Abstract
The ketogenic diet (KD) has recently gained increasing popularity. This high-fat, adequate-protein, and carbohydrate-poor eating pattern leads to nutritional ketosis. The KD has long been known for its antidiabetic and antiepileptic effects and has been used therapeutically in these contexts. Recently, the KD, due to its effectiveness in inducing weight loss, has also been proposed as a possible approach to treat obesity. Likewise, a KD is currently explored as a supporting element in the treatment of obesity-associated metabolic disorders and certain forms of cancer. Here, we discuss the metabolic and biochemical mechanisms at play during the shift of metabolism to fatty acids and fatty acid–derived ketone bodies as main fuel molecules, in the substitution of carbohydrates, in ketogenic nutrition. Different sources of ketone bodies and KDs as alternatives to glucose and carbohydrates as main energy substrates are discussed, together with an attempt to weigh the benefits and risks posed by the chronic use of a KD in the context of weight loss, and also considering the molecular effects that ketone bodies exert on metabolism and on the endocrine system.
Keywords: beta-hydroxybutyrate, inflammatory response, ketogenic diet, ketone bodies, lipid metabolism, obesity
INTRODUCTION
According to the World Health Organization, the incidence of obesity worldwide has nearly tripled since 1975. Lifestyle changes, including the nutritional shift to highly processed foods rich in saturated fats and carbohydrates,1 increased sedentary lifestyle and urbanization,2 reduced physical activity,2,3 poor sleep habits,4 and increased stress,5 are all predisposing factors for weight gain. Globally, with the exception of some sub-Saharan African regions and Asian regions, the incidence of obesity is higher than the incidence of underweight.6
Obesity is defined as excessive fat accumulation and is a more serious condition than overweight. The most commonly used index for the classification of overweight/obesity in adults is body mass index (BMI; kg/m2)—the ratio of body mass to the square of body height. A BMI in the 25–30 kg/m2 range corresponds to a status of overweight, while a BMI >30 kg/m2 defines obesity and a BMI >35 kg/m2 is indicative of morbid obesity.7 BMI does not univocally inform on the ratio between fat and lean mass, a measure that can be obtained by dual-energy X-ray absorptiometry (DXA). Yet, BMI is still strongly correlated to DXA and is an acceptable predictor of obesity-related morbidities at the population level.8 Obesity, in turn, is a risk factor for coronary heart disease or stroke, musculoskeletal diseases such as osteoarthritis, type 2 diabetes, hypertension, gallbladder disease, dyslipidemia, sleep apnea and breathing problems,9–11 reduced fertility,12,13 and multiple types of cancers including endometrial, breast, ovarian, prostate, liver, gallbladder, kidney, and colon cancer.14–16 Many studies also suggest that people with obesity are more prone to mental illness conditions, such as clinical depression or anxiety.17–19 Because of these detrimental effects on health, weight-loss strategies in the population with obesity can be viewed as a preventive approach to alleviate the incidence of obesity-associated disorders.
In recent years, the ketogenic diet (KD), which aims at providing a normal-protein, high-fat, and low-carbohydrate (<50 g/d) intake, has been proposed as a weight-loss strategy. A KD, which is highly restricted in carbohydrates, was used from the 18th century as a remedy for diabetes.20 Likewise, the KD has been used for over a century as an effective anti-epileptic treatment21 and is still used today to treat pharmacologically refractory forms of epilepsy,22,23 or rare genetic conditions such as glucose transporter 1 (GLUT1) deficiency syndromes.24 In these specific situations, there are no alternatives to the KD, and patients must follow this diet continuously.
In weight-loss approaches, the usefulness of a KD has yet to be firmly established, and discordant studies provide different results on the matter, not least because of the multiple experimental protocols applied, the intrinsic variability in the KD administered in each study, and the occurrence of potential side effects. Here, several open questions related to the use of a KD to attain weight loss are discussed, the first of which is whether fatty acids can almost completely substitute carbohydrates as body fuel. Then, the evidence supporting the association between the obese state and multiple morbidities in humans is discussed, and finally focusing on the outcomes of studies investigating the use of a KD to curb obesity, with particular attention to the experimental protocols and their duration, KD composition, and the quality of fats, which are all factors affecting study outcomes.
METHODOLOGY OF THE LITERATURE SEARCH
Medline and Scopus databases were searched for original articles and reviews using the following key words and terms, used alone or in combination: anti-cancer therapy, ATP, cancer, carbohydrates, cardiovascular diseases, diabetes, endocrine diseases, energy production, inflammation, intermittent fasting, KD, ketone bodies, ketolysis, ketogenesis, lipids, obesity, musculoskeletal diseases, PCOS, proteins, supplementation, therapeutic potential. Clinical trials were searched in the National Institutes of Health (NIH) register (clinicaltrials.gov) and in the European Council EU Clinical Trials Register (https://www.clinicaltrialsregister.eu).
COULD FAT BE THE MACRONUTRIENT OF CHOICE TO PRODUCE ENERGY AND CURB OBESITY?
The optimal dietary macronutrient balance has been a longstanding topic of discussion, with current guidelines suggesting that adults derive 55%–70% of energy from carbohydrates, 15%–20% from fats, and 7%–20% from proteins.25 Poor nutritional education often leads people to rely on highly processed foods to ensure a supply of calories and a quick feeling of satiety. However, these highly processed foods, such as salty snacks and sugar-sweetened beverages, are characterized by a high glycemic index (GI) and deliver quickly absorbed nutrients that cause dopamine-triggered satisfaction and contribute to metabolic health issues and addictive eating behaviors.26,27
Fats are highly energy-dense, containing 9.0 kcal/g, more than double the energy provided by carbohydrates and proteins, which yield 4.0 kcal/g. This makes fat an efficient energy source providing a sustained release of energy that supports longer-lasting satiety compared with more rapidly metabolized carbohydrates. This satiety effect can aid weight management and in supporting calorie control. However, the role of fat in weight management is strongly related to the ratio between fat and carbohydrate intake, and ketone body production conditions. Physiologically, carbohydrate intake inhibits fat utilization. An excess of carbohydrates induces hyperinsulinemia and serves as lipogenic substrate, promoting fat accumulation and obesity, lending credibility to the proposed carbohydrate-insulin model (CIM) as a theoretical model explaining the development of obesity.28 Hence, shifting the metabolism into fat oxidation and fatty acid–derived ketone bodies is possible only after dietary carbohydrate deprivation, which causes nutritional ketosis (ketone bodies >0.5 mmol/L) with a concomitant increase in dietary fat consumption over a period of several weeks.29–31 In these circumstances, ketone bodies replace most of the glucose required by the brain, while liver gluconeogenesis provides the limited amount of energy needed by glucose-dependent tissues such as red blood cells, retina, and the renal medulla.32
During intestinal transit, fats tend to delay gastric emptying, thereby slowing down the digestion and absorption of carbohydrates. This means that a high-fat meal will have a lower glycemic effect than a low-fat meal, even if both contain the same amount and type of carbohydrates. A study by Lilly et al33 found that supplementation with peanut butter reduced blood glucose spikes and the overall glycemic response to a high-GI meal, potentially representing a beneficial approach to prevent blood glucose spikes.
The type of fat consumed plays a significant role in determining its impact on metabolic health, obesity, and cardiovascular risks. The debate often centers around the effects of different types of fats, notably trans-fats (also known as trans-unsaturated fats) and cis-fats (commonly found in most naturally occurring unsaturated fats).34,35 Understanding the distinctions between these types of fats and their metabolic effects is crucial to evaluating whether fat can indeed serve as a beneficial macronutrient for energy homeostasis and obesity management (Table 1).36–45
Table 1.
Key Points of Comparison of trans-Fats vs cis-Fats
| Chemical/metabolic feature | trans-Unsaturated fats | cis-Unsaturated fats | References |
|---|---|---|---|
| Structure | Chemically altered to remain solid at room temperature | Naturally occurring; no need for hydrogenation | 36, 37 |
| Primary sources | Processed foods (margarines, baked goods) | Olive oil, avocados, nuts, and seeds | 36 |
| Metabolic impact | Decrease insulin sensitivity, increase visceral fat | Improve insulin sensitivity, support metabolic health | 38 |
| Effect on inflammation | Proinflammatory, linked to increased cytokines | Anti-inflammatory, shown to reduce CRP levels | 39, 40, 41 |
| Heart health | Increase LDL and reduce HDL | Increase HDL, reduce LDL and total cholesterol | 42 |
| Obesity and weight management | Linked to increased risk of obesity and belly fat | Potentially aid in weight loss by enhancing satiety | 43 |
| Energy utilization | Inefficient, contribute to fat storage | Efficient, utilized as a steady energy source | 37, 44, 45 |
Abbreviations: CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Additionally, fats influence the body’s hormonal response, including the release of hormones such as ghrelin, often called the “hunger hormone,” and leptin, which helps regulate appetite and energy balance. Diets higher in healthy fats can modulate these hormones in a way that promotes satiety and reduces caloric intake, potentially making fat an effective macronutrient for managing obesity.46,47 In conclusion, while fat can indeed be a valuable macronutrient for energy production and weight management, the type of fat is crucial in determining its effects on health and obesity.
KETONE BODIES ARE A LAST-RESORT FUEL
Lipid metabolism begins with the hydrolysis of triglycerides into free fatty acids and glycerol (Figure 1). Fatty acids undergo β-oxidation to form acetyl coenzyme A (acetyl-CoA), or acetyl-CoA and propionyl-CoA for fatty acids with an odd number of carbon atoms.48 When excessive acetyl-CoA is produced, overloading the Krebs cycle, acetyl-CoA is shunted to ketogenesis. The 3 main circulating ketone bodies are d-3-hydroxybutyrate—also named β-hydroxybutyrate (BHB), acetoacetate (AcAc), and acetone (Ac). They are continuously produced in small amounts, along with glycolysis, reaching physiological plasma concentrations of 0.05–0.25 mmol/L in a circadian manner. During ketosis, ketone body production is augmented in parallel with decreased carbohydrate availability, making ketones the main metabolic fuel. In pathological conditions, such as diabetic ketoacidosis, ketone body levels exceeding 15 mmol/L may become life-threatening.49,50
Figure 1.
Metabolism of Fatty Acids (FAs). (A) Ketogenesis is initiated in order to make available energy stored in fatty acids. Once formed in the mitochondria, acetyl coenzyme A (acetyl-CoA) is reversibly converted to acetoacetyl-CoA, what is catalyzed by acetyl-CoA transferase 1 (ACAT1). Next, HMG (hydroxymethylglutaryl)-CoA synthase condenses acetyl-CoA with acetoacetyl-CoA to form HMG-CoA. HMG-CoA lyase catalyzes the formation of acetoacetate from HMG-CoA. The acetoacetate (AcAc) is then reversibly converted to β-hydroxybutyrate (BHB) by β-hydroxybutyrate dehydrogenase 1 (BDH1). (B) Fatty acids are present in the blood due to prior disruption of lipids (lipolysis) and are used as substrates in the β-oxidation process. Following hepatic ketogenesis, the ketone bodies are transferred back to the blood, where BHB as a signaling metabolite can be further transferred to extrahepatic tissues and AcAc can be degraded spontaneously into acetone (Ac), which will then be exhaled. (C) Ketolysis encompasses a set of reactions designed to recover energy through the oxidation of ketone bodies, which takes place in the mitochondria. The main ketone body distributed to the extrahepatic tissues—BHB—undergoes the reversible conversion to AcAc by BDH1. AcAc is then incorporated into acetoacetyl-CoA in a reaction catalyzed by succinyl-CoA-3-ketoacid-coenzyme A transferase (SCOT). Acetoacetyl-CoA can be reversibly converted by ACAT1 to acetyl-CoA, which is used in the tricarboxylic acid (TCA) cycle. The figure was created with BioRender.com
METABOLIC CONTROL OF KETONE BODY LEVELS
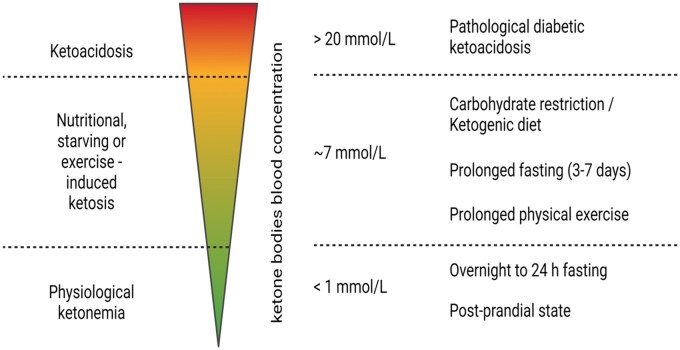
The balance between basal metabolism, physical activity, and food intake contributes to the long-term determination of body weight. In accordance with the Energy Balance Model (EBM), a negative energy balance causes weight loss, while a positive energy balance leads to storage of the excessive energy accumulation, mainly in the adipose tissue, promoting obesity.51 Stored fat may, however, be mobilized to induce a state of ketosis during starvation, exercise, or with a KD. Under these different conditions, in which the common thread is an initial depletion of the glucose and glycogen stores, fatty acids are mobilized from adipose tissue, which is constituted by more than 80% of fatty acids, and transported to the liver for conversion into ketone bodies. However, induction of ketogenesis has its limitations, and modulation of ketone levels can be very risky to health if not handled properly. The optimal flow of fuel for the brain and muscles depends on the level of serum ketone bodies, more specifically BHB (Figure 2).
Figure 2.
Changes in the Ketone Body Levels Depending on Ketone Body Sources and Metabolic Conditions. In the physiological state, concentrations of BHB are below 1 mmol/L. Overnight fasting and 24-hour fasting generate physiological ketonemia. Longer fasting, from 3 to 7 days, results in overt ketosis and the induction of ketosis through the ketogenic diet may increase the concentration of ketone bodies to up to 7 mmol/L. Starvation leads to an approximately 6–8 mmol/L blood BHB concentration, when maintained for more than 10 days. Beyond this range (up to 20 mmol/L), pathological conditions, such as untreated diabetes and ketoacidosis, occur. The figure was created with BioRender.com
Prolonged Fasting and Starvation
Starvation is defined as a prolonged fasting (>7 days) and can theoretically be prolonged for weeks (or even months) in humans.52 At the beginning of a starvation period, glucose is obtained from the breakdown of glycogen stores.53 Once glycogen stores are depleted, usually after 2–3 days of fasting, fats stored in adipose tissues are hydrolyzed into free fatty acids and glycerol, with glycerol being a gluconeogenic precursor, and free fatty acids substrates for β-oxidation and ketogenesis.
Upon prolonged fasting, protein degradation is also initiated. This phase is considered as the beginning of starvation. Protein degradation, mainly from skeletal muscle tissues, also provides precursors for ketone body synthesis, which can reach concentrations of approximately 8 mmol/L. Excessive protein deprivation will affect the functioning of vital organs, which is a life-threatening condition. Obviously, a weight-loss program must not be pushed to such limits.
Physical Activity
Skeletal muscle accounts for the largest fraction of total ketone body utilization at rest.54 Exercise increases the absolute rate of skeletal muscle ketone uptake and oxidation,55 similarly to a short-term starvation or ketone body infusion.56 Ketone body catabolism in human skeletal muscle increases up to 5 times during exercise.54 Immediately after the start of exercise, a decrease in ketone bodies—mainly BHB—is observed, with a concomitant increase in skeletal muscle ketone uptake and oxidation and an elevated ketone body metabolic clearance rate (MCR). The MCR measures the ability of tissues to remove ketones from the blood. During exercise, the MCR represents an index of the ability of exercise to stimulate the ability of contracting muscles to uptake and catabolize ketone bodies.54 Under a low ketonemia (<1 mmol/L) resulting from an overnight fast, resting MCR is up to 4 times greater than during prolonged fasting (>72 hours). Low- to moderate-intensity endurance exercise training after an overnight fast leads to an increase in MCR by 50%–70%. This shows that working muscles have an increased ability to extract ketones from blood compared with the resting state.54 However, the exercise-induced increase in ketone body uptake reaches a plateau when plasma ketone body concentrations exceed approximately 3–4 mmol/L, indicating that saturation of ketone uptake has been achieved.56 Two-hour treadmill exercise at 50% of maximum oxygen uptake (VO2max) was shown to stimulate whole-body absorption of ketones along with induced hyperketonemia (∼5.5–6 mmol/L) by exogenously delivered ketone bodies. Importantly, this was only observed in overnight-fasted subjects, but not in those who achieved similar levels of hyperketonemia by short-term fasting.57 The oxidation rate of the ketone body was found to increase by approximately 3- to 5-fold during 30 minutes of cycling at 60% VO2max in fasting subjects. Furthermore, it was observed that the ketone oxidation rate increased from approximately 0.44 mmol/L per minute at rest to approximately 2.1 mmol/L per minute (or ∼13 g/h) in the early stages of exercise, but then decreased to approximately 1.4 mmol/L per minute (or ∼9 g/h).58 Physical exercise itself has a short-term effect on increasing ketone levels. Together with a proper nutrition regimen, physical exercise accelerates weight reduction and may have a positive effect on morbidities associated with excessive body weight.
SUPPLEMENTATION OF KETONE BODIES OR KETONE BODY PRECURSORS
As the adaptation to the KD, also termed “keto-induction,” may be unpractical and unpalatable, other methods to induce ketosis have been introduced. Leucine supplementation was suggested to be beneficial in increasing ketone bodies (especially BHB) as leucine, together with lysine, is the most ketogenic amino acid. Leucine supplementation was studied in 30% calorie-restricted rats and, at 6 weeks of administration, increased consumption of leucine resulted in retained lean body mass and protein mass while improving protein anabolism. Leucine supplementation also increased the amount of leptin and ketone bodies, but no changes in cholesterol or triglyceride levels were observed.59 One study in humans has shown that butyric acid is even more ketogenic than either leucine or 8-carbon monoglycerides.60
Under real-life nutritional conditions to reach high BHB levels, great emphasis is placed on the supplementation of ketogenic medium-chain fatty acids (MCFAs).61 MCFAs encompass 6–12 carbon chains, including caproic (C6), caprilic (C8), capric (C10), and lauric (C12) acid. MCFAs induce both ketonemia and, in the long-term, ketogenesis, increasing BHB in a linear, dose-dependent manner.61 Ketosis is induced relatively quickly after MCFA ingestion, as MCFAs are absorbed directly into the liver's portal vein instead of transiting via the lymphatic system. As a consequence, MCFAs are swiftly converted to ketones after β-oxidation in the liver.62
An alternative way to promote ketonemia is the use of exogenous ketone esters and ketone salts in the form of “ketone drinks.” Dietary ketone salts are usually a racemic mixture of the BHB stereoisomers R-BHB and S-BHB,63 but also ketone esters or the ketone bodies precursor 1,3-butandiol.64 Soto-Mota et al65 analyzed the safety and tolerability of sustained (28 days) exogenous ketosis developed by using ketone drinks, providing information that such supplementation is safe for healthy adults with no changes in kidney function, blood glucose, triglycerides, cholesterol, or electrolytes. In addition, body weight and composition also remained the same. In this study, participants consumed a normal, balanced diet, with the exception of 3 participating athletes who were also on a KD. Another study reported that, although exogenous ketones increase serum BHB (up to 6 mmol/L), they are not ketogenic and may, in fact, inhibit the production of endogenous ketones from endogenous lipid depots. In other words, exogenous ketones directly promote ketonemia but do not stimulate ketone body biosynthesis in the liver.61 In a 14-day study, supplementation of a ketone monoester (KME) drink containing BHB prior to each meal to obese participants lowered postprandial and 24-hour average blood glucose levels, and increased brachial artery flow-mediated dilation—supporting a vascular benefit of the KME. However, the short study duration did not allow drawing conclusions on a potential effect on weight loss.66 Exogenously provided ketones may not directly induce weight loss, but they reportedly improved energy yield during exercise (eg, in healthy male athletes exercising for 1 hour on a bike at 75% of maximum exercise intensity, BHB levels increased from 0.1 to 3.4 mmol/L after consuming a ketone drink) and improved mental health.67 This study suggests that ketone body supplementation, while not curative against obesity, may serve as a preventive and supportive factor in obesity treatment.67
KETOGENIC DIETARY STRATEGIES
A high-fat, low-carbohydrate diet is the most efficient source of ketone bodies. Research on a low-carbohydrate diet as a weight-loss approach dates back to the 1970s.68 The reduction in quickly digestible starches and sugars has been reported as a key factor in reducing the risk of obesity, diabetes, and cardiovascular disorders.69 Studies have shown that a ketogenic dietary approach is superior to others in terms of rapid weight loss in the first 6–12 months. A first randomized controlled trial aiming at evaluating the efficacy of a low-carbohydrate, high-protein, high-fat diet (of the Atkins diet type) in comparison to a classical diet showed significant weight loss at 3 and 6 months, but no significant difference at 12 months.70 The 2-year Dietary Intervention Randomized Controlled Trial (DIRECT) compared (1) a low-fat diet, restricted in calories; (2) a Mediterranean diet, restricted in calories; and (3) a low-carbohydrate diet, nonrestricted in calories. Among the 3 diets, and at a 2-year follow-up, the greater weight loss was observed in the low-carbohydrate diet, with a parallel favorable affect in lipid profiles.71 In a 20-week study, 164 participants were randomly assigned a low-carbohydrate (20%), medium-carbohydrate (40%), or high-carbohydrate (60%) diet. In those who followed the low-carbohydrate diet, energy expenditure increased by approximately 200–250 kcal per day,72 suggesting that carbohydrate restriction may be more effective than fat restriction in treating obesity and maintaining weight loss. Moreover, a study of 262 adults with type 2 diabetes who followed a 2-year low-carbohydrate diet showed an average weight loss of 11.9 kg, as well as a reduction of glycosylated hemoglobin (HbA1c) by 1.0%.73 Another study of 316 children and adults with type 1 diabetes following a very-low-carbohydrate diet documented a mean HbA1c of 5.7%, low rates of hypoglycemia and ketoacidosis, and also improved cardiovascular disease risk profile.74 To date, several different varieties of low-carbohydrate diets have been proposed, such as the South Beach diet, Zone diet, Atkins diet, and KD; however, only the KD induces a sustained shift towards biosynthesis and use of ketone bodies.
Ketogenic Diet: The Most Efficient Nutritional Pattern to Induce Ketone Body Production
Currently, one of the most investigated eating patterns that substantially alter metabolic energy production is the KD, which is based on a minimal amount of carbohydrates (<5% in calories) and nutritionally optimal amount of protein (10%–20% of calories), with a simultaneous high supply of fats (∼80–90% of calories). Limiting carbohydrates to less than 50 g per day causes a loss of glycogen and the hepatic production of ketone bodies from fat stored in adipose tissue. Dietary ketosis, defined by BHB levels greater than 0.5 mmol/L in blood or urine, is induced in the first 2–5 days after starting the diet.60 This “initiation phase” can cause side effects such as constipation, halitosis, muscle spasms, diarrhea, skin rash, and more. Such symptoms, however, resolve within a few weeks of following the KD.
In addition to the standard KD, a number of different variants have been proposed due to several ways of inducing a state of ketosis. This includes the very-low-carbohydrate KD (VLCKD), medium-chain-triglyceride KD (MCTKD), the calorie-restricted KD (CRKD), the cyclic KD, the targeted KD, and the high-protein KD.75–79 Because of this variability, it must be underlined that a standardized and universal formulation of the KD does not exist (as it also does not exist for any other kind of diet). The compositions of the main types of KD are summarized in Table 2.76, 80–82
Table 2.
Composition of the Main Types of Ketogenic Diet
| Ketogenic diets | Total calorie intake | Fat calories, % of total | Lipid intake | Protein intake | Carbohydrate intake | References |
|---|---|---|---|---|---|---|
| “Classic” KD | No restriction, or caloric restriction depending on the study | 70 | 70% (total calories) | 25% (total calories) | 5% (total calories) | 80 |
| VLCKD | 780 kcal/d | 40 | 35 (g/d) | 90 (g/d) | 26 (g/d) | 81 |
| MCTD | Adapted to individual body weight | 40 | 71% (total calories) | 11% (total calories) | 10% (total calories) | 76 |
| Modified Atkins diet (mostly used for epileptic patients) | Adapted to individual body weight | 70 | 70% (total calories) | 25% (total calories) | 5% (total calories) | 83 |
Examples of different ketogenic diets are provided, with the implicit understanding that precise diet compositions vary among studies.
Abbreviations: KD, ketogenic diet; MCTD, medium-chain triglycerides diet; VLCKD, very-low-calorie ketogenic diet.
Gibson and colleagues83 compared, in a systematic review, very-low-energy diets (VLEDs) and KDs. The VLED users were found to be less hungry and more satiated, and KD users reported a decreased desire to eat. These results were relevant because of the typical increase in appetite in obese people. Overall, these studies suggest that ketosis appears to be effective in suppressing the appetite. While several studies show that a high-fat, low-carbohydrate diet is beneficial in losing weight and improving overall body parameters in the short–medium term,84 the most clinically relevant evidence must come from long-term efficacy. In this respect, a prospective clinical trial demonstrated persistent weight loss after 2 years on a low-carbohydrate diet.85 The same study, however, showed comparably persistent body-weight loss with a low-fat diet, underscoring the notion that the primary driver of weight loss is more attributable to the balance between caloric intake and caloric expenditure, supporting the EBM model, rather than dietary macronutrient composition. Both diets were, however, not associated with significant improvements in endothelial vascular function as measured by flow-mediated vasodilation.86
ALTERNATIVE DIETARY STRATEGIES TO ATTAIN ENHANCED KETONE BODY PRODUCTION—INTERMITTENT FASTING
Intermittent fasting is among the eating patterns that has recently received much attention for supporting weight loss while increasing the concentration of ketone bodies.87 During circadian fasting (ie, overnight), serum ketone levels increase naturally, but on a daily basis their concentration can be modulated through dietary routine. Intermittent fasting is a time-limited feeding period, usually based on the “16/8 system,” consisting of 16 hours of fasting and an 8-hour “eating window.” Intermittent fasting is typically associated with the consumption of a balanced diet with normal daily calorie intake in healthy individuals. Intermittent fasting also includes “every other day fasting,” in which fasting is extended to a 24-hour duration every second day.88
Intermittent fasting associated with energy restriction caused weight loss in obese people.89 This finding supported previous studies,90,91 in which intermittent fasting was even more beneficial than continued energy restriction in promoting insulin sensitivity. Interestingly, lean mass and resting energy expenditure were found to be the same regardless of continued energy restriction or the use of intermittent fasting.89 In a pilot study, the use of 2-day intermittent fasting resulted in a similar improvement in glycemic control in individuals with type 2 diabetes as compared with continued energy restriction, providing an alternative treatment strategy.92
There are 3 main theories for reducing metabolic and cardiovascular risk factors by intermittent fasting: the oxidative stress hypothesis, the circadian rhythm, and the ketogenic state. The oxidative stress hypothesis suggests that mitochondria produce fewer free radicals due to reduced energy intake, resulting in reduced inflammation. The circadian rhythm approach evolved with regard to timing of eating patterns. Properly planned meal times can allow for optimization of the peripheral clocks of the liver, adipose tissue, and skeletal tissue. Physical, mental, and behavioral changes are influenced by factors such as increases and decreases in blood pressure and secretion of hormones depending on the time of day and exposure to light. Hence, circadian regulation appears to be at the core of all biological processes in the body. Therefore, daily misalignment leads to an increased risk of chronic diseases, as proven by a higher rate of cardiovascular disease in shift workers. Some studies suggest that ketone bodies can affect the circadian rhythm by modulating the expression of clock genes.93,94
Intermittent fasting and the KD share some metabolic similarities, as these 2 eating patterns seem to have the most promising effects in terms of increasing ketone levels and reducing body fat. However, in a case report, prolonged fasting after a KD resulted in “starvation ketoacidosis,” which is a life-threatening condition.95 In the implementation of a KD to maintain ketosis at an optimal and safe level, fasting and eating times must be carefully planned. This requires support from experienced physicians and/or nutritionists and good compliance by the patient. Hence, as with any restrictive diet, intermittent fasting with an associated KD should not be used by pregnant women, children, and those engaged in heavy physical labor.
THE ROLE OF KETONE BODIES IN OBESITY AND OBESITY-RELATED DISEASES
Research into the antidiabetic potential of the KD dates back to the 17th century,20 and was then introduced by pediatrician Hugh Conklin in the treatment of drug-resistant epilepsy in the 1920s.96,97 This diet has proven its usefulness in achieving a lasting seizure-free condition, with a return to the traditional diet resulting in relapse.98 Recent studies show that the KD can successfully reverse metabolic abnormalities in diabetic patients by improving blood glucose levels while reducing insulin requirements, and significantly participate in the attenuation of inflammatory response.99 In addition, the potential of the use of a KD in the treatment of hyperlipidemic patients has also been hypothesized.100 The most important molecular effects exerted by ketone bodies are summarized in Figure 3.
Figure 3.
Molecular Responses to Induction of Ketosis. Abbreviations: IL, interleukin; GPR109A, hydroxycarboxylic acid receptor 2, niacin receptor 1; MCFA, medium-chain fatty acid; NF-κB, nuclear factor–kappa B; NLR, Nod-like receptor protein family; NLRP3, NLR pyrin domain-containing protein 3; Nrf2, nuclear factor erythroid 2-related factor 2; ROS, reactive oxygen species; TNFα, tumor necrosis factor alpha; TLR4, Toll-like receptor 4. The figure was created with BioRender.com
Obesity is associated with the release of proinflammatory mediators such as interleukins or tumor necrosis factor-α, but also reduced production of anti-inflammatory adiponectin. In this respect, the KD has been administered in patients with obesity and as obesity-related conditions, such as chronic kidney disease, cancer, or cardiovascular diseases, which are also characterized by a proinflammatory environment; several clinical trials have explored the usefulness of the KD in these pathologies.
THE EFFECT OF THE KD ON METABOLIC DISORDERS
Cardiovascular Diseases
The accumulation of visceral fat is recognized as a major cardiometabolic risk factor promoting the production of proinflammatory cytokines and adipokines with cardio-depressant and pro-atherosclerotic properties.101–103 Overweight is also associated with hypertension, the dominant cause of heart failure.104,105 A 5% weight gain leads to a 20%–30% increase in the incidence of hypertension.106 In physiological conditions, the largest consumer of energy (∼400 kcal/kg/d) of all tissues is the adult human heart, which uses all energy sources, with particular emphasis on fatty acids (both in the nonesterified and esterified form) and carbohydrates.107 Physiologically, the heart is a “metabolic omnivore” as it has the metabolic flexibility to switch from 1 substrate to another, depending on its availability.108 Thus, cardiomyocytes use ketone bodies in proportion to their supply, making the heart muscle the largest consumer of ketone bodies per unit mass.109 Such metabolic flexibility is lost in pathological conditions and, along with altered substrate utilization, is correlated with changes in mechanical function. In early stages of heart disease, the use of substrates shifts from fatty acids to glucose, leading to a reduction in oxidative metabolism. Such reprogramming underlies myocardial energy starvation, contributing to the pathogenesis of heart failure. Interestingly, while fatty acid oxidation is reduced, a failing heart appears to reprogram the metabolism to rely even more on ketone bodies as an energy supply.110
Studies have shown that circulating ketone bodies and their use in the myocardium are increased in both heart failure with a reduced ejection fraction and a conserved ejection fraction.111 As signaling molecules, ketone bodies can suppress systemic inflammation and cardiac inflammation, suggesting that a deliberate increase in circulating ketone bodies may be beneficial in the event of heart failure.112,113 This can be achieved by ingesting ketone body precursors such as 1,3-butanediol or medium-chain triglycerides (MCTs) as well as ketone salts and ketone esters. A study by Tzenios et al114 found that, in overweight patients, body composition and cardiometabolic markers improved after 140 days on a VLCKD.
Diabetes
Obesity increases the likelihood of type 2 diabetes or insulin resistance.115 One of the most serious and even life-threatening complications of diabetes is diabetic ketoacidosis, which appears in conjunction with hyperglycemia when glucose uptake by tissues is failing because of a lack of insulin (in type 1 diabetes) or insulin resistance. When tissues cannot internalize glucose, the body response consists of switching to hepatic ketone body synthesis as an alternative energy source. When circulating ketone bodies become excessive, blood becomes more acidic (venous blood pH <7.3), leading to diabetic ketoacidosis. To avoid this, in patients with diabetes it is important to measure not only blood sugar levels but also ketones.116,117 The main metabolic parameters of a KD and diabetic ketoacidosis are shown in Table 3.
Table 3.
Metabolic Blood Level Parameters During a Normal Diet, Ketogenic Diet, and Diabetic Ketoacidosis
| Metabolic parameters | Normal diet | Ketogenic diet | Diabetic ketoacidosis |
|---|---|---|---|
| Glucose (mg/dL) | 80–120 | 65–80 | >300 |
| Insulin (µU/L) | 6-23 | 6.6-9.4 | ∼0 |
| Ketone bodies (mmol/L) | 0.1 | 7/8 | >25 |
| pH | 7.4 | 7.4 | <7.3 |
| Total cholesterol (mg/dL) | <200 | 300 | ∼1600 |
Musculoskeletal Diseases, Osteoarthritis
The impact of obesity and metabolic syndrome on the musculoskeletal system (muscles, bones, and joints) is pervasive and ranges from obesity-linked sarcopenia (where individuals gain fat mass but lose lean muscle mass) to diabetic osteoporosis and arthritis.118,119 All of these disturbances result from degenerative and systemic inflammatory conditions that accompany obesity,120 including osteoarthritis (OA). Osteoarthritis is a multi-tissue disease of joints, characterized by cartilage degradation, subchondral bone sclerotization, ectopic bone outgrowths on joints (osteophytes), and moderate synovial membrane inflammation. The main risk factors for OA are aging and trauma, but, as mentioned previously, an additional important and compounding risk factor is obesity. First, excessive body weight places extra pressure on lower body joints—every 5 kg of weight gain confers a 36% increase in the risk of the knee osteoarthritis121 and an 11% increased risk of hip osteoarthritis.122 Second, a pro-inflammatory adipokine profile linked to dyslipidemia and abnormal fat tissue metabolism worsens OA progression.123,124 Third, systemic low-grade inflammation contributes to cartilage degeneration and synovial tissue inflammation.125
Currently, the global prevalence of osteoarthritis is escalating due to both the aging population and the epidemic of obesity.126 Based on the etiology of the disease, the body-weight factor seems to be of importance during OA treatment as obesity causes mechanical stress and metabolic inflammation resulting in accelerated cartilage damage and, ultimately, premature and severe OA. Intermittent fasting should be considered in weight-loss programs and its benefits are proven for the nonsurgical management of OA.127,128 The benefits of fasting in OA can be explained by changes in advanced glycation end-products (AGEs) and their receptors (RAGEs), both identified as important elements in the pathology of OA. In a cohort of 37 patients with OA who underwent fasting for 8 days, a significant, but non-sustained, reduction in RAGE levels in serum but not of AGEs was found; however, no correlation with improvement in clinical endpoints of OA was found.129 Low-carbohydrate diets have been reported to provide relief from osteoarthritis of the knee,130 but it is unknown whether a high-fat and low-carbohydrate diet can achieve the same result. Initial experiments by Masino and Ruskin132 demonstrated that such a dietary regimen, approximating a KD, can reduce inflammation and thus pain associated with inflammation.131 However, further investigations will be necessary to determine whether circulating ketone bodies contribute to OA symptom improvement after intermittent fasting.
In connection with this open question, Felson et al133 analyzed whether fatty acid levels affected the development of OA. It is known that saturated and n–6 fatty acids increase inflammation, whereas n–3 fatty acids reduce inflammation. Despite previously described effects on systemic inflammation, it was found that blood levels of fatty acids were not associated with risk of later knee OA or other OA outcomes. These studies, focused on the role of a KD in rheumatoid arthritis (RA), revealed its low efficacy.134,135 It was found that fasting, but not a KD, significantly decreased serum interleukin-6 (IL-6) levels and alleviated clinical symptoms in patients with RA. However, in these studies, KD administration was limited to 7 days, reproducing the effects of acute fasting rather than a prolonged weight-loss dietary intervention. Future prospective studies will be needed to elucidate the potential beneficial effects of a KD on specific domains and clinical outcomes in patients with inflammatory arthritis.
Polycystic Ovary Syndrome
Obesity is associated with several endocrine diseases and may itself be a consequence of hormonal disorders, especially in the case of polycystic ovary syndrome (PCOS). Indeed, PCOS is associated with many obesity-related comorbidities, such as insulin resistance (50%–90% incidence), type 2 diabetes, dyslipidemia, nonalcoholic fatty liver disease, hyperinsulinemia, and glucose intolerance.136,137 At present, PCOS cannot be cured, but its symptoms may be managed with medications (eg, metformin) and lifestyle changes. Since a weight loss of as little as 5% can lead to a significant improvement in PCOS, weight-loss interventions constitute a promising approach. A clinical study by Li et al138 observed that an 8-hour time-limited feeding improved the hormonal and metabolic profiles of patients with PCOS.
Studies by Paoli et al139 have shown that the KD is also beneficial for overweight women with PCOS, leading to a significant body-weight reduction and lowering blood glucose, insulin levels, and testosterone levels. Li et al140 showed that a 12-week period on a KD along with conventional pharmaceutical treatment improved the menstrual cycle. However, the question remains as to how the KD should be composed in terms of its lipid constituents. In fact, some studies have shown that the consumption of significant amounts of trans-unsaturated fats by women resulted in a 2-fold greater risk of ovulation disorders.141,142 This emphasizes that the composition of the KD must be adjusted to accommodate the patient’s needs, especially when the regimen is planned over the long term.
Cancer
According to research from the American Cancer Society, excessive body fat may be responsible for approximately 11% of cancers in women and 5% of cancers in men in the United States.143 Overall, approximately 1 in 20 cancers are associated with obesity.144 Excess body fat is linked to a higher risk of developing multiple types of cancer, including meningioma, adenocarcinoma of the esophagus, multiple myeloma, kidney, uterus, thyroid, breast, liver, gallbladder, upper stomach, ovarian, pancreatic, colon, and rectal cancers.145 Standard cancer treatments, such as radiation therapy, chemotherapy, and immunotherapy, have limitations and often have side effects. For this reason, many new strategies for reducing cancer have emerged. Special emphasis is placed on energy management by cancer cells that prefer glucose as their primary energy source. For example, leukemic cells create a diabetes-like state by reducing the availability of glucose to normal cells, leading to greater use of glucose by leukemic cells.146 This sparked the debate as to whether reducing carbohydrates and replacing them with lipids could be a new window in cancer treatment. Not all of the controversy surrounding some of these issues has yet been resolved. The switch to ketone bodies as an alternative main fuel seems promising, especially by virtue of the anti-inflammatory properties of ketone bodies. Also, there are studies confirming that cancer cells are unable to use ketone bodies due to acquired metabolic inflexibility. Certain tumor types show decreased levels of β-hydroxybutyrate dehydrogenase (BDH1) and succinyl-CoA-3-ketoacid-CoA transferase (SCOT1), and even undetectable acetyl-CoA acetyltransferase 1 (ACAT1). Nevertheless, the ketolytic enzyme expression seems to be cell/tissue-specific. For example, in cervical cancer–derived HeLa cells, BDH1/SCOT1 expression was high, but not in pancreatic cancer PANC-1 cells.147 Moreover, supplementation with ketone bodies (especially BHB) has proven harmful in cancer cells due to abnormalities in the structure of the mitochondria.148
Lin et al149 reported that dietary restriction alone reduces the density of microvessels, leading to an anti-tumor effect in lung and breast cancer. Another study by Allen et al150 has shown that following a KD increases the sensitivity of A549 lung cancer xenografts to carboplatin and reduces proliferation when combined with radiotherapy. This nutritional pattern has also been reported to be an effective adjuvant therapy in radiotherapy for the treatment of brain tumors.151 In a case report, a 65-year-old patient with glioblastoma multiforme treated with radiotherapy and chemotherapy and following a KD displayed tumor regression, supporting the potential effectiveness of an adjuvant KD in this type of cancer.152 In colon cancer, a KD with concomitant omega-3 fatty acid and MCT supplementation resulted in significant tumor growth inhibition.153 A study performed in women with ovarian or endometrial cancer showed that elevated serum BHB may reflect a metabolic environment inhospitable to cancer proliferation.154 During the 12-week study duration, the KD did not negatively affect quality of life of the patients and improved patients’ physical function end energy expenditure while diminishing food cravings. Also, the KD had no adverse effects on blood lipids, suggesting that ketone bodies are a safe and achievable component of treatment for some patients with cancer.155,156
On the other hand, some studies have shown the limitations of following a KD in patients with cancer. Porper et al157 have shown that high levels of nutritional ketosis were not achievable in 13 adults with glioblastoma treated with a KD for 8 weeks. In other studies, this dietary regimen even showed pro-cancer effects or the results were inconclusive. Chu-Shore and Thiele158 reported that, in 3 out of 5 children of patients with tuberous sclerosis complex, the implementation of a KD resulted in the progression of an existing tumor or the formation of a new tumor. Zahra et al159 then investigated the possible effects of a KD in patients with locally advanced non–small cell lung cancer and pancreatic cancer. The studies were conducted with simultaneous radiotherapy and chemotherapy. The difficulty of patients to follow a KD under these conditions was noticeable, giving an inconclusive result possibly due to low compliance to the diet. This area of research requires more in-depth investigation with an emphasis on treatment duration and long-term follow-up.
CONCLUSION
The evidence supporting the KD (or ketone body supplementation) in the regulation of metabolism and body weight remains incomplete. While there is research supporting certain benefits, individual responses to ketogenic nutrition can vary, and the long-term effects of a KD are still being studied. Table 4 presents the supportive evidence for the use of a KD, as well as potential drawbacks.160–169
Table 4.
Summary of the Effects of Ketogenic Diets on Weight Control and Physiological/Pathological Situations Affected by Body Weight
| Supportive evidence | Considerations | |
|---|---|---|
| Weight loss and obesity | Effective for weight loss: A significant number of studies have shown that a KD can be effective at least for short-term weight loss, making a KD a potential tool for managing obesity and overweight conditions.65,66,72,73,161 | Long-term adherence to a KD can be challenging, with weight-loss maintenance a concern. Specific risks include (1) gallstone formation, especially for those with gallbladder issues, due to rapid weight loss; (2) digestive issues, particularly for those with gastrointestinal disorders, due to low fiber content, which may exacerbate symptoms like constipation; (3) potential stress on the pancreas and liver from the high-fat content.112,162 |
| Athletic performance | Varied effects: The impact of KDs on athletic performance is a topic of debate. While some athletes report benefits, others report a decreased performance, especially during high-intensity activities. Individual factors and the type of physical activity are key considerations.66,163,164 | Nutrient deficiency risk: Restricting certain food groups in a KD may lead to deficiencies in essential nutrients, vitamins, and minerals if not carefully planned.165 |
| Blood sugar control and diabetes | Improved blood sugar control: Some studies suggest that a KD may improve insulin sensitivity and blood sugar control, making it potentially beneficial for individuals with type 2 diabetes.65,66,73,166 | Risk of diabetic ketoacidosis: Individual responses may vary, and the impact on blood sugar levels can depend on various factors. Patients with type 1 diabetes may face an increased risk of diabetic ketoacidosis.167,168 |
| Cardiovascular health | Improved cardiovascular risk factors: It was shown that a KD may improve cardiovascular risk factors, such as triglycerides and HDL cholesterol, while others raise concerns about potential negative effects on LDL cholesterol.115,169 | Saturated fat intake: (1) The impact on cardiovascular health may depend on the composition of the diet and individual health status; (2) certain versions of the KD may involve a high intake of saturated fats, which could impact lipid profiles and cardiovascular health, especially in those with pre-existing cardiovascular issues. |
| Metabolic disorders | Improved metabolic markers: There are studies showing that a KD may lead to improvements in cardiometabolic markers.115,169 | Carnitine deficiency: The KD may lower carnitine levels, posing issues for individuals with metabolic disorders involving impaired carnitine metabolism.170 |
| Inflammation | Anti-inflammatory potential: Studies in preclinical models suggest that a KD may have anti-inflammatory effects.112 | Insufficient research: The relationship between diet, inflammation, and individual response is complex, and more research is needed. |
| Cancer | Adjunctive cancer therapy: Some preclinical studies suggest potential anti-cancer effects of a KD, but more research is needed to establish its efficacy and safety in humans.156,157,159 | Insufficient research: The role of a KD in cancer prevention and treatment is still widely investigated. There are issues with low dietary compliance and also related to reaching nutritional ketosis.158,160 |
| Polycystic ovary syndrome (PCOS) | Hormonal regulation: KDs improve insulin sensitivity in individuals with PCOS, addressing some symptoms of the condition.97,139,140,141 | Very few small-scale clinical trials have been completed to date.139,140 |
Abbreviations: HDL, high-density lipoprotein; KD, ketogenic diet; LDL, low-density lipoprotein.
Overall, the currently available evidence supports the potential for ketogenesis-inducing dietary habits as an aid to induce weight loss and treat obesity-related diseases, at least in a transitory manner, perhaps through reduced appetite and reduced calorie intake. At present, the long-term safety of ketosis in weight-loss interventions remains unknown, and it is of the utmost importance to only switch to ketogenic regimens after appropriate medical consulting.
Acknowledgments
The authors apologize to colleagues whose scientific work has not been cited because of space constraints.
Contributor Information
Marta Biesiekierska, Department of Oncobiology and Epigenetics, Faculty of Biology and Environmental Protection, University of Lodz, 90-236 Lodz, Poland.
Maura Strigini, University Jean Monnet Saint-Etienne, INSERM, Mines Saint Etienne, SAINBIOSE U1059, F-42023 Saint-Etienne, France.
Agnieszka Śliwińska, Department of Nucleic Acid Biochemistry, Medical University of Lodz, 92-213 Lodz, Poland.
Luciano Pirola, INSERM Unit 1060, CarMeN Laboratory, Lyon 1 University, F-69495 Pierre Bénite, France.
Aneta Balcerczyk, Department of Oncobiology and Epigenetics, Faculty of Biology and Environmental Protection, University of Lodz, 90-236 Lodz, Poland.
Author Contributions
Conceptualization: A.B. and L.P.; data collection and data interpretation: all authors; writing—original draft preparation: M.B, M.S., A.Ś., L.P., and A.B.; figures: M.B., L.P., and A.B.; supervision and project administration: A.B.; funding acquisition: A.B., M.S. and L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Polish National Science Centre, Project Grant NCN Harmonia, No. 2018/30/M/NZ3/00682. M.S. was supported by intramural funding from University Jean Monnet (UJM) Saint-Etienne and INSERM, the AAP 2017 of UJM Saint-Etienne, and the French Rheumatology Society (SFR 2020). L.P. and A.B. were supported by the 2021 EFSD-Boehringer Ingelheim funding program.
Conflicts of Interest
None declared.
Data Availability
Not applicable.
REFERENCES
- 1. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30:67-77.e3. 10.1016/j.cmet.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Curran F, Davis ME, Murphy K, et al. Correlates of physical activity and sedentary behavior in adults living with overweight and obesity: a systematic review. Obes Rev. 2023;24:e13615. 10.1111/obr.13615 [DOI] [PubMed] [Google Scholar]
- 3. Bray GA, Frühbeck G, Ryan DH, Wilding JPH. Management of obesity. Lancet. 2016;387:1947-1956. 10.1016/S0140-6736(16)00271-3 [DOI] [PubMed] [Google Scholar]
- 4. Yoong SL, Chai LK, Williams CM, Wiggers J, Finch M, Wolfenden L. Systematic review and meta‐analysis of interventions targeting sleep and their impact on child body mass index, diet, and physical activity. Obesity (Silver Spring). 2016;24:1140-1147. 10.1002/oby.21459 [DOI] [PubMed] [Google Scholar]
- 5. van Baak MA, Mariman ECM. Obesity-induced and weight-loss-induced physiological factors affecting weight regain. Nat Rev Endocrinol. 2023;19:655-670. 10.1038/s41574-023-00887-4 [DOI] [PubMed] [Google Scholar]
- 6. Jaacks LM, Vandevijvere S, Pan A, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7:231-240. 10.1016/S2213-8587(19)30026-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Purnell JQ. Definitions, classification, and epidemiology of obesity. In: Feingold KR, Anawalt B, Blackman MR, et al. , eds. Endotext [Internet]. MDText.com, Inc.; 2000. [PubMed] [Google Scholar]
- 8. Achamrah N, Colange G, Delay J, et al. Comparison of body composition assessment by DXA and BIA according to the body mass index: a retrospective study on 3655 measures. PLoS One. 2018;13:e0200465. 10.1371/journal.pone.0200465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schnall PL, Dobson M, Landsbergis P. Globalization, work, and cardiovascular disease. Int J Health Serv. 2016;46:656-692. 10.1177/0020731416664687 [DOI] [PubMed] [Google Scholar]
- 10. Scherer PE, Hill JA. Obesity, diabetes, and cardiovascular diseases. Circ Res. 2016;118:1703-1705. 10.1161/CIRCRESAHA.116.308999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beak JS, Kim YT, Lee SH. Predisposing factors for posttraumatic osteoarthritis after malleolus fracture fixation in patients younger than 50 years. Foot Ankle Int. 2022;43:389-397. 10.1177/10711007211050039 [DOI] [PubMed] [Google Scholar]
- 12. Hazlehurst JM, Singh P, Bhogal G, Broughton S, Tahrani AA. How to manage weight loss in women with obesity and PCOS seeking fertility? Clin Endocrinol (Oxf). 2022;97:208-216. 10.1111/cen.14726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hunter E, Avenell A, Maheshwari A, Stadler G, Best D. The effectiveness of weight‐loss lifestyle interventions for improving fertility in women and men with overweight or obesity and infertility: a systematic review update of evidence from randomized controlled trials. Obes Rev. 2021;22:e13325. 10.1111/obr.13325 [DOI] [PubMed] [Google Scholar]
- 14. Adnan Y, Ali SMA, Awan MS, et al. Body mass index and diabetes mellitus may predict poorer overall survival of oral squamous cell carcinoma patients: a retrospective cohort from a tertiary-care centre of a resource-limited country. Clin Med Insights Oncol. 2022;16:11795549221084832. 10.1177/11795549221084832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bou Malhab LJ, Abdel-Rahman WM. Obesity and inflammation: colorectal cancer engines. Curr Mol Pharmacol. 2022;15:620-646. 10.2174/1874467214666210906122054 [DOI] [PubMed] [Google Scholar]
- 16. Sakai NS, Taylor SA, Chouhan MD. Obesity, metabolic disease and the pancreas—quantitative imaging of pancreatic fat. Br J Radiol. 2018;91:20180267. 10.1259/bjr.20180267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. 2019;24:18-33. 10.1038/s41380-018-0017-5 [DOI] [PubMed] [Google Scholar]
- 18. Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression. Arch Gen Psychiatry. 2010;67:220-229. 10.1001/archgenpsychiatry.2010.2 [DOI] [PubMed] [Google Scholar]
- 19. Mazereel V, Detraux J, Vancampfort D, van Winkel R, De Hert M. Impact of psychotropic medication effects on obesity and the metabolic syndrome in people with serious mental illness. Front Endocrinol (Lausanne). 2020;11:573479. 10.3389/fendo.2020.573479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lennerz BS, Koutnik AP, Azova S, Wolfsdorf JI, Ludwig DS. Carbohydrate restriction for diabetes: rediscovering centuries-old wisdom. J Clin Invest. 2021;131:e142246. 10.1172/JCI142246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cooder HR. Epilepsy in children: with particular reference to the ketogenic diet. Cal West Med. 1933;39:169-173. PMID: 18742621 [PMC free article] [PubMed] [Google Scholar]
- 22. IJff DM, Postulart D, Lambrechts DAJE, et al. Cognitive and behavioral impact of the ketogenic diet in children and adolescents with refractory epilepsy: a randomized controlled trial. Epilepsy Behav. 2016;60:153-157. 10.1016/j.yebeh.2016.04.033 [DOI] [PubMed] [Google Scholar]
- 23. Ros Pérez P, Zamarrón Cuesta I, Aparicio Meix M, Sastre Gallego A. Evaluation of the effectiveness of the ketogenic diet with medium-chain triglycerides, in the treatment of refractory epilepsy in children. Apropos of a series of cases. An Esp Pediatr. 1989;30:155-158. PMID: 2729781 [PubMed] [Google Scholar]
- 24. Sandu C, Burloiu CM, Barca DG, Magureanu SA, Craiu DC. Ketogenic diet in patients with GLUT1 deficiency syndrome. Maedica (Bucur). 2019;14:93-97. 10.26574/maedica.2019.14.2.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Institute of Medicine (US); Subcommittee on Interpretation and Uses of Dietary Reference Intakes; Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. DRI Dietary Reference Intakes: Applications in Dietary Assessment. National Academies Press; 2000. 10.17226/9956 [DOI] [PubMed] [Google Scholar]
- 26. Yalcin T, Al A, Rakıcıoğlu N. The effects of meal glycemic load on blood glucose levels of adults with different body mass indexes. Indian J Endocrinol Metab. 2017;21:71-75. 10.4103/2230-8210.195995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wallace CW, Fordahl SC. Obesity and dietary fat influence dopamine neurotransmission: exploring the convergence of metabolic state, physiological stress, and inflammation on dopaminergic control of food intake. Nutr Res Rev. 2022;35:236-251. 10.1017/S0954422421000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Speakman JR, Hall KD. Carbohydrates, insulin, and obesity. Science. 2021;372:577-578. 10.1126/science.aav0448 [DOI] [PubMed] [Google Scholar]
- 29. Schugar RC, Crawford PA. Low-carbohydrate ketogenic diets, glucose homeostasis, and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2012;15:374-380. 10.1097/MCO.0b013e3283547157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spriet LL. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. 2014;44(Suppl 1):S87-S96. 10.1007/s40279-014-0154-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Volek JS, Noakes T, Phinney SD. Rethinking fat as a fuel for endurance exercise. Eur J Sport Sci. 2015;15:13-20. 10.1080/17461391.2014.959564 [DOI] [PubMed] [Google Scholar]
- 32. Shah AM, Wondisford FE. Tracking the carbons supplying gluconeogenesis. J Biol Chem. 2020;295:14419-14429. 10.1074/jbc.REV120.012758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lilly LN, Heiss CJ, Maragoudakis SF, Braden KL, Smith SE. The effect of added peanut butter on the glycemic response to a high–glycemic index meal: a pilot study. J Am Coll Nutr. 2019;38:351-357. 10.1080/07315724.2018.1519404 [DOI] [PubMed] [Google Scholar]
- 34. De Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351:H3978. 10.1136/bmj.h3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Y, Hruby A, Bernstein AM, et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective cohort study. J Am Coll Cardiol. 2015;66:1538-1548. 10.1016/j.jacc.2015.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lemeshow AR, Rimm EB, Hasin DS, et al. Food and beverage consumption and food addiction among women in the Nurses’ Health Studies. Appetite. 2018;121:186-197. 10.1016/j.appet.2017.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jaworowska A, Blackham T, Davies IG, Stevenson L. Nutritional challenges and health implications of takeaway and fast food. Nutr Rev. 2013;71:310-318. 10.1111/nure.12031 [DOI] [PubMed] [Google Scholar]
- 38. Lovejoy JC, Smith SR, Champagne CM, et al. Effects of diets enriched in saturated (palmitic), monounsaturated (oleic), or trans (elaidic) fatty acids on insulin sensitivity and substrate oxidation in healthy adults. Diabetes Care. 2002;25:1283-1288. 10.2337/diacare.25.8.1283 [DOI] [PubMed] [Google Scholar]
- 39. Oteng A-B, Kersten S. Mechanisms of action of trans fatty acids. Adv Nutr. 2020;11:697-708. 10.1093/advances/nmz125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mozaffarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr. 2009;63(Suppl 2):S22-33. 10.1038/sj.ejcn.1602976 [DOI] [PubMed] [Google Scholar]
- 41. Zhu XF, Hu YQ, Dai ZC, Li XJ, Zhang J. Associations between trans fatty acids and systemic immune-inflammation index: a cross-sectional study. Lipids Health Dis. 2024;23:122. 10.1186/s12944-024-02109-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wanders AJ, Brouwer IA, Siebelink E, Katan MB. Effect of a high intake of conjugated linoleic acid on lipoprotein levels in healthy human subjects. PLoS One. 2010;5:e9000. 10.1371/journal.pone.0009000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matthan NR, Lovato L, Petersen KS, et al. Effect of daily avocado consumption for 6 mo compared with habitual diet on red blood cell fatty acid profiles and association with cardiometabolic risk factors in individuals with abdominal obesity: a randomized trial. Am J Clin Nutr. 2024;120:794-803. 10.1016/j.ajcnut.2024.08.002 [DOI] [PubMed] [Google Scholar]
- 44. Mann GV. Metabolic consequences of dietary trans fatty acids. Lancet. 1994;343:1268-1271. 10.1016/s0140-6736(94)92157-1 [DOI] [PubMed] [Google Scholar]
- 45. Scholz A, Navarrete-Muñoz EM, García-de-la-Hera M, et al. Association between trans fatty acid intake and overweight including obesity in 4 to 5-year-old children from the INMA study. Pediatr Obes. 2019;14:e12528. 10.1111/ijpo.12528 [DOI] [PubMed] [Google Scholar]
- 46. Sun L, Goh HJ, Govindharajulu P, Leow MK, Henry CJ. Differential effects of monounsaturated and polyunsaturated fats on satiety and gut hormone responses in healthy subjects. Foods. 2019;8:634. 10.3390/foods8120634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stevenson JL, Paton CM, Cooper JA. Hunger and satiety responses to high-fat meals after a high-polyunsaturated fat diet: a randomized trial. Nutrition. 2017;41:14-23. 10.1016/j.nut.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 48. Fukao T, Lopaschuk GD, Mitchell GA. Pathways and control of ketone body metabolism: on the fringe of lipid biochemistry. Prostaglandins Leukot Essent Fatty Acids. 2004;70:243-251. 10.1016/j.plefa.2003.11.001 [DOI] [PubMed] [Google Scholar]
- 49. Nelson AB, Queathem ED, Puchalska P, Crawford PA. Metabolic messengers: ketone bodies. Nat Metab. 2023;5:2062-2074. 10.1038/s42255-023-00935-3 [DOI] [PubMed] [Google Scholar]
- 50. Dąbek A, Wojtala M, Pirola L, Balcerczyk A. Modulation of cellular biochemistry, epigenetics and metabolomics by ketone bodies. Implications of the ketogenic diet in the physiology of the organism and pathological states. Nutrients. 2020;12:788. 10.3390/nu12030788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011;121:2133-2141. 10.1172/JCI46043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Owen OE, Felig P, Morgan AP, Wahren J, Cahill GF. Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969;48:574-583. 10.1172/JCI106016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jensen J, Rustad PI, Kolnes AJ, Lai Y-C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front Physiol. 2011;2:112. 10.3389/fphys.2011.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol. 2017;595:2857-2871. 10.1113/JP273185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mikkelsen KH, Seifert T, Secher NH, Grøndal T, van Hall G. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-β-hydroxybutyratemia in post-absorptive healthy males. J Clin Endocrinol Metab. 2015;100:636-643. 10.1210/jc.2014-2608 [DOI] [PubMed] [Google Scholar]
- 56. Fery F, Balasse EO. Effect of exercise on the disposal of infused ketone bodies in humans. J Clin Endocrinol Metab. 1988;67:245-250. 10.1210/jcem-67-2-245 [DOI] [PubMed] [Google Scholar]
- 57. Balasse EO, Féry F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev. 1989;5:247-270. 10.1002/dmr.5610050304 [DOI] [PubMed] [Google Scholar]
- 58. Balasse EO, Fery F, Neef MA. Changes induced by exercise in rates of turnover and oxidation of ketone bodies in fasting man. J Appl Physiol Respir Environ Exerc Physiol. 1978;44:5-11. 10.1152/jappl.1978.44.1.5 [DOI] [PubMed] [Google Scholar]
- 59. Pedroso JAB, Nishimura LS, de Matos‐Neto EM, Donato J, Tirapegui J. Leucine improves protein nutritional status and regulates hepatic lipid metabolism in calorie‐restricted rats. Cell Biochem Funct. 2014;32:326-332. 10.1002/cbf.3017 [DOI] [PubMed] [Google Scholar]
- 60. Harvey CJdC, Schofield GM, Williden M. The use of nutritional supplements to induce ketosis and reduce symptoms associated with keto-induction: a narrative review. PeerJ. 2018;6:e4488. 10.7717/peerj.4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Harvey CJdC, Schofield GM, Williden M, McQuillan JA. The effect of medium chain triglycerides on time to nutritional ketosis and symptoms of keto-induction in healthy adults: a randomised controlled clinical trial. J Nutr Metab. 2018;2018:2630565-2630569. 10.1155/2018/2630565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jensen NJ, Wodschow HZ, Nilsson M, Rungby J. Effects of ketone bodies on brain metabolism and function in neurodegenerative diseases. Int J Mol Sci. 2020;21:8767. 10.3390/ijms21228767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stubbs BJ, Cox PJ, Evans RD, et al. On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:848. 10.3389/fphys.2017.00848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Poff AM, Koutnik AP, Egan B. Nutritional ketosis with ketogenic diets or exogenous ketones: features, convergence, and divergence. Curr Sports Med Rep. 2020;19:251-259. 10.1249/JSR.0000000000000732 [DOI] [PubMed] [Google Scholar]
- 65. Soto-Mota A, Vansant H, Evans RD, Clarke K. Safety and tolerability of sustained exogenous ketosis using ketone monoester drinks for 28 days in healthy adults. Regul Toxicol Pharmacol. 2019;109:104506. 10.1016/j.yrtph.2019.104506 [DOI] [PubMed] [Google Scholar]
- 66. Walsh JJ, Neudorf H, Little JP. 14-Day ketone supplementation lowers glucose and improves vascular function in obesity: a randomized crossover trial. J Clin Endocrinol Metab. 2021;106:1738-1754. 10.1210/clinem/dgaa925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293-315. 10.1016/j.brainresrev.2008.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yang MU, Van Itallie TB. Composition of weight lost during short-term weight reduction. Metabolic responses of obese subjects to starvation and low-calorie ketogenic and nonketogenic diets. J Clin Invest. 1976;58:722-730. 10.1172/JCI108519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Brouns F. Overweight and diabetes prevention: is a low-carbohydrate–high-fat diet recommendable? Eur J Nutr. 2018;57:1301-1312. 10.1007/s00394-018-1636-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082-2090. 10.1056/NEJMoa022207 [DOI] [PubMed] [Google Scholar]
- 71. Shai I, Schwarzfuchs D, Henkin Y, et al. ; Dietary Intervention Randomized Controlled Trial (DIRECT) Group. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229-241. 10.1056/NEJMoa0708681 [DOI] [PubMed] [Google Scholar]
- 72. Ebbeling CB, Feldman HA, Klein GL, et al. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. BMJ. 2018;363: K 4583. 10.1136/bmj.k4583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol (Lausanne). 2019;10:348. 10.3389/fendo.2019.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lennerz BS, Barton A, Bernstein RK, et al. Management of type 1 diabetes with a very low–carbohydrate diet. Pediatrics. 2018;142:e20181536B. 10.1542/peds.2017-3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bueno NB, de Melo ISV, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. 10.1017/S0007114513000548 [DOI] [PubMed] [Google Scholar]
- 76. Liu YC. Medium‐chain triglyceride (MCT) ketogenic therapy. Epilepsia. 2008;49(Suppl 8):33-36. 10.1111/j.1528-1167.2008.01830.x [DOI] [PubMed] [Google Scholar]
- 77. Vidić V, Ilić V, Toskić L, Janković N, Ugarković D. Effects of calorie restricted low carbohydrate high fat ketogenic vs. non-ketogenic diet on strength, body-composition, hormonal and lipid profile in trained middle-aged men. Clin Nutr. 2021;40:1495-1502. 10.1016/j.clnu.2021.02.028 [DOI] [PubMed] [Google Scholar]
- 78. Kysel P, Haluzíková D, Doležalová RP, et al. The influence of cyclical ketogenic reduction diet vs. nutritionally balanced reduction diet on body composition, strength, and endurance performance in healthy young males: a randomized controlled trial. Nutrients. 2020;12:2832. 10.3390/nu12092832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr. 2008;87:44-55. 10.1093/ajcn/87.1.44 [DOI] [PubMed] [Google Scholar]
- 80. Zoccali C, Bellizzi V, Minutolo R, Mallamaci F, Conte G, De Nicola L. The effect of a ketogenic diet on weight loss in CKD: a randomized controlled trial in obese stage G1-3a CKD patients. Clin Kidney J. 2023;16:2309-2313. 10.1093/ckj/sfad176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Basciani S, Camajani E, Contini S, et al. Very-low-calorie ketogenic diets with whey, vegetable, or animal protein in patients with obesity: a randomized pilot study. J Clin Endocrinol Metab. 2020;105:dgaa336. 10.1210/clinem/dgaa336 [DOI] [PubMed] [Google Scholar]
- 82. Molteberg E, Thorsby PM, Kverneland M, et al. Stress biomarkers in adult patients with drug-resistant epilepsy on a modified Atkins diet: a prospective study. Epilepsia Open. 2023;8:1331-1339. 10.1002/epi4.12808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gibson AA, Seimon RV, Lee CMY, et al. Do ketogenic diets really suppress appetite? A systematic review and meta‐analysis. Obes Rev. 2015;16:64-76. 10.1111/obr.12230 [DOI] [PubMed] [Google Scholar]
- 84. Hession M, Rolland C, Kulkarni U, Wise A, Broom J. Systematic review of randomized controlled trials of low‐carbohydrate vs. low‐fat/low‐calorie diets in the management of obesity and its comorbidities. Obes Rev. 2009;10:36-50. 10.1111/j.1467-789X.2008.00518.x [DOI] [PubMed] [Google Scholar]
- 85. Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet. Ann Intern Med. 2010;153:147-157. 10.7326/0003-4819-153-3-201008030-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mohler ER, Sibley AA, Stein R, et al. Endothelial function and weight loss: comparison of low‐carbohydrate and low‐fat diets. Obesity (Silver Spring). 2013;21:504-509. 10.1002/oby.20055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Arora N, Pulimamidi S, Yadav H, et al. Intermittent fasting with ketogenic diet: a combination approach for management of chronic diseases. Clin Nutr ESPEN. 2023;54:166-174. 10.1016/j.clnesp.2023.01.024 [DOI] [PubMed] [Google Scholar]
- 88. Ganesan K, Habboush Y, Sultan S. Intermittent fasting: the choice for a healthier lifestyle. Cureus. 2018;10:e2947. 10.7759/cureus.2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Harvie M, Howell A. Potential benefits and harms of intermittent energy restriction and intermittent fasting amongst obese, overweight and normal weight subjects—a narrative review of human and animal evidence. Behav Sci. 2017;7:4. 10.3390/bs7010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727. 10.1038/ijo.2010.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Harvie M, Wright C, Pegington M, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110:1534-1547. 10.1017/S0007114513000792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Carter S, Clifton PM, Keogh JB. The effect of intermittent compared with continuous energy restriction on glycaemic control in patients with type 2 diabetes: 24-month follow-up of a randomised noninferiority trial. Diabetes Res Clin Pract. 2019;151:11-19. 10.1016/j.diabres.2019.03.022 [DOI] [PubMed] [Google Scholar]
- 93. Dong TA, Sandesara PB, Dhindsa DS, et al. Intermittent fasting: a heart healthy dietary pattern? Am J Med. 2020;133:901-907. 10.1016/j.amjmed.2020.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gangitano E, Gnessi L, Lenzi A, Ray D. Chronobiology and metabolism: is ketogenic diet able to influence circadian rhythm? Front Neurosci. 2021;15:756970. 10.3389/fnins.2021.756970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Blanco JC, Khatri A, Kifayat A, Cho R, Aronow WS. Starvation ketoacidosis due to the ketogenic diet and prolonged fasting—a possibly dangerous diet trend. Am J Case Rep. 2019;20:1728-1731. 10.12659/AJCR.917226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Conklin HW. Cause and treatment of epilepsy. J Am Osteopathic Assoc. 1922;26:11-14. [Google Scholar]
- 97. Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49(Suppl 8):3-5. 10.1111/j.1528-1167.2008.01821.x [DOI] [PubMed] [Google Scholar]
- 98. Sampaio LPdB. Ketogenic diet for epilepsy treatment. Arq Neuropsiquiatr. 2016;74:842-848. 10.1590/0004-282X20160116 [DOI] [PubMed] [Google Scholar]
- 99. O’Neill B, Raggi P. The ketogenic diet: pros and cons. Atherosclerosis. 2020;292:119-126. 10.1016/j.atherosclerosis.2019.11.021 [DOI] [PubMed] [Google Scholar]
- 100. Keith L, Seo CA, Rowsemitt C, et al. Ketogenic diet as a potential intervention for lipedema. Med Hypotheses. 2021;146:110435. 10.1016/j.mehy.2020.110435 [DOI] [PubMed] [Google Scholar]
- 101. Tchernof A, Després J-P. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359-404. 10.1152/physrev.00033.2011 [DOI] [PubMed] [Google Scholar]
- 102. Ballak DB, Stienstra R, Tack CJ, Dinarello CA, van Diepen JA. IL-1 family members in the pathogenesis and treatment of metabolic disease: focus on adipose tissue inflammation and insulin resistance. Cytokine. 2015;75:280-290. 10.1016/j.cyto.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Frayn KN, Karpe F, Fielding BA, Macdonald IA, Coppack SW. Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord. 2003;27:875-888. 10.1038/sj.ijo.0802326 [DOI] [PubMed] [Google Scholar]
- 104. Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension. Circ Res. 2015;116:991-1006. 10.1161/CIRCRESAHA.116.305697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Benjamin EJ, Virani SS, Callaway CW, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67-e492. 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 106. Shariq OA, McKenzie TJ. Obesity-related hypertension: a review of pathophysiology, management, and the role of metabolic surgery. Gland Surg. 2020;9:80-93. 10.21037/gs.2019.12.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Murashige D, Jang C, Neinast M, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. 2020;370:364-368. 10.1126/science.abc8861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Abdul Kadir A, Clarke K, Evans RD. Cardiac ketone body metabolism. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165739. 10.1016/j.bbadis.2020.165739 [DOI] [PubMed] [Google Scholar]
- 109. Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304:H1060-76. 10.1152/ajpheart.00646.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Aubert G, Martin OJ, Horton JL, et al. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133:698-705. 10.1161/CIRCULATIONAHA.115.017355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yurista SR, Chong C-R, Badimon JJ, Kelly DP, de Boer RA, Westenbrink BD. Therapeutic potential of ketone bodies for patients with cardiovascular disease. J Am Coll Cardiol. 2021;77:1660-1669. 10.1016/j.jacc.2020.12.065 [DOI] [PubMed] [Google Scholar]
- 112. Takahara S, Soni S, Maayah ZH, Ferdaoussi M, Dyck JRB. Ketone therapy for heart failure: current evidence for clinical use. Cardiovasc Res. 2022;118:977-987. 10.1093/cvr/cvab068 [DOI] [PubMed] [Google Scholar]
- 113. Pirola L, Ciesielski O, Balcerczyk A. Fat not so bad? The role of ketone bodies and ketogenic diet in the treatment of endothelial dysfunction and hypertension. Biochem Pharmacol. 2022;206:115346. 10.1016/j.bcp.2022.115346 [DOI] [PubMed] [Google Scholar]
- 114. Tzenios N, Lewis ED, Crowley DC, Chahine M, Evans M. Examining the efficacy of a very-low-carbohydrate ketogenic diet on cardiovascular health in adults with mildly elevated low-density lipoprotein cholesterol in an open-label pilot study. Metab Syndr Relat Disord. 2022;20:94-103. 10.1089/met.2021.0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cashen K, Petersen T. Diabetic ketoacidosis. Pediatr Rev. 2019;40:412-420. 10.1542/pir.2018-0231 [DOI] [PubMed] [Google Scholar]
- 117. Dhatariya KK. Defining and characterising diabetic ketoacidosis in adults. Diabetes Res Clin Pract. 2019;155:107797. 10.1016/j.diabres.2019.107797 [DOI] [PubMed] [Google Scholar]
- 118. Tallis J, James RS, Seebacher F. The effects of obesity on skeletal muscle contractile function. J Exp Biol. 2018;221:16384. 10.1242/jeb.163840 [DOI] [PubMed] [Google Scholar]
- 119. Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL; IOF Bone and Diabetes Working Group. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13:208-219. 10.1038/nrendo.2016.153 [DOI] [PubMed] [Google Scholar]
- 120. Iyer A, Fairlie DP, Prins JB, Hammock BD, Brown L. Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol. 2010;6:71-82. 10.1038/nrendo.2009.264 [DOI] [PubMed] [Google Scholar]
- 121. Bliddal H, Leeds AR, Christensen R. Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons—a scoping review. Obes Rev. 2014;15:578-586. 10.1111/obr.12173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. King LK, March L, Anandacoomarasamy A. Obesity & osteoarthritis. Indian J Med Res. 2013;138:185-193. PMID: 24056594 [PMC free article] [PubMed] [Google Scholar]
- 123. Zapata-Linares N, Eymard F, Berenbaum F, Houard X. Role of adipose tissues in osteoarthritis. Curr Opin Rheumatol. 2021;33:84-93. 10.1097/BOR.0000000000000763 [DOI] [PubMed] [Google Scholar]
- 124. Fan J, Zhu J, Sun L, Li Y, Wang T, Li Y. Causal association of adipokines with osteoarthritis: a Mendelian randomization study. Rheumatology (Oxford). 2021;60:2808-2815. 10.1093/rheumatology/keaa719 [DOI] [PubMed] [Google Scholar]
- 125. Wang T, He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38-50. 10.1016/j.cytogfr.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 126. Mathus-Vliegen EMH, Basdevant A, Finer N, et al. ; Obesity Management Task Force of the European Association for the Study of Obesity. Prevalence, pathophysiology, health consequences and treatment options of obesity in the elderly: a guideline. Obes Facts. 2012;5:460-483., 10.1159/000341193 [DOI] [PubMed] [Google Scholar]
- 127. Babu S, Vaish A, Vaishya R, Agarwal A. Can intermittent fasting be helpful for knee osteoarthritis? J Clin Orthop Trauma. 2021;16:70-74. 10.1016/j.jcot.2020.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Muthuri SG, Hui M, Doherty M, Zhang W. What if we prevent obesity? Risk reduction in knee osteoarthritis estimated through a meta-analysis of observational studies. Arthritis Care Res (Hoboken). 2011;63:982-990. 10.1002/acr.20464 [DOI] [PubMed] [Google Scholar]
- 129. Drinda S, Franke S, Schmidt S, et al. AGE-RAGE interaction does not explain the clinical improvements after therapeutic fasting in osteoarthritis. Complement Med Res. 2018;25:167-172. 10.1159/000486237 [DOI] [PubMed] [Google Scholar]
- 130. Strath LJ, Jones CD, Philip George A, et al. The effect of low-carbohydrate and low-fat diets on pain in individuals with knee osteoarthritis. Pain Med. 2020;21:150-160. 10.1093/pm/pnz022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Masino SA, Ruskin DN. Ketogenic diets and pain. J Child Neurol. 2013;28:993-1001. 10.1177/0883073813487595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Marques Miranda C, de Lima Campos M, Leite-Almeida H. Diet, body weight and pain susceptibility—a systematic review of preclinical studies. Neurobiol Pain. 2021;10:100066. 10.1016/j.ynpai.2021.100066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Felson DT, Misra D, LaValley M, et al. Fatty acids and osteoarthritis: the MOST study. Osteoarthritis Cartilage. 2021;29:973-978. 10.1016/j.joca.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Fraser DA, Thoen J, Bondhus S, et al. Reduction in serum leptin and IGF-1 but preserved T-lymphocyte numbers and activation after a ketogenic diet in rheumatoid arthritis patients. Clin Exp Rheumatol. 2000;18:209-214. [PubMed] [Google Scholar]
- 135. Fraser DA, Thoen J, Djøseland O, Førre O, Kjeldsen-Kragh J. Serum levels of interleukin-6 and dehydroepiandrosterone sulphate in response to either fasting or a ketogenic diet in rheumatoid arthritis patients. Clin Exp Rheumatol. 2000;18:357-362. [PubMed] [Google Scholar]
- 136. Deswal R, Narwal V, Dang A, Pundir C. The prevalence of polycystic ovary syndrome: a brief systematic review. J Hum Reprod Sci. 2020;13:261-271. 10.4103/jhrs.JHRS_95_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Barber TM, Hanson P, Weickert MO, Franks S. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin Med Insights Reprod Health. 2019;13:1179558119874042. 10.1177/1179558119874042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Li C, Xing C, Zhang J, Zhao H, Shi W, He B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J Transl Med. 2021;19:148. 10.1186/s12967-021-02817-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Paoli A, Mancin L, Giacona MC, Bianco A, Caprio M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18:104. 10.1186/s12967-020-02277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Li J, Bai W-P, Jiang B, et al. Ketogenic diet in women with polycystic ovary syndrome and liver dysfunction who are obese: a randomized, open‐label, parallel‐group, controlled pilot trial. J Obstet Gynaecol Res. 2021;47:1145-1152. 10.1111/jog.14650 [DOI] [PubMed] [Google Scholar]
- 141. Kulak D, Polotsky AJ. Should the ketogenic diet be considered for enhancing fertility? Maturitas. 2013;74:10-13. 10.1016/j.maturitas.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 142. Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Dietary fatty acid intakes and the risk of ovulatory infertility. Am J Clin Nutr. 2007;85:231-237. 10.1093/ajcn/85.1.231 [DOI] [PubMed] [Google Scholar]
- 143.Accessed November 2024. https://www.cancer.org/cancer/cancer-causes/diet-physical-activity/body-weight-and-cancer-risk/effects.html
- 144.Accessed November 2024. https://www.worldwidecancerresearch.org/news-opinion/2020/october/what-is-the-link-between-obesity-and-cancer/
- 145.Accessed November 2024. https://www.cdc.gov/cancer/obesity/index.htm
- 146. Ye H, Adane B, Khan N, et al. Subversion of systemic glucose metabolism as a mechanism to support the growth of leukemia cells. Cancer Cell. 2018;34:659-673.e6. 10.1016/j.ccell.2018.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhang J, Jia P-P, Liu Q-L, et al. Low ketolytic enzyme levels in tumors predict ketogenic diet responses in cancer cell lines in vitro and in vivo. J Lipid Res. 2018;59:625-634. 10.1194/jlr.M082040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Feng S, Wang H, Liu J, AA J, Zhou F, Wang G. Multi-dimensional roles of ketone bodies in cancer biology: opportunities for cancer therapy. Pharmacol Res. 2019;150:104500. 10.1016/j.phrs.2019.104500 [DOI] [PubMed] [Google Scholar]
- 149. Lin B-Q, Zeng Z-Y, Yang S-S, Zhuang C-W. Dietary restriction suppresses tumor growth, reduces angiogenesis, and improves tumor microenvironment in human non-small-cell lung cancer xenografts. Lung Cancer. 2013;79:111-117. 10.1016/j.lungcan.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 150. Allen BG, Bhatia SK, Buatti JM, et al. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin Cancer Res. 2013;19:3905-3913. 10.1158/1078-0432.CCR-12-0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Woolf EC, Syed N, Scheck AC. Tumor metabolism, the ketogenic diet and β-hydroxybutyrate: novel approaches to adjuvant brain tumor therapy. Front Mol Neurosci. 2016;9:122. 10.3389/fnmol.2016.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zuccoli G, Marcello N, Pisanello A, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case Rrport. Nutr Metab (Lond). 2010;7:33. 10.1186/1743-7075-7-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hao G-W, Chen Y-S, He D-M, Wang H-Y, Wu G-H, Zhang B. Growth of human colon cancer cells in nude mice is delayed by ketogenic diet with or without omega-3 fatty acids and medium-chain triglycerides. Asian Pac J Cancer Prev. 2015;16:2061-2068. 10.7314/APJCP.2015.16.5.2061 [DOI] [PubMed] [Google Scholar]
- 154. Cohen CW, Fontaine KR, Arend RC, Gower BA. A ketogenic diet is acceptable in women with ovarian and endometrial cancer and has no adverse effects on blood lipids: a randomized, controlled trial. Nutr Cancer. 2020;72:584-594. 10.1080/01635581.2019.1645864 [DOI] [PubMed] [Google Scholar]
- 155. Cohen C, Fontaine K, Arend R, Soleymani T, Gower B. Favorable effects of a ketogenic diet on physical function, perceived energy, and food cravings in women with ovarian or endometrial cancer: a randomized, controlled trial. Nutrients. 2018;10:1187. 10.3390/nu10091187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Cohen CW, Fontaine KR, Arend RC, et al. A ketogenic diet reduces central obesity and serum insulin in women with ovarian or endometrial cancer. J Nutr. 2018;148:1253-1260. 10.1093/jn/nxy119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Porper K, Zach L, Shpatz Y, et al. Dietary-induced ketogenesis: adults are not children. Nutrients. 2021;13:3093. 10.3390/nu13093093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Chu-Shore CJ, Thiele EA. Tumor growth in patients with tuberous sclerosis complex on the ketogenic diet. Brain Dev. 2010;32:318-322. 10.1016/j.braindev.2009.04.009 [DOI] [PubMed] [Google Scholar]
- 159. Zahra A, Fath MA, Opat E, et al. Consuming a ketogenic diet while receiving radiation and chemotherapy for locally advanced lung cancer and pancreatic cancer: the University of Iowa experience of two phase 1 clinical trials. Radiat Res. 2017;187:743-754. 10.1667/RR14668.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Tragni E, Vigna L, Ruscica M, et al. Reduction of cardio-metabolic risk and body weight through a multiphasic very-low calorie ketogenic diet program in women with overweight/obesity: a study in a real-world setting. Nutrients. 2021;13:1804. 10.3390/nu13061804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Arslan N, Guzel O, Kose E, et al. Is ketogenic diet treatment hepatotoxic for children with intractable epilepsy? Seizure. 2016;43:32-38. 10.1016/j.seizure.2016.10.024 [DOI] [PubMed] [Google Scholar]
- 162. Vargas-Molina S, García-Sillero M, Bonilla DA, Petro JL, García-Romero J, Benítez-Porres J. The effect of the ketogenic diet on resistance training load management: a repeated-measures clinical trial in trained participants. J Int Soc Sports Nutr. 2024;21:2306308. 10.1080/15502783.2024.2306308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Cho W, Jung H, Hong S, et al. The effect of a short-term ketogenic diet on exercise efficiency during graded exercise in healthy adults. J Int Soc Sports Nutr. 2023;20:2264278. 10.1080/15502783.2023.2264278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. McKay AKA, Pyne DB, Burke LM, Peeling P. Iron metabolism: interactions with energy and carbohydrate availability. Nutrients. 2020;12:3692. 10.3390/nu12123692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Li S, Yuan S, Lin G, Zhang J. Effects of a two meals-a-day ketogenic diet on newly diagnosed obese patients with type 2 diabetes mellitus: a retrospective observational study. Medicine (Baltimore). 2023;102: E 35753. 10.1097/MD.0000000000035753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Watanabe M, Tuccinardi D, Ernesti I, et al. Scientific evidence underlying contraindications to the ketogenic diet: an update. Obes Rev. 2020;21:E13053. 10.1111/obr.13053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Leow ZZX, Guelfi KJ, Davis EA, Jones TW, Fournier PA. The glycaemic benefits of a very‐low‐carbohydrate ketogenic diet in adults with type 1 diabetes mellitus may be opposed by increased hypoglycaemia risk and dyslipidaemia. Diabet Med. 2018;35:1258-1263. 10.1111/dme.13663 [DOI] [PubMed] [Google Scholar]
- 168. Graybeal AJ, Kreutzer A, Moss K, Shah M. Changes in the chronic and postprandial blood lipid profiles of trained competitive cyclists and triathletes following a ketogenic diet: a randomized crossover trial. BMC Sports Sci Med Rehabil. 2024;16:19. 10.1186/s13102-023-00801-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Berry-Kravis E, Booth G, Sanchez AC, Woodbury-Kolb J. Carnitine levels and the ketogenic diet. Epilepsia. 2001;42:1445-1451. 10.1046/j.1528-1157.2001.18001.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.