Abstract
Background
Cancer cells, which rely heavily on mitochondria for their energy demands and oncometabolites, have a high mitochondrial load, often associated with an aggressive, invasive, and metastatic phenotype. Mitochondrial ROS (mtROS), which play a causal role in cancer, represent the Achilles’ heel of cancer since excessive mtROS causes protein misfolding/aggregation, resulting in cell death via proteotoxic stress. Furthermore, the detailed underlying mechanism(s) of mitochondrial oxidative stress-induced cell death remain obscure.
Methods
Cell growth was estimated by MTT assay, clonogenic assay, live-cell imaging and flow cytometry. Intracellular ROS, mtROS, glutathione and antioxidant levels were studied by spectrophotometry. RNAseq and Western blotting were performed to elucidate the underlying mechanism(s). In vivo efficacy was evaluated using a syngeneic mouse model.
Results
We employed a mitochondria-targeted agent to disrupt the mitochondrial redox balance. Among tumors of different origins, such as lung, breast, prostate, bone, skin, cervical and liver, triple-negative breast cancer (TNBC) exhibited the highest sensitivity to mitochondrial oxidative stress. Compared with the parent compound, mitochondria-targeted agent showed 39-fold more effectiveness in killing TNBCs. We observed a possible correlation between the mitochondrial load in different cancer cell lines and their sensitivity to mitochondrial oxidative stress. Transcriptomic analysis revealed an enrichment of biological response to unfolded and/or misfolded proteins, which are regulated by two key proteases, Lon peptidase 1 (LONP1) and Caseinolytic protease P (CLPP), that control mitochondrial proteostasis. Bioinformatics analyses revealed enhanced expression and a strong positive correlation between these proteases in breast cancer patients, with highest expression observed in TNBC. Additionally, an early relapse was observed in breast cancer patients over-expressing both LONP1 and CLPP. Mitochondrial oxidative stress triggered a decrease in the native functional forms and an increase in the aggregated forms of LONP1 and CLPP, thereby disrupting mitochondrial proteostasis. Interestingly, no such changes were observed in normal cells. Mechanistically, excess mtROS induced proteotoxic stress and mitochondrial dysfunction, culminating in growth inhibition both in vitro and in vivo.
Conclusion
Our studies, for the first time, show that the mitochondrial load and induction of mtROS for concomitant inhibition of LONP1 and CLPP to induce proteotoxic stress, could be novel therapeutic targets for cancer.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-025-02127-w.
Keywords: Mitochondria, Oxidative stress, Proteotoxic stress, Unfolded protein response, Mitochondrial proteases
Introduction
Mitochondria, referred to as the powerhouse of the cell, are involved in several essential cellular functions. These include producing adenosine triphosphate (ATP) through oxidative phosphorylation (OXPHOS), regulating calcium levels, synthesizing iron-sulfur (Fe-S) clusters and heme, and carrying out Krebs cycle reactions [1–3]. Mitochondria also play a key role in regulating critical decisions pertaining to cell fate (survival versus programmed cell death) in the presence of various cellular stressors [4, 5]. Additionally, mitochondria are major sources of reactive oxygen species (ROS), such as superoxide radicals, which are by-products of premature reactions of electrons with oxygen at complexes I, II and III during electron transport chain (ETC) reactions [6]. Hence, mitochondria not only play a key role in physiological ROS-mediated redox signaling but are also most susceptible to oxidative stress and damage [6]. Therefore, maintaining redox homeostasis is crucial for both mitochondrial and overall cellular health.
Cancer cells heavily rely on mitochondrial metabolism for their energy requirements and oncometabolite biosynthesis [7, 8]. Several studies have confirmed the fundamental role of mitochondria in carcinogenesis, metastasis, and resistance to cancer therapies. For example, respiratory-deficient breast and osteosarcoma cell lines fail to exhibit anchorage-independent growth and demonstrate increased sensitivity to platinum-based cytotoxic drugs [9]. In another study, metastatic melanoma and breast adenocarcinoma cells devoid of mitochondrial DNA showed slower tumor growth in vivo [10]. Increased mitochondrial biogenesis was observed in endometrial cancer compared with normal endometrial tissues [11]. Additionally, an increase in mitochondrial mass and DNA content was observed as the disease progressed from benign to hyperplasia to carcinoma of the endometrium [12]. A subset of breast cancer cells with higher mitochondrial mass expressed cancer stem cell-specific markers and showed increased biological activity and resistance to paclitaxel treatment, emphasizing the key role of mitochondria in chemoresistance [13]. In an in vivo 4T1 mouse breast tumor model, the motility and invasiveness of circulating cancer cells depended on the enhanced OXPHOS, ATP generation, a higher mitochondrial oxygen consumption rate, and a higher mitochondrial DNA content compared with primary tumor cells [14]. Melanoma patients with tumors that had higher mitochondrial mass and increased expression of genes involved in mitobiogenesis had a worse prognosis and lesser overall survival than those with tumors bearing less mitochondrial mass and lower mitobiogenesis [15].
The aforementioned reports clearly highlight the critical role of both, mitochondrial mass and mitochondrial redox homeostasis, in cancer progression and metastasis. Hence, targeting the mitochondrial redox balance of cancer cells could serve as a potential anti-cancer strategy. Furthermore, the molecular mechanism(s) involved in cell death induced by mitochondrial oxidative stress are not fully understood. Moreover, since the mitochondrial load varies across different cancer types, it would be a prudent approach to ascertain if these differences impact the sensitivity of different cancers to alterations in the mitochondrial redox balance. To test these hypotheses, we specifically induced oxidative stress in mitochondria using a mitochondria-targeted agent (mitocurcumin; MC), which exploits the structural and functional differences in the mitochondria and specifically localizes to this organelle. These agents exploit the difference in the mitochondrial membrane potential between cancer cells (∼ − 220 mV) and normal cells (∼ − 140 mV), allowing them to preferentially accumulate inside the mitochondria of cancer cells (~ 100 to 1000-fold more) [16, 17]. Previous studies have demonstrated that MC is specifically taken up by the mitochondria and exhibits enhanced cytotoxic efficacy as compared to curcumin in cancer cells. They also showed that, treatment of MCF-7 cells with MC significantly reduced the phosphorylation of Akt and STAT3, while increasing ERK1/2 phosphorylation, and upregulated the pro-apoptotic protein BNIP3 through DNA methylation changes [18, 19].
In the present study, we evaluated the effect of mitochondrial oxidative stress on cancer cell lines from different tissue origins and, for the first time, showed that their varying sensitivities to mitochondrial oxidative stress-mediated cytotoxicity correlate with their mitochondrial load. We also identified the molecular mechanisms regulating mitochondrial oxidative stress-induced cell death. Our findings clearly demonstrated that the disruption of mitochondrial proteostasis, regulated by the mitochondrial unfolded protein response (UPRmt), plays a primary role in mitochondrial oxidative stress-induced cell death. Furthermore, we show that the mitochondrial ATP-dependent proteases, Caseinolytic protease P (CLPP) and Lon peptidase 1 (LONP1), are critical players in this UPRmt.
Materials and methods
Materials
MC was synthesized and characterized as described previously [18]. Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum (FBS), trypsin and antibiotic–antimycotic solution were procured from Himedia Laboratories Pvt. Lt., MTT reagent (3-(4,5-dimethyl-2-thiazolyl)−2,5-diphenyl-2H-tetrazolium bromide), dimethyl sulfoxide (DMSO), a caspase 3 assay Kit (CASP3F) and polyethylene glycol-superoxide dismutase (PEG-SOD) were purchased from Sigma‒Aldrich, USA. The IncuCyte® Annexin V Red dye was obtained from Sartorius, Germany. Tetraphenylethene maleimide (TPE-MI) dye (HY-143218) and Mitoquinone mesylate (MitoQ) were purchased from MedChemExpress, USA. CellTiter-Glo® assay kit was purchased from Promega, USA. All the other chemicals used in this study were procured from reputed local manufacturers.
Cell line and culture conditions
Normal mammary epithelial cell line (MCF-10A), breast cancer cell lines (MDA-MB-231, 4T1, and MCF-7), melanoma cell lines (B16 melanoma 4A5 and B16-F10), a lung cancer cell line (A549), a liver cancer cell line (HepG2), an osteosarcoma cell line (U-2 OS), a prostate cancer cell line (PC-3) and a cervical cancer cell line (HeLa) were purchased from the European Collection of Authenticated Cell Cultures and grown in the recommended cell culture media at 37 °C in a humidified atmosphere (5% CO2 and 95% air). The cells were maintained as a monolayer and routinely passaged three times a week when 80–90% confluent.
Cytotoxicity assay
In vitro cytotoxicity was evaluated using the MTT cell viability assay as described previously [20]. Briefly, exponentially growing cells (3 × 103 cells/well) were seeded in a 96-well culture plate, allowed to attach overnight, and treated with the indicated concentrations of MC or Curcumin (Cur) for 72 h. Then, MTT reagent (10 μl of 5 mg/ml per well) was added, followed by 4 h incubation. Afterwards, the solution from each well was aspirated, and the formazan crystals were solubilized by adding 100 µl of DMSO. Absorbance (Abs) was measured at 570 nm using a microplate reader (Synergy H1 Hybrid, USA). The percentage of viable cells was calculated using the formula: % Viability = [(Abs sample—Abs blank)/ (Abs control—Abs blank)] × 100. The half maximal inhibitory concentration (IC50) of MC for each cell line was determined using GraphPad Prism software.
In another experiment, cells were pretreated with 1 mM dithiothreitol (DTT)/ 5 mM glutathione (GSH)/ 50 U/ml PEG-SOD or 1.25 nM MitoQ for 2 h and then treated with MC for 72 h to estimate cytotoxicity.
Estimation of mitochondrial mass
Measurement of the mitochondrial mass in MDA-MB-231, HeLa and A549 cells was performed using two different methods, as described previously [21, 22]. Briefly, equal numbers of MDA-MB-231, HeLa and A549 cells were harvested, washed with 1X PBS and stained with the cell permeable MitoTracker Red CMXRos dye (MTR) (Invitrogen, USA), which stains active mitochondria in live cells and emits fluorescence. This fluorescence was measured spectrophotometrically at λex/em = 560/599 nm. The second method involved the isolation of high-purity mitochondrial fractions from equal numbers of MDA-MB-231, A549 and HeLa cells via differential centrifugation using the Qproteome Mitochondria isolation kit according to the manufacturer’s instructions (Qiagen, Germany). Mitochondrial mass was measured in terms of the amount of mitochondrial proteins in the isolated mitochondrial fraction.
Clonogenic assay
As described earlier [19], exponentially growing cells were seeded in 6-well culture plates and allowed to adhere overnight, followed by MC treatment. After 12 days of treatment, the colonies that formed were fixed and stained in methanol and crystal violet, respectively. The colonies (containing at least 50 cells) were manually counted.
Cell cycle analysis by propidium iodide (PI) assay
Exponentially growing cells were treated with either vehicle control or different concentrations of MC for 24 or 72 h. The cells were harvested and stained with PI solution [1X PBS solution containing RNase, sodium citrate (1 mg/ml), Triton X-100 (0.1%), and PI (50 μg/ml)] and incubated overnight at 4 °C. A total of 20,000 cells were acquired using a flow cytometer (BD FACS Melody, USA), and the proportion of cells in different phases of the cell cycle was enumerated by analysis in FlowJo software [23].
Mitochondrial membrane potential (MMP) estimation
The effect of mitocurcumin on MMP was studied using a lipophilic cationic dye JC-10, which accumulates in healthy mitochondria with a normal MMP as reversible aggregates emitting red fluorescence (λ ex/em = 540/590 nm). However, a decrease in or loss of the MMP results in the release of JC-10 from the mitochondria to the cytosol, where it exists in the monomeric form and emits green fluorescence (λex/em = 490/525 nm). Thus, the JC-10 red/green fluorescence ratio directly reflects the MMP status [24, 25]. MDA-MB-231 cells (104 cells/ well in a 96-well culture plate) were treated with different MC concentrations for 24 h, followed by JC-10 dye staining (37 °C for 30 min) and measurement of red and green fluorescence.
Determination of ATP levels
MDA-MB-231 cells and MCF-10A cells (10,000 cells/well) were treated with different doses of MC (0, 0.312, 0.625, 1.25, 2.5, 5, 10 µM) for 24 h. ATP levels were determined using CellTiter-Glo® assay kit (Promega, USA) according to the manufacturer’s protocol.
Measurement of caspase-3 enzyme activity
Caspase-3 activity was determined using a fluorometric caspase-3 assay kit (Sigma‒Aldrich, USA).
Real-time quantification of cell proliferation and apoptosis
Non-invasive, label-free, real-time monitoring of the growth and morphology of overnight-adhered cells (4000 cells/well in a 96-well culture plate) following treatment with MC was performed (IncuCyte S3 Live Cell Imaging System, Sartorius, Germany). To assess apoptosis induction, MC-treated cells were incubated with IncuCyte® Annexin V Red Dye for apoptosis (Sartorius, Germany) and imaged every 2 h to quantify the number of cells undergoing apoptosis using the IncuCyte S3 analysis software [23].
Estimation of intracellular ROS
MDA-MB-231 cells were treated with MC (0.625, 1.25, 2.5 or 5 µM) for 1 or 2 h, followed by staining with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) for 20 min and the fluorescence was measured (λex/em = 485/535 nm) [19, 26].
Estimation of mitochondrial ROS
Cells were treated with varying concentrations of MC (0.625, 1.25, 2.5 or 5 µM) for 1 or 2 h, followed by staining with MitoSOX Red, a mitochondria-targeted, superoxide-specific fluorogenic dye (20 min), and fluorescence was measured (λ ex/em = 510/580 nm) [27].
Measurement of glutathione levels
Intracellular glutathione (GSH + GSSG) levels were assessed using a Glutathione assay kit (Sigma‒Aldrich, USA). Cells were treated with different concentrations of MC for 24 h, harvested, and deproteinized with 5% 5-sulfosalicylic acid solution, and the total glutathione levels in the samples were estimated from the standard GSH plot and normalized by protein concentration for each group.
Measurement of total antioxidant capacity (TAC)
Cells were treated with MC (0.625 to 5 µM) for 24 h, and the levels of small molecule antioxidants (SMA) and TAC (SMA + protein antioxidants) were estimated in the cell lysates using the Total Antioxidant Capacity assay kit (Sigma‒Aldrich, USA).
RNA isolation, library preparation and sequencing
For transcriptome analysis, MDA-MB-231 cells were treated with 5 µM MC for 9 h. RNA extraction was carried out using TRIzol method. The concentration of the isolated RNA was assessed using Qubit 4 fluorometer. The integrity of the isolated RNA was analyzed on an Agilent Tapestation 4200 (Agilent, CA, USA) as per the manufacturer’s protocol. Samples with RNA integrity number (RIN) values > 9 were subjected to library preparation and deep sequencing. The RNA sequencing libraries were prepared with an Illumina-compatible kit, the KAPA mRNA Hyper Prep Kit from Roche (#KK8541). mRNA capture was performed using Oligo(dT) beads (#KK8441) followed by fragmentation at 85 °C for 5 min. After fragmentation, first strand cDNA was synthesized using random primers. The cDNA was then converted to double-stranded form, followed by A-tailing and adapter ligation. The ligated library was amplified using limited PCR cycles (11). The amplified library was purified using KAPA Pure beads. The obtained library was verified using Agilent 4200 Tapestation and quantified using Qubit 4 fluorometer. The qualified libraries were sequenced using NovaSeq X Plus (2 × 150 bp PE chemistry) to generate ~ 30 million reads (~ 4.5 GB/sample) with Q30 > 90%. The raw data generated from the sequencer is in the FASTQ format which was loaded into StrandNGS (version 4.0) for all bioinformatics analysis. After removing adapters, only high-quality reads (average base quality greater than 37) were used for further analysis and mapped to the reference genome, hg38. Quantification was performed by counting the number of raw reads that aligned to a particular gene and then normalized using TMM. Differential gene expression analysis (DGEA) was performed by running the edgeR (version 3.18.1) function. Genes were considered significantly differentially expressed when log2 fold change was > + 1 or < −1 and adjusted p-value was less than 0.05. Volcano plot was generated for the differentially expressed gene list using their normalized expression levels. Gene Ontology (GO) enrichment analysis was carried out with the human background database and a false discovery rate (FDR) cutoff of 0.05 using ShinyGO gene-set enrichment tool (version 0.80).
Gene expression data analysis of LONP1 & CLPP in breast cancer patients & cell lines
Data from the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) database was obtained via the cBioPortal (http://www.cbioportal.org/). TCGA RNA-seq data was retrieved from GDC data portal using R, followed by processing and normalization as described previously [28]. Gene expression data for correlation calculation between LONP1 and CLPP was downloaded from UALCAN cancer database of Dana-Farber Cancer Institute [29, 30]. Gene expression data for different human cancer cell lines and data for genetic dependencies based on RNAi screening was accessed from DepMap portal (https://depmap.org/portal) [31]. Affymetrix Human U133a GeneChips microarray data for time to relapse event in lymph node negative breast cancer patients was obtained from Genbank GEO database GSE2034 [32]. To determine optimal cut-off for categorization into high vs low gene expression category cut-off finder was used. Survival analysis between high and low sub-groups was conducted following quantile normalization of gene expression data. Kaplan–Meier survival curves representing relapse time in months were plotted using GraphPad prism 9.0.
Measurement of total cellular unfolded protein levels
The amount of unfolded proteins was estimated by using TPE-MI dye as described previously [33]. TPE-MI dye emits fluorescence only upon reaction with cysteine thiol residues, which are typically buried in the core of properly folded globular proteins but are exposed in unfolded proteins [33]. Briefly, the cells were treated with MC for 8 h, followed by staining with 50 µM TPE-MI, and TPE fluorescence was measured (λex/em = 350/470 nm).
Western blotting
Western blotting was performed as described previously [27]. Briefly, cells were treated with MC or curcumin, and the cell lysates were subjected to SDS‒PAGE. The separated protein bands were transferred to nitrocellulose membranes, and the membranes were blocked with 5% milk and probed with primary antibodies against CLPP, LONP1, Caseinolytic mitochondrial matrix peptidase chaperone subunit X (ClpX), heat shock protein 60 (HSP60) or activation transcription factor 5 (ATF5) (1: 1000) (Abcam, Cambridge, UK) overnight at 4 °C. Human β-actin antibody (1: 10,000) (Sigma‒Aldrich, USA) served as a loading control. The blots were then washed with Tris-buffered saline with Tween 20 (TBST), incubated with IR dye-conjugated secondary antibodies (1: 10,000) for 90 min, washed, and visualized using LI-COR Odyssey FC imaging system (LI-COR Biosciences, USA). The bands were quantified using ImageJ software.
In another experiment, MDA-MB-231 cells were pretreated with the following antioxidants: 1 mM DTT/ 10 mM GSH/ 5 mM N-acetyl L-cysteine (NAC)/ 80 U/ml PEG-SOD or mitochondria-targeted antioxidant (MitoQ; 25 nM), for 2 h, followed by treatment with MC for 24 h. In another experiment, detergent soluble and insoluble protein fractions of MC-treated MDA-MB-231 cells were prepared as described by Hu et al. (2019), with slight modifications [34]. Briefly, the cells were incubated in digitonin lysis buffer (50 mM Tris HCl pH 8, 150 mM NaCl, 1X protease inhibitor cocktail and 1% digitonin) for 1 h on ice, followed by centrifugation at 20,000 g (4 °C) for 20 min. The resulting supernatant was the digitonin-soluble protein fraction, while the pellet was washed and resuspended in digitonin lysis buffer containing 1% sodium dodecyl sulfate, then subjected to 3 rounds of incubation (100 °C for 3 min) followed by vortexing (20 s). The resulting mixture was then centrifuged (20,000 g at 4 °C for 20 min) to obtain the supernatant as the digitonin-insoluble protein fraction. The levels of CLPP and LONP1 proteins in the digitonin-soluble and insoluble fractions were then detected by western blotting.
In vivo studies
The anti-tumor efficacy of MC was evaluated in a syngeneic 4T1 murine breast tumor model as described previously [35]. Briefly, exponentially growing 4T1 breast cancer cells were harvested, washed with 1X PBS, and 7.5 × 104 cell suspension in 50 µl 1X PBS was subcutaneously injected into the dorsal region of 8-week-old female BALB/c mice (n = 14). After 10 days of 4T1 injection, visible palpable tumors with 50mm3 average tumor volumes were formed. The mice were randomly segregated into 2 groups (7 mice/group). Then, 100 µl of 2.5 mg/kg body weight of MC prepared in DMSO was administered to one group of mice via intraperitoneal (I.P.) route, whereas the control group was intraperitoneally administered equal volumes of DMSO. MC was administered thrice a week on alternate days, and mice weight and tumor dimensions, measured using a Vernier caliper, were recorded. The tumor volume was calculated by the formula: V = 0.5236 × L1 × (L2)2, where L1 and L2 are the long and short axes of the tumor dimension, respectively [35]. On day 24, tumor dimensions were measured, the mice were euthanized, and the tumors formed were excised, photographed, and weighed.
Statistical analysis
The statistical analyses were carried out using GraphPad Prism 8.0.1 software. All data points represent mean ± SEM of at least three biological replicates, and the number of independent experiments is specified in the figure legends. Statistically significant differences were evaluated using ordinary one-way analysis of variance (ANOVA) followed by the Tukey‒Kramer test for multiple comparisons or Student’s t test, ns: p > 0.05, *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001. Log-rank (Mantel-Cox) test was used for the comparison of survival curves. Pearson’s correlation coefficient was calculated using IBM SPSS version 21.
Results
Mitochondria-targeted curcumin effectively induces cytotoxicity in several cancer cell lines
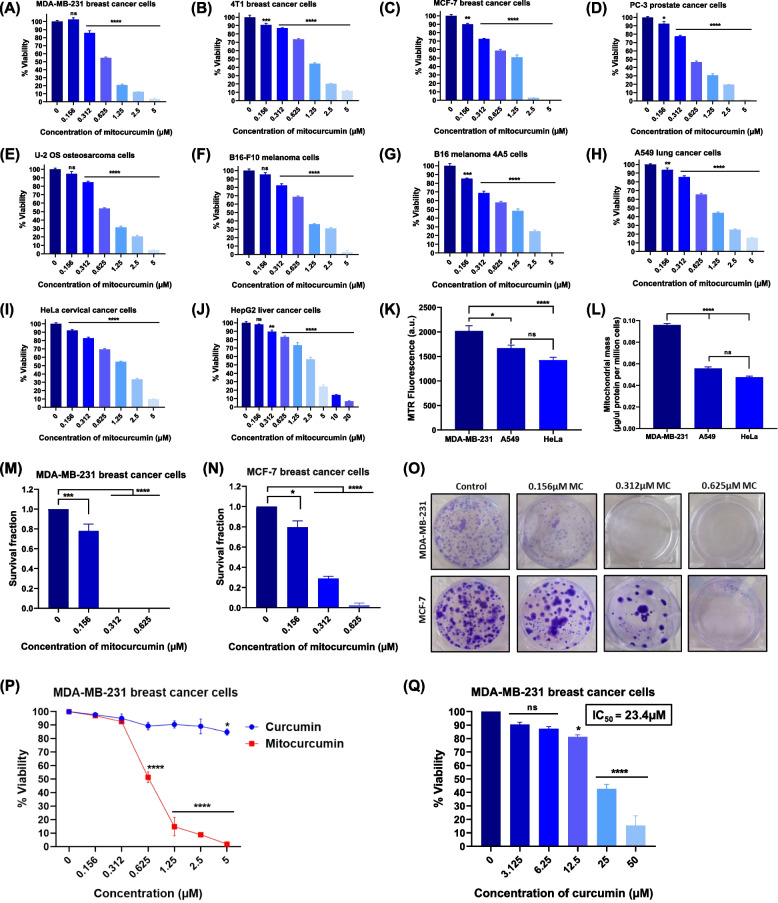
As shown in Fig. 1A-J, MC treatment significantly induced cytotoxicity and reduced the cell proliferative ability of MDA-MB-231, 4T1 and MCF-7 (breast), PC-3 (prostate), U-2 OS (bone), B16-F10 and B16 melanoma 4A5 (skin), A549 (lung), HeLa (Cervix) and HepG2 (liver) cancer cell lines in a concentration-dependent manner. The IC50 for MC, obtained from the dose‒response curve for these cancer cells ranged from 0.61 µM to 2.69 µM, indicating varying sensitivity of these cells toward MC (Table 1). MDA-MB-231 cells were found to be most sensitive to MC-induced toxicity, with the lowest IC50 value (0.608 µM), while the IC50 for A549 lung cancer cells was 1.161 µM (1.9-fold higher than that for MDA-MB-231), and for the cervical cancer cell line HeLa, the IC50 value was 1.684 µM (2.8-fold higher than that for MDA-MB-231). To understand the mechanism responsible for the difference in the sensitivity of these cell lines towards MC treatment, we estimated the mitochondrial mass of these cell lines, as MC is targeted to the mitochondria of cancer cells [18]. Interestingly, we found that MDA-MB-231 cells had significantly higher content of mitochondria compared with A549 and HeLa. This was shown by the higher fluorescence in MDA-MB-231 cells stained with the MitoTracker Red (MTR) dye (Fig. 1K), as well as higher mitochondrial protein content in the mitochondrial fraction from MDA-MB-231 cells compared to A549 and HeLa cells (Fig. 1L). Therefore, the higher sensitivity of MDA-MB-231 cells to MC-induced cell death might be due to their higher mitochondrial content, allowing more MC to accumulate in these cells compared to A549 and HeLa cells. Furthermore, clonogenic assay was performed to evaluate the effect of MC on the reproductive viability of cancer cells. Treatment with MC resulted in a significant decrease in survival fraction of MDA-MB-231 and MCF-7 cells (Figs. 1M-O), indicating a role of mitochondrial oxidative balance in regulating the reproductive viability of cancer cells. Given the aggressive and highly invasive nature of MDA-MB-231 cells and the greater sensitivity of these cells to mitochondrial oxidative stress-induced cytotoxicity (Table 1), further mechanistic studies were carried out in MDA-MB-231 cells.
Fig. 1.
Evaluation of the anti-cancer effects of MC in human and murine tumor cell lines. A MDA-MB-231 TNBCs (B) 4T1 breast cancer cells (C) MCF-7 breast cancer cells (D) PC-3 prostate cancer cells (E) U-2 OS osteosarcoma cells (F) B16-F10 melanoma cells (G) B16 melanoma 4A5 (H) A549 lung cancer cells (I) HeLa cervical cancer cells (J) HepG2 liver cancer cells were incubated with different doses of mitocurcumin (0.156–20 µM) for 72 h, and cell viability was estimated by MTT assay. Each data point represents the mean ± SEM from at least four replicates, and at least three such independent experiments were conducted. ns: p > 0.05, *p < 0.05; **p < 0.005; ***p < 0.001, ****p < 0.0001; as compared with vehicle-treated control. MDA-MB-231 cells possess higher mitochondrial mass than A549 and HeLa cancer cells. K Mitochondrial mass in terms of fluorescence from equal numbers of MDA-MB-231, A549 and HeLa cells stained with 100 nM active mitochondria-specific MTR dye for 30 min at 37 °C. L Mitochondrial mass in terms of amount of mitochondrial proteins/million cells in the high-purity mitochondrial fractions isolated from MDA-MB-231, A549 and HeLa cells. The data points represent mean ± SEM from at least four replicates and three such independent experiments were performed. ns p > 0.05, *p ≤ 0.05; ****p ≤ 0.0001. Survival fraction of (M) MDA-MB-231 and (N) MCF-7 cancer cells incubated with different doses of MC, as estimated by clonogenic assay. The data points represent mean ± SEM of three independent experiments. *p < 0.05; ***p < 0.001, ****p < 0.0001; compared with the control. O Representative images demonstrating the effect of MC on the clonogenic potential of the indicated cancer cell lines. P Effects of MC and curcumin on the viability of MDA-MB-231 cells, as determined by MTT assay post 72 h of treatment. Q Cell viability of MDA-MB-231 cells treated with curcumin for 72 h. The data points represent mean ± SEM of three independent experiments; ns: p > 0.05, *p < 0.05; ****p < 0.0001; compared with the control
Table 1.
IC50 values of mitocurcumin for different cancer cell lines post 72 h of treatment
| Sr. No. | Origin | Cancer cell line | IC50 value (µM) mean ± SEM |
|---|---|---|---|
| 1 | Breast | MDA-MB-231 | 0.608 ± 0.038 |
| 2 | Prostate | PC-3 | 0.696 ± 0.069 |
| 3 | Osteosarcoma | U-2 OS | 0.738 ± 0.045 |
| 4 | Breast | 4T1 | 1.016 ± 0.038 |
| 5 | Skin | B16-F10 | 1.122 ± 0.062 |
| 6 | Lung | A549 | 1.161 ± 0.130 |
| 7 | Breast | MCF-7 | 1.348 ± 0.046 |
| 8 | Skin | B16 melanoma 4A5 | 1.528 ± 0.069 |
| 9 | Uterus (Cervix) | HeLa | 1.684 ± 0.206 |
| 10 | Liver | HepG2 | 2.692 ± 0.112 |
To better understand how mitochondrial oxidative stress contributes to cancer cell death, we compared the cytotoxic efficacy of mitocurcumin with its parent compound, curcumin. Earlier studies showed that there was no accumulation of curcumin in the mitochondria of breast cancer cells [18]. Interestingly, curcumin did not cause cell death even at concentrations up to 2.5 µM (Fig. 1P). When cells were treated with higher concentrations of curcumin, the IC50 of curcumin for MDA-MB-231 cells was calculated to be 23.4 µM (Fig. 1Q), which was much higher than IC50 of MC (0.608 µM). Therefore, MC was about 39-fold more effective in killing MDA-MB-231 cells compared with curcumin.
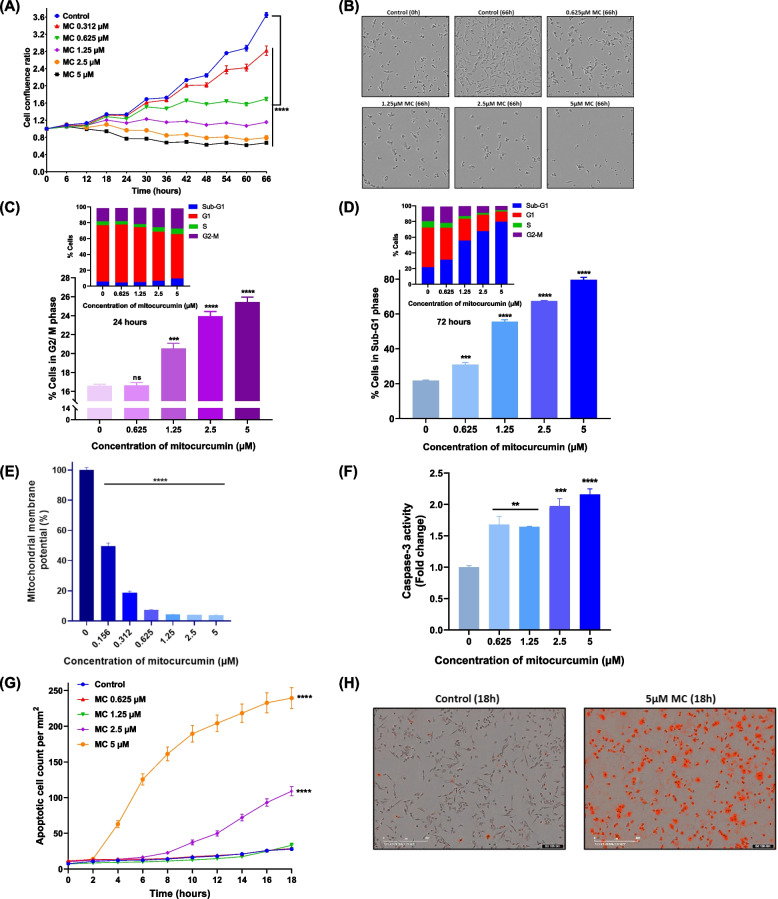
Mitocurcumin perturbs cell cycle progression and induces apoptosis in cancer cells
Real-time monitoring of growth dynamics and morphological changes induced by MC was performed using a live cell imaging system (Fig. 2A). We observed that treatment with MC affected the morphology and retarded the growth of MDA-MB-231 cells in a dose and time-dependent manner (Figs. 2A & B). Cell cycle analysis was carried out to evaluate the effects of MC on cell cycle progression and cell death. Treatment of MDA-MB-231 cells with MC for 24 h resulted in a concentration-dependent arrest of cells in the G2/M phase of the cell cycle (Fig. 2C & S8A). Prolonged treatment (72 h) with MC led to a dose-dependent increase in cell death (Fig. 2D & S8B). This increase in cell death prompted us to investigate changes in the MMP and caspase-3 activation, both of which are hallmarks of cell death signaling [36]. Treatment with MC led to a dose-dependent loss of MMP, indicating the induction of bioenergetic stress and cell death (Fig. 2E). Moreover, MC treatment significantly reduced ATP levels in MDA-MB-231 cells, whereas, treatment of MCF-10A cells with MC induced a small but significant decrease in ATP production (Fig. S9A & B). Additionally, there was a concentration- and time-dependent increase in caspase-3 enzyme activity in the MC-treated groups (Fig. 2F & S10A). These results indicate caspase-3-dependent pathway as one of the mechanisms of MC-induced death. This was further supported by studying the externalization of phosphatidylserine, which is a hallmark of apoptotic death and requires active caspases [37]. MC treatment resulted in a dose- and time-dependent increase in apoptotic cells, confirming the induction of apoptotic pathway (Figs. 2G & H).
Fig. 2.
Effect of mitochondrial oxidative stress on cell growth, cell cycle progression, MMP and apoptosis in MDA-MB-231. A Real-time monitoring of the effect of MC on the growth of MDA-MB-231 cells. The data points represent mean ± SEM from at least four replicates and three such independent experiments were performed. B Representative phase contrast images of control and MC-treated MDA-MB-231 cells at the indicated time points. C, D Effects of MC on cell cycle progression in MDA-MB-231 cells. The cells were treated with MC for 24 h (C) or 72 h (D) and stained with PI dye, and the percentage of cells in different phases of the cell cycle was estimated by flow cytometry. The data represent the mean percentage of cells ± SEM from three replicates, and two such independent experiments were conducted. E Effect of MC on the mitochondrial membrane potential of MDA-MB-231 cells. The cells were incubated with different concentrations of MC (0.156–5 µM) for 24 h, and the MPP was measured by staining with JC-10 dye. Data represents mean % ± SEM from four replicates and three such independent experiments were carried out. F Effect of MC on caspase 3 activity in MDA-MB-231 cells treated with MC for 18 h. The data represent the mean fold change in caspase 3 activity ± SEM from three replicates, and three such independent experiments were conducted. G Mitocurcumin induced apoptosis in MDA-MB-231 cells in a dose and time-dependent manner, as revealed by staining with IncuCyte Annexin V Red Reagent for apoptosis. The data represent the mean apoptotic cell count/mm2 ± SEM from three replicates and two such independent experiments were performed. H Representative images depicting apoptotic cells stained with IncuCyte Annexin V Red Reagent emitting red fluorescence upon MC treatment. ns: p > 0.05, *p < 0.05; **p < 0.005; ***p < 0.001, ****p < 0.0001; compared with the vehicle-treated control
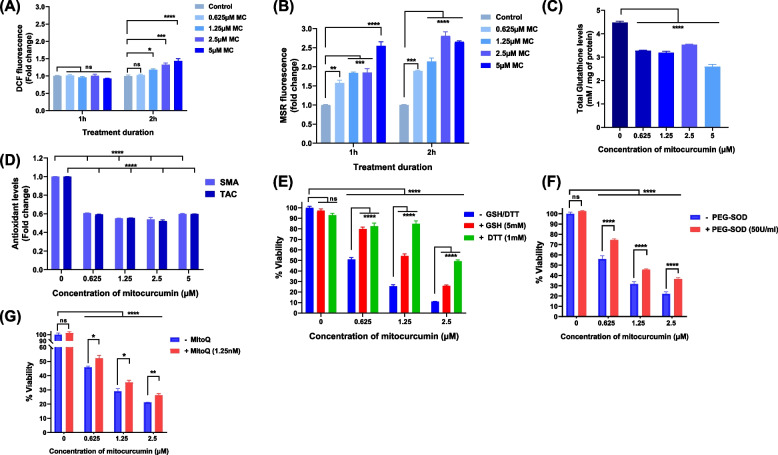
Role of oxidative stress in mitocurcumin-mediated cell death
To unravel the molecular mechanisms behind apoptosis triggered by MC, we studied the effect of MC on the cellular redox status. We measured the total intracellular ROS levels and observed a dose- and time-dependent increase in the intracellular ROS level after treatment with MC (Fig. 3A). Since mitocurcumin is known to accumulate in the mitochondria of cancer cells [18], the primary source of ROS in cells [38], we measured mitochondrial ROS (mtROS) levels in MC-treated cells. After MC treatment, we observed a significant increase in MitoSOX* (MSR) fluorescence intensity, indicating elevated mtROS levels (Fig. 3B and S10B). However, the fold increase in the levels of mtROS was notably higher than the increase in the cellular ROS levels. Moreover, MC treatment also reduced the levels of total intracellular glutathione (Fig. 3C), small molecule antioxidants and the total antioxidant capacity of the cancer cells (Fig. 3D). This increase in ROS levels, along with the decrease in antioxidant levels suggests that MC creates redox imbalance in cancer cells. To confirm the role of redox imbalance in MC-induced cell death, the cells were pre-treated with thiol antioxidants (GSH and DTT) or a non-thiol antioxidant (PEG-SOD) and a mitochondria-targeted antioxidant (MitoQ). The loss of viability was significantly reduced in the presence of antioxidants (Figs. 3E-G), confirming that oxidative stress plays a key role in the cytotoxic effects of MC in MDA-MB-231 cells.
Fig. 3.
Role of oxidative stress in mitocurcumin-induced cytotoxicity. A Fold change in intracellular ROS levels in MDA-MB-231 cells treated with MC for the indicated time intervals as estimated by H2DCF-DA staining. (B) Fold change in mitochondrial ROS levels in MDA-MB-231 cells incubated with different doses of MC for the indicated durations, as measured by MitoSOX red staining. C Total intracellular glutathione levels (mM/mg protein) in MDA-MB-231 cells following 24 h of treatment with MC. D Small molecule antioxidant (SMA) levels and total antioxidant capacity (TAC) in MDA-MB-231 cells treated with MC for 24 h. E-G Effects of antioxidants on the cytotoxic effects of MC. MDA-MB-231 cells were pre-incubated with (E) 5 mM glutathione (GSH) or 1 mM dithiothreitol (DTT) or (F) 50 U/ml polyethylene glycol-superoxide dismutase (PEG-SOD) or (G) 1.25 nM mitoquinone mesylate (MitoQ) for 2 h, followed by MC treatment for 72 h, and cell viability was assessed via the MTT assay. Each bar indicates the mean ± SEM from at least three replicates and 2 independent experiments were performed. ns: p > 0.05, *p < 0.05; **p < 0.005; ***p < 0.001, ****p < 0.0001; compared with the vehicle-treated control
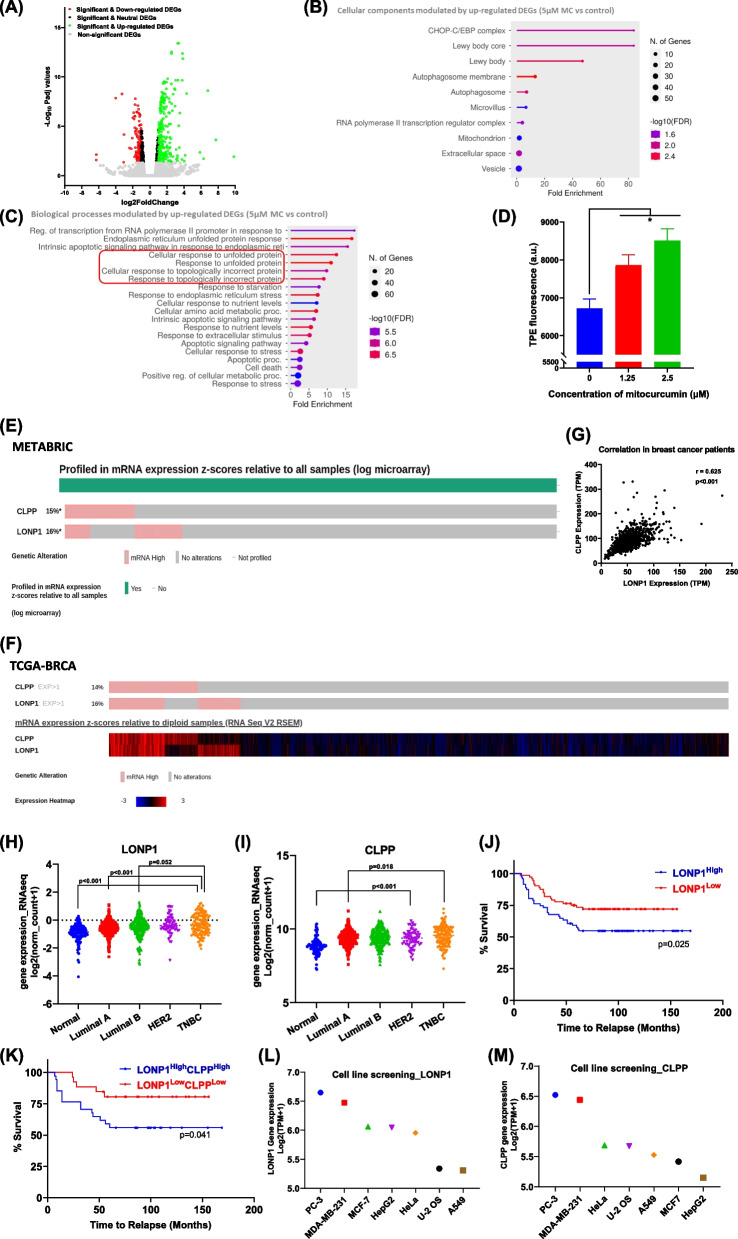
Oxidative stress induces proteostasis imbalance
To decipher the molecular mechanisms responsible for oxidative stress-induced cell death, we performed whole-transcriptome sequencing. Our analysis revealed 236 significant and up-regulated DEGs (padj value < 0.05 and log2 fold change > 1) and 108 significant and down-regulated DEGs (padj value < 0.05 and log2 fold change < −1) in MDA-MB-231 cells treated with MC compared to vehicle treated control (5 µM for 9 h) (Fig. 4A). GO enrichment analysis of the significantly upregulated DEGs showed notable enrichments, including 83.8-fold for the Lewy body core and CHOP-C/EBP complex, 47-fold for Lewy body and 1.85-fold for the mitochondrion (cellular components) (Fig. 4B and Additional file 1). The enriched biological processes included cellular response to glucose starvation (13.7-fold), regulation of DNA-templated transcription in response to stress (15.5-fold), cellular response to nutrient levels (7-sevenfold), cellular response to stress (2.6-fold) and the intrinsic apoptotic signaling pathway (6.3-fold) (Fig. 4C and Additional file 1). STRING enrichment analysis confirmed the enrichment of biological processes regulating cellular response to oxygen-containing compound and cellular response to nitrogen starvation (Additional file 1). These RNA-seq results support our earlier findings of oxidative stress, bioenergetic stress and the activation of the apoptotic pathway in MC-treated cells. Other biological processes, such as integrated stress response signaling (15.7-fold), the cellular response to unfolded proteins (12.5-fold) and topologically incorrect proteins (9.8-fold) were also up-regulated (Fig. 4C and Additional file 1). Together, these data suggest that MC treatment disrupts protein homeostasis by causing the accumulation of unfolded or misfolded proteins in cells.
Fig. 4.
Transcriptional profiling of MC-treated MDA-MB-231 cells. A Volcano plot illustrating changes in the gene expression pattern in MDA-MB-231 cells treated with 5 µM MC for 9 h compared with the vehicle-treated control. Each circle denotes a single gene. B Gene Ontology (GO) enrichment analysis indicating the cellular components controlled by the significantly up-regulated DEGs in MC-treated MDA-MB-231 cells versus control cells. C Graph illustrating biological processes GO terms modulated by up-regulated DEGs in MC-treated MDA-MB-231 cells. D Effect of MC on cellular protein homeostasis in MDA-MB-231 cells. Each bar indicates the mean ± SEM of fluorescence intensity of TPE-MI-stained cells from three replicates and 2 independent experiments were performed; Student’s t test, *p < 0.05 versus the control. LONP1 and CLPP are overexpressed in breast cancer patients and are associated with early relapse. E mRNA expression z-scores relative to all samples from METABRIC database (log microarray) obtained from cBioportal. F mRNA expression z-scores relative to diploid samples from TCGA-BRCA Firehose Legacy database with expression heatmap obtained from cBioportal. G Scatter plot representing the gene co-expression (transcripts per million) between LONP1 and CLPP with Pearson’s correlation coefficient and significance level. Scatter plot shows log2 transformed normalized gene expression from TCGA-BRCA RNAseq database for normal tissue (n = 114), Luminal A (n = 422), Luminal B (n = 194), HER2 (n = 67), and TNBC (n = 142) of LONP1 (H) and CLPP (I) respectively. Kaplan–Meier survival curve for time to relapse along with the Log-Rank (Mantel-Cox) test p-value for LONP1High vs LONP1Low (J) or LONP1HighCLPPHigh vs LONP1LowCLPPLow (K). Plot represents the log2 transformed mRNA abundance expressed in transcripts per million of LONP1 (L) and CLPP (M) for the indicated human cancer cell lines obtained from DepMap portal
To validate this, we performed an in vitro assay using TPE-MI dye to measure unfolded protein levels. A dose-dependent increase in unfolded proteins was observed in MC-treated cells confirming the induction of proteotoxic stress (Fig. 4D).
LONP1 and CLPP show strong co-expression and have prognostic value in breast cancer
Based on the biological processes related to protein folding and mitochondrial oxidative stress, we studied mitochondrial proteostasis, which is regulated by the UPRmt and is essential for mitochondrial functions and tumor survival [39]. CLPP and LONP1 are key ATP-dependent serine proteases that help degrade misfolded and damaged proteins in the mitochondrial matrix, maintaining protein quality [40]. Oncoprint analysis of breast cancer datasets reveal that mRNA levels of CLPP are elevated in 15% of patients in the METABRIC cohort (n = 2506) (Fig. 4E) and 14% in the TCGA-BRCA Firehose Legacy cohort (n = 1108) (Fig. 4F). Similarly, LONP1 mRNA levels were elevated in 16% of METABRIC patients (Fig. 4E) and 16% of TCGA-BRCA Firehose Legacy patients (Fig. 4F). The expression heatmap showing mRNA expression z-scores (−3 to + 3) relative to all samples among these patients indicate significant differences in gene expression among breast cancer patients (Fig. 4F). Interestingly, LONP1 and CLPP gene expression levels were strongly correlated, with a Pearson’s correlation coefficient of 0.63 (p < 0.001) in breast cancer patients (n = 1100) (Fig. 4G).
Further, previous studies have reported that LONP1 and CLPP are overexpressed in primary tumors compared to tissue normal [40]. Analysis of TCGA-BRCA dataset revealed that both LONP1 and CLPP had the highest expression in triple negative breast cancers followed by HER2-positive, Luminal A/B subtypes, and normal tissue (Figs. 4H and I). Kaplan–Meier survival analysis showed that breast cancer patients with high LONP1 expression (LONP1High) had a mean relapse interval of 104.88 ± 8.51 months, whereas patients with low LONP1 expression (LONP1Low) had a longer time to relapse (120.95 ± 6.71 months). Thus, LONP1High patients experience disease relapse 16 months earlier than LONP1Low patients (p = 0.025) (Fig. 4J). Given the strong correlation between LONP1 and CLPP gene expression, we compared the relapse interval between LONP1High/CLPPHigh breast cancer patients with LONP1Low/CLPPLow sub-group. It was observed that breast cancer patients, over-expressing both LONP1 and CLPP relapsed nearly 26 months earlier (Fig. 4K).
Next, we examined the expression of LONP1 and CLPP in human cell lines used in our study using the DepMap portal database. Interestingly, results of cell line screening performed using MC matched with expression status of LONP1 and CLPP in these cell lines. MDA-MB-231 showed significantly higher expression of both LONP1 and CLPP compared with rest of the cell lines, while PC-3 cells had similar mRNA levels. However, RNAi screen data indicated moderate dependency of MDA-MB-231 cells (D2 combined gene dependency score, −0.527) over PC-3 (−0.322) cells for LONP1, validating our selection of this cell line (Figs. 4L and M).
Targeting the mitochondrial redox balance selectively impairs mitochondrial protein quality control mechanisms in TNBCs
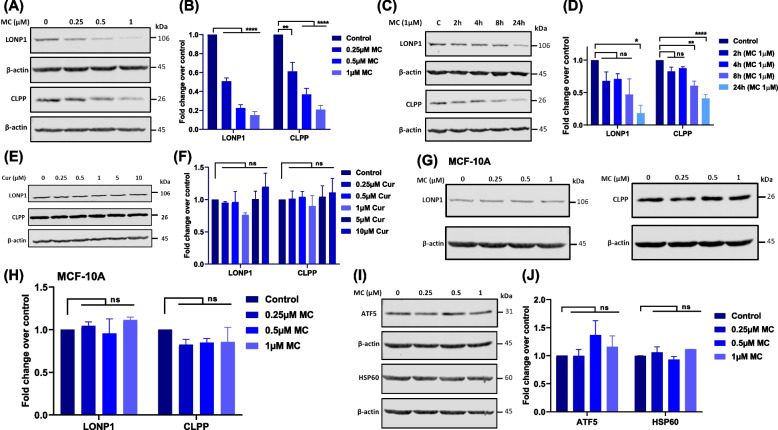
We examined the effects of MC on LONP1 and CLPP proteins and observed that increasing concentrations of MC, led to a decrease in the protein levels of LONP1 and CLPP (Figs. 5A & B). Time kinetic studies revealed a time-dependent decrease in LONP1 and CLPP protein levels with MC treatment (Figs. 5C, 5D, S11C & S11D). Further, we also studied the effect of MC on ClpX, which functions as a recognition protein for the proteolytic ClpXP complex. We observed that MC treatment led to a decrease in ClpX levels (Fig. S11A & B). Interestingly, treatment of cells with the parent compound curcumin, even at tenfold higher concentrations, did not lead to any change in the levels of LONP1 and CLPP (Figs. 5E & F). Additionally, there was no significant difference in CLPP or LONP1 protein levels in the non-tumorigenic MCF-10A mammary epithelial cell line after MC treatment (Figs. 5G & H). These findings suggest that mitochondrial oxidative stress, induced by MC, decreases the level of mitochondrial matrix proteases, LONP1 and CLPP proteins, exclusively in cancer cells but not in normal cells, contributing to proteotoxic stress.
Fig. 5.
Effects of mitocurcumin on the levels of proteins involved in the mitochondrial unfolded protein response. A, B Overnight-adhered MDA-MB-231 cells were treated with MC (0–1 µM) for 24 h, and LONP1 and CLPP protein levels were then analyzed by Western blotting. C, D MDA-MB-231 cells were treated with 1 µM MC for 2, 4, 8 and 24 h, followed by analysis of LONP1 and CLPP protein by Western blotting. E, F MDA-MB-231 cells were treated with the indicated concentrations of curcumin for 24 h, and CLPP and LONP1 protein levels were determined by Western blotting. G, H MCF-10A normal breast epithelial cells were incubated with MC (0–1 µM) for 24 h, and CLPP and LONP1 protein levels were determined by Western blotting. I, J Effects of MC on ATF5 and HSP60 protein expression in MDA-MB-231 cells exposed to MC (0–1 µM) for 24 h. The respective protein levels are presented as the fold change over the control (B, D, F, H, J), which were determined via densitometry of the immunoblots using ImageJ software. β-actin was used as the loading control in all of the above immunoblotting experiments. All data points represent the mean ± SEM of 3 independent experiments. ns: p > 0.05, *p < 0.05; **p < 0.005, ****p < 0.0001; compared with the control
ATF5, a transcription factor activated under conditions of mitochondrial stress and adaptive UPRmt, induces expression of cytoprotective genes to restore mitochondrial protein homeostasis [41]. ATF5 can induce the expression of mitochondrial chaperone such as HSP60 and the proteases, CLPP and LONP1, during mitochondrial stress [42]. Therefore, we checked the levels of ATF5 and HSP60 in MC-treated cells. However, we did not observe any change in ATF5 or HSP60 protein levels in MC-treated cancer cells (Figs. 5I & J).
Antioxidants abrogate mitocurcumin-mediated decrease in the levels of LONP1 and CLPP proteases
To further confirm the role of oxidative stress in the disruption of UPRmt, we studied the effects of different antioxidants (GSH, DTT, NAC, PEG-SOD and MitoQ) on the MC-induced decrease in the levels of LONP1 and CLPP proteins. Pre-treatment of cells with DTT, GSH, NAC or PEG-SOD restored LONP1 and CLPP protein levels in MC treated groups compared to the MC-alone treatment group (Figs. 6A-H). Additionally, treatment with the mitochondria-targeted antioxidant MitoQ also significantly restored the levels of both proteins, indicating the involvement of mitochondrial redox imbalance in their degradation (Figs. 6I & J). These results clearly indicate the involvement of oxidative stress in inducing proteotoxic stress in the mitochondria by decreasing the levels of LONP1 and CLPP proteins.
Fig. 6.
Effect of antioxidants on mitocurcumin-mediated decrease in CLPP and LONP1 levels. Representative western blots and graphs depicting LONP1 and CLPP protein levels in cell lysates obtained from MDA-MB-231 cells pre-treated with either (A, B) 10 mM GSH, (C, D) 1 mM DTT, (E, F) 5 mM NAC, (G, H) 80 U/ml PEG-SOD or (I, J) 25 nM MitoQ for 2 h followed by treatment with 1 µM MC for 24 h. β-actin was used as a loading control. Effect of MC on the folding status of the LONP1 and CLPP proteins. K Representative immunoblot and (L) bar graphs showing the relative levels of CLPP protein in digitonin-soluble and digitonin-insoluble fractions obtained from MDA-MB-231 cells treated with the indicated concentrations of MC for 24 h. M Representative immunoblot and (N) bar graphs displaying relative levels of LONP1 protein in digitonin soluble and insoluble fractions prepared from MDA-MB-231 cells treated with the indicated concentrations of MC for 24 h. β-actin was used as the loading control. The data points represent mean ± SEM of 3 independent experiments. ns p > 0.05, *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001
Mitochondrial redox imbalance promotes the aggregation of LONP1 and CLPP
Several mitochondrial proteins are sensitive to oxidative stress, which can disrupt their folding and function, causing them to accumulate as insoluble aggregates [43]. Given that oxidative stress decreases the levels of LONP1 and CLPP protein, we hypothesized that this decrease might be due to oxidative stress-induced misfolding and aggregation of these proteins. Misfolded proteins form large protein aggregates or inclusions that are often found to be insoluble in lysis buffers containing mild detergents, such as NP-40, digitonin or triton X-100, whereas the corresponding wild-type protein, which is not prone to aggregation and is in the native conformation, is soluble in these lysis buffers [44]. This differential detergent solubility was exploited to detect the accumulation of misfolded protein aggregates by SDS-PAGE and immunoblot analysis of the detergent insoluble fraction. We observed a dose-dependent reduction in the protein levels of LONP1 and CLPP in the digitonin-soluble fraction upon MC treatment, indicating a loss of their native folded forms (Figs. 6K-N). Interestingly, we observed a concomitant increase in the levels of these proteins in the digitonin-insoluble fraction obtained from MC-treated cells compared to control (Figs. 6K-N). This accumulation of LONP1 and CLPP in the detergent-insoluble fractions strongly indicates that oxidative stress is driving the formation of CLPP and LONP1 protein aggregates. Since LONP1 and CLPP selectively as well as cooperatively maintain mitochondrial proteostasis [40], their aggregation would lead to the accumulation of misfolded proteins, impairing mitochondrial function, contributing to proteotoxic stress, and ultimately cell death.
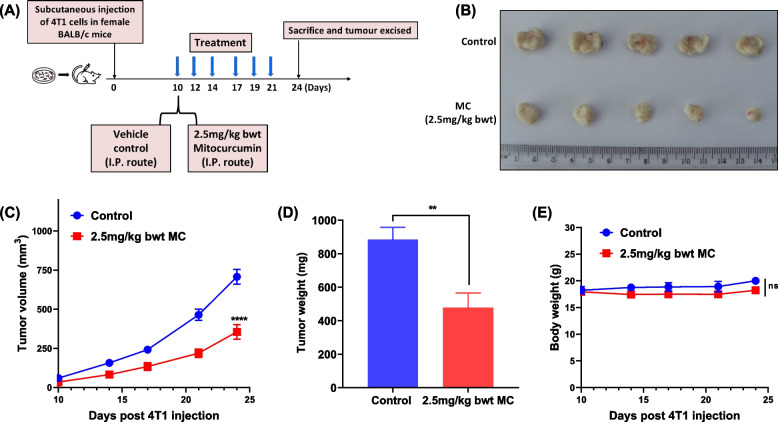
Mitocurcumin demonstrates potent in vivo anti-tumor efficacy
We also tested the in vivo anti-tumor effects of mitocurcumin in a syngeneic 4T1 murine breast tumor model, as shown in the schematic in Fig. 7A. Compared to the vehicle control, administering 2.5 mg/kg body weight of MC led to a significant reduction in tumor volume (Figs. 7B & C) and tumor weight (Fig. 7D). Importantly, MC administration displayed no apparent in vivo toxicity, as there was no significant change in body weight (Fig. 7E).
Fig. 7.
Mitocurcumin treatment inhibits the in vivo growth of 4T1 tumors in female BALB/c mice. (A) Schematic illustration of 4T1 breast tumor model induction and mitocurcumin treatment regimen. (B) Photographs of 4T1 tumors excised from 2.5 mg/kg bwt MC-administered mice and vehicle-administered control mice on day 24 post 4T1 SC injection. (C) Graph illustrating the mean tumor volumes (mm3) ± SEM of control and MC-administered mice at different time points. ****p ≤ 0.0001. (D) Tumor weights measured on day 24 following 4T1 injection (Student’s t test **p ≤ 0.01). (E) Mice body weights were recorded on the indicated days. ns: p > 0.05
Discussion
Cancer is a multifactorial disease and the second leading cause of death worldwide [45]. According to global cancer statistics for the year 2022, nearly 20 million new cases of cancer and closer to 10 million cases of cancer mortality were reported, with lung cancer being the most commonly diagnosed cancer, followed by breast cancer [46]. Despite significant progress in cancer diagnostics and treatment, challenges such as normal tissue toxicity, adverse side effects, drug resistance, metastasis and relapse remain major concerns [47]. Therefore, there is a pressing need to develop new, more effective, and targeted anti-cancer therapeutics.
In this context, mitochondria are closely linked to multiple hallmarks of cancer, such as cell death evasion, altered bioenergetics, genomic instability, tumor-stimulating inflammation, and metastasis. This makes them attractive targets for the development of new anti-cancer therapeutics [48]. Specifically, mtROS have been shown to play a direct role in tumorigenesis and cancer progression. Elevated mtROS can cause mutations in nuclear or mitochondrial DNA that promote neoplastic transformation and also accelerate the accumulation of additional mutations that lead to metastasis [49]. However, excessive mtROS can also oxidize proteins, causing their misfolding and aggregation, resulting in proteotoxic stress that can be detrimental to cell survival. In normal cells, proteotoxic stress in the mitochondria is managed by activating the UPRmt. In cancer cells, which are rapidly dividing and facing increased oxidative stress, the UPRmt plays a vital role in maintaining the integrity of the mitochondria and facilitating tumor growth. Interestingly, the UPRmt is often hyperactive, and its components are upregulated in various types of cancers [50]. Since mitochondria are the primary source of ROS in cells, they are particularly vulnerable to oxidative stress. Therefore, targeted disruption of the mitochondrial redox balance and the UPRmt to induce proteotoxic stress represents a promising avenue for cancer therapy [50].
In the present study, we employed a mitochondrial-targeted agent, mitocurcumin, which is known to disrupt the mitochondrial redox balance by increasing mtROS and decreasing mitochondrial glutathione levels [19]. Consistent with previous findings in lung and breast cancer cells [19], MC exhibited potent cytotoxic effects across various cancer types, including breast, cervical, lung, osteosarcoma, prostate, melanoma and liver cancer cell lines (Fig. 1). Notably, we observed that MDA-MB-231 breast cancer cells were most sensitive to mitocurcumin-induced cell death, with MC being 39-fold more effective than the parent compound curcumin, in inducing cell death in these cells (Fig. 1 and Table 1). Treatment with MC inhibited the clonogenic potential of MDA-MB-231 cells. It also led to cell cycle arrest in G2/M phase, loss of MMP, decrease in ATP levels, and caspase-3 activation, triggering mitochondria-dependent apoptosis (Figs. 1 and 2). MDA-MB-231 cells are categorized as the most aggressive basal triple-negative breast cancer cell line (ER-, PR-, HER2-), exhibiting features similar to the claudin-low TNBC subtype in vivo. They are least differentiated with low expression of E-cadherin, claudin-3, and claudinin-4/7. These also show greatest degree of stemness, a more mesenchymal-like appearance, higher invasiveness, and increased drug resistance and relapse potential [51–53]. Previous studies have highlighted the role of increased mitochondrial biogenesis and activity in cancer cells compared to normal cells, and in circulating cancer stem cells compared with primary tumor cells. These findings suggest a positive correlation between mitochondrial mass and cancer progression, aggressiveness, and metastasis [11–14]. However, the relationship between the mitochondrial load and the response of cancer cells from different tissues to mitochondria-targeted drugs has not been fully explored. In this study, we report the differential sensitivities of various cancer cells to mitochondrial oxidative stress. Our findings show that the most sensitive cell line, MDA-MB-231, has the highest mitochondrial mass compared with A549 and HeLa cells, which exhibited lower sensitivity to MC (Figs. 1K and L). These results align with a recent study by Thomas K J et al., which showed that A549 cells (lung adenocarcinoma) had higher mitochondrial mass than Calu1 cells (lung squamous cell carcinoma) [54]. Interestingly, both of these cancer cell lines had significantly higher mitochondrial mass than the lung epithelial cell line (NL20) [54]. This study is the first to establish a potential correlation between mitochondrial load in different cancer cell lines and their sensitivity to a mitochondria-targeted anti-cancer compound. Our findings suggest that tumors with higher mitochondrial load and reliance on mitochondria can be effectively targeted and eliminated by employing mitochondria-targeted analogs of anti-cancer drugs that induce oxidative stress. These drugs may accumulate more in cancer cells with high mitochondrial content, leading to increased cytotoxicity.
As mentioned earlier, cancer cells are generally under mild oxidative stress due to high metabolic rates, and maintaining mitochondrial integrity is vital for cancer progression and metastasis. To cope with oxidative stress and other cellular stresses, such as nutrient deprivation and genotoxic stress, cells have evolved a sophisticated proteostasis network that includes chaperones, the ubiquitin proteasome system, the endoplasmic reticulum-associated degradation (ERAD) pathway, aggrephagy and proteases. This network helps prevent the accumulation of toxic misfolded or aggregated proteins [55]. Transcriptomic analysis of MDA-MB-231 cells treated with MC revealed upregulation of cellular/biological processes related to the accumulation of unfolded and/or misfolded proteins and the induction of proteotoxic stress (Fig. 4). Since we targeted mitochondrial oxidative stress using MC, we hypothesized that proteins involved in maintaining mitochondrial proteostasis might be affected. In this direction, it is reported that CLPP and LONP1 are primary regulators of mitochondrial protein quality control. These proteases work selectively and cooperatively to maintain protein quality, regulating the activity of several proteins involved in the TCA cycle, OXPHOS, and metabolism of lipids and amino acid [40]. Recent studies have highlighted the crucial role of CLPP and LONP1 in cancer progression and mitochondrial homeostasis. The expression and activity of both CLPP and LONP1 have been shown to be substantially elevated in several human cancers, such as prostate, bladder, kidney, colon, thyroid, lung, uterine and breast cancer, compared to normal tissues [40]. A study by Luo et al. demonstrated a marked increase in CLPP expression in breast cancer tissues, which was associated with poor recurrence-free survival. Moreover, CLPP protein levels were also found to be elevated in a panel of breast cancer cell lines compared with normal mammary cell lines. Knockdown of CLPP inhibited cell proliferation, migration, and invasion and induced apoptosis in MDA-MB-231 and ZR-75–1 breast cancer cells [56]. Silencing of CLPP in acute myeloid leukemia cells also resulted in the loss of cell viability associated with the accumulation of misfolded succinate dehydrogenase subunit A of respiratory complex II, dysfunctional OXPHOS and disrupted mitochondrial metabolism [57]. Similarly, silencing LONP1 in melanoma cells caused decreased ATP levels, increased ROS levels and mitochondrial fragmentation, loss of MMP and impaired respiratory complex functions, resulting in mitochondrial dysfunction, cell cycle arrest and cellular senescence [58]. Both LONP1 and CLPP have been shown to protect cancer cells exposed to oxidative stress. LONP1 helps degrade oxidatively damaged proteins under oxidative stress [59, 60], and a recent study showed that LONP1 protects against p53-dependent apoptotic cell death under oxidative stress in oral squamous carcinoma cells [61]. Pryde et al., demonstrated that the CLPP‒LONP1 protease axis prevents the accumulation of toxic ROS in depolarized mitochondria by degrading the ROS-generating domain of respiratory complex I in cervical and neuroblastoma cell lines [62]. A recent study also found that simultaneous knockdown of CLPP and LONP1 in prostate cancer cells drastically reduced colony-forming ability of prostate cancer cells [40]. This also led to increased accumulation of misfolded and aggregated proteins, disrupted mitochondrial proteostasis, impaired mitochondrial bioenergetics, and elevated mtROS levels, autophagy markers, and UPRmt gene expression [40]. This dual knockdown increased cancer cells sensitivity to metabolic stress elicited by starvation and oxidative stress [40].
Our findings revealed that LONP1 and CLPP mRNA levels are elevated in almost 15% breast cancer patients, with these patients showing nearly a three-fold increase in gene expression compared to other patients. This defines a subset of breast cancer patients with LONP1/CLPP addiction. The strong correlation between LONP1 and CLPP expression underscores their interdependence and overexpression in aggressive breast cancer subtype, such as TNBC. This pattern suggests that LONP1/CLPP over-expression is associated with more aggressive breast cancers and poor prognosis. Kaplan–Meier survival analysis further supports the clinical significance of LONP1 overexpression. It shows that breast cancer patients with high levels of LONP1 or dual over-expression of LONP1/CLPP experience significantly shorter relapse-free intervals (16 to 26 months) compared to patients with low expression, indicating a higher risk of early disease-related mortality (Figs. 4E-M). Collectively, these results underscore the potential of LONP1 and CLPP as prognostic markers and therapeutic targets, suggesting the development of targeted therapies for LONP1 and CLPP that could improve patient outcomes.
In view of the aforementioned reports, one critical question that remains is the identity of the sensors/transducers that mediate oxidative stress-induced UPRmt and proteotoxic stress-induced cytotoxicity in cancer cells. Interestingly, we observed a time- and concentration-dependent decrease in the LONP1 and CLPP levels with MC treatment, which was a result of oxidative stress-induced misfolding of these proteins (Figs. 5 & 6). We also observed a decrease in the levels of ClpX in MC-treated cells (Fig. S11A & B). This indicates that mitochondrial oxidative damage can also decrease the levels of ClpX and lead to the inhibition of the ClpXP complex's ability to recognize and degrade misfolded proteins leading to proteotoxic stress.
We detected the formation of LONP1 and CLPP protein aggregates, leading to the accumulation of cellular misfolded proteins, misassembled proteins and inclusion bodies, which contribute to proteotoxic stress and cell death (Figs. 4–6). The ability of antioxidants to abrogate the effects of MC on LONP1/CLPP and cancer cell cytotoxicity further confirms the role of mtROS in inducing cell death via proteotoxic stress (Figs. 3E-G, 6A-J). To the best of our knowledge, no anti-cancer strategy currently targets both LONP1 and CLPP in cancer cells simultaneously. Interestingly, decrease in the levels of both LONP1 and CLPP levels by MC was observed only in MDA-MB-231 cells and not in the normal mammary cell line (MCF-10A) (Fig. 5), consistent with our earlier observations showing that MC had no cytotoxic effects in normal cells (MCF-10A, normal human lung cell lines and peripheral blood mononuclear cells [PBMNC]) [18, 63]. Moreover, the parent compound, curcumin, even at 10-fold higher concentrations, did not change the levels of LONP1 and CLPP (Fig. 5), likely due to its inability to reach the mitochondria, as shown previously [18]. Additionally, MC also showed significant anti-tumor activity in a mouse breast tumor model, indicating the in vivo potential of this approach (Fig. 7). Further in vivo experiments need to carried out using xenograft model to study the efficacy of MC in preventing the growth of human tumors and to confirm the role of LONP1 and CLPP in tumor progression. Additionally, while this study identifies a promising therapeutic strategy targeting LONP1 and CLPP, the long-term effects and potential resistance mechanisms associated with such treatments remain to be fully explored.
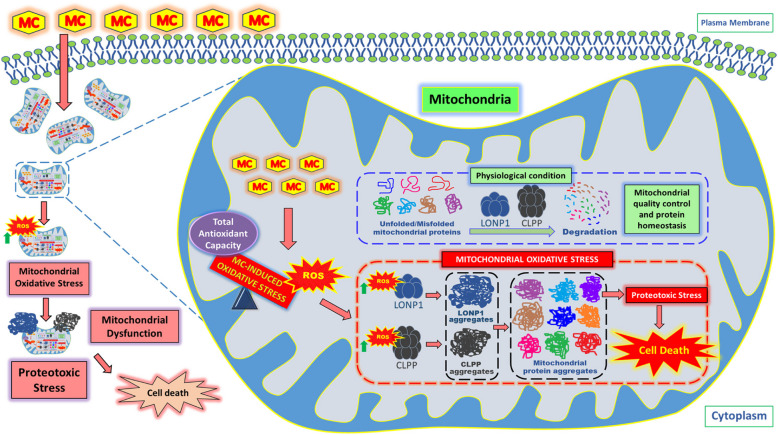
Conclusion
The UPRmt, which is crucial for maintaining mitochondrial integrity under oxidative stress, is also the Achilles’ heel of cancer cells and could therefore be targeted to develop novel anti-cancer therapeutics. Additionally, concomitant inhibition of LONP1 and CLPP, two key regulators of the UPRmt, through selective induction of mtROS offers a promising new therapeutic strategy for cancer (Fig. 8).
Fig. 8.
Mechanism of mitochondrial oxidative stress-induced cell death via induction of proteotoxic stress in human triple negative breast cancer cells
Supplementary Information
Additional file 1: S1. List of all DEGs. S2. List of significant DEGs (padj value < 0.05). S3. List of significant and up-regulated DEGs (padj value < 0.05 & log2 fold change > 1). S4. List of significant and down-regulated DEGs (padj value < 0.05 & log2 fold change < -1). S5. List of cellular components modulated by significantly up-regulated DEGs and S6. List of biological processes modulated by significantly up-regulated DEGs obtained via GO enrichment analyses using ShinyGO gene-set enrichment tool (version 0.80). S7. List of biological processes modulated by significantly up-regulated DEGs obtained from STRING enrichment analysis.
Additional file 2: Figure S8. Representative flow cytometric histograms of MDA-MB-231 cells treated with MC for (A) 24 h and (B) 72 h and stained with PI. Figure S9. ATP levels in (A) MDA-MB-231 cells and (B) MCF-10A cells treated with the indicated concentrations of MC for 24 h. Each data point represents the mean ± SEM from at least four replicates, and three such independent experiments were conducted. ns: p > 0.05, ****p < 0.0001; as compared with vehicle-treated control. Figure S10. (A) Caspase-3 activity in MDA-MB-231 cells treated with 5 μM MC for indicated time-points. Each data point represents the mean ± SEM from at least three replicates, and two such independent experiments were conducted. ns: p > 0.05, ****p < 0.0001; as compared with vehicle-treated control. (B) Representative images of MitoSOX Red-stained MDA-MB-231 cells treated with 2.5 µM MC for 2 h. Figure S11. (A, B) MDA-MB-231 cells were treated with 1 µM MC for 2, 4, 8 and 24 h, followed by ClpX protein expression analysis by western blotting. (C, D) MDA-MB-231 cells were treated with 5 µM or 10 µM MC for 2 h or 5 µM MC for 4 h, followed by LONP1 and CLPP protein expression analysis by western blotting. Data points represent the mean ± SEM of 3 independent experiments. ns: p > 0.05, *p < 0.05; **p < 0.005; compared with the control.
Acknowledgements
The authors would like to acknowledge the technical assistance provided by Ms Binita K Kumar, Mr Deepak Kathole and Mr. B A Naidu.
Abbreviations
- Abs
Absorbance
- ANOVA
Analysis of Variance
- ATF5
Activation Transcription Factor 5
- ATP
Adenosine Triphosphate
- CLPP
Caseinolytic protease P
- ClpX
Caseinolytic mitochondrial matrix peptidase chaperone subunit X
- Cur
Curcumin
- DEGs
Differentially expressed genes
- DGEA
Differential gene expression analysis
- DMEM
Dulbecco's Modified Eagle Medium
- DMSO
Dimethyl sulfoxide
- DTT
Dithiothreitol
- ETC
Electron Transport Chain
- FBS
Fetal Bovine Serum
- FDR
False discovery rate
- GO
Gene ontology
- GSH
Glutathione
- H2DCF-DA
2′,7′-Dichlorodihydrofluorescein diacetate
- HSP60
Heat shock protein 60
- IC50
Half maximal inhibitory concentration
- IP
Intraperitoneal
- LONP1
Lon peptidase 1
- MC
Mitocurcumin
- MitoQ
Mitoquinone mesylate
- MMP
Mitochondrial membrane potential
- MTR
MitoTracker Red CMXRos dye
- mtROS
Mitochondrial ROS
- NAC
N-acetyl L-cysteine
- OXPHOS
Oxidative phosphorylation
- PEG-SOD
Polyethylene glycol-Superoxide dismutase
- PI
Propidium Iodide
- ROS
Reactive oxygen species
- SMA
Small molecule antioxidants
- TAC
Total Antioxidant Capacity.
- TBST
Tris Buffered Saline with Tween 20
- TCA
Tri carboxylic acid
- TNBC
Triple negative breast cancer
- TPE-MI
Tetraphenylethene maleimide
- UPRmt
Mitochondrial unfolded protein response
Authors’ contributions
SRN: Methodology, Investigation, Formal analysis, Data curation, Visualization, Writing- Original draft preparation, Writing- Reviewing and Editing. RC: Conceptualization, Supervision, Investigation, Data curation, Methodology, Visualization, Writing- Original draft preparation, Writing- Reviewing and Editing. RSP: Investigation, Data curation. DS Supervision, Data curation, Writing- Reviewing and Editing. SKS: Conceptualization, Supervision, Writing- Reviewing and Editing. All authors read, reviewed and approved the final manuscript.
Funding
The financial support from the Department of Atomic Energy, Government of India, is kindly acknowledged.
Data availability
The RNA-seq data have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE276992 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE276992).
Declarations
Ethics approval and consent to participate
All mice experiments were conducted in accordance with the guidelines, regulations and approved protocols of the Institutional Animal Ethics Committee (IAEC) of Bhabha Atomic Research Centre, Mumbai (Project No: BAEC/10/2020).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Santosh K. Sandur, Email: sskumar@barc.gov.in
Rahul Checker, Email: rchecker@barc.gov.in.
References
- 1.Brand MD, Orr AL, Perevoshchikova IV, Quinlan CL. The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br J Dermatol. 2013;169(Suppl 2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vakifahmetoglu-Norberg H, Ouchida AT, Norberg E. The role of mitochondria in metabolism and cell death. Biochem Biophys Res Commun. 2017;482:426–31. [DOI] [PubMed] [Google Scholar]
- 3.Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol. 2018;20:745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen TT, Wei S, Nguyen TH, Jo Y, Zhang Y, Park W, Gariani K, Oh CM, Kim HH, Ha KT, et al. Mitochondria-associated programmed cell death as a therapeutic target for age-related disease. Exp Mol Med. 2023;55:1595–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21:85–100. [DOI] [PubMed] [Google Scholar]
- 6.Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163:560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2:e1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu XD, Shao SX, Jiang HP, Cao YW, Wang YH, Yang XC, Wang YL, Wang XS, Niu HT. Warburg effect or reverse Warburg effect? A review of cancer metabolism. Oncol Res Treat. 2015;38:117–22. [DOI] [PubMed] [Google Scholar]
- 9.Cavalli LR, Varella-Garcia M, Liang BC. Diminished tumorigenic phenotype after depletion of mitochondrial DNA. Cell Growth Differ. 1997;8:1189–98. [PubMed] [Google Scholar]
- 10.Tan AS, Baty JW, Dong LF, Bezawork-Geleta A, Endaya B, Goodwin J, Bajzikova M, Kovarova J, Peterka M, Yan B, et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 2015;21:81–94. [DOI] [PubMed] [Google Scholar]
- 11.Cormio A, Guerra F, Cormio G, Pesce V, Fracasso F, Loizzi V, Cantatore P, Selvaggi L, Gadaleta MN. The PGC-1alpha-dependent pathway of mitochondrial biogenesis is upregulated in type I endometrial cancer. Biochem Biophys Res Commun. 2009;390:1182–5. [DOI] [PubMed] [Google Scholar]
- 12.Cormio A, Guerra F, Cormio G, Pesce V, Fracasso F, Loizzi V, Resta L, Putignano G, Cantatore P, Selvaggi LE, Gadaleta MN. Mitochondrial DNA content and mass increase in progression from normal to hyperplastic to cancer endometrium. BMC Res Notes. 2012;5:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farnie G, Sotgia F, Lisanti MP. High mitochondrial mass identifies a sub-population of stem-like cancer cells that are chemo-resistant. Oncotarget. 2015;6:30472–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, de Carvalho FM, Damascena A, Domingos Chinen LT, Rocha RM, et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16(992–1003):1001–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang G, Frederick DT, Wu L, Wei Z, Krepler C, Srinivasan S, Chae YC, Xu X, Choi H, Dimwamwa E, et al. Targeting mitochondrial biogenesis to overcome drug resistance to MAPK inhibitors. J Clin Invest. 2016;126:1834–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zielonka J, Joseph J, Sikora A, Hardy M, Ouari O, Vasquez-Vivar J, Cheng G, Lopez M, Kalyanaraman B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem Rev. 2017;117:10043–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael D Forrest PD. Why cancer cells have a more hyperpolarised mitochondrial membrane potential and emergent prospects for therapy. bioRxiv. 2015:025197.
- 18.Reddy CA, Somepalli V, Golakoti T, Kanugula AK, Karnewar S, Rajendiran K, Vasagiri N, Prabhakar S, Kuppusamy P, Kotamraju S, Kutala VK. Mitochondrial-targeted curcuminoids: a strategy to enhance bioavailability and anticancer efficacy of curcumin. PLoS ONE. 2014;9:e89351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayakumar S, Patwardhan RS, Pal D, Singh B, Sharma D, Kutala VK, Sandur SK. Mitochondrial targeted curcumin exhibits anticancer effects through disruption of mitochondrial redox and modulation of TrxR2 activity. Free Radic Biol Med. 2017;113:530–8. [DOI] [PubMed] [Google Scholar]
- 20.Checker R, Pal D, Patwardhan RS, Basu B, Sharma D, Sandur SK. Modulation of Caspase-3 activity using a redox active vitamin K3 analogue, plumbagin, as a novel strategy for radioprotection. Free Radic Biol Med. 2019;143:560–72. [DOI] [PubMed] [Google Scholar]
- 21.Bashari MH, Fan F, Vallet S, Sattler M, Arn M, Luckner-Minden C, Schulze-Bergkamen H, Zörnig I, Marme F, Schneeweiss A, et al. Mcl-1 confers protection of Her2-positive breast cancer cells to hypoxia: therapeutic implications. Breast Cancer Res. 2016;18:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–24. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim MK, Nandha SR, Patil AS, Sathaye S, Degani MS, Kumar B, Checker R, Sharma D, Sandur SK. Mitochondria-targeted derivative of pterostilbene, a dietary phytoestrogen, exhibits superior cancer cell cytotoxicity via mitochondrial superoxide mediated induction of autophagy. Advances in Redox Research. 2023;8:100071. [Google Scholar]
- 24.Kamiloglu S, Sari G, Ozdal T, Capanoglu E. Guidelines for cell viability assays. Food Frontiers. 2020;1:332–49. [Google Scholar]
- 25.Gerencser AA, Chinopoulos C, Birket MJ, Jastroch M, Vitelli C, Nicholls DG, Brand MD. Quantitative measurement of mitochondrial membrane potential in cultured cells: calcium-induced de- and hyperpolarization of neuronal mitochondria. J Physiol. 2012;590:2845–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Checker R, Sharma D, Sandur SK, Subrahmanyam G, Krishnan S, Poduval TB, Sainis KB. Plumbagin inhibits proliferative and inflammatory responses of T cells independent of ROS generation but by modulating intracellular thiols. J Cell Biochem. 2010;110:1082–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Checker R, Bhilwade HN, Nandha SR, Patwardhan RS, Sharma D, Sandur SK. Withaferin A, a steroidal lactone, selectively protects normal lymphocytes against ionizing radiation induced apoptosis and genotoxicity via activation of ERK/Nrf-2/HO-1 axis. Toxicol Appl Pharmacol. 2023;461:116389. [DOI] [PubMed] [Google Scholar]
- 28.Network CGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia. 2017;19:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne U, et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia. 2022;25:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsherniak A, Vazquez F, Montgomery PG, Weir BA, Kryukov G, Cowley GS, Gill S, Harrington WF, Pantel S, Krill-Burger JM, et al. Defining a Cancer Dependency Map. Cell. 2017;170:564-576.e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–9. [DOI] [PubMed] [Google Scholar]
- 33.Chen MZ, Moily NS, Bridgford JL, Wood RJ, Radwan M, Smith TA, Song Z, Tang BZ, Tilley L, Xu X, et al. A thiol probe for measuring unfolded protein load and proteostasis in cells. Nat Commun. 2017;8:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu D, Sun X, Liao X, Zhang X, Zarabi S, Schimmer A, Hong Y, Ford C, Luo Y, Qi X. Alpha-synuclein suppresses mitochondrial protease ClpP to trigger mitochondrial oxidative damage and neurotoxicity. Acta Neuropathol. 2019;137:939–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tailor D, Going CC, Resendez A, Kumar V, Nambiar DK, Li Y, Dheeraj A, LaGory EL, Ghoochani A, Birk AM, et al. Novel Aza-podophyllotoxin derivative induces oxidative phosphorylation and cell death via AMPK activation in triple-negative breast cancer. Br J Cancer. 2021;124:604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng F, Liao M, Qin R, Zhu S, Peng C, Fu L, Chen Y, Han B. Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct Target Ther. 2022;7:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomes MT, Palasiewicz K, Gadiyar V, Lahey K, Calianese D, Birge RB, Ucker DS. Phosphatidylserine externalization by apoptotic cells is dispensable for specific recognition leading to innate apoptotic immune responses. J Biol Chem. 2022;298:102034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Povea-Cabello S, Brischigliaro M, Fernández-Vizarra E. Emerging mechanisms in the redox regulation of mitochondrial cytochrome c oxidase assembly and function. Biochem Soc Trans. 2024;52:873–85. [DOI] [PubMed] [Google Scholar]
- 39.Inigo JR, Chandra D. The mitochondrial unfolded protein response (UPR. J Hematol Oncol. 2022;15:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee YG, Kim HW, Nam Y, Shin KJ, Lee YJ, Park DH, Rhee HW, Seo JK, Chae YC. LONP1 and ClpP cooperatively regulate mitochondrial proteostasis for cancer cell survival. Oncogenesis. 2021;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiorese CJ, Schulz AM, Lin YF, Rosin N, Pellegrino MW, Haynes CM. The Transcription Factor ATF5 Mediates a Mammalian Mitochondrial UPR. Curr Biol. 2016;26:2037–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng P, Haynes CM. Mitochondrial dysfunction in cancer: Potential roles of ATF5 and the mitochondrial UPR. Semin Cancer Biol. 2017;47:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaus A, Sareen D. ALS Patient Stem Cells for Unveiling Disease Signatures of Motoneuron Susceptibility: Perspectives on the Deadly Mitochondria, ER Stress and Calcium Triad. Front Cell Neurosci. 2015;9:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lystad AH, Simonsen A. Assays to monitor aggrephagy. Methods. 2015;75:112–9. [DOI] [PubMed] [Google Scholar]
- 45.Marino P, Mininni M, Deiana G, Marino G, Divella R, Bochicchio I, Giuliano A, Lapadula S, Lettini AR, Sanseverino F. Healthy Lifestyle and Cancer Risk: Modifiable Risk Factors to Prevent Cancer. Nutrients. 2024;16(6):800. 10.3390/nu16060800. [DOI] [PMC free article] [PubMed]
- 46.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-63. [DOI] [PubMed]
- 47.Khan SU, Fatima K, Aisha S, Malik F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun Signal. 2024;22:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kenny TC, Manfredi G, Germain D. The Mitochondrial Unfolded Protein Response as a Non-Oncogene Addiction to Support Adaptation to Stress during Transformation in Cancer and Beyond. Front Oncol. 2017;7:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai X, Cheng H, Bai Z, Li J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J Cancer. 2017;8:3131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y, Zhang H, Merkher Y, Chen L, Liu N, Leonov S, Chen Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J Hematol Oncol. 2022;15:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas KJ, Jacobson MR. Defects in mitochondrial fission protein dynamin-related protein 1 are linked to apoptotic resistance and autophagy in a lung cancer model. PLoS ONE. 2012;7:e45319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brancolini C, Iuliano L. Proteotoxic Stress and Cell Death in Cancer Cells. Cancers. 2020;12(9):2385. 10.3390/cancers12092385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo J, Zeng B, Tao C, Lu M, Ren G. ClpP regulates breast cancer cell proliferation, invasion and apoptosis by modulating the Src/PI3K/Akt signaling pathway. PeerJ. 2020;8:e8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cole A, Wang Z, Coyaud E, Voisin V, Gronda M, Jitkova Y, Mattson R, Hurren R, Babovic S, Maclean N, et al. Inhibition of the Mitochondrial Protease ClpP as a Therapeutic Strategy for Human Acute Myeloid Leukemia. Cancer Cell. 2015;27:864–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quirós PM, Español Y, Acín-Pérez R, Rodríguez F, Bárcena C, Watanabe K, Calvo E, Loureiro M, Fernández-García MS, Fueyo A, et al. ATP-dependent Lon protease controls tumor bioenergetics by reprogramming mitochondrial activity. Cell Rep. 2014;8:542–56. [DOI] [PubMed] [Google Scholar]
- 59.Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–80. [DOI] [PubMed] [Google Scholar]
- 60.Pinti M, Gibellini L, Liu Y, Xu S, Lu B, Cossarizza A. Mitochondrial Lon protease at the crossroads of oxidative stress, ageing and cancer. Cell Mol Life Sci. 2015;72:4807–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sung YJ, Kao TY, Kuo CL, Fan CC, Cheng AN, Fang WC, Chou HY, Lo YK, Chen CH, Jiang SS, et al. Mitochondrial Lon sequesters and stabilizes p53 in the matrix to restrain apoptosis under oxidative stress via its chaperone activity. Cell Death Dis. 2018;9:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pryde KR, Taanman JW, Schapira AH. A LON-ClpP Proteolytic Axis Degrades Complex I to Extinguish ROS Production in Depolarized Mitochondria. Cell Rep. 2016;17:2522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumari S, Jayakumar S, Gupta GD, Bihani SC, Sharma D, Kutala VK, Sandur SK, Kumar V. Antibacterial activity of new structural class of semisynthetic molecule, triphenyl-phosphonium conjugated diarylheptanoid. Free Radic Biol Med. 2019;143:140–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: S1. List of all DEGs. S2. List of significant DEGs (padj value < 0.05). S3. List of significant and up-regulated DEGs (padj value < 0.05 & log2 fold change > 1). S4. List of significant and down-regulated DEGs (padj value < 0.05 & log2 fold change < -1). S5. List of cellular components modulated by significantly up-regulated DEGs and S6. List of biological processes modulated by significantly up-regulated DEGs obtained via GO enrichment analyses using ShinyGO gene-set enrichment tool (version 0.80). S7. List of biological processes modulated by significantly up-regulated DEGs obtained from STRING enrichment analysis.
Additional file 2: Figure S8. Representative flow cytometric histograms of MDA-MB-231 cells treated with MC for (A) 24 h and (B) 72 h and stained with PI. Figure S9. ATP levels in (A) MDA-MB-231 cells and (B) MCF-10A cells treated with the indicated concentrations of MC for 24 h. Each data point represents the mean ± SEM from at least four replicates, and three such independent experiments were conducted. ns: p > 0.05, ****p < 0.0001; as compared with vehicle-treated control. Figure S10. (A) Caspase-3 activity in MDA-MB-231 cells treated with 5 μM MC for indicated time-points. Each data point represents the mean ± SEM from at least three replicates, and two such independent experiments were conducted. ns: p > 0.05, ****p < 0.0001; as compared with vehicle-treated control. (B) Representative images of MitoSOX Red-stained MDA-MB-231 cells treated with 2.5 µM MC for 2 h. Figure S11. (A, B) MDA-MB-231 cells were treated with 1 µM MC for 2, 4, 8 and 24 h, followed by ClpX protein expression analysis by western blotting. (C, D) MDA-MB-231 cells were treated with 5 µM or 10 µM MC for 2 h or 5 µM MC for 4 h, followed by LONP1 and CLPP protein expression analysis by western blotting. Data points represent the mean ± SEM of 3 independent experiments. ns: p > 0.05, *p < 0.05; **p < 0.005; compared with the control.
Data Availability Statement
The RNA-seq data have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE276992 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE276992).