Abstract
Hemangiomas are benign tumors of the vascular endothelium and are the most common tumors of infancy. These tumors are characterized by an initial phase of rapid proliferation, which is followed, in most cases, by spontaneous involution. Although most lesions resolve without complication, there are some cases in which hemangiomas can be life threatening when occurring near a vital structure. Treatment for these aggressive tumors represents an unmet clinical need. In addition, this characteristic progression of hemangiomas through distinct phases provides a unique opportunity for studying endothelial cell biology and angiogenesis. Using DNA microarrays representing approximately 10,000 human genes, we identified insulin-like growth factor 2 (IGF-2) as a potentially important regulator of hemangioma growth. IGF-2 was highly expressed during the proliferative phase and substantially decreased during involution. This finding was confirmed at the message level by quantitative reverse transcription–PCR and at the protein level by immunohistochemistry. IGF-2 protein was localized primarily to tumor vessels or vascular channels. Using a human hemangioma explant model, we show that IGF-2 promotes sprouting from intact hemangioma tissue. In addition, several angiogenesis-related factors, including integrins αvβ3 and α5β1, are present in proliferating hemangiomas. During the involuting phase, an increase in several IFN-induced genes was observed. These studies identify potential regulators of hemangioma growth and involution and provide a foundation on which to build further mechanistic investigations into angiogenesis, using hemangiomas as a model.
Hemangiomas are the most common tumors of infancy. They are benign vascular tumors that generally grow rapidly in the first months of life, enter a plateau phase, and in the majority of cases, involute spontaneously over the course of several years (1). These lesions are thought to arise from the microvascular endothelium and usually follow a benign course with adverse effects generally limited to transient cosmetic concerns. However, in some cases, hemangiomas can become vision- or life-threatening when occurring near the eye, airway, or other vital structures. Hemangiomas, particularly facial lesions, can be disfiguring and also often carry a significant psychosocial component for the patient and family.
Systemic or intralesional corticosteroids are often effective treatments for hemangiomas and are a first-line therapy for those requiring intervention. In proliferative hemangiomas, corticosteroids arrest growth with response rates of 30–90% (2). However, side effects such as delayed skeletal growth, depressed immune function, and personality changes limit their usefulness, particularly when long-term treatment is required. In life-threatening cases that have not responded to corticosteroids, IFN-α has been used as an alternative (3, 4). IFN-α is believed to inhibit proliferation of endothelial cells; however, its use has been limited by reports of severe and irreversible neurotoxicity in as many as 20% of patients treated (5, 6).
Despite advances in the fields of angiogenesis and endothelial cell biology, the pathogenesis of hemangiomas remains unclear. Investigations into the molecular factors controlling the growth and subsequent involution of hemangiomas have identified several factors that have phase-specific expression patterns and thus may have roles in regulating these lesions. Using several angiogenesis-related markers, immunohistochemical analysis has identified proliferating cell nuclear antigen, type IV collagenase, and vascular endothelial growth factor (VEGF) as being expressed during hemangioma proliferation (7). Tissue inhibitor of metalloproteinase 1, a reported inhibitor of new blood vessel formation, was shown to be expressed exclusively in the involution phase, whereas basic fibroblast growth factor (bFGF) and urokinase were expressed during both proliferation and involution. Another study has also implicated VEGF and bFGF as important in hemangioma progression and has suggested that an imbalance in positive and negative regulators of angiogenesis is associated with hemangioma development (8). IFN-β was also identified as a potential endogenous inhibitor of hemangioma proliferation, because it was found to be expressed in the epidermis overlying involuted hemangiomas, whereas epidermis overlying proliferating tumors showed no expression. Other studies have shown increased expression of clusterin/ApoJ (9) and cytochrome b (10) during hemangioma involution. Using cultured endothelial cells isolated from hemangiomas, the angiopoietin/Tie2 signaling pathway has been implicated in hemangioma pathogenesis by showing increased expression of Tie2 and increased cellular response to angiopoietin-1 in hemangioma-derived cells compared with normal endothelial cells (11). Although these studies have described biological characteristics of hemangiomas in different phases and identified pathways that may be involved, the causative factors in hemangioma growth and involution remain to be clearly identified.
Studies into molecular regulators of hemangioma progression have generally focused on a preselected group of genes known to have roles in angiogenesis in other contexts (7, 8, 11–14). In one report, differential display analysis was used to identify mRNAs that undergo changes in expression during the different phases (9). Unlike other studies focusing on factors known to be involved in angiogenesis, this approach has the advantage of indiscriminate expression analysis but is limited by the resolution of electrophoretic separation. The present study, using DNA microarrays representing approximately 10,000 human genes with known functions, also has the advantage of indiscriminate expression analysis but has a higher degree of sensitivity and is more quantitative. The power of this approach lies in the ability to analyze gene expression within a complex tissue undergoing defined stages of proliferation and involution. From such an analysis, one can identify a workable number of targets on which to focus efforts to develop safe effective treatments for an important disease. In a broader context, it is anticipated that these studies will provide information into the mechanisms of neovascularization in angiogenesis-dependent diseases.
Methods
Specimens.
Twenty specimens were obtained by surgical resection of lesions from children ranging in age from 3 mo to 7 yr. Procedures conformed to National Institutes of Health guidelines regarding use of human subjects and received Institutional Review Board approval from participating institutions. All specimens were obtained from tissue that would otherwise have been discarded and were collected from Children's Hospital, San Diego, CA. Cases were classified as proliferating, plateau or involuting on the basis of clinical appearance and age of the patient.
DNA Microarray Analysis.
After resection, tissue was immediately placed in RNAlater (Ambion, Austin, TX) and frozen at −80°C. Skin or other surrounding tissue was carefully removed before homogenization. Total RNA was isolated from specimens using Trizol (GIBCO/BRL). Isolated RNA was further purified with the RNeasy kit (Qiagen, Valencia, CA). Integrity of the RNA was verified before reverse transcription by visualization of the 28S and 18S ribosomal RNA bands on an agarose gel. cDNA was then in vitro transcribed with incorporation of labeled ribonucleotides. cRNA was then fragmented and hybridized to Affymetrix U95Av2 arrays (Santa Clara, CA). Hybridization quality was checked by measuring the ratio of hybridization intensities of the 3′ to 5′ regions of control genes. Data analysis was performed by using genespring software (Silicon Genetics, Redwood City, CA). Normalization was used to allow chip-to-chip comparisons by dividing the expression values for each gene by the median gene expression value for each chip. Three independent cases from each clinical phase were used for analysis.
Quantitative Reverse Transcription–PCR (RT-PCR).
RNA isolated as described above for DNA microarray analysis was reverse transcribed by using poly-d(T) primers and the Bulk First-Strand cDNA Reaction Mix (Amersham Pharmacia). Quantitative RT-PCR reactions were performed with IGF-2-specific primers designed using Primer Express (Perkin–Elmer). The forward primer had the sequence 5′-CCGTGCTTCCGGACAACTT-3′ and the reverse primer, 5′-TGGACTGCTTCCAGGTGTCA-3′. All reactions were performed in triplicate with equal quantities of starting RNA.
Immunohistochemistry.
Tissue was placed in ice-cold 20% sucrose/PBS immediately after resection. Tissue was then embedded in OCT compound, frozen, and sectioned. After blocking, sections were incubated overnight with primary antibody followed by incubation with fluorophore-conjugated secondary antibody. Imaging was done with a multiphoton confocal microscope (Bio-Rad). Integrin αvβ3 (LM609) and type IV collagen (AB748) antibodies were obtained from Chemicon. Antibody against α5β1 was generously provided by EOS Biotechnology (South San Francisco, CA). IGF-2 protein was detected with clone S7F2 from Research Diagnostics (Flanders, NJ). CD31 was detected with clone JC/70A anti-human CD31 (platelet–endothelial cell adhesion molecule, PECAM) mAb from Biocare Medical (Walnut Creek, CA).
Hemangioma Explants.
Resected hemangioma specimens were prepared for culture as described by Tan et al. (15). Briefly, fresh hemangioma tissue was cut into fragments (1–2 mm) and embedded into a fibrin gel (3 mg/ml of fibrin/1 mg/ml of streptomycin/100 units/ml of penicillin/10 mg/ml of ɛ-amino caproic acid/20 μg/ml of aprotinin/0.5 units/ml of thrombin in MCDB131 medium). Gels were then covered with MDCB131 medium and incubated at 37°C in 5% CO2. Recombinant IGF-2 protein (Calbiochem; ED50 = 10 ng/ml) was added at 50 ng/ml to the cultures daily, and medium was changed every 5 days. Sprouting cells were stained with isolectin IB4 from Griffonia simplicifolia conjugated with Alexa Flour 594 (Molecular Probes).
Results
Microarray Data.
Each of the three hemangioma phases, as determined by clinical classification, was analyzed in triplicate. One thousand one hundred and thirty-eight genes were expressed above a relatively stringent arbitrarily assigned level (raw value = 1,000). Forty-four genes with expression over the assigned level in any of the three phases underwent at least a 3-fold decrease in expression during the progression from proliferation to involution (see Table 1, which is published as supporting information on the PNAS web site, www.pnas.org), whereas 68 genes underwent at least a 3-fold increase under these conditions (see Table 2, which is published as supporting information on the PNAS web site).
Hemangiomas Express Angiogenic Factors and Markers of Proliferating Endothelial Cells.
To validate the results obtained from the microarray experiments, we examined the expression profiles of genes previously associated with angiogenesis, and in particular, a selection of genes reported to be expressed in hemangiomas. Studies have demonstrated that the angiogenic growth factors VEGF and basic fibroblast growth factor are expressed in proliferating hemangiomas (7, 8). Angiogenesis-related enzymes, type IV collagenase and urokinase, and tissue inhibitor of metalloproteinase 2 have also been reported to be expressed in hemangiomas (7). Microarray analysis confirmed expression of all of these genes at the mRNA level; however, the expression levels of these genes were relatively low and did not change significantly throughout hemangioma progression. Type IV collagen, a major component of endothelial basement membranes, has been reported to be expressed in hemangiomas (14). Microarray analysis demonstrated that type IV collagen was expressed at high levels during the proliferative phase and decreased 3.5-fold as the tumors progressed toward involution (Fig. 1a). Immunohistochemical analysis confirmed that type IV collagen was expressed at the protein level and demonstrated lower levels in involuting tumors compared with proliferating tumors (Fig. 2 i and j).
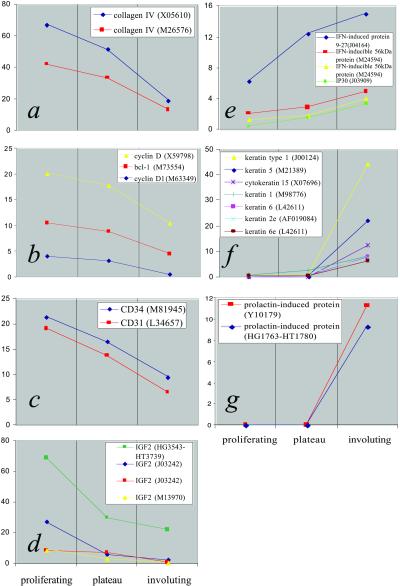
Figure 1.
Gene expression profiles of selected genes generated through DNA microarray analysis. Data represent average values obtained from three independent cases from each clinical phase. Genes are identified in the Insets, followed by their GenBank accession numbers (in parentheses). Some genes are represented by multiple probe sets on the arrays and are shown here. y axes represent normalized expression levels. (a–c) Expression of type IV collagen (a), cyclin genes (b), and CD31 and CD34 (c) decrease as hemangiomas progress toward involution, reflecting reduced endothelial proliferation in involuting tumors. (d) High IGF-2 message levels are found in proliferating hemangiomas, decreasing substantially in involuting tumors. (e–g) IFN-induced genes (e), keratins (f), and prolactin-induced protein (g) show increased expression during hemangioma involution.
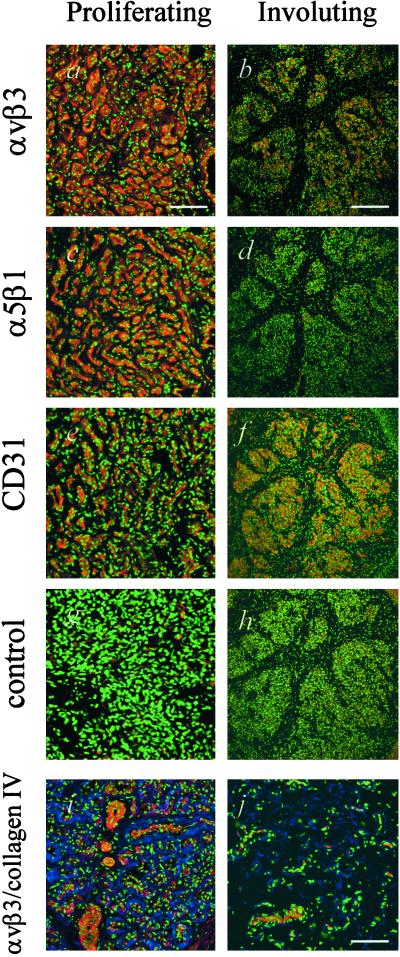
Figure 2.
Immunohistochemical analysis of hemangiomas. All sections were costained with DAPI to visualize nuclei (green). (a–h) αvβ3 integrin (red) is highly expressed in proliferating tumors (Bar = 100 μm) (a) and in the islands of hemangioma tissue remaining during involution (Bar = 300 μm) (b), α5β1 integrin (red) is expressed on the endothelium of proliferating hemangiomas (c) and to a lesser degree, in involuting tumors (d). Endothelium is identified by CD31 staining (red; e and f). Corresponding negative controls are shown (g and h). a, c, e, and g represent serial sections although a single specimen. b, d, f, and h similarly represent serial sections although a single specimen. (i and j) Collagen IV (blue) is highly expressed in proliferative lesions and is distributed around groups of αvβ3-positive (red) cells (i). Involuting tumors show fewer αvβ3-positive cells and less collagen IV (j). (Bar = 100 μm.)
Among the genes undergoing significant interphase changes in expression were those associated with regulation of mitosis, a finding that supports the concept that proliferating hemangiomas are populated largely by dividing endothelial cells. The cyclin family of proteins regulates the cell cycle, and selected members were found to be differentially expressed in hemangiomas of different phases (Fig. 1b). For example, cyclin D, which controls the G1/S transition, is found to undergo substantial reduction in expression between the proliferating and involuting phases.
As hemangiomas progress toward involution, endothelial cells comprise a decreasing proportion of the lesion. This observation is reflected in the data where platelet–endothelial cell adhesion molecule (PECAM) (CD31), an endothelial marker, is found to be expressed in proliferating cases at levels three times that of involuting cases. Another endothelial cell marker, CD34, follows a similar pattern with reduced expression during involution (Fig. 1c). By demonstrating that hemangiomas express previously identified genes related to angiogenesis and proliferating endothelial cells, microarray analysis is validated as an approach to investigate other genes that are important in regulating hemangioma growth and involution.
Integrins αvβ3 and α5β1 Are Expressed on the Endothelium of Proliferating Hemangiomas.
A subset of integrin receptors are markers of proliferating endothelial cells. Integrin αvβ3 has been shown to have important roles in angiogenesis by regulating endothelial proliferation, migration, and apoptosis (16, 17). More recently, α5β1 has been shown to be involved in regulating similar functions, possibly by modulating αvβ3 function (18). Microarray analysis identified message for these integrin subunits as being expressed (data not shown). To determine whether the heterodimers are in fact present, immunohistochemical analysis was performed on sections of human hemangiomas. Strong expression of the αvβ3 integrin was demonstrated throughout the tumor in proliferating cases (Fig. 2a). By using serial sections and an antibody specific for PECAM (CD31), it is shown that the αvβ3-positive regions are also positive for this endothelial cell marker (Fig. 2e). A similar pattern of expression was seen with the α5β1 integrin (Fig. 2c). Both integrins were present in involuting tumors but appear to have reduced expression levels and were more localized, likely marking a minority of cells that are still proliferating (Fig. 2 b and d). Hemangiomas did not, however, stain positive for another angiogenesis-associated integrin, αvβ5 (data not shown).
Proliferating Hemangiomas Express High Levels of IGF-2 mRNA.
Large-scale analysis of gene expression revealed high levels of IGF-2 transcript in proliferating tumors (Fig. 1d). Mean IGF-2 levels were 18,950 (using Affymetrix average difference values) or 69-fold higher than the median expression level, corresponding to expression in the top 0.1% of genes represented on the arrays. Expression was detected by multiple probe sets on the array. These redundant probe sets recognize different regions of the IGF-2 gene and, by showing similar expression patterns, serve as internal confirmation of the results. Mean expression levels from phase replicates were greater than 10-fold higher in proliferating hemangiomas compared with involuting tumors, with plateau tumors showing intermediate expression. To independently confirm the IGF-2 results obtained from the microarray experiments, we performed quantitative reverse transcription–PCR by using IGF-2-specific primer sets. Data from this series of experiments strongly agreed with the microarray data (see Fig. 5, which is published as supporting information on the PNAS web site).
IGF-2 Protein Is Present in Proliferating Hemangiomas and Localizes with Tumor Vessels.
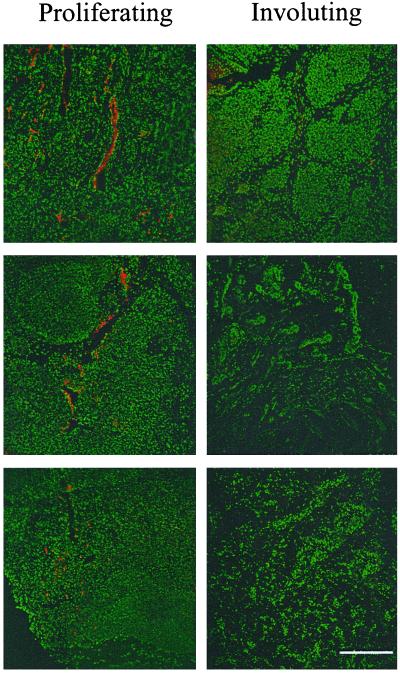
To determine that high levels of IGF-2 message detected in proliferating hemangiomas correlated with protein expression, we performed immunohistochemical analysis for IGF-2 protein on sections of human hemangiomas. IGF-2 protein was detected in proliferating tumors, but not in involuting hemangiomas (Fig. 3). Immunohistochemistry for IGF-2 also provided information on the localization of the protein. IGF-2 localized to vessels within the tumor, as confirmed by endothelial staining with CD31 (not shown) and nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI) (Fig. 3). The protein was also found in small foci scattered across the specimens. These findings clearly corroborate, at the protein level, those from the microarray and reverse transcription–PCR experiments and strengthen the idea that IGF-2 is important in regulating the proliferative phase of hemangiomas.
Figure 3.
IGF-2 protein is expressed at high levels in proliferating hemangiomas. Immunohistochemical analysis demonstrates that IGF-2 protein (red) localizes to blood vessels or vascular channels in proliferating, but not involuting, tumors. Nuclei are stained with DAPI (green). Six independent cases are shown. (Bar = 300 μm.)
IGF-2 Stimulates Outgrowth of Sprouts from Human Hemangioma Explants.
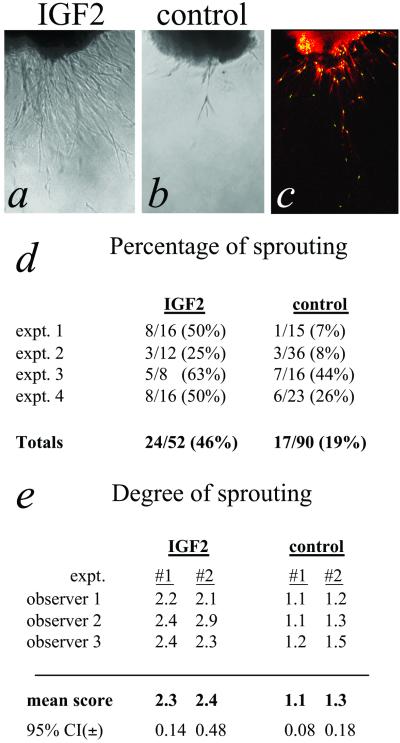
Recently, Tan et al. showed that freshly resected hemangioma tissue placed into fibrin gels develop outgrowths or “microvessels” within days after placing into culture (15). Using this system, we demonstrated that IGF-2 stimulated significant sprouting from the intact tissue explant (Fig. 4a). Evidence that these sprouting outgrowths contain endothelial cells was provided by showing positive staining with G. simplicifolia isolectin (Fig. 4c), a documented endothelial stain (19–21). Cells were also positive for staining with antibodies against CD31 and CD34. For these markers, positive cells were observed only in the area immediately adjacent to the explant tissue (not shown), suggesting that these cells may dedifferentiate and down-regulate these proteins as they acquire a migratory phenotype. Treated explants sprouted with a higher frequency after 7 days in culture compared with control explants (Fig. 4d). Among the sprouting explants, the IGF-2-treated group was dramatically more advanced in terms of number and length of sprouts than the control group (Fig. 4e).
Figure 4.
IGF-2 promotes sprouting of outgrowths from cultured hemangioma explant tissue. (a) Representative phase-contrast image of sprouts arising from explant cultures of hemangioma tissue shows significant enhancement in the presence of IGF-2 compared with control (b). (Bar = 1 mm.) (c) Cells migrating from explanted tissue are stained with G. simplicifolia lectin (red). Nuclei are stained green with DAPI. (d) Percentages of sprouting explants after 7 days in culture compiled from four independent experiments (P < 0.0001). (e) Semiquantitative measurement of the degree of sprouting in those explants that showed observable sprouting. A scale of 1–3 was used to describe degree of sprouting, where 1 represents minimum sprouting and 3, the most advanced sprouting observed in that experiment. Mean scores from three blinded observers scoring two independent experiments are shown. P < 0.0001 for combined means from both experiments.
Apoptosis-Related Genes Are Up-Regulated in Involuting Hemangiomas.
We expected to find increased expression of apoptosis-related genes in involuting tumors because apoptosis characterizes this phase (22, 23). Several IFN-induced genes did, in fact, increase during the involuting phase (Fig. 1e). Interestingly, IFN genes themselves were not expressed at significant levels within the tumor, consistent with previous reports that IFN-β is produced in the skin overlying cutaneous hemangiomas (8). Although not believed to have roles in apoptosis, several types of keratin were up-regulated during involution (Fig. 1f). Keratins may be components of the fibro-fatty residuum observed after hemangiomas resolve. Prolactin-induced protein (GCDFP-15, gp17), which was not expressed at detectable levels during proliferation, was expressed at significant levels in involuting tumors (Fig. 1g).
Discussion
We have used large-scale expression analysis to identify a number of genes that may be important to growth and regression of hemangiomas. The findings were indiscriminately generated by simultaneous analysis of approximately 10,000 human genes. IGF-2 has been implicated in a number of malignancies including breast cancer (24) and Wilm's tumor (25). There is also evidence that IGF-2 may have roles in angiogenesis but has not previously been associated with hemangiomas. IGF-2 is a reasonable putative regulator of hemangioma proliferation, because it is a known mitogen and suppresses apoptosis. The function of this protein has been characterized (26, 27), as has its primary functional receptor, IGF1R (28, 29). The IGF-2/mannose 6-phosphate receptor may also be important in hemangioma pathogenesis, because it had been shown to regulate angiogenesis in other contexts (30). Although its expression profile was not as dramatic, IGF-1 cannot be ruled out as a potential stimulator of hemangioma growth, and it is possible in fact, that IGF-1 and IGF-2 have cooperative effects because they share IGF1R as a major receptor.
The precise role of VEGF in hemangioma growth and involution is not clear. Our analysis supports the observation that VEGF is present (7, 14), although at low levels. Its expression, however, may be a downstream effect of high IGF levels, because IGFs have been demonstrated to induce expression of VEGF in a number of cell types (31–33). There is evidence that VEGF requires IGFs for its angiogenic activity (34, 35). Our data are consistent with this idea and suggest that, in fact, IGFs may be critical factors in this context because their expression is more highly correlated to hemangioma growth phase than is VEGF. GLUT1 expression (36) could be another example of a downstream effect, because IGFs are also known to increase cell surface expression of GLUT1 (37–39). Expression of type IV collagen has been demonstrated previously in hemangiomas (14), an observation confirmed in this report at both the message and protein levels. Immunohistochemistry shows that type IV collagen appears to be produced up to the point of endothelial apoptosis, suggesting that reduced levels are an effect, rather than a cause, of involution.
The distinct phases of hemangioma progression suggest that separate mechanisms are responsible for the growth of these tumors and their subsequent involution. This is contrasted to the idea of a single factor causing endothelial cell growth during the proliferative phase and its removal initiating involution by eliminating the growth/survival stimulus. Microarray analysis supports the multiple mechanism hypothesis by identifying groups of genes (i.e., IFN-induced genes) that undergo increases in expression during the involuting phase. IFNs themselves were not detected at significant levels, indicating that the source of these factors lies outside of the tumor. This notion is supported by the finding that IFN-β is expressed in the skin overlying cutaneous involuting hemangiomas (8). One of the three involuting cases studied with microarray analysis showed strong induction of a panel of IFN-related genes, whereas the increases in expression were more restricted and less dramatic in the other cases studied. This is a likely reinforcement of the concept that hemangioma growth and recession are a continuum of events rather than well-demarcated phases. This idea is in line with the clinical observation that hemangiomas vary in terms of time to involution, and thus all involuting specimens would not necessarily be expected to express IFN-induced genes.
Our data suggest that at least two approaches to therapeutic intervention of aggressive hemangiomas may be possible. IGF-2 or its receptors represent promising targets for early intervention. Antagonists of IGF-2 could prevent or halt the proliferation of endothelial cells and subsequent events downstream activated by IGF-2. An alternative approach would involve inducing premature involution of the tumors. One way to accomplish this would be with antagonists of αvβ3 and/or α5β1 integrins, which have been shown to induce apoptosis in other systems involving proliferating endothelial cells (40, 41). Specific fragments of fibronectin are known to block the function of α5β1 and αvβ3 integrins, inducing apoptosis of endothelial cells (42). It has also recently been reported that prolactin-induced protein, a novel aspartyl proteinase found to be dramatically up-regulated in involuting hemangiomas, specifically cleaves the extracellular matrix protein fibronectin (43), the major ligand for the α5β1 integrin. IFN-induced proteins have been identified as potential targets for the latter therapeutic approach by demonstrating that they are up-regulated in at least some involuting hemangiomas. These findings, along with those of Bielenberg et al. (8), provide a biological justification for the clinical use of IFN in the treatment of hemangiomas. However, the risk of severe side effects produced by IFN therapy minimizes its utility and necessitates the identification of new treatment options.
These data suggest a model of hemangioma progression in which the key element is a relationship between IGFs and IFNs. In this model, the initial event is overexpression of IGF-2 by the vascular endothelium or associated cells. There is a large literature on the role of imprinting in IGF-2 expression describing mechanisms involving relaxed suppression of one allele resulting in increased IGF-2 protein production (44, 45). Alternatively, hemangiomas may originate from placentally derived cells. Several markers associated with placental vessels were expressed in juvenile hemangiomas but not other types of vascular lesions (46), indicating that hemangiomas might arise from invading angioblasts or placental cells. Because IGF-2 is highly expressed and important during embryonic development, the findings from our study provide some support for this hypothesis. It is therefore possible that these placental cells produce IGFs and respond in an autocrine fashion by proliferating and at the same time are afforded protection from apoptosis by IGFs. High IGF levels could be expected to increase expression of VEGF, resulting in an increasingly favorable local environment for endothelial cell proliferation. Recent studies indicate that hemangiomas result from clonal expansion of endothelial cells (47, 48), conforming to the idea that a single angioblast expressing high levels of IGFs could be at the source of these lesions. The progeny of this cell would continue to express markers characteristic of placental vessels. It has been reported that IGFs induce expression of IFN-γ (49). In this scenario, sustained IGF levels could shift the balance of regulators in favor of involution. Not only can IFNs induce apoptosis, a known feature of involuting hemangiomas, but interestingly, they are also known to down-regulate the expression of IGF-2 and other growth factors (50, 51). Taken together, these events could complete a self-destructive program characteristic of hemangioma behavior.
These studies have confirmed some of what is known about hemangiomas at the molecular level and have identified new areas for investigation. We have used an explant model in this study to test the role of one newly identified factor in hemangioma progression. Although potentially important circulating factors are eliminated, this model has a significant advantage in that intact tissue from clinically characterized hemangiomas was used, thus preserving the cellular and extracellular milieu that exists in vivo. The observation that outgrowths contain cells that stain positively with G. simplicifolia isolectin suggests, but does not conclusively prove, that these sprouts are composed of endothelial cells. These studies do conclusively demonstrate, however, that cells within intact hemangioma tissue are responsive to IGF-2 and support the idea that IGF-2 is important in hemangioma proliferation.
The results of this study indicate that hemangiomas probably rely on several well-studied angiogenic themes but suggest that other factors, less commonly associated with angiogenesis, may also be important regulators. IGFs have been shown to be critical regulators in retinal angiogenesis, and inhibition of IGF1R suppresses VEGF-induced angiogenesis (34). These findings, combined with the current data, suggest the possibility of an emerging paradigm where IGFs and their primary receptor play important regulatory roles in angiogenic signaling pathways.
Supplementary Material
Acknowledgments
We thank Drs. Thomas Veccione, Ralph Holmes, and Steve Cohen (Children's Hospital, San Diego, CA) for kindly providing specimens. We acknowledge members of The Scripps Research Institute DNA Microarray Core Facility and Steve Head for expert assistance. We thank David Friedlander, Stacey Hanekamp, and Joshua Ney for assistance with this project. This work was supported by National Eye Institute Grant R01 EY11254, a National Eye Institute Administrative Supplement for Microarray Studies and by a National Eye Institute Core Grant for Vision Research (P30 EY12598) (M.F.). M.R.R. is supported by National Research Service Award Fellowship F32 EY13916.
Abbreviations
- IGF
insulin-like growth factor
- VEGF
vascular endothelial growth factor
- IGF1R
insulin-like growth factor 1 receptor
- DAPI
4′,6-diamidino-2-phenylindole
References
- 1.Mulliken J B, Glowacki J. Plast Reconstr Surg. 1982;69:412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Boon L M, MacDonald D M, Mulliken J B. Plast Reconstr Surg. 1999;104:1616–1623. doi: 10.1097/00006534-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Ezekowitz A, Mulliken J, Folkman J. Br J Haematol. 1991;79 Suppl. 1:67–68. doi: 10.1111/j.1365-2141.1991.tb08123.x. [DOI] [PubMed] [Google Scholar]
- 4.Chang E, Boyd A, Nelson C C, Crowley D, Law T, Keough K M, Folkman J, Ezekowitz R A, Castle V P. J Pediatr Hematol Oncol. 1997;19:237–244. doi: 10.1097/00043426-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Barlow C F, Priebe C J, Mulliken J B, Barnes P D, Mac Donald D, Folkman J, Ezekowitz R A. J Pediatr. 1998;132:527–530. doi: 10.1016/s0022-3476(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 6.Worle H, Maass E, Kohler B, Treuner J. Eur J Pediatr. 1999;158:344. doi: 10.1007/s004310051089. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Mulliken J B, Kozakewich H P, Rogers R A, Folkman J, Ezekowitz R A. J Clin Invest. 1994;93:2357–2364. doi: 10.1172/JCI117241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bielenberg D R, Bucana C D, Sanchez R, Mulliken J B, Folkman J, Fidler I J. Int J Oncol. 1999;14:401–408. doi: 10.3892/ijo.14.3.401. [DOI] [PubMed] [Google Scholar]
- 9.Hasan Q, Ruger B M, Tan S T, Gush J, Davis P F. Hum Pathol. 2000;31:691–697. doi: 10.1053/hupa.2000.7638. [DOI] [PubMed] [Google Scholar]
- 10.Hasan Q, Tan S T, Gush J, Davis P F. Plast Reconstr Surg. 2001;108:1471–1476. doi: 10.1097/00006534-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Yu Y, Varughese J, Brown L F, Mulliken J B, Bischoff J. Am J Pathol. 2001;159:2271–2280. doi: 10.1016/S0002-9440(10)63077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin-Padura I, De Castellarnau C, Uccini S, Pilozzi E, Natali P G, Nicotra M R, Ughi F, Azzolini C, Dejana E, Ruco L. J Pathol. 1995;175:51–57. doi: 10.1002/path.1711750109. [DOI] [PubMed] [Google Scholar]
- 13.Kraling B M, Razon M J, Boon L M, Zurakowski D, Seachord C, Darveau R P, Mulliken J B, Corless C L, Bischoff J. Am J Pathol. 1996;148:1181–1191. [PMC free article] [PubMed] [Google Scholar]
- 14.Tan S T, Velickovic M, Ruger B M, Davis P F. Plast Reconstr Surg. 2000;106:529–538. doi: 10.1097/00006534-200009030-00001. [DOI] [PubMed] [Google Scholar]
- 15.Tan S T, Hasan Q, Velickovic M, Ruger B M, Davis R P, Davis P F. Mod Pathol. 2000;13:92–99. doi: 10.1038/modpathol.3880014. [DOI] [PubMed] [Google Scholar]
- 16.Brooks P C, Clark R A, Cheresh D A. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 17.Friedlander M, Theesfeld C L, Sugita M, Fruttiger M, Thomas M A, Chang S, Cheresh D A. Proc Natl Acad Sci USA. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Harris M, Varner J A. J Biol Chem. 2000;275:33920–33928. doi: 10.1074/jbc.M003668200. [DOI] [PubMed] [Google Scholar]
- 19.Faraggiana T, Crescenzi A, Marinozzi V. Histochem J. 1989;21:235–240. doi: 10.1007/BF01747526. [DOI] [PubMed] [Google Scholar]
- 20.Sahagun G, Moore S A, Fabry Z, Schelper R L, Hart M N. Am J Pathol. 1989;134:1227–1232. [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen-Smith F, Egginton S, Zhou A L, Hudlicka O. Microvasc Res. 2001;62:1–14. doi: 10.1006/mvre.2001.2308. [DOI] [PubMed] [Google Scholar]
- 22.Iwata J, Sonobe H, Furihata M, Ido E, Ohtsuki Y. J Pathol. 1996;179:403–408. doi: 10.1002/(SICI)1096-9896(199608)179:4<403::AID-PATH604>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 23.Razon M J, Kraling B M, Mulliken J B, Bischoff J. Microcirculation. 1998;5:189–195. [PubMed] [Google Scholar]
- 24.Stewart A J, Johnson M D, May F E, Westley B R. J Biol Chem. 1990;265:21172–21178. [PubMed] [Google Scholar]
- 25.Ogawa O, Eccles M R, Szeto J, McNoe L A, Yun K, Maw M A, Smith P J, Reeve A E. Nature (London) 1993;362:749–751. doi: 10.1038/362749a0. [DOI] [PubMed] [Google Scholar]
- 26.Baker J, Liu J P, Robertson E J, Efstratiadis A. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 27.O'Dell S D, Day I N. Int J Biochem Cell Biol. 1998;30:767–771. doi: 10.1016/s1357-2725(98)00048-x. [DOI] [PubMed] [Google Scholar]
- 28.LeRoith D, Werner H, Beitner-Johnson D, Roberts C T., Jr Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 29.Sepp-Lorenzino L. Breast Cancer Res Treat. 1998;47:235–253. doi: 10.1023/a:1005955017615. [DOI] [PubMed] [Google Scholar]
- 30.Volpert O, Jackson D, Bouck N, Linzer D I. Endocrinology. 1996;137:3871–3876. doi: 10.1210/endo.137.9.8756559. [DOI] [PubMed] [Google Scholar]
- 31.Warren R S, Yuan H, Matli M R, Ferrara N, Donner D B. J Biol Chem. 1996;271:29483–29488. doi: 10.1074/jbc.271.46.29483. [DOI] [PubMed] [Google Scholar]
- 32.Punglia R S, Lu M, Hsu J, Kuroki M, Tolentino M J, Keough K, Levy A P, Levy N S, Goldberg M A, D'Amato R J, et al. Diabetes. 1997;46:1619–1626. doi: 10.2337/diacare.46.10.1619. [DOI] [PubMed] [Google Scholar]
- 33.Miele C, Rochford J J, Filippa N, Giorgetti-Peraldi S, Van Obberghen E. J Biol Chem. 2000;275:21695–216702. doi: 10.1074/jbc.M000805200. [DOI] [PubMed] [Google Scholar]
- 34.Smith L E, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, Robinson G, Driver S, Bischoff J, Zhang B, et al. Nat Med. 1999;5:1390–1395. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- 35.Hellstrom A, Perruzzi C, Ju M, Engstrom E, Hard A L, Liu J L, Albertsson-Wikland K, Carlsson B, Niklasson A, Sjodell L, et al. Proc Natl Acad Sci USA. 2001;98:5804–5808. doi: 10.1073/pnas.101113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.North P E, Waner M, Mizeracki A, Mihm M C., Jr Hum Pathol. 2000;31:11–22. doi: 10.1016/s0046-8177(00)80192-6. [DOI] [PubMed] [Google Scholar]
- 37.Bilan P J, Mitsumoto Y, Ramlal T, Klip A. FEBS Lett. 1992;298:285–290. doi: 10.1016/0014-5793(92)80078-u. [DOI] [PubMed] [Google Scholar]
- 38.Wilson C M, Mitsumoto Y, Maher F, Klip A. FEBS Lett. 1995;368:19–22. doi: 10.1016/0014-5793(95)00589-2. [DOI] [PubMed] [Google Scholar]
- 39.Fladeby C, Bjonness B, Serck-Hanssen G. J Cell Physiol. 1996;169:242–247. doi: 10.1002/(SICI)1097-4652(199611)169:2<242::AID-JCP3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 40.Brooks P C, Montgomery A M, Rosenfeld M, Reisfeld R A, Hu T, Klier G, Cheresh D A. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 41.Kim S, Bell K, Mousa S A, Varner J A. Am J Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukai F, Mashimo M, Akiyama K, Goto T, Tanuma S, Katayama T. Exp Cell Res. 1998;242:92–99. doi: 10.1006/excr.1998.4076. [DOI] [PubMed] [Google Scholar]
- 43.Caputo E, Manco G, Mandrich L, Guardiola J. J Biol Chem. 2000;275:7935–7941. doi: 10.1074/jbc.275.11.7935. [DOI] [PubMed] [Google Scholar]
- 44.Reik W, Constancia M, Dean W, Davies K, Bowden L, Murrell A, Feil R, Walter J, Kelsey G. Int J Dev Biol. 2000;44:145–150. [PubMed] [Google Scholar]
- 45.Burns J L, Jackson D A, Hassan A B. FASEB J. 2001;15:1694–1703. doi: 10.1096/fj.010085rev. [DOI] [PubMed] [Google Scholar]
- 46.North P E, Waner M, Mizeracki A, Mrak R E, Nicholas R, Kincannon J, Suen J Y, Mihm M C., Jr Arch Dermatol. 2001;137:559–570. [PubMed] [Google Scholar]
- 47.Boye E, Yu Y, Paranya G, Mulliken J B, Olsen B R, Bischoff J. J Clin Invest. 2001;107:745–752. doi: 10.1172/JCI11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walter J W, North P E, Waner M, Mizeracki A, Blei F, Walker J W, Reinisch J F, Marchuk D A. Genes Chromosomes Cancer. 2002;33:295–303. doi: 10.1002/gcc.10028. [DOI] [PubMed] [Google Scholar]
- 49.Tu W, Cheung P T, Lau Y L. Pediatr Res. 1999;46:748–754. doi: 10.1203/00006450-199912000-00019. [DOI] [PubMed] [Google Scholar]
- 50.Martin D M, Carlson R O, Feldman E L. J Neurosci Res. 1993;34:489–501. doi: 10.1002/jnr.490340502. [DOI] [PubMed] [Google Scholar]
- 51.Der S D, Zhou A, Williams B R, Silverman R H. Proc Natl Acad Sci USA. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.