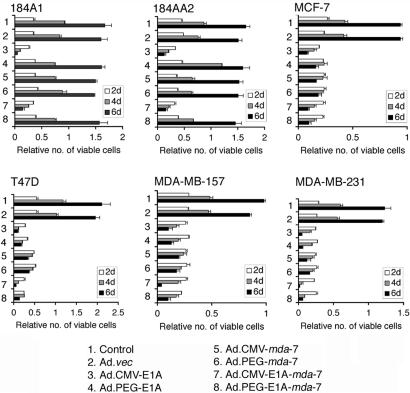
Studies were performed next to establish potential selective effects on growth and viability of normal and breast cancer cells when replication was controlled by the PEG-Prom vs. the CMV promoter. In 184A1 and 184AA2 cells, infection with only Ad.CMV-E1A or Ad.CMV-E1A-mda-7, but not with Ad.PEG-E1A, Ad.CMV-mda-7, Ad.PEG-mda-7, or Ad.PEG-E1A-mda-7, induced profound growth inhibition (Fig. 3). In contrast, in all breast cancer cells, Ad.CMV-E1A-mda-7, Ad.PEG-E1A-mda-7, Ad.CMV-E1A, and Ad.PEG-E1A infection resulted in significant growth inhibition. Infection with Ad.CMV-mda-7 and Ad.PEG-mda-7 also inhibited growth of the breast cancer cells. These findings indicate that the PEG-Prom allows Ad replication specifically in cancer cells, protecting normal cells from growth inhibition because of Ad replication. The observation that mda-7/IL-24 exerted no direct growth-inhibitory effect on normal cells confirms the cancer cell-selectivity of this therapeutic approach (23, 28). Of interest, the effect of the various viruses was similar in all four breast cancer cell lines, suggesting that the difference in final levels of MDA-7/IL-24 protein did not impact on antiproliferative and antisurvival effect of these viruses (Fig. 3).
Fig. 3.
PEG-Prom driven CRCA selectively kills breast cancer cells. The indicated cells were uninfected (control) or infected with the indicated Ad (as described at the bottom of the figure) at a multiplicity of infection of 100 plaque-forming units/cell. Cell viability was analyzed by standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay after 2, 4, and 6 d of infection. The data represent mean ± SD
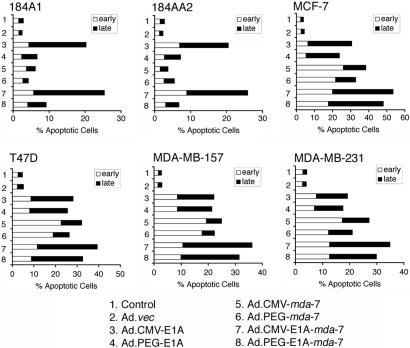
To investigate the mechanism of growth inhibition, Annexin V staining assays, which permit differentiation between apoptotic and necrotic cells, were performed (Fig. 4). Infection with only Ad.CMV-E1A and Ad.CMV-E1A-mda-7 elevated the percentage of early apoptotic and late apoptotic (necrotic) 184A1 and 184AA2 cells. However, all of the Ads, except for Ad.vec, resulted in significant apoptosis in the breast cancer cell lines. Infection with the replication competent Ads resulted in predominantly necrosis as evidenced by an increase in late apoptotic cells, whereas infection with Ad.CMV-mda-7 and Ad.PEG-mda-7 resulted in predominantly apoptosis as evidenced by an increase in early apoptotic cells. As observed for antiproliferative and antisurvival effects, the various viruses induced similar changes in all four breast cancer cell lines, again, suggesting that the difference in final levels of MDA-7/IL-24 protein also did not impact on apoptosis and necrosis induction in vitro (Fig. 4).
Fig. 4.
PEG-Prom driven CRCA selectively induces apoptosis in breast cancer cells. The indicated cells were treated as in Fig. 3. Annexin V staining was analyzed by flow cytometry 48 h after infection
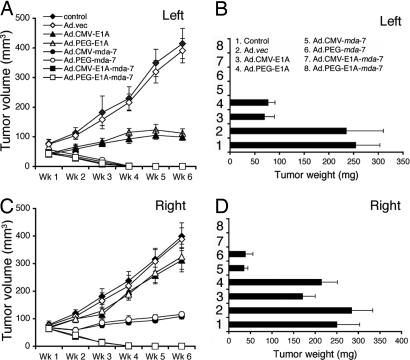
To expand on the in vitro studies, in vivo assays were performed by using nude mice containing established T47D s.c. xenografts on both right and left flanks. After palpable tumors of ≈75 mm3 developed, in ≈4–5 d, seven intratumoral injections with different Ads, three times per week for the first week and two times per week for an additional 2 wk, were administered to the tumors on the left flank at a dose of 1 × 108 plaque-forming units in 100 μl. No injections were given to the right-sided tumors. The experiment was terminated after 6 wk with injections of Ad.CMV-E1A-mda-7 or Ad.PEG-E1A-mda-7 because tumors on both sides showed regression after only three injections, and with seven injections they were completely eradicated (Fig. 5). T47D tumor xenografts grow slowly in the absence of estrogen and in our studies by using male nude mice at 6 wk, the control and Ad.vec injected tumors reached a weight of ≈250–300 mg, which agrees with a previous report (39). Although Ad.CMV-E1A or Ad.PEG-E1A inhibited the growth of tumors on the left flank they had some inhibitory effect on tumors on the right side, which was not statistically significant. Ad.CMV-mda-7 or Ad.PEG-mda-7 eradicated tumors on the left flanks and significantly inhibited tumor growth on the right flanks. The observation that intratumoral injection of Ad.PEG-E1A-mda-7 completely eradicated the primary and distant tumors (comparable with a metastasis) provides confidence that this strategy could prove amenable for successfully treating aggressive cancers.
Fig. 5.
CRCAs eradicate primary and distant tumors. s.c. tumor xenografts from T47D cells were established in athymic nude mice in both right and left flanks, and only tumors on the left side were injected with PBS (control) or with the indicated Ad for 3 wk (total of seven injections). (A and C) Measurement of tumor volume. The data represent mean ± SD with a minimum of five mice per group. (B and D) Measurement of tumor weight at the end of the study. The data represent mean ± SD with at least five mice per group. Qualitatively similar results were obtained in an additional study
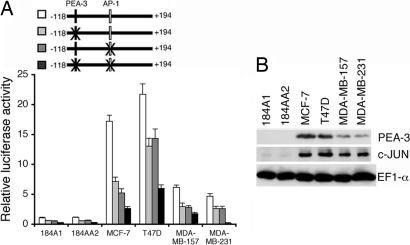
In the minimum effective element of the PEG-Prom (-118 to +194), there are two important transcription factor-binding sites, one for PEA-3 at -104 and another at +8 for AP-1 (Fig. 6A). These two transcription factors play an essential role in regulating PEG-Prom activity in prostate cancer cell lines and in rodent cell culture systems (12, 16, 17). Based on this consideration, the potential involvement of PEA-3 and AP-1 in regulating PEG-Prom activity was evaluated in breast cancer cell lines. PEG-Prom activity was ≈5- to 20-fold higher in breast cancer cells than in 184A1 or 184AA2 cells. Mutation in the PEA-3 site reduced promoter activity by ≈50% in all cell types. Mutation in the AP-1 site also reduced promoter activity by ≈50%, whereas mutation in both the PEA-3 and AP-1 sites reduced activity by ≈75% in all of the cell lines. To further strengthen the role of PEA-3 and AP-1 in regulating PEG-Prom activity, the relative abundance of these proteins was analyzed in breast cancer cell lines and 184A1 and 184AA2 cells. Elevated expression levels of PEA-3 and c-JUN correlated with PEG-Prom activity in the different breast cancer cell lines, with highest activity in MCF-7 and T47D cells that contain the greatest elevation in PEA-3 as well as elevations in c-JUN. These observations provide a mechanistic explanation for the cancer cell-selective activity of the PEG-Prom in breast cancer cells.
Fig. 6.
PEA-3 and AP-1 confer cancer-cell selective activity of the PEG-Prom. (A) The various cell types were transfected with the indicated plasmids and a β-galactosidase expression plasmid, and luciferase and β-galactosidase activities were measured 48 h after transfection. The luciferase activity was normalized by β-galactosidase activity. The data represent mean ± SD. (B) The expressions of PEA-3, c-JUN, and EF1-α proteins were analyzed by Western blot in the indicated cells
Discussion
In principle, viral-based gene therapies should be effective options in treating neoplastic diseases. However, in reality, these approaches have not proven as successful as originally intended or envisioned. We have currently addressed these problems and endeavored to produce a viral vector that would obviate major gene therapy hurdles of cancer-specific targeting and poor tumor distribution. To achieve these objectives, we reasoned that a dual therapeutic approach resulting in cancer-specific virus replication with simultaneous production of a potent antitumor agent with profound antitumor bystander activity would culminate in an efficacious reagent for treating both primary and distant tumors. We presently describe such a virus, Ad.PEG-E1A-mda-7, that selectively replicates in tumor cells and simultaneously produces the antitumor cytokine mda-7/IL-24. When administered to human breast tumor xenografts established in one flank of a nude mouse, potent antitumor activity is evident, and remarkably this effect is also manifested in tumors established on the opposite flank of these animals. Moreover, this approach results in a complete eradication of tumors in animals promoting a cure of both primary and distant tumors.
Why are gene therapy approaches using a CRCA not as effective in treating cancer as anticipated? This result may occur because simply having a CRCA that only replicates in cancer cells is not potent enough to treat distant tumors (metastases), and actually achieving a complete destruction of distant tumors might require an additional gene therapy component. We have examined this possibility and developed a bipartite CRCA that also expresses mda-7/IL-24, a cytokine gene cloned in our laboratory that displays broad-spectrum direct and bystander antitumor activity without affecting normal cells (23, 28, 30, 32). Replication-competent Ads were able to migrate to distant sites in an animal and replicate. However, although administration of a replication-competent Ad alone induced significant growth inhibition in primary tumors, no significant effect was apparent in distant tumors, indicating that at those new tumor sites replication of Ad may not be sufficiently robust to be bioactive. As such, an additional antitumor strategy combined with the replication-competent Ad would be required to provide therapeutic benefit. Administration of Ad.CMV-mda-7 or Ad.PEG-mda-7 completely eradicated the primary tumors and these Ads significantly inhibited the growth of the distant tumors, without eliciting a complete cure of these distant tumors. In contrast, the combination of Ad replication and mda-7/IL-24 expression resulting from Ad.CMV-E1A-mda-7 and Ad.PEG-E1A-mda-7 induced complete purging of both the primary and distant tumors. The effectiveness of both Ad.CMV-E1A-mda-7 and Ad.PEG-E1A-mda-7 further strengthens the importance of using the PEG-Prom in this therapeutic strategy to protect normal cells from the adverse effects of Ad replication.
Tissue-specific and cancer cell-selective promoters can facilitate conditional Ad replication (40). The human telomerase promoter has been used successfully for this purpose (41). For breast cancer, estrogen and hypoxia-responsive promoters have been used to drive the expression of E1A (42, 43). The advantage of using the PEG-Prom in these cancer contexts is its apparent ubiquitous cancer specificity. PEA-3 and AP-1 transactivation and subsequently PEG-Prom activity are positively regulated by the ras-dependent signaling cascade (18, 19, 44). Because activation of the ras pathway is a frequent event in diverse cancers, including breast cancer, the ability of the PEG-Prom to drive transgene expression in these cancers will be robust and specific (45, 46). Additionally, PEA-3 and AP-1 are frequently overexpressed in a wide spectrum of cancers thereby promoting apparent universal cancer-specific activity of the PEG-Prom. These findings suggest that in addition to successfully treating breast cancer, Ad.PEG-E1A-mda-7 may also provide tangible benefit to an expanded patient population with additional types of cancer.
How does mda-7/IL-24 exert its potent inhibitory effects on distant tumors? Unlike a classical cytokine that acts by binding to its receptors and activating the Janus Kinase-Signal Transducer and Activator of Transcription (JAK-STAT) pathway, an intracellular mode of action, especially its localization in the endoplasmic reticulum, has been shown to be a primary mechanism of mda-7/IL-24-induced apoptosis when this gene is administered by Ad infection, plasmid transfection, or by means of a GST-MDA-7 fusion protein (47–49). However, mda-7/IL-24, as a secreted cytokine, also possesses potent bystander antitumor activity, which is exerted by its ability to interact with its cognate IL-20/IL-22 receptors although the signal transduction pathway(s) involved in this bystander activity is unclear (31, 32). Another potential mechanism underlying inhibition of cancer cell growth by mda-7/IL-24 in distant tumors in an in vivo context might be its activation of the immune system. mda-7/IL-24 expression is restricted to melanocytes, and those tissues associated with the immune system such as spleen, thymus, and peripheral blood mononuclear cells and its expression is induced in peripheral blood mononuclear cells upon treatment with phytohemagglutinin or LPS (23, 34, 50, 51). Treatment of peripheral blood mononuclear cells with purified recombinant MDA-7/IL-24 protein, results in the induction of IL-6, IFN-γ, TNF-α, IL-1β, IL-12, and granulocyte/macrophage colony-stimulating factor, all of which are potent immunomodulatory agents (25, 34). These secondary cytokines induced by mda-7/IL-24 might activate antigen-presenting cells to present tumor antigens, thereby triggering an antitumor immune response. Studies in a phase I clinical trial involving intratumoral injection of Ad.mda-7 (INGN 241) suggest that these in vitro effects are recapitulated in the context of patients, supporting the immune modulating properties of this cytokine (25). Although current studies were performed in athymic nude mice that are immunocompromised, these mice still have a spleen and a liver and display potent natural killer cell activity (52). Based on an intact immune system, it is hypothesized that Ad.PEG-E1A-mda-7 would prove even more effective in an immunocompetent animal. In this context, the balance between clearance of Ad by the immune system and the modulation of the immune system by mda-7/IL-24 represent major determinants in the antitumor potency of Ad.PEG-E1A-mda-7 in patients. The robust activity of this Ad suggests a need for only limited administration, which in principle will preclude the activation of the immune system promoting viral clearance. Additionally, the observation that neutralizing anti-Ad Abs do not inhibit replicating Ads indicates that Ad.PEG-E1A-mda-7 might be extremely effectual in inducing complete eradication of primary and metastatic cancers (9).
In summary, although impressive progress continues to be made in the diagnosis and therapy of organ-confined primary cancers, few if any approaches have proven universally successful in ameliorating the negative prognosis associated with cancer progression culminating in metastasis. We presently describe an innovative dual cancer-specific targeting therapeutic approach, which obviates two fundamental limitations of current gene therapy strategies, namely confining gene expression uniquely in cancer cells and evoking a potent antitumor bystander effect, which offers promise for potentially promoting a cure for primary and metastatic cancer. Further studies are essential in established animal models of breast cancer that mimic the human disease followed by appropriate clinical trials to effectively translate this approach into a mainstream, viable therapy for primary and metastatic breast and other cancers.
Acknowledgments
This work is dedicated to Susan Goodman in her struggle to defeat breast cancer. She is an inspiration to us all. This work was supported in part by National Institutes of Health Grants CA035675, CA097318, CA098712, and P01 CA104177; Army Department of Defense Breast Cancer Fellowship Grant DAMD17-03-1-0290; the Samuel Waxman Cancer Research Foundation; and the Chernow Endowment. P.B.F. is the Michael and Stella Chernow Urological Cancer Research Scientist and a Samuel Waxman Cancer Research Foundation Investigator.
Author contributions: D.S. and P.B.F. designed research; D.S., Z.-z.S., N.V., and E.S.P. performed research; D.S. and P.G. contributed new reagents/analytical tools; D.S. and P.B.F. analyzed data; and D.S. and P.B.F. wrote the paper.
Abbreviations: CRCA, conditionally replication competent adenovirus; Ad, adenoviral.
© 2005 by The National Academy of Sciences of the USA
References
- 1.Hortobagyi, G. N. (1998) N. Engl. J. Med. 339, 974-984. [DOI] [PubMed] [Google Scholar]
- 2.El-Aneed, A. (2004) Eur. J. Pharmacol. 498, 1-8. [DOI] [PubMed] [Google Scholar]
- 3.Fang, B. & Roth, J. A. (2003) Curr. Opin. Mol. Ther. 5, 475-482. [PubMed] [Google Scholar]
- 4.Huber, C. H. & Wolfel, T. (2004) J. Cancer Res. Clin. Oncol. 130, 367-374. [DOI] [PubMed] [Google Scholar]
- 5.McCormick, F. (2001) Nat. Rev. Cancer 1, 130-141. [DOI] [PubMed] [Google Scholar]
- 6.Wu, Q., Moyana, T. & Xiang, J. (2001) Curr. Gene Ther. 1, 101-122. [DOI] [PubMed] [Google Scholar]
- 7.Haviv, Y. S. & Curiel, D. T. (2001) Adv. Drug Delivery Rev. 53, 135-154. [DOI] [PubMed] [Google Scholar]
- 8.Haviv, Y. S. & Curiel, D. T. (2003) Curr. Gene Ther. 3, 357-835. [DOI] [PubMed] [Google Scholar]
- 9.Tsai, V., Johnson, D. E., Rahman, A., Wen, S. F., LaFace, D., Philopena, J., Nery, J., Zepeda, M., Maneval, D. C., Demers, G. W., et al. (2004) Clin. Cancer Res. 10, 7199-7206. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff, J. R., Kirn, D. H., Williams, A., Heise, C., Horn, S., Muna, M., Ng, L., Nye, J. A., Sampson-Johannes, A., Fattaey, A., et al. (1996) Science 274, 373-376. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, L., Shen, A., Boyle, L., Kunich, J., Pandey, K., Lemmon, M., Hermiston, T., Giedlin, M., McCormick, F. & Fattaey, A. (2002) Cancer Cell 1, 325-337. [DOI] [PubMed] [Google Scholar]
- 12.Su, Z.-z., Sarkar, D., Emdad, L., Duigou, G. J., Young, C. S., Ware, J., Randolph, A., Valerie, K. & Fisher, P. B. (2005) Proc. Natl. Acad. Sci. USA 102, 1059-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su, Z.-z., Shi, Y. & Fisher, P. B. (1997) Proc. Natl. Acad. Sci. USA 94, 9125-9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su, Z.-z., Goldstein, N. I., Jiang, H., Wang, M. N., Duigou, G. J., Young, C. S. & Fisher, P. B. (1999) Proc. Natl. Acad. Sci. USA 96, 15115-15120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su, Z.-z., Emdad, L., Sarkar, D., Randolph, A., Valerie, K., Yacoub, A., Dent, P. & Fisher, P. B. (2005) Oncogene 24, 2247-2255. [DOI] [PubMed] [Google Scholar]
- 16.Su, Z.-z., Shi, Y. & Fisher, P. B. (2000) Oncogene 19, 3411-3421. [DOI] [PubMed] [Google Scholar]
- 17.Su, Z.-z., Shi, Y., Friedman, R., Qiao, L., McKinstry, R., Hinman, D., Dent, P. & Fisher, P. B. (2001) Nucleic Acids Res. 29, 1661-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Launoit, Y., Baert, J. L., Chotteau, A., Monte, D., Defossez, P. A., Coutte, L., Pelczar, H. & Leenders, F. (1997) Biochem. Mol. Med. 61, 127-135. [DOI] [PubMed] [Google Scholar]
- 19.Eferl, R. & Wagner, E. F. (2003) Nat. Rev. Cancer 3, 859-868. [DOI] [PubMed] [Google Scholar]
- 20.Jiang, H., Lin, J. J., Su, Z.-z., Goldstein, N. I. & Fisher, P. B. (1995) Oncogene 11, 2477-2486. [PubMed] [Google Scholar]
- 21.Jiang, H., Su, Z.-z, Lin, J. J., Goldstein, N. I., Young, C. S. & Fisher, P. B. (1996) Proc. Natl. Acad. Sci. USA 93, 9160-9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su, Z.-z., Madireddi, M. T., Lin, J. J., Young, C. S., Kitada, S., Reed, J. C., Goldstein, N. I. & Fisher, P. B. (1998) Proc. Natl. Acad. Sci. USA 95, 14400-14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher, P. B., Gopalkrishnan, R. V., Chada, S., Ramesh, R., Grimm, E. A., Rosenfeld, M. R., Curiel, D. T. & Dent, P. (2003) Cancer Biol. Ther. 2, S23-S37. [PubMed] [Google Scholar]
- 24.Sarkar, D., Su, Z.-z., Lebedeva, I. V., Sauane, M., Gopalkrishnan, R. V., Valerie, K., Dent, P. & Fisher, P. B. (2002) Proc. Natl. Acad. Sci. USA 99, 10054-10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong, A. W., Nemunaitis, J., Su, D., Zhang, Y., Cunningham, C., Senzer, N., Netto, G., Rich, D., Mhashilkar, A., Parker, K., et al. (2005) Mol. Ther. 11, 160-172. [DOI] [PubMed] [Google Scholar]
- 26.Lebedeva, I. V., Su, Z.-z., Chang, Y., Kitada, S., Reed, J. C. & Fisher, P. B. (2002) Oncogene 21, 708-718. [DOI] [PubMed] [Google Scholar]
- 27.Lebedeva, I. V., Su, Z.-z., Sarkar, D., Kitada, S., Dent, P., Waxman, S., Reed, J. C. & Fisher, P. B. (2003) Cancer Res. 63, 8138-8144. [PubMed] [Google Scholar]
- 28.Lebedeva, I. V., Sauane, M., Gopalkrishnan, R. V., Sarkar, D., Su, Z.-z., Gupta, P., Nemunaitis, J., Cunningham, C., Yacoub, A., Dent, P., et al. (2005) Mol. Ther. 11, 4-18. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham, C. C., Chada, S., Merritt, J. A., Tong, A., Senzer, N., Zhang, Y., Mhashilkar, A., Parker, K., Vukelja, S., Richards, D., et al. (2005) Mol. Ther. 11, 149-159. [DOI] [PubMed] [Google Scholar]
- 30.Su, Z.-z., Lebedeva, I. V., Gopalkrishnan, R. V., Goldstein, N. I., Stein, C. A., Reed, J. C., Dent, P. & Fisher, P. B. (2001) Proc. Natl. Acad. Sci. USA 98, 10332-10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramesh, R., Mhashilkar, A. M., Tanaka, F., Saito, Y., Branch, C. D., Sieger, K., Mumm, J. B., Stewart, A. L., Boquoi, A., Dumoutier, L., et al. (2003) Cancer Res. 63, 5105-5113. [PubMed] [Google Scholar]
- 32.Su, Z.-z., Emdad, L., Sauane, M., Lebedeva, I. V., Sarkar, D., Gupta, P., James, C. D., Randolph, A., Valerie, K., Walter, M. R., et al. (2005) Oncogene, in press. [DOI] [PubMed]
- 33.Saeki, T., Mhashilkar, A., Swanson, X., Zou-Yang, X. H., Sieger, K., Kawabe, S., Branch, C. D., Zumstein, L., Meyn, R. E., Roth, J. A., et al. (2002) Oncogene 21, 4558-4566. [DOI] [PubMed] [Google Scholar]
- 34.Caudell, E. G., Mumm, J. B., Poindexter, N., Ekmekcioglu, S., Mhashilkar, A. M., Yang, X. H., Retter, M. W., Hill, P., Chada, S. & Grimm, E. A. (2002) J. Immunol. 168, 6041-6046. [DOI] [PubMed] [Google Scholar]
- 35.Jemal, A., Murray, T., Ward, E., Samuels, A., Tiwari, R. C., Ghafoor, A., Feuer, E. J. & Thun, M. J. (2005) CA Cancer J. Clin. 55, 10-30. [DOI] [PubMed] [Google Scholar]
- 36.Danthinne, X. (2001) BioTechniques 30, 612- 6, 618-619. [DOI] [PubMed] [Google Scholar]
- 37.Holmes, M., Rosenberg, E. & Valerie, K. (2003) Methods in Molecular Biology (Humana, Totowa, NJ).
- 38.Madireddi, M. T., Su, Z.-z., Young, C. S., Goldstein, N. I. & Fisher, P. B. (2000) Adv. Exp. Med. Biol. 465, 239-261. [DOI] [PubMed] [Google Scholar]
- 39.Sartorius, C. A., Shen, T. & Horwitz, K. B. (2003) Breast Cancer Res. Treat. 79, 287-299. [DOI] [PubMed] [Google Scholar]
- 40.Saukkonen, K. & Hemminki, A. (2004) Expert Opin. Biol. Ther. 4, 683-696. [DOI] [PubMed] [Google Scholar]
- 41.Gu, J. & Fang, B. (2003) Cancer Biol. Ther. 2, S64-S70. [PubMed] [Google Scholar]
- 42.Hernandez-Alcoceba, R., Pihalja, M., Wicha, M. S. & Clarke, M. F. (2000) Hum. Gene. Ther. 11, 2009-2024. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez-Alcoceba, R., Pihalja, M., Qian, D. & Clarke, M. F. (2002) Hum. Gene Ther. 13, 1737-1750. [DOI] [PubMed] [Google Scholar]
- 44.Park, J. S., Qiao, L., Su, Z.-z., Hinman, D., Willoughby, K., McKinstry, R., Yacoub, A., Duigou, G. J., Young, C. S., Grant, S., et al. (2001) Oncogene 20, 3266-3280. [DOI] [PubMed] [Google Scholar]
- 45.Adjei, A. A. (2001) J. Natl. Cancer Inst. 93, 1062-1074. [DOI] [PubMed] [Google Scholar]
- 46.von Lintig, F. C., Dreilinger, A. D., Varki, N. M., Wallace, A. M., Casteel, D. E. & Boss, G. R. (2000) Breast Cancer Res. Treat. 62, 51-62. [DOI] [PubMed] [Google Scholar]
- 47.Sauane, M., Gopalkrishnan, R. V., Choo, H. T., Gupta, P., Lebedeva, I. V., Yacoub, A., Dent, P. & Fisher, P. B. (2004) Oncogene 23, 7679-7690. [DOI] [PubMed] [Google Scholar]
- 48.Sauane, M., Gopalkrishnan, R. V., Lebedeva, I., Mei, M. X., Sarkar, D., Su, Z.-z., Kang, D. C., Dent, P., Pestka, S. & Fisher, P. B. (2003) J. Cell Physiol. 196, 334-345. [DOI] [PubMed] [Google Scholar]
- 49.Sauane, M., Lebedeva, I. V., Su, Z.-z., Choo, H. T., Randolph, A., Valerie, K., Dent, P., Gopalkrishnan, R. V. & Fisher, P. B. (2004) Cancer Res. 64, 2988-2993. [DOI] [PubMed] [Google Scholar]
- 50.Huang, E. Y., Madireddi, M. T., Gopalkrishnan, R. V., Leszczyniecka, M., Su, Z.-z., Lebedeva, I. V., Kang, D., Jiang, H., Lin, J. J., Alexandre, D., Chen, Y., et al. (2001) Oncogene 20, 7051-7063. [DOI] [PubMed] [Google Scholar]
- 51.Pestka, S., Krause, C. D., Sarkar, D., Walter, M. R., Shi, Y. & Fisher, P. B. (2004) Annu. Rev. Immunol. 22, 929-979. [DOI] [PubMed] [Google Scholar]
- 52.Smyth, M. J., Hayakawa, Y., Takeda, K. & Yagita, H. (2002) Nat. Rev. Cancer 2, 850-861. [DOI] [PubMed] [Google Scholar]