Abstract
Background
Malaria transmission hinges on infected Anopheles mosquitoes biting humans, with carbon dioxide (CO2), host odor, and body heat acting as key attractants. Along the Thai–Myanmar border, Anopheles minimus (the Funestus Group), a primary malaria vector, exhibits a stronger preference for human hosts than species of the Maculatus Group. Elucidating the genetic basis of this feeding behavior is essential for improving malaria control strategies.
Methods
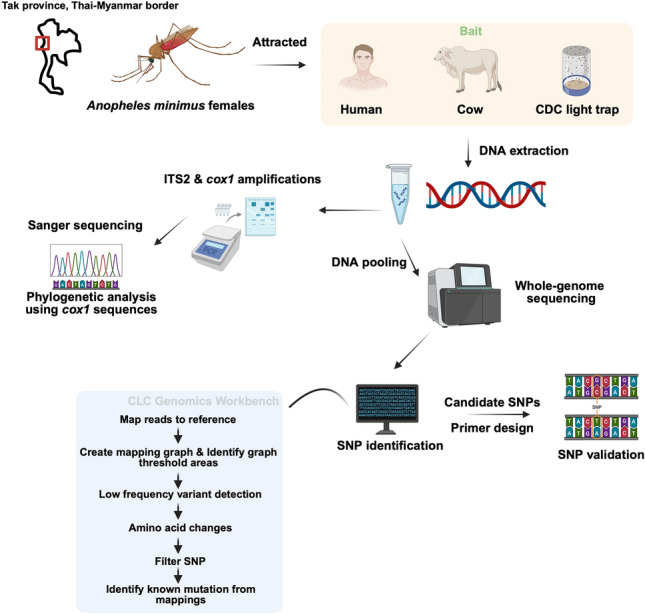
Wild Anopheles mosquitoes were collected in Tha Song Yang district, Tak province, Thailand, from July 2019 to November 2020, using cow-baited traps, human landing catches, and Center for Disease Control (CDC) light traps. Specimens were identified morphologically and confirmed by Sanger sequencing of the cytochrome c oxidase subunit 1 (cox1) gene. We then performed whole-genome sequencing on An. minimus females categorized by host-seeking behavior: cow-baited collection (COW), human landing indoor (HLI), and human landing outdoor (HLO) to investigate the genetic determinants of host preference.
Results
Anopheles minimus females accounted for 25% of total samples (504/1,997). Cox1 sequencing revealed 143 unique haplotypes among 287 specimens, forming two major phylogenetic lineages, A (181 sequences) and B (106 sequences), suggestive of potential cryptic diversity. Whole-genome sequencing of An. minimus Lineage A from COW, HLI, and HLO groups yielded 12,659,785 variants. After filtering, 68,975 non-synonymous single-nucleotide polymorphisms (nsSNPs) remained. Comparing allele frequencies across the three pooled groups (FDR-adjusted p-value < 0.001) yielded 2,629, 2,948, and 4,369 significant nsSNPs, respectively. Gene Ontology (GO) analysis of genes harboring these nsSNPs showed strong enrichment for olfaction-related terms. The top six nsSNPs with olfactory annotations from each group comparison were selected for validation; Sanger sequencing confirmed their association with host-seeking preference. The VectorBase gene IDs for these candidate nsSNPs are AMIN001807, AMIN001339, AMIN003886, AMIN000912, AMIN003926, AMIN011060, AMIN002342, and AMIN015480.
Conclusions
The observed significant genomic variance in field-collected An. minimus females, categorized by collection methods (reflecting host-seeking behavior), proposes a genetic underpinning for these behavioral variations. Differential nsSNPs within olfactory pathway genes might be functionally linked to host-seeking in this important malaria vector.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-025-07029-x.
Keywords: Mosquito, Whole-genome sequencing, Single-nucleotide polymorphism, Olfaction
Background
Malaria, caused by Plasmodium spp., Generally results in 600,000 deaths every year [1]. Certain female Anopheles mosquitoes transmit such pathogen to humans by biting [2]. The need of essential nutrients for egg development obligates them to feed on blood from hosts [3]. Thus, their innate biting behavior play a significant role in the disease transmission. To locate the hosts, they rely heavily on their robust olfactory organization [4, 5]. Carbon dioxide (CO2), host odor, and body heat are utilized as main cues, integrated with other sensory signals derived from humidity, mechanical effect, and visual inputs [6, 7]. Mosquitoes and other insects perceive those cues using peripheral appendages covered with sensilla [8]. Several forms of sensilla have been established on antennae, maxillary palps, proboscis, and tarsi of mosquito vectors [9–17]. Moreover, they are innervated by neurons that express olfactory receptors [18–22]. Zeroing in on insect olfactory receptors, these ligand-gated ion channels are encoded by three main gene classes: odorant receptors (ORs), ionotropic receptors (IRs), and gustatory receptors (GRs). Conventional ORs form a heterotetrameric complex with the highly conserved co-receptor Orco [23–27]. Ionotropic receptors are a variant subfamily of ionotropic glutamate receptors comprising IR tuning receptors and one of the three identified co-receptors, Ir8a, Ir25a, and Ir76b [28, 29]. A spectrum of odorants elicits responses from ORs and IRs [30, 31]. Strikingly, some IRs also detect temperature and humidity shifts [32–35]. In contrast to the previously mentioned receptors, the GR coreceptors have not yet been observed. The coreceptor-independent GRs are highly sensitive to CO2 by creating a three-GR complex [36–39]. Information via olfactory sensory neurons is encoded into an odor map inside the glomeruli within the antennal lobe and the higher brain centers, respectively [4].
Apart from well-studied anthropophilic vectors such as the dengue fever mosquito, Aedes aegypti [40] and the African malaria mosquito, Anopheles gambiae [41], most mosquito species tend to prefer animals as blood sources [42]. The increased expression of olfactory receptors assists mosquitoes in distinguishing between human and non-human hosts more precisely [42–46]. Hence, disruption of this fascinating system has received more attention over the past decade. Mutating the functional receptors to modulate host preference has been implemented by interrupting the expression of Orco [47], IR co-receptor genes (Ir8a, Ir25a, or Ir76b) [29, 48], and Gr3 [49]. The results from these works show a moderate loss of attraction to humans, but mutant mosquitoes still retain their anthropophilia. In field settings, environmental conditions profoundly affect mosquito feeding behavior, from generalists, who feed on a wide range of vertebrates, to host-specific mosquitoes or specialists [50–52]. Such adaptive feeding behavior could overcome the inherent traits in unfavorable situations, including the availability of preferred hosts [53, 54], the coverage of insecticide-treated interventions [55], and the parasite manipulation [56, 57].
Little is known about the underlying mechanisms in host-seeking behavior in field-collected mosquitoes, which could possibly be the game changer for new vector control strategies. In this study, we conducted a comparative analysis of An. minimus genomes from natural populations with different types of host preferences based on collection methods. Female An. minimus was chosen due to its strong anthropophilic behavior and vector competence compared with other vector species at the Thai–Myanmar border [58]. We performed a phylogenetic analysis, on the basis of cox1, and detected a new lineage of An. minimus, suggesting a possible unknown species. To assess the presence of the single-nucleotide polymorphisms (SNPs) within the olfactory-related genes, whole-genome sequencing was performed to generate genomic data.
Methods
Study site
The mosquitoes were collected within three hilly villages in Tha Song Yang district, Tak province: Suan Oi (SO, 17° 32′ 26.484'' N, 97° 56′ 16.908'' E), Pha Man (PM, 17° 32′ 22.596'' N, 97° 56′ 22.416'' E), and Komonae (KN, 17° 32′ 4.236'' N, 97° 57′ 9.684'' E) at the Thai–Myanmar border. The temperature typically ranges between 16 °C and 36 °C, and the average annual precipitation is 74.2 mm [59]. Malaria is endemic in these neighborhoods. Most of the indigenous cases are dominated by Plasmodium vivax while P. falciparum infections are rare and typically defined as imported cases [60]. The principal malaria vectors in this region are An. minimus, An. maculatus, and An. dirus [61–63].
Mosquito collection
To investigate the phenotypic host preference of Anopheles mosquitoes in malaria-endemic areas, collections of mosquitoes were carried out by three methods, cow bait, human bait, and Center for Disease Control (CDC) light traps. We tried to mimic the natural conditions by assigning the collection method in the study sites to represent the host seeking behavior of collected mosquitoes. Considering this is more convenient strategy to carry out the behavioral study in the field trials, there are several external factors (e.g., seasonal dynamics and weather conditions) that could affect the host preference and should not be overlooked. Notably, the availability of the preferred hosts is one of the significant factors that consistently concerns us in field studies. To minimize these confounding effects, the collections were conducted simultaneously, and all baits were placed exclusively outside the village boundaries at standardized distances.
Collections were conducted four times a month (on 5 consecutive nights) between 18.00 and 06.00 h from June 2019 to January 2020, and October to December 2020. These periods showed the highest abundance of An. minimus [61]. Collections were carried out by well-trained villagers as paid-volunteers. All participants were given a full explanation of the test procedure both verbally and in written form prior to human landing catch. With the aim of obtaining anthropophilic mosquitoes from the field settings, each volunteer was assigned as human bait inside (HLI) or outside the house (HLO). The distance between HLI and HLO was 20 m apart. They sat on the floor and exposed their legs. Each collection time was 45 min with a 15-min break. Mosquitoes that landed on the skin were caught with an aspirator, and placed in net-covered cups, provided with sugar-soaked cotton. The cups were labeled by date, location, and hour of collection. For cow-baited collection (COW), a cow was tethered overnight inside a net (3.6 × 3.5 × 2 m) with a zippered opening, 300 and 400 m from the villages. Blood-engorged females resting inside the net were collected by an aspirator and transferred to mosquito cups in the following morning. These samples were classified as zoophilic. In addition, CDC Light traps and 6-V battery with dry ice (n = 28, BioQuip model 2836BQ, USA) were utilized to collect mosquitoes from selected houses (n = 14). The traps were hung approximately 1.5 m above the ground, either indoors (LTI) or 10–20 m away from the houses (LTO). Mosquitoes in the traps were transferred to mosquito cups each morning. The mosquito cups were delivered to the laboratory in the field station for morphological identification using a taxonomic key [64].
DNA extraction and molecular identification
Genomic DNA was extracted from the whole body of each An. minimus female using the PureLink™ Genomic DNA Mini Kit (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. DNA quantity was evaluated using the Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA). To distinguish An. minimus s.s. from other sibling species in the Minimus Complex, the ITS2 region was amplified as previously described [65]. The partial cox1 gene was used for DNA barcoding and haplotype mapping. The specific primers and protocol were described previously with minor modifications [66]. The amplified products were electrophoresed on 1.5% agarose gel and stained using Ultrapower™ dye (BioTeke, Bejing, China). Positive cox1 PCR products were sent for Sanger sequencing at First BASE Laboratories (Selangor, Malaysia).
Phylogenetic analyses
The sequences of cox1 gene were then analyzed to determine the intra- and interspecific variation within the An. minimus populations. This mitochondrial gene is posited as a standard gene for DNA barcoding in invertebrates, and its resolution prevails over other nuclear genes [67]. The raw sequences were assembled and aligned using Geneious Prime version 2021.0.3 and compared with cox1 references using BLAST in NCBI. Haplotype diversity was determined by DnaSP6 [68]. The mosquito reference sequences including An. minimus (GenBank accession HQ877373) and An. harrisoni (HQ877375) were aligned with consensus sequences to construct a phylogenetic tree in MEGA version 7.0 with MUSCLE under default parameters [69]. The Kimura 2-parameter (K2P) model was applied for estimating genetic distances [70]. The jModelTest version 2.1.10 was used to assess the best-fit evolutionary model, which was HKY + I + G, for phylogenetic inference based on the maximum likelihood (ML) method with 1,000 bootstrap replicates [71].
Library preparation and whole-genome sequencing
Intrinsic factors including the variation of olfactory genes could be noticeable in wild mosquitoes, which perpetually confront dynamic environment. To begin to justify this assumption, we carried out WGS using pooled An. minimus samples from the lineage A on the basis of the phylogenetic analysis. Given Pool-seq (whole-genome sequencing of pools of individuals) is more unbiased and cost-effective than sequencing individuals separately for population genomic analyses, the influence of the pool size and the limitations are discussed [72, 73]. The equal amount of DNA from each sample should be optimized before pooling to thoroughly represent individuals in the pool [74]. Very small pools show significant bias in allele frequency estimates [72]. Its accuracy increases with a large pool size and high sequencing coverage [75]. In this study, we pooled 10 individuals for WGS, which is empirically sufficient to assess colony-level phenotypes in Cataglyphis niger ants [76]. Since natural Anopheles mosquitoes exhibit high behavioral plasticity, we pooled samples to reduce allele frequency bias compared with individual sequencing and to derive a consensus for each group. However, larger pool sizes and greater sequencing depth are required to minimize inherent allele frequency estimation errors in pooled sequencing.
DNA quality and quantity of each individual mosquito were assessed using Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA) and the Qubit™ fluorometer (Invitrogen, Carlsbad, CA). DNA integrity was checked by agarose gel electrophoresis. Samples that passed quality control from the COW, HLI, and HLO groups were pooled by mixing equal amounts of DNA (~ 100 ng per sample) to create three pools of 10 samples each. The nine pooled DNA samples were adjusted to a final DNA amount of ~ 1 µg and sent to Admera Health Biopharma Services (New Jersey, USA) for library preparation and whole-genome sequencing. Briefly, libraries were constructed using the KAPA Hyper Prep kit without PCR (Roche, Basel, Switzerland). The quality and quantity of the libraries were determined on the Agilent TapeStation (Agilent Technologies, Santa Clara, CA) and in the QuantStudio System (Applied Biosystems, Foster City, CA). The Libraries were sequenced on the Illumina NovaSeq 6000 sequencer (Illumina, San Diego, CA) and 150-bp paired-end reads were generated.
Sequence read alignment and SNP identification
FASTQ reads were trimmed and adapters removed by Trimmomatic version 0.32 to a Phred score of 30 [77]. Then, the reads were filtered on the basis of their quality using the Illumina pipeline function to trim those of low-quality reads and filter out failed ones in the CLC Genomics Workbench version 12.0.3 software (CLCbio, Aahus, Denmark). Cleaned reads of each pooled sample were mapped to the An. minimus reference genome, AminM1.9, GCA_000349025.1 (http://www.vectorbase.org) under default parameters in the CLC Genomics Workbench. Variant calling was carried out on a mapped read using the Low Frequency Variant Detection tool in the CLC Genomics Workbench. It applied modified parameters as follows: required significance = 1%, ignore positions with coverage above = 2,700,000, ignore broken pairs = yes, minimum coverage = 540 (based on 90 samples with at least 6 reads for each sample), minimum count = 54, minimum frequency = 10%, base quality filter = no, read direction filter = no, relative read direction filter = yes, read position filter = no, remove pyro-error variants = no. Single nucleotide polymorphisms (SNPs) with coverage < 10,000 were chosen. Non-synonymous variants were extracted using the Amino Acid Changes tool in the CLC Genomics Workbench and considered as a known variant template. The allele frequency of each pool was generated using the Identify Known Mutations from mappings tool in the CLC Genomics Workbench, and the variant file as described above was employed. The parameters were adjusted as follows: minimum coverage = 10, detection frequency = 20%, ignore broken pairs = no, ignore nonspecific matches = no. The excel files of resulting data were exported. The read counts and coverages from each pooled sample collected by the same method were combined in Microsoft Excel. SNPs with coverage > 1,000 were filtered out and then imported into R for Fisher’s exact test [78]. Differential SNPs were annotated with AminM1_mRNA and AminM1_gene_ontology using merge() and left_join(), respectively, in the R base version 4.4.2 [78]. Gene ontology (GO) analysis was conducted using the web-based tool ShinyGO version 0.82 (http://bioinformatics.sdstate.edu/go/) [79].
SNP validation and multiple correspondence analyses (MCA)
Significantly differential SNPs that existed in both COW versus HLI and COW versus HLO were identified using inner_join() in the R base version 4.4.2 [78]. These variants were sorted in ascending order on the basis of the FDR-adjusted p-values. The top six SNPs associated with olfactory function were selected for genotyping. To validate the allele frequency of the chosen SNPs within individual samples, specific primers were designed by Primer3 version 4.1.0 [80]. Target regions were extracted from the reference Genome using SeqKit version 2.5.1 [81]. Each PCR was carried out in a total volume of 20 µL containing 0.4 U of Platinum™ Taq DNA polymerase (Invitrogen, Carlsbad, CA), 1X of PCR buffer, 2 mM of MgCl2, 0.2 mM of dNTPs, 0.2 µM of each primer, and 1 µL of DNA template. The conditions of amplification consisted of initial denaturation at 94 °C for 2 min, 35 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s, and extension at 72 °C for 30 s, with a final extension at 72 °C for 5 min. After performing DNA gel electrophoresis, amplified products were sent to First BASE Laboratories (Selangor, Malaysia) for Sanger sequencing. The FactoMineR version 2.11 [82] and factoextra version 1.0.7 [83] packages were chosen for performing MCA.
Statistical analyses
Fisher’s exact tests were conducted to examine the differences in variant allele frequency between three groups using fisher.test() function in stats package version 3.6.2 [78]. Statistical significance was determined at p < 0.001. The false discovery rate (FDR) adjusted p-value was computed by p.adjust (p-values, method = “BH”) function using the Benjamini Hochberg method [84].
Results
Female An. minimus exhibits the highest anthropophilic behavior based on human landing catch method
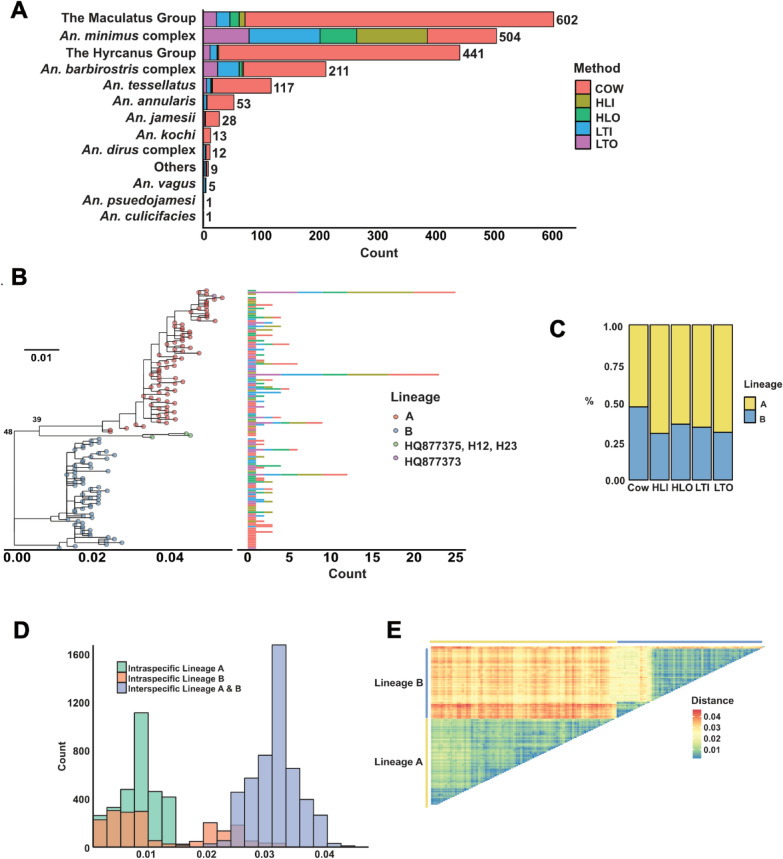
A total of 1,997 females of Anopheles spp. were collected, with the Maculatus Group comprising 30% and the An. minimus complex 25% of the catches (Fig. 1A and Table S1). Of 228 human-baited samples, 184 (81%) were morphologically identified as members of the An. minimus complex. In contrast, only 9% of animal-baited samples belonged to this complex, compared with 38% for the Maculatus Group and 30% for the Hyrcanus Group. There were statistically significant associations between the number of captured An. minimus complex and collection method (Fisher’s exact test, two-tailed p < 0.05), except for indoor versus outdoor CDC light trap comparison. Conversely, the Maculatus Group exhibited a strong preference for animal hosts, with the highest number of cow-collected samples among the methods (Fisher’s exact test, two-tailed p < 0.05).
Fig. 1.
Anopheles composition and cox1 sequence analyses A A stacked bar chart displaying species abundance of Anopheles female samples with collection methods. B Maximum likelihood phylogenetic tree based on cox1 Gene showing 143 haplotypes and two lineages that are lineage A (red dots) and lineage B (blue dots). HQ877375 (An. harrisoni), H12 (haplotype No. 12), and H23 (haplotype No. 23) are indicated in green nodes while HQ877373 (An. minimus) is denoted in purple. The scale bar represents 0.01 substitution of the nucleotide. A side stacked bar chart represents the number of each haplotype with collection methods. C The proportion of lineages A and B in each collection method. D Histogram showing the intra- and interspecific distances of haplotypes. E Heatmap showing the intra- and interspecific distances of haplotypes. COW cow-baited collection, HLI human landing indoor, HLO human landing outdoor, LTI light trap indoor, LTO light trap outdoor.
The plausible two lineages are present in the An. minimus population
Our initial analyses revealed no significant variation of ITS2 sequences of An. minimus specimens in the area (unpublished data), but high diversity was observed in the cox1 sequences. For phylogenetic analysis, 287 randomly selected An. minimus samples underwent cox1 sequencing, yielding 143 haplotypes from 616-bp alignment (Table S2). The phylogenetic tree showed three unique clades, consisting of two major ones flanking a minor one (Fig. 1B and Fig. 1C). Most haplotypes fell in lineage A or Lineage B. Lineage A was detected in 181 sequences, whereas Lineage B had a smaller number with 106 sequences. The H12 and H23 haplotypes were closely related with An. harrisoni (HQ877375) with 0.9 and 1% Genetic distances, respectively. The mean intraspecific distance of each was less than 1.5% while the mean interspecific value was above 3% (Fig. 1D, E). We also noted that the maximum intraspecific distance within lineage B group (3.9%) exceeded the minimum interspecific value (2%), resulting in the subtle overlapping area between intraspecific zone of lineage B and interspecific group, and also the ambiguity in the cox1 interpretation.
Whole-genome analysis reveals significant SNPs in olfactory pathway genes between each group comparison
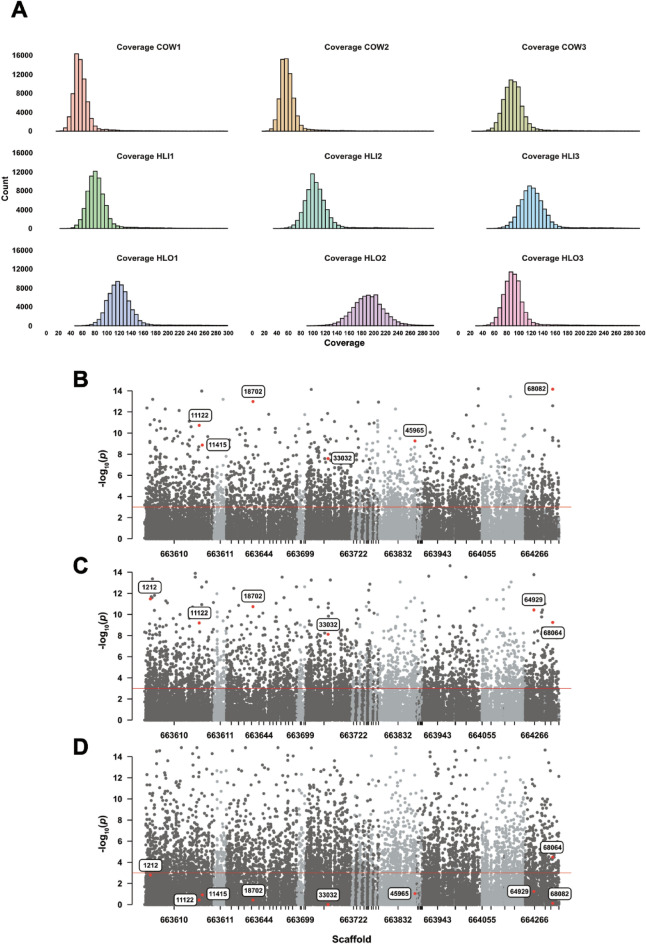
Cleaned reads of each pooled sample were mapped to the reference genome (AminM1.9, GCA_000349025.1) simultaneously and 1,813,223,170 paired-end reads were Generated, resulting in an averaged and individual coverage depth of 676.6 × and 7.5 × , respectively. Individual mappings were also performed by mapping individual cleaned reads to the AminM1.9 (Table 1 and Fig. 2A). Normalized combined reads were employed for variant calling, which identified 12,659,785 variants. Of these, 78,635 were non-synonymous and located in the coding regions, eliciting amino acid changes. Since non-synonymous single-nucleotide polymorphisms (nsSNPs) were our target variants, synonymous (sSNP) variants with coverage < 10,000 were filtered out, resulting in 68,975 nsSNPs. SNP rates were filtered using the allele frequency of 0.10 (Table 1). To identify known nsSNPs from each pooled sample, prior ones were used as a variant track to call them from individual mappings, thus creating nine variant files. Read counts and coverages were extracted and combined into three groups (g1 = COW, g2 = HLI, g3 = HLO) on the basis of the collection methods. The nsSNPs with coverage > 1,000 were removed. After computing the p-values using Fisher’s exact tests, we discovered 2,629, 2,948, and 4,369 differential nsSNPs from g1 versus g2, g1 versus g3, and g2 versus g3, respectively (Fig. 2B–D and Tables S3–S5).
Table 1.
Summary of whole-genome sequencing of An. minimus with different biting behaviors
| Group | Replicate | Raw read | Cleaned read | Mapped read | Mean SNP count (95% CI) | Mean SNP coverage (95% CI) | SNP rate (95%CI) | Mapped percentage (%) | Coverage depth (1x) |
|---|---|---|---|---|---|---|---|---|---|
| COW | I | 252,112,574 | 182,377,695 | 112,620,117 | 26.48 (26.18–26.78) | 63.60 (63.17–64.03) | 0.50 (0.49–0.52) | 61.80 | 4.2 |
| II | 262,733,338 | 194,882,349 | 117,219,841 | 27.16 (26.85–27.47) | 65.64 (65.17–66.11) | 0.50 (0.49–0.51) | 60.10 | 4.4 | |
| III | 335,299,620 | 241,732,431 | 177,521,836 | 43.58 (43.09–44.06) | 103.55 (102.84–104.27) | 0.51 (0.49–0.52) | 73.40 | 6.6 | |
| HLI | I | 263,387,232 | 189,878,989 | 157,731,517 | 38.92 (38.51–39.32) | 92.95 (92.35–93.55) | 0.51 (0.49–0.52) | 83.10 | 5.9 |
| II | 354,147,544 | 244,819,317 | 202,737,145 | 49.69 (49.19–50.20) | 118.60 (117.84–119.35) | 0.51 (0.50–0.53) | 82.80 | 7.6 | |
| III | 421,074,322 | 295,386,826 | 242,756,494 | 58.78 (58.16–58.97) | 139.87 (138.95–140.79) | 0.51 (0.50–0.53) | 82.20 | 9.1 | |
| HLO | I | 415,798,774 | 302,739,867 | 240,335,443 | 58.34 (57.71–58.97) | 138.78 (137.85–139.71) | 0.51 (0.50–0.53) | 79.40 | 9 |
| II | 661,669,770 | 469,656,355 | 383,986,668 | 92.51 (91.57–93.45) | 220.76 (219.36–222.17) | 0.52 (0.51–0.54) | 81.80 | 14.3 | |
| III | 305,731,560 | 218,547,031 | 178,314,109 | 42.87 (42.43–43.30) | 102.50 (101.83–103.17) | 0.51 (0.49–0.52) | 81.60 | 6.7 |
COW cow-baited collection, HLI human landing indoor, HLO human landing outdoor
Fig. 2.
Whole-genome sequencing analyses and SNP discovery A The distribution of single nucleotide variant (SNP) coverage in the nine pooled An. minimus mosquitoes. Three replicates were conducted in each group. B–D Manhattan plots of differential non-synonymous single-nucleotide polymorphisms in each group comparison: B COW versus HLI. C COW versus HLO. D HLI versus HLO. The candidate SNPs are highlighted in bold. The red horizontal line indicates a significance threshold of p-value = 1 × 10–3. COW cow-baited collection, HLI human landing indoor, HLO human landing outdoor.
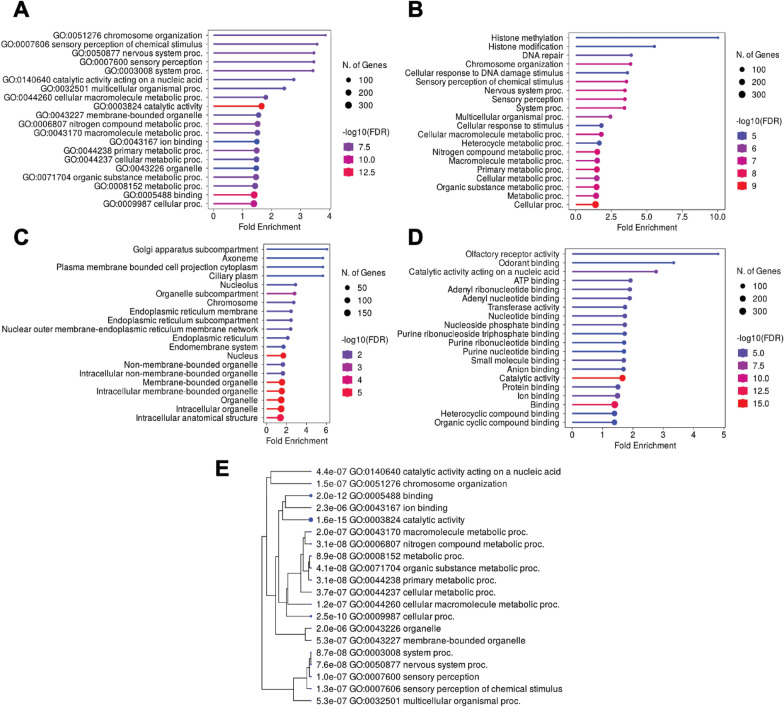
Among 798 mutual nsSNPs between g1 versus g2 and g1 versus g3 comparisons, 602 unique protein-coding genes were identified. To investigate the functional attributes and activities of these genes, we categorized the gene characteristics into Gene Ontology (GO) terms and performed GO enrichment analysis with a cutoff of FDR < 0.05 using ShinyGO version 0.82. The top three most enriched GO categories in all given data sets were GO:0051276 chromosome organization, followed by GO:0007606 sensory perception of chemical stimulus and GO:0050877 nervous system process, respectively (Fig. 3A, E and Table S6). While defining GO in three aspects (Biological Process: BP, Cellular Component: CC, and Molecular Function: MF), there were 152 BP, 46 CC, 88 MF enrichments, respectively (Fig. 3B–D and Tables S7–S9). Intriguingly, we noted that GO:0004984 olfactory receptor activity and GO:0005549 odorant binding were the most highly enriched in molecular function category (Fig. 3D). This points to the magnitude of olfactory-related gene families such as olfactory receptor and odorant-binding protein genes in the context of host-seeking behavior driven by the genetic polymorphisms.
Fig. 3.
Gene ontology (GO) enrichment analyses of mutual nsSNPs between COW versus HLI and COW versus HLO comparisons (602 unique protein-coding genes). A–D Lollipop charts showing top 20 pathways of all gene sets (A), biological processes (B), cellular components (C), and molecular functions (D), respectively. E The hierarchical clustering tree of the correlation of top 20 pathways. The size of the blue nodes indicates the significant level of p-value.
The nsSNPs annotated with olfactory receptors (ORs, IRs, and GRs) accounted for 4.3% (113) and 5.0% (148) of the total in g1 versus g2 and g1 versus g3, respectively, whereas g2 versus g3 contained 4.9% (213) of such SNPs. In addition, we found 60 mutual nsSNPs between g1 versus g2 and g1 versus g3. To validate the occurrence of significant variants in each individual sample, mutual nsSNPs were ranked in ascending order according to p-values, and the top six nsSNPs were chosen for Sanger sequencing. The following VectorBase gene IDs were used: AMIN001807, AMIN001339, AMIN003886, AMIN000912, AMIN003926, AMIN011060, AMIN002342, and AMIN015480 (Table 2). We noted that in HLI versus HLO comparison, most of selected nsSNPs fell under the threshold except for SNP No. 68064 (Fig. 2D).
Table 2.
The selected SNPs associated with host-seeking preference in An. minimus for Sanger sequencing
| SNP No. | Scaffold | Region | Gene ID | Amino acid change | Gene description | Ortholog/similar protein |
|---|---|---|---|---|---|---|
| 68082 | KB664277 | 3390629 | AMIN001807 | Tyr104Phe | Or18 | – |
| 18702 | KB663644 | 679476 | AMIN001339 | Thr428Met | Or9 |
- ACMO_003324 odorant receptor Or1-like (An. coluzzii) - AGAP008894 Or65 (An. gambiae) |
| 11122 | KB663610 | 24859250 | AMIN003886 | Val430Ile | Ir139 |
- ASTE003155 Ir139 (An. stephensi) - AFUN004207 Ir139 (An. funestus) |
| 45965 | KB663832 | 16299463 | AMIN000912 | Ala264Gly | Ionotropic receptor |
- ACUA025671 (An. culicifacies) - ASTE004650 (An. stephensi) |
| 11415 | KB663610 | 26266674 | AMIN003926 | Asp74His | Or410 | - AGAP002639 Or39 (An. gambiae) |
| 33032 | KB663721 | 10410166 | AMIN011060 | His126Pro | Or33 | - ACUA020367 Or33 (An. culicifacies) |
| 1212 | KB663610 | 2623713 | AMIN002342 | Asp59Gly | Gr60 | - ASTE005723 Gr60 (An. stephensi) |
| 64929 | KB664266 | 3985239 | AMIN015480 | Arg119His | Gustatory receptor |
- AGAP028572 (An. gambiae) - ACON028572 (An. coluzzii) |
| 68064 | KB664277 | 3389955 | AMIN001807 | Leu250Phe | Or18 | – |
Gene description is derived from VEuPathDB. Ortholog and similar protein section is based on protein similarity (> 50%) in the well-known anopheline members from OrthoMCL and UniRef databases.
Multiple correspondence analysis (MCA) of candidate SNPs segregates cow-collected mosquitoes from human-collected mosquitoes
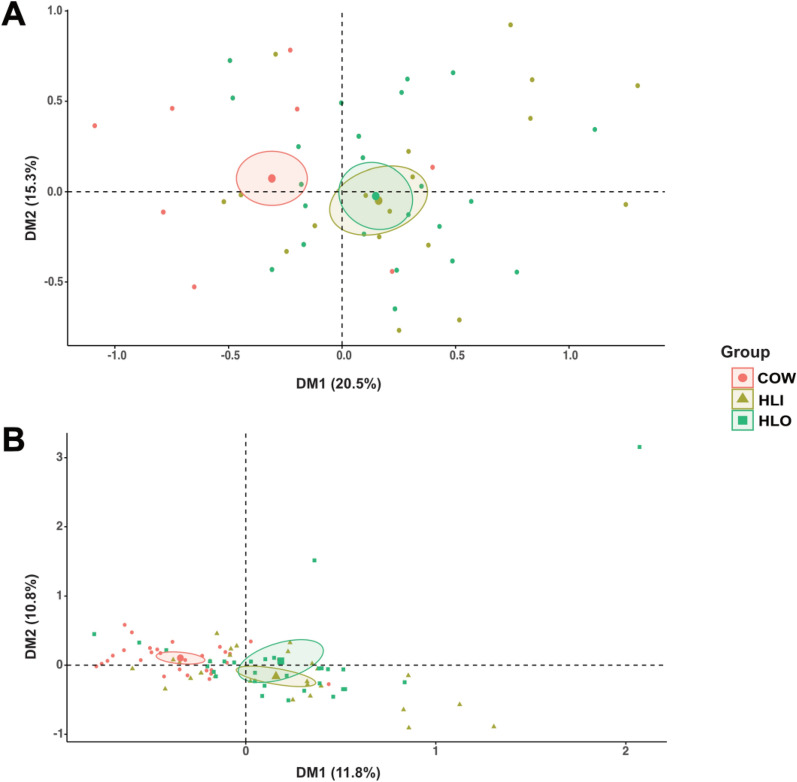
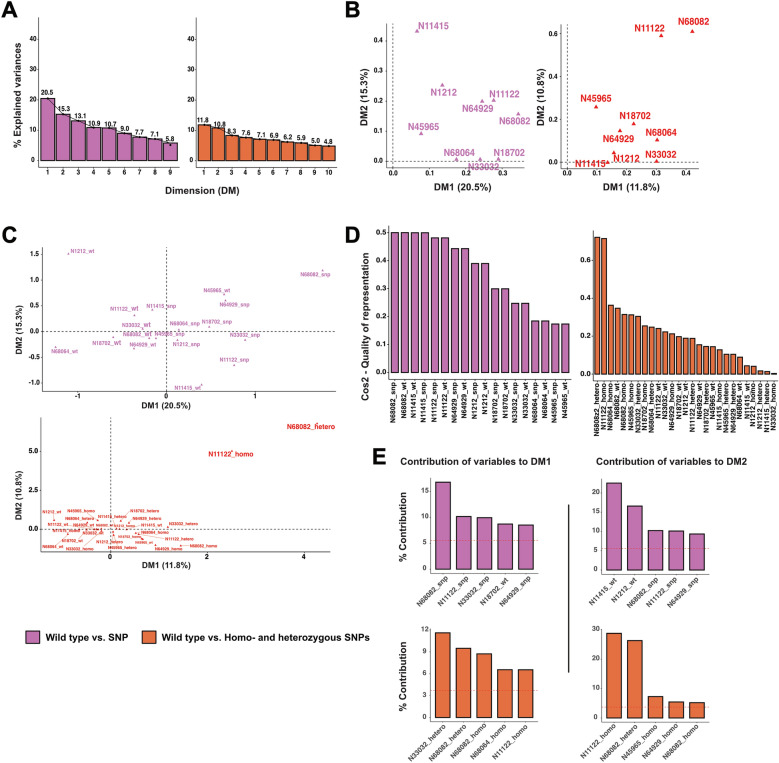
To validate the whole-genome sequencing results, we designed specific primers targeting the identified nsSNPs and confirmed these variants by Sanger sequencing of the resulting amplicons (Tables S10 and S11). We then applied multiple correspondence analysis (MCA) to explore relationships among categorical SNPs and collection methods in the sequenced samples (Table 3). When coding each locus as wild type (WT) or SNP, individuals collected with human bait (HLI and HLO) clustered more closely together than those from the cow-baited group, as indicated by the confidence intervals (Fig. 4A). The first two MCA dimensions (DM1, dimension 1; DM2, dimension 2) explained 20.5% and 15.3% of the variance, respectively (Fig. 5A). In the correlation plot of variables versus dimensions (Fig. 5B), SNP68082, SNP11122, and SNP18702 showed the strongest association with DM1, while SNP11415, SNP1212, and SNP11122 were most closely linked to DM2. The variable-category coordinates (Fig. 5C) revealed that similar categories group together, with most WT points appearing in opposite quadrants, reflecting their negative correlations with the dimensions. We assessed the quality of representation for each variable category via the squared cosine (cos2) values (Fig. 5D); categories with a total cos2 near one are well represented by the first two dimensions. Notably, SNP68064 and SNP45965 had lower cos2 values, suggesting caution when interpreting their positions. To quantify each category’s contribution to the dimensions, we plotted percentage contributions in bar charts (Fig. 5E): SNP68082, SNP11122, and SNP33032 contributed most to DM1, whereas WT11415, WT1212, and SNP68082 were the top contributors to DM2. Finally, by replotting the MCA using three genotype categories (WT, homozygous SNP, heterozygous SNP; Fig. 4B), we observed a similar clustering pattern: HLI and HLO remained close, while the COW group formed a distinct cluster. In this plot, the dimensions accounted for 11.8% (DM1) and 10.8% (DM2) of the variance (Fig. 5A). Overall, these results support an association between selected olfactory gene SNPs and mosquito collection methods; however, this association requires further functional validation.
Table 3.
Multiple correspondence analysis (MCA) results of variables and variable categories in the dimension 1 (DM1) and dimension 2 (DM2)
| WT versus SNP | WT versus Homo- and heterozygous SNPs | |
|---|---|---|
| % Eigenvalues of DM1 | 20.5 | 11.8 |
| % Eigenvalues of DM2 | 15.3 | 10.8 |
| Correlation between variables and DM1 | SNP68082, SNP11122, SNP18702 | SNP68082, SNP11122, SNP33032 |
| Correlation between variables and DM2 | SNP11415, SNP1212, SNP11122 | SNP68082, SNP11122, SNP45965 |
| Most contributed categories of DM1 | SNP68082, SNP11122, SNP33032 | SNP33032_hetero, SNP68082_hetero, SNP68082_homo |
| Most contributed categories of DM2 | WT11415, WT1212, SNP68082 | SNP11122_homo, SNP68082_hetero, SNP45965_homo |
Fig. 4.
Multiple correspondence analysis (MCA) plot of candidate SNPs in 90 individual samples showing confidence ellipse around the mean point of variable categories. A Wild type versus mutated. B Wild type versus Homozygous SNP versus heterozygous SNP. COW cow-baited collection, HLI human landing indoor, HLO human landing outdoor.
Fig. 5.
Multiple correspondence analysis (MCA) of candidate SNPs by wild type versus SNP (pink) and Wild type versus Homozygous SNP versus Heterozygous SNP (orange). A Eigenvalues of each dimension. B Correlation between candidate SNPs and principal dimensions. C Coordinates of candidate SNPs. D Bar charts showing quality of representation of variable categories using squared cosine (cos2) in descending order. E Bar charts showing top five contributions (%) of variable categories to dimension 1 (DM1) and dimension 2 (DM2).
Discussion
This study represents the first investigation of genomic variation associated with biting behavior in An. minimus on the Thai–Myanmar border. Behaviorally, An. minimus showed the strongest anthropophilic tendency among local malaria vectors, as evidenced by its higher capture rate in human landing catches. It has been proposed to be the most anthropophilic Anopheles species in this area [58, 61, 85]. Although An. minimus populations in the study area shared similar ITS2 sequences, their cox1 sequences were highly divergent, clustering into two sympatric lineages (A and B). This pattern likely reflects mitochondrial introgression within the complex, facilitating the transfer of novel alleles between taxa [86–90]. The low to moderate levels of genetic differentiation between lineages in this study are reminiscent of the past works in An. arabiensis [91], An. maculatus [92], An. minimus [66], An. sinensis [93], and An. subpictus [94]. They speculated the lack of geographical barriers resulting in gene flow between groups might describe this phenomenon. Further studies are needed to determine whether the two cox1 lineages of An. minimus populations are conspecific or distinct biological species.
The lack of information on blood-meal origin may hinder our understanding of how specific olfactory receptor genes drive host-seeking behavior. Blood engorgement in mosquitoes induces dramatic physiological and behavioral changes, including selective downregulation of AgOr1 mRNA expression in An. gambiae at 12 h post-blood feeding [95] and altered chemosensory gene expression profiles in post-blood fed Ae. aegypti [96]. Determining the prior blood meals of field-collected specimens remains challenging when focusing on the recency of host-seeking behavior. Moreover, multiple blood feedings in a single gonotrophic cycle and high level of behavioral plasticity are common in Anopheles mosquitoes [97–100]. To control those factors, exploitation of an olfactometer to quantify host preference of An. minimus females from both field and lab strains will be essential for studying this complex behavior.
The idea that genes influence host-seeking behavior has arisen, and previous studies in other mosquitoes posits this hypothesis. In laboratory research, most findings point to the interruption of olfactory genes can alter the host preference in model mosquitoes under controlled conditions as previously mentioned. Field studies are more challenging due to numerous uncontrolled variables. Nonetheless, genetic differentiation associated with distinct feeding phenotypes has been documented in the An. gambiae complex, including chromosomal inversions linked to anthropophily [101–104]. Our findings demonstrate the differential nsSNPs between behavioral traits in the realm of host selection. Moreover, we identified over 2,000 significant nsSNPs in the sample genomes and the strong enrichment of olfactory-related gene families, calling into question the correlation between the presence of such SNPs and host-seeking behavior in this mosquito. Consistent with this hypothesis, our genome-wide results are relative to the findings of SNP markers in An. darlingi with different biting behavior (indoor versus outdoor and dusk versus dawn) [105]. The scaffold-level assembly of the An. minimus genome currently limits genome-wide analyses. A fully assembled chromosome-level reference would enhance future investigations.
We demonstrated that the allele frequencies of differential nsSNPs differ markedly among biting phenotypes. Because most selected SNPs and genes for validation were newly identified in this population, comprehensive gene annotation with orthologs was performed to characterize these genes and obtain detailed functional information. The three orthologous odorant receptors, Or1-like, Or65, and Or39, have all been experimentally shown to respond to specific ligands found in host-derived compounds. AgOr1 and AcolOR39 have been shown to be responsive to 4-methylphenol [106] and sulcatone [107], respectively. These compounds can be found in human body odor [108, 109]. The highly tuned receptor Or65 elicits a strong response to 2-ethylphenol, a component of animal urine [104]. Variation in olfactory gene families may underlie the evolution of host preference in insects [21, 110–114]. The Or4 from field-collected Ae. aegypti is upregulated significantly in the human-biting colonies compared with the animal-preferring subspecies [52, 115]. This odorant receptor is also sensitive to sulcatone found in human odor. Another example of genetic changes involved in host preference is seen in the An. farauti complex, where amino acid changes in olfactory genes including Ir8a and Or75 owing to selective pressure are associated with the shift from anthropophily to zoophily [116]. Many studies also suggest genes encoding odorant-binding proteins and antennal proteins, which are crucial in insect olfaction as the first recognition site and the carrier of odorant molecules [117–120]. Exploring the gene expression of candidate olfactory genes and other families along with SNP knockout or allele-specific editing will further elucidate the genetic mechanisms governing host-seeking behavior.
Conclusions
Our findings provide the genetic and behavioral insights involved in host-seeking behavior in the primary vector of malaria, An. minimus, in Thailand. Polymorphisms in genome, especially olfactory genes, may contribute to altering mosquito feeding behavior. Nonetheless, impact of the surrounding environment has yet to be addressed. Further studies should be undertaken to determine the expression of olfactory genes in parallel with functional validation. This knowledge could form the foundation for the novel vector control management.
Supplementary Information
Supplementary material 1. Table S1. Anopheles abundance collected from this study.
Supplementary material 2. Table S2. The unique haplotypes from cox1 sequencing with accession numbers.
Supplementary material 3. Tables S3–S5. List of significant differential nsSNPs in each group comparison.
Supplementary material 4. Tables S6–S9. List of significantly enriched GO terms of mutual nsSNPs between COW versus HLI and COW versus HLO comparisons.
Supplementary material 5. Table S10. List of novel designed primer sequences used for SNP validation.
Supplementary material 6. Table S11. SNP validation data by Sanger sequencing.
Acknowledgements
We thank all health care staff and volunteers in Tha Song Yang district, Tak province, Thailand, for their assistance with mosquito collections.
Abbreviations
- COW
Cow-baited collection
- GO
Gene ontology
- GR
Gustatory receptor
- HLI
Human landing indoor
- HLO
Human landing outdoor
- IR
Ionotropic receptor
- LTI
Light trap indoor
- LTO
Light trap outdoor
- MCA
Multiple correspondence analysis
- nsSNP
Non-synonymous single-nucleotide polymorphism
- OR
Odorant receptor
- SNP
Single-nucleotide polymorphism
- WGS
Whole-genome sequencing
Author contributions
Conceptualization, PS (Patchara Sriwichai), AS, and GY; methodology, KP, DZ, and PS (Patchara Sriwichai); software, KP and DZ; validation, KP, DZ, and GY; formal analysis, KP and DZ; investigation, KP, DZ, PS (Patchara Sriwichai), and YS; resources, KP, DZ, and GY; data curation, KP and DZ; visualization, KP; writing—original draft preparation, KP; writing—review and editing, KP, DZ, PS (Patchara Sriwichai), YS, AS, KA, PS (Pradya Somboon), AJ, SPW, JS (Jassada Saingamsook), JS (Jetsumon Sattabongkot), LC, and GY; supervision, DZ, PS (Patchara Sriwichai), YS, AS, KA, PS (Pradya Somboon), AJ, SPW, JS (Jassada Saingamsook), JS (Jetsumon Sattabongkot), LC, and GY; project administration, JS (Jetsumon Sattabongkot) and LC; funding acquisition, KP, PS (Patchara Sriwichai), AS, JS (Jetsumon Sattabongkot), and LC. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Institutes of Health (NIH) (https://www.nih.gov) grant U19 AI089672 to LC and U19 AI181583 to JS (Jetsumon Sattabongkot). It was also supported by Specific League Funds 2021 from Mahidol University to PS (Patchara Sriwichai). KP and AS received support from the National Research Council of Thailand under the Royal Golden Jubilee Ph.D. program (Grant No. PHD/0113/2560).
Data availability
Supplementary files are attached to this paper. The raw whole-genome sequencing data are available in the NCBI Sequence Read Archive repository, BioProject ID PRJNA1187263. The unique haplotypes from cox1 sequencing are deposited in GenBank (accession numbers PV174626–PV174768). SNP filtering and statistical analysis scripts are available on GitHub at https://github.com/kpusawang/minimus-wgs. Additional raw data, plots, and scripts are also freely available at https://github.com/kpusawang/minimus-wgs.
Declarations
Ethics approval and consent to participate
The protocol for this study was approved by the Research Ethics Committee (Animals: Protocol Number 16/2562; Humans: 06820/2020) of the Faculty of Medicine, Chiang Mai University, Chiang Mai province, Thailand.
Consent for publication
All authors have read and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Atiporn Saeung, Email: atisaeung.noi@gmail.com.
Guiyun Yan, Email: guiyuny@hs.uci.edu.
References
- 1.World Health Organization. World malaria report 2024. 2024. https://www.who.int/publications/i/item/9789240104440.
- 2.Macdonald G. The epidemiology and control of malaria. Oxford: Oxford University Press; 1957. [Google Scholar]
- 3.Allan SA, Day JF, Edman JD. Visual ecology of biting flies. Annu Rev Entomol. 1987;32:297–314. [DOI] [PubMed] [Google Scholar]
- 4.Riabinina O, Task D, Marr E, Lin C-C, Alford R, O’brochta DA, et al. Organization of olfactory centres in the malaria mosquito Anopheles gambiae. Nat Commun. 2016;7:13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herre M, Goldman OV, Lu T-C, Caballero-Vidal G, Qi Y, Gilbert ZN, et al. Non-canonical odor coding in the mosquito. Cell. 2022;185:3104–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso San Alberto D, Rusch C, Zhan Y, Straw AD, Montell C, Riffell JA. The olfactory gating of visual preferences to human skin and visible spectra in mosquitoes. Nat Commun. 2022;13:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutinho-Abreu IV, Riffell JA, Akbari OS. Human attractive cues and mosquito host-seeking behavior. Trends Parasitol. 2022;38:246–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mclver SB. Sensilla of mosquitoes (diptera: Culicidae). J Med Entomol. 1982;19:489–535. [DOI] [PubMed] [Google Scholar]
- 9.Slifer EH, Sekhon SS. The fine structure of the sense organs on the antennal flagellum of the yellow fever mosquito Aedes aegypti (Linnaeus). J Morphol. 1962;111:49–67. [DOI] [PubMed] [Google Scholar]
- 10.McIver S, Siemicki R. Fine structure of tarsal sensilla of Aedes aegypti (L.) (Diptera: Culicidae). J Morphol. 1978;155:137–55. [DOI] [PubMed] [Google Scholar]
- 11.McIver S. Structure of sensilla trichodea of female Aedes aegypti with comments on innervation of antennal sensilla. J Insect Physiol. 1978;24:383–90. [Google Scholar]
- 12.McIver S, Siemicki R. Fine structure of antennal sensilla of male Aedes aegypti (L.). J Insect Physiol. 1979;25:21–8. [Google Scholar]
- 13.Pitts RJ, Zwiebel LJ. Antennal sensilla of two female anopheline sibling species with differing host ranges. Malar J. 2006;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taai K, Harbach RE, Aupalee K, Srisuka W, Yasanga T, Otsuka Y, et al. An effective method for the identification and separation of Anopheles minimus, the primary malaria vector in Thailand, and its sister species Anopheles harrisoni, with a comparison of their mating behaviors. Parasit Vectors. 2017;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taai K, Harbach R, Somboon P, Sriwichai P, Aupalee K, Srisuka W, et al. A method for distinguishing the important malaria vectors Anopheles dirus and An. cracens (Diptera: Culicidae) based on antennal sensilla of adult females. Trop Biomed. 2019;36:926–37. [PubMed] [Google Scholar]
- 16.Jatuwattana W, Saeung A, Taai K, Srisuka W, Thongsahuan S, Aupalee K, et al. Systematic studies of Anopheles (Cellia) kochi (Diptera: Culicidae): morphology, cytogenetics, cross-mating experiments, molecular evidence and susceptibility level to infection with nocturnally subperiodic Brugia malayi. Acta Trop. 2020;205:105300. [DOI] [PubMed] [Google Scholar]
- 17.Pusawang K, Sriwichai P, Aupalee K, Yasanga T, Phuackchantuck R, Zhong D, et al. Antennal morphology and sensilla ultrastructure of the malaria vectors, Anopheles maculatus and An. sawadwongporni (Diptera: Culicidae). Arthropod Struct Dev. 2023;76:101296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melo ACA, Rützler M, Pitts RJ, Zwiebel LJ. Identification of a chemosensory receptor from the yellow fever mosquito, Aedes aegypti, that is highly conserved and expressed in olfactory and gustatory organs. Chem Senses. 2004;29:403–10. [DOI] [PubMed] [Google Scholar]
- 19.Pitts RJ, Fox AN, Zwiebel LJ. A highly conserved candidate chemoreceptor expressed in both olfactory and gustatory tissues in the malaria vector Anopheles gambiae. Proc Natl Acad Sci U S A. 2004;101:5058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews BJ, McBride CS, DeGennaro M, Despo O, Vosshall LB. The neurotranscriptome of the Aedes aegypti mosquito. BMC Genom. 2016;17:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Athrey G, Cosme LV, Popkin-Hall Z, Pathikonda S, Takken W, Slotman MA. Chemosensory gene expression in olfactory organs of the anthropophilic Anopheles coluzzii and zoophilic Anopheles quadriannulatus. BMC Genom. 2017;18:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saveer AM, Pitts RJ, Ferguson ST, Zwiebel LJ. Characterization of chemosensory responses on the labellum of the malaria vector mosquito, Anopheles coluzzii. Sci Rep. 2018;8:5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–14. [DOI] [PubMed] [Google Scholar]
- 24.Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–6. [DOI] [PubMed] [Google Scholar]
- 25.Rinker DC, Zhou X, Pitts RJ, Rokas A, Zwiebel LJ. Antennal transcriptome profiles of anopheline mosquitoes reveal human host olfactory specialization in Anopheles gambiae. BMC Genomics. 2013;14:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Del Mármol J, Yedlin MA, Ruta V. The structural basis of odorant recognition in insect olfactory receptors. Nature. 2021;597:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endersby-Harshman NM, Ali A, Alhumrani B, Alkuriji MA, Al-Fageeh MB, Al-Malik A, et al. Voltage-sensitive sodium channel (Vssc) mutations associated with pyrethroid insecticide resistance in Aedes aegypti (L.) from two districts of Jeddah, Kingdom of Saudi Arabia: baseline information for a Wolbachia release program. Parasit Vectors. 2021;14:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silbering AF, Rytz R, Grosjean Y, Abuin L, Ramdya P, Jefferis GS, et al. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J Neurosci. 2011;31:13357–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Obaldia ME, Morita T, Dedmon LC, Boehmler DJ, Jiang CS, Zeledon EV, et al. Differential mosquito attraction to humans is associated with skin-derived carboxylic acid levels. Cell. 2022;185:4099–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–60. [DOI] [PubMed] [Google Scholar]
- 31.Rytz R, Croset V, Benton R. Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem Mol Biol. 2013;43:888–97. [DOI] [PubMed] [Google Scholar]
- 32.Knecht ZA, Silbering AF, Ni L, Klein M, Budelli G, Bell R, et al. Distinct combinations of variant ionotropic glutamate receptors mediate thermosensation and hygrosensation in Drosophila. Elife. 2016;5:e17879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni L, Klein M, Svec KV, Budelli G, Chang EC, Ferrer AJ, et al. The ionotropic receptors IR21a and IR25a mediate cool sensing in Drosophila. Elife. 2016;5:e13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greppi C, Laursen WJ, Budelli G, Chang EC, Daniels AM, Van Giesen L, et al. Mosquito heat seeking is driven by an ancestral cooling receptor. Science. 2020;367:681–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laursen WJ, Budelli G, Tang R, Chang EC, Busby R, Shankar S, et al. Humidity sensors that alert mosquitoes to nearby hosts and egg-laying sites. Neuron. 2023;111:874–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill CA, Fox AN, Pitts RJ, Kent LB, Tan PL, Chrystal MA, et al. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298:176–8. [DOI] [PubMed] [Google Scholar]
- 37.Jones WD, Cayirlioglu P, Grunwald Kadow I, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. [DOI] [PubMed] [Google Scholar]
- 38.Robertson HM, Kent LB. Evolution of the gene lineage encoding the carbon dioxide receptor in insects. J Insect Sci. 2009;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tauxe GM, MacWilliam D, Boyle SM, Guda T, Ray A. Targeting a dual detector of skin and CO2 to modify mosquito host seeking. Cell. 2013;155:1365–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott TW, Chow E, Strickman D, Kittayapong P, Wirtz RA, Lorenz LH, et al. Blood-feeding patterns of Aedes aegypti (Diptera: Culicidae) collected in a rural Thai village. J Med Entomol. 1993;30:922–7. [DOI] [PubMed] [Google Scholar]
- 41.White G. Anopheles gambiae complex and disease transmission in Africa. Trans R Soc Trop Med Hyg. 1974;68:278–98. [DOI] [PubMed] [Google Scholar]
- 42.Wolff GH, Riffell JA. Olfaction, experience and neural mechanisms underlying mosquito host preference. J Exp Biol. 2018;221:jeb157131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou X, Rinker DC, Pitts RJ, Rokas A, Zwiebel LJ. Divergent and conserved elements comprise the chemoreceptive repertoire of the nonblood-feeding mosquito Toxorhynchites amboinensis. Genome Biol Evol. 2014;6:2883–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McBride CS. Genes and odors underlying the recent evolution of mosquito preference for humans. Curr Biol. 2016;26:R41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dekel A, Yakir E, Bohbot JD. The evolutionarily conserved indolergic receptors of the non-hematophagous elephant mosquito Toxorhynchites amboinensis. Insect Biochem Mol Biol. 2019;110:45–51. [DOI] [PubMed] [Google Scholar]
- 46.Gomulski LM, Manni M, Carraretto D, Nolan T, Lawson D, Ribeiro JM, et al. Transcriptional variation of sensory-related genes in natural populations of Aedes albopictus. BMC Genom. 2020;21:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C, et al. Orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 2013;498:487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raji JI, Melo N, Castillo JS, Gonzalez S, Saldana V, Stensmyr MC, et al. Aedes aegypti mosquitoes detect acidic volatiles found in human odor using the IR8a pathway. Curr Biol. 2019;29:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell. 2014;156:1060–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. 2013;58:433–53. [DOI] [PubMed] [Google Scholar]
- 51.Stephenson EB, Murphy AK, Jansen CC, Peel AJ, McCallum H. Interpreting mosquito feeding patterns in Australia through an ecological lens: an analysis of blood meal studies. Parasit Vectors. 2019;12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rose NH, Sylla M, Badolo A, Lutomiah J, Ayala D, Aribodor OB, et al. Climate and urbanization drive mosquito preference for humans. Curr Biol. 2020;30:3570–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takken W, Stuke K, Klowden MJ. Biological differences in reproductive strategy between the mosquito sibling species Anopheles gambiae sensu stricto and An. quadriannulatus. Entomol Exp Appl. 2002;103:83–9. [Google Scholar]
- 54.Abella-Medrano CA, Ibáñez-Bernal S, Carbó-Ramírez P, Santiago-Alarcon D. Blood-meal preferences and avian malaria detection in mosquitoes (Diptera: Culicidae) captured at different land use types within a neotropical montane cloud forest matrix. Parasitol Int. 2018;67:313–20. [DOI] [PubMed] [Google Scholar]
- 55.Bogh C, Pedersen EM, Mukoko DA, Ouma JH. Permethrin-impregnated bednet effects on resting and feeding behaviour of lymphatic filariasis vector mosquitoes in Kenya. Med Vet Entomol. 1998;12:52–9. [DOI] [PubMed] [Google Scholar]
- 56.Lacroix R, Mukabana WR, Gouagna LC, Koella JC. Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol. 2005;3:e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smallegange RC, van Gemert GJ, van de Vegte-Bolmer M, Gezan S, Takken W, Sauerwein RW, et al. Malaria infected mosquitoes express enhanced attraction to human odor. PLoS ONE. 2013;8:e63602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edwards HM, Sriwichai P, Kirabittir K, Prachumsri J, Chavez IF, Hii J. Transmission risk beyond the village: entomological and human factors contributing to residual malaria transmission in an area approaching malaria elimination on the Thailand-Myanmar border. Malar J. 2019;18:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thai Meteorological Department. Thailand monthly rainfall 2022. 2022. https:// data.tmd.go.th/api/ThailandMonthlyRainfall/v1/index.php?uid1⁄4api&ukey1⁄4api12345.
- 60.Ministry of Public Health. The status of malaria cases in Thailand. 2022. http://malaria.ddc.moph.go.th/malariaR10/index_newversion.php.
- 61.Sriwichai P, Samung Y, Sumruayphol S, Kiattibutr K, Kumpitak C, Payakkapol A, et al. Natural human Plasmodium infections in major Anopheles mosquitoes in western Thailand. Parasit Vectors. 2016;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sriwichai P, Karl S, Samung Y, Kiattibutr K, Sirichaisinthop J, Mueller I, et al. Imported Plasmodium falciparum and locally transmitted Plasmodium vivax: cross-border malaria transmission scenario in northwestern Thailand. Malar J. 2017;16:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pusawang K, Sattabongkot J, Saingamsook J, Zhong D, Yan G, Somboon P, et al. Insecticide susceptibility status of Anopheles and Aedes mosquitoes in malaria and dengue endemic areas, Thai-Myanmar border. Insects. 2022;13:1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Somboon P, Rattanarithikul R. Mosquito surveys, rearing, preservation of mosquito specimens and identification of Anopheles in Thailand. Nonthaburi: Ministry of Public Health; 2013. p. 79–153. [Google Scholar]
- 65.Garros C, Koekemoer LL, Coetzee M, Coosemans M, Manguin S. A single multiplex assay to identify major malaria vectors within the African Anopheles funestus and the Oriental An. minimus groups. Am J Trop Med Hyg. 2004;70:583–90. [PubMed] [Google Scholar]
- 66.Bunmee K, Thaenkham U, Saralamba N, Ponlawat A, Zhong D, Cui L, et al. Population genetic structure of the malaria vector Anopheles minimus in Thailand based on mitochondrial DNA markers. Parasit Vectors. 2021;14:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hebert PD, Cywinska A, Ball SL, DeWaard JR. Biological identifications through DNA barcodes. Proc R Soc Lond B Biol Sci. 2003;270:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 2017;34:3299–302. [DOI] [PubMed] [Google Scholar]
- 69.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–20. [DOI] [PubMed] [Google Scholar]
- 71.Darriba D, Taboada GL, Doallo R, Posada D. JModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Futschik A, Schlötterer C. The next generation of molecular markers from massively parallel sequencing of pooled DNA samples. Genetics. 2010;186:207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gautier M, Foucaud J, Gharbi K, Cézard T, Galan M, Loiseau A, et al. Estimation of population allele frequencies from next-generation sequencing data: pool-versus individual-based genotyping. Mol Ecol. 2013;22:3766–79. [DOI] [PubMed] [Google Scholar]
- 74.Zhu Y, Bergland AO, González J, Petrov DA. Empirical validation of pooled whole genome population re-sequencing in Drosophila melanogaster. PLoS ONE. 2012;7:e41901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schlötterer C, Tobler R, Kofler R, Nolte V. Sequencing pools of individuals—mining genome-wide polymorphism data without big funding. Nat Rev Genet. 2014;15:749–63. [DOI] [PubMed] [Google Scholar]
- 76.Inbar S, Cohen P, Yahav T, Privman E. Comparative study of population genomic approaches for mapping colony-level traits. PLoS Comput Biol. 2020;16:e1007653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.R Core Team. R: a language and environment for statistical computing; R foundation for statistical computing. 2013. http://www.R-project.org/.
- 79.Ge SX, Jung D, Yao R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics. 2020;36:2628–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shen W, Le S, Li Y, Hu F. SeqKit: a cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS ONE. 2016;11:e0163962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Husson F, Josse J, Le S, Mazet J. FactoMineR: multivariate exploratory data analysis and data mining with R. R package version 2.11. 2013. https://cran.r-project.org/web/packages/FactoMineR/index.html.
- 83.Kassambara A, Mundt F. Factoextra: extract and visualize the results of multivariate data analyses. R package version 1.0.7. 2016. https://cran.r-project.org/web/packages/factoextra/index.html.
- 84.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 85.Tisgratog R, Tananchai C, Juntarajumnong W, Tuntakom S, Bangs MJ, Corbel V, et al. Host feeding patterns and preference of Anopheles minimus (Diptera: Culicidae) in a malaria endemic area of western Thailand: baseline site description. Parasit Vectors. 2012;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Besansky NJ, Powell JR, Caccone A, Hamm DM, Scott JA, Collins FH. Molecular phylogeny of the Anopheles gambiae complex suggests genetic introgression between principal malaria vectors. Proc Natl Acad Sci U S A. 1994;91:6885–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Besansky NJ, Lehmann T, Fahey GT, Fontenille D, Braack LE, Hawley WA, et al. Patterns of mitochondrial variation within and between African malaria vectors, Anopheles gambiae and An. arabiensis, suggest extensive gene flow. Genetics. 1997;147:1817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thelwell N, Huisman R, Harbach R, Butlin R. Evidence for mitochondrial introgression between Anopheles bwambae and Anopheles gambiae. Insect Mol Biol. 2000;9:203–10. [DOI] [PubMed]
- 89.Walton C, Handley JM, Collins FH, Baimai V, Harbach RE, Deesin V, et al. Genetic population structure and introgression in Anopheles dirus mosquitoes in South-east Asia. Mol Ecol. 2001;10:569–80. [DOI] [PubMed] [Google Scholar]
- 90.Amaya Romero JE, Chenal C, Ben Chehida Y, Miles A, Clarkson CS, Pedergnana V, et al. Mitochondrial variation in Anopheles gambiae and Anopheles coluzzii: phylogeographic legacy and mitonuclear associations with metabolic resistance to pathogens and insecticides. Genome Biol Evol. 2024;16:evae172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nyanjom S, Chen H, Gebre-Michael T, Bekele E, Shililu J, Githure J, et al. Population genetic structure of Anopheles arabiensis mosquitoes in Ethiopia and Eritrea. J Hered. 2003;94:457–63. [DOI] [PubMed] [Google Scholar]
- 92.Rongnoparut P, Rodpradit P, Kongsawadworakul P, Sithiprasasna R, Linthicum KJ. Population genetic structure of Anopheles maculatus in Thailand. J Am Mosq Control Assoc. 2006;22:192–7. [DOI] [PubMed] [Google Scholar]
- 93.Ma Y, Yang M, Fan Y, Wu J, Ma Y, Xu J. Population structure of the malaria vector Anopheles sinensis (Diptera: Culicidae) in China: two gene pools inferred by microsatellites. PLoS ONE. 2011;6:e22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weeraratne TC, Surendran SN, Walton C, Karunaratne SP. Genetic diversity and population structure of malaria vector mosquitoes Anopheles subpictus, Anopheles peditaeniatus, and Anopheles vagus in five districts of Sri Lanka. Malar J. 2018;17:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fox AN, Pitts RJ, Robertson HM, Carlson JR, Zwiebel LJ. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down-regulation in response to blood feeding. Proc Natl Acad Sci U S A. 2001;98:14693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hill SR, Ghaninia M, Ignell R. Blood meal induced regulation of gene expression in the maxillary palps, a chemosensory organ of the mosquito Aedes aegypti. Front Ecol Evol. 2019;7:336. [Google Scholar]
- 97.Klowden MJ, Briegel H. Mosquito gonotrophic cycle and multiple feeding potential: contrasts between Anopheles and Aedes (Diptera: Culicidae). J Med Entomol. 1994;31:618–22. [DOI] [PubMed] [Google Scholar]
- 98.Ramasamy M, Srikrishnaraj K, Hadjirin N, Perera S, Ramasamy R. Physiological aspects of multiple blood feeding in the malaria vector Anopheles tessellatus. J Insect Physiol. 2000;46:1051–9. [DOI] [PubMed] [Google Scholar]
- 99.Scott TW, Takken W. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol. 2012;28:114–21. [DOI] [PubMed] [Google Scholar]
- 100.Jeyaprakasam NK, Low VL, Liew JWK, Pramasivan S, Wan-Sulaiman W-Y, Saeung A, et al. Blood meal analysis of Anopheles vectors of simian malaria based on laboratory and field studies. Sci Rep. 2022;12:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Coluzzi M, Sabatini A, Petrarca V, di Angela Deco M. Behavioural divergences between mosquitoes with different inversion karyotypes in polymorphic populations of the Anopheles gambiae complex. Nature. 1977;266:832–3. [DOI] [PubMed] [Google Scholar]
- 102.Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979;73:483–97. [DOI] [PubMed] [Google Scholar]
- 103.Guelbeogo WM, Sagnon NF, Liu F, Besansky NJ, Costantini C. Behavioural divergence of sympatric Anopheles funestus populations in Burkina Faso. Malar J. 2014;13:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Main BJ, Lee Y, Ferguson HM, Kreppel KS, Kihonda A, Govella NJ, et al. The genetic basis of host preference and resting behavior in the major African malaria vector, Anopheles arabiensis. PLoS Genet. 2016;12:e1006303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Campos M, Alonso DP, Conn JE, Vinetz JM, Emerson KJ, Ribolla PEM. Genetic diversity of Nyssorhynchus (Anopheles) darlingi related to biting behavior in western Amazon. Parasit Vectors. 2019;12:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hallem EA, Nicole Fox A, Zwiebel LJ, Carlson JR. Mosquito receptor for human-sweat odorant. Nature. 2004;427:212–3. [DOI] [PubMed] [Google Scholar]
- 107.Hinze A, Pelletier J, Ghaninia M, Marois E, Hill SR, Ignell R. Knockout of OR39 reveals redundancy in the olfactory pathway regulating the acquisition of host seeking in Anopheles coluzzii. Proc R Soc B. 2023;290:20232092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cork A, Park K. Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med Vet Entomol. 1996;10:269–76. [DOI] [PubMed] [Google Scholar]
- 109.Zhao Z, Zung JL, Hinze A, Kriete AL, Iqbal A, Younger MA, et al. Mosquito brains encode unique features of human odour to drive host seeking. Nature. 2022;605:706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matsuo T, Sugaya S, Yasukawa J, Aigaki T, Fuyama Y. Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia. PLoS Biol. 2007;5:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McBride CS. Rapid evolution of smell and taste receptor genes during host specialization in Drosophila sechellia. Proc Natl Acad Sci U S A. 2007;104:4996–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McBride CS, Arguello JR. Five Drosophila genomes reveal nonneutral evolution and the signature of host specialization in the chemoreceptor superfamily. Genetics. 2007;177:1395–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vieira FG, Sánchez-Gracia A, Rozas J. Comparative genomic analysis of the odorant-binding protein family in 12 Drosophila genomes: purifying selection and birth-and-death evolution. Genome Biol. 2007;8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heilig M, Sturiale SL, Marzec S, Holzapfel CM, Bradshaw WE, Meuti ME, et al. Phenotypic variation in biting behavior associated with differences in expression of olfactory genes in the vector mosquito, Aedes albopictus (Diptera: Culicidae). J Med Entomol. 2024;61:367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McBride CS, Baier F, Omondi AB, Spitzer SA, Lutomiah J, Sang R, et al. Evolution of mosquito preference for humans linked to an odorant receptor. Nature. 2014;515:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ambrose L, Popovic I, Hereward J, Ortiz-Barrientos D, Beebe NW. Comparisons of chemosensory gene repertoires in human and non-human feeding Anopheles mosquitoes link olfactory genes to anthropophily. iScience. 2022;25:104521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li ZX, Pickett JA, Field LM, Zhou JJ. Identification and expression of odorant-binding proteins of the malaria-carrying mosquitoes Anopheles gambiae and Anopheles arabiensis. Arch Insect Biochem Physiol. 2005;58:175–89. [DOI] [PubMed] [Google Scholar]
- 118.Li S, Picimbon JF, Ji S, Kan Y, Chuanling Q, Zhou JJ, et al. Multiple functions of an odorant-binding protein in the mosquito Aedes aegypti. Biochem Biophys Res Commun. 2008;372:464–8. [DOI] [PubMed] [Google Scholar]
- 119.Schymura D, Forstner M, Schultze A, Kröber T, Swevers L, Iatrou K, et al. Antennal expression pattern of two olfactory receptors and an odorant binding protein implicated in host odor detection by the malaria vector Anopheles gambiae. Int J Biol Sci. 2010;6:614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pelletier J, Dawit M, Ghaninia M, Marois E, Ignell R. A mosquito-specific antennal protein is critical for the attraction to human odor in the malaria vector Anopheles gambiae. Insect Biochem Mol Biol. 2023;159:103988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1. Table S1. Anopheles abundance collected from this study.
Supplementary material 2. Table S2. The unique haplotypes from cox1 sequencing with accession numbers.
Supplementary material 3. Tables S3–S5. List of significant differential nsSNPs in each group comparison.
Supplementary material 4. Tables S6–S9. List of significantly enriched GO terms of mutual nsSNPs between COW versus HLI and COW versus HLO comparisons.
Supplementary material 5. Table S10. List of novel designed primer sequences used for SNP validation.
Supplementary material 6. Table S11. SNP validation data by Sanger sequencing.
Data Availability Statement
Supplementary files are attached to this paper. The raw whole-genome sequencing data are available in the NCBI Sequence Read Archive repository, BioProject ID PRJNA1187263. The unique haplotypes from cox1 sequencing are deposited in GenBank (accession numbers PV174626–PV174768). SNP filtering and statistical analysis scripts are available on GitHub at https://github.com/kpusawang/minimus-wgs. Additional raw data, plots, and scripts are also freely available at https://github.com/kpusawang/minimus-wgs.