Abstract
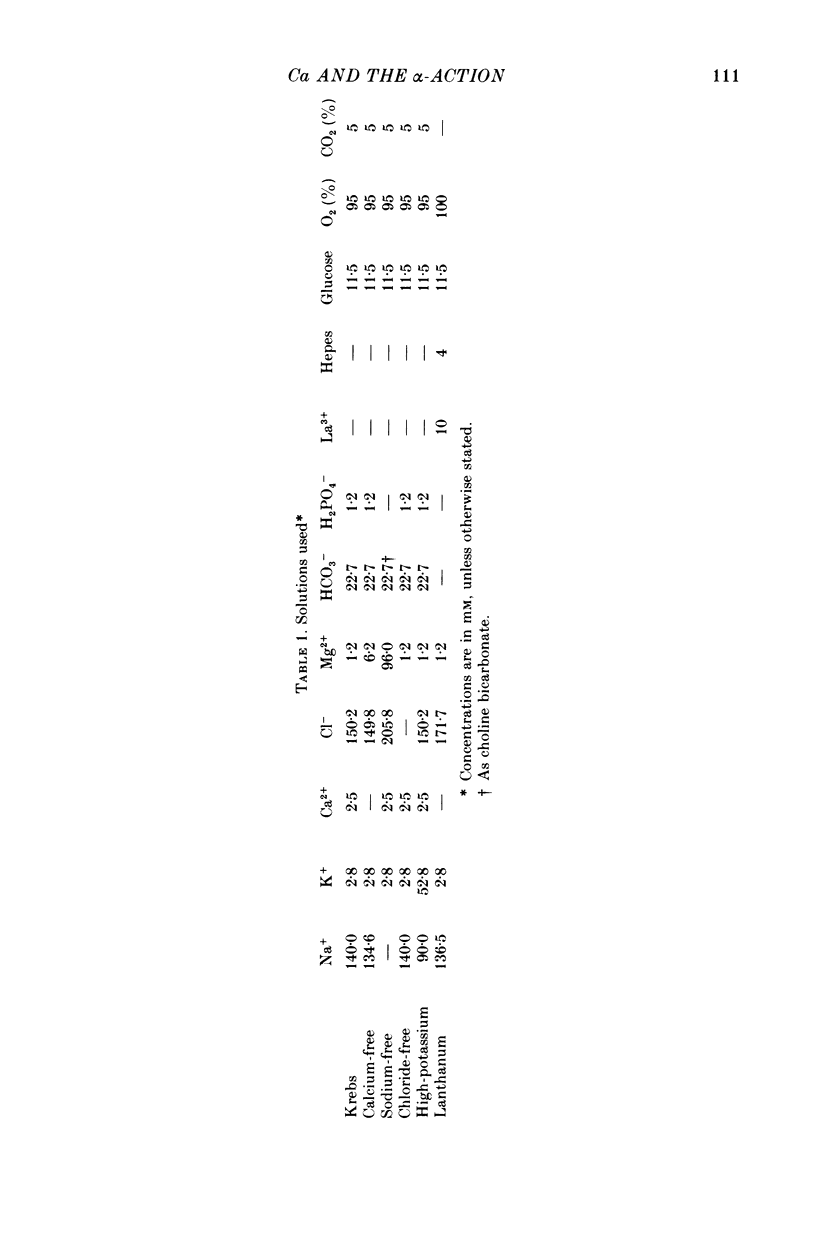
1. The involvement of calcium in the α-action of adrenaline on guinea-pig taenia caeci was studied by measuring the changes in membrane potential and muscle contraction, using the sucrose-gap method, and by determining the 42K efflux, in the presence of a β-blocker (propranolol, 1·8 × 10-6 m).
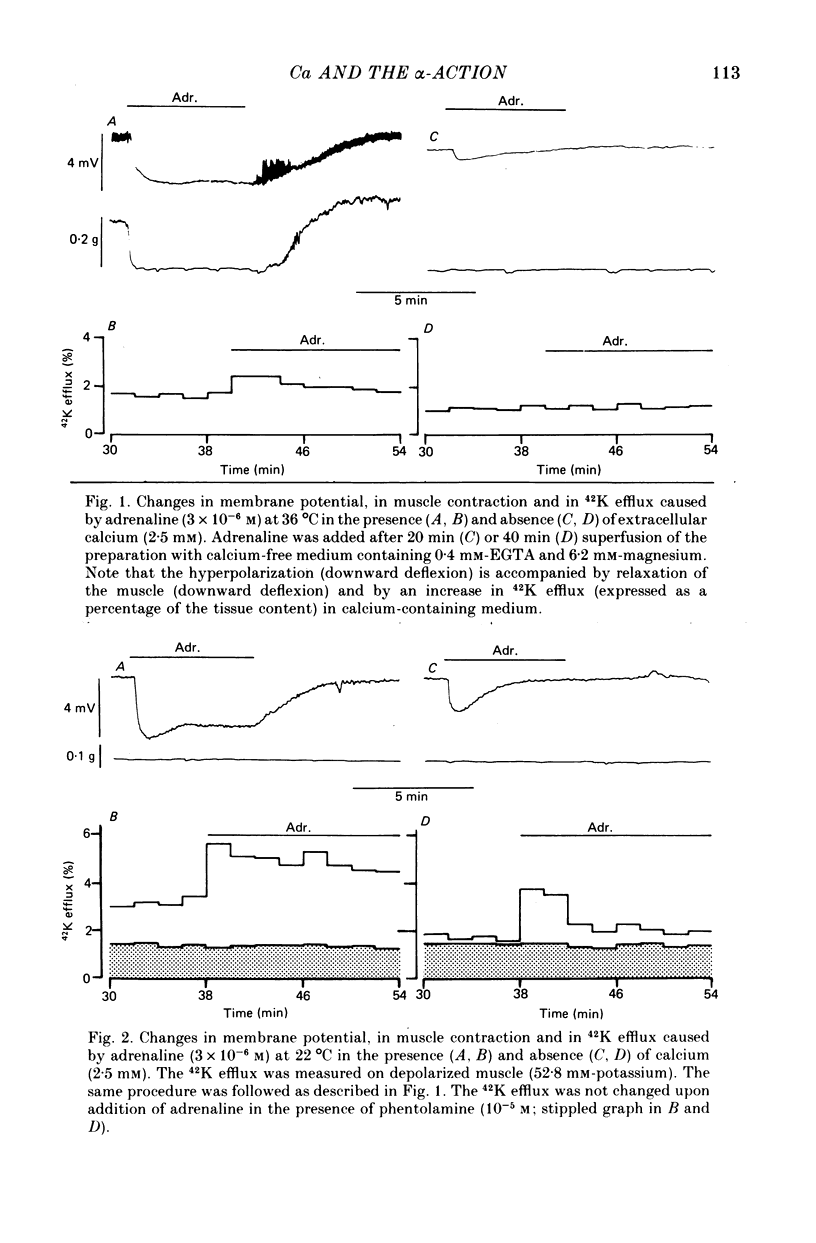
2. In the presence of extracellular calcium, the hyperpolarization caused by adrenaline (3 × 10-6 m) was sustained during the period of its application (5 min), both in active preparations (at 36 °C) and in quiescent muscle (22 °C). In the absence of calcium, adrenaline caused a transient hyperpolarization which was smaller at 36 °C than at 22 °C and passed off within 5 min, while adrenaline was present.
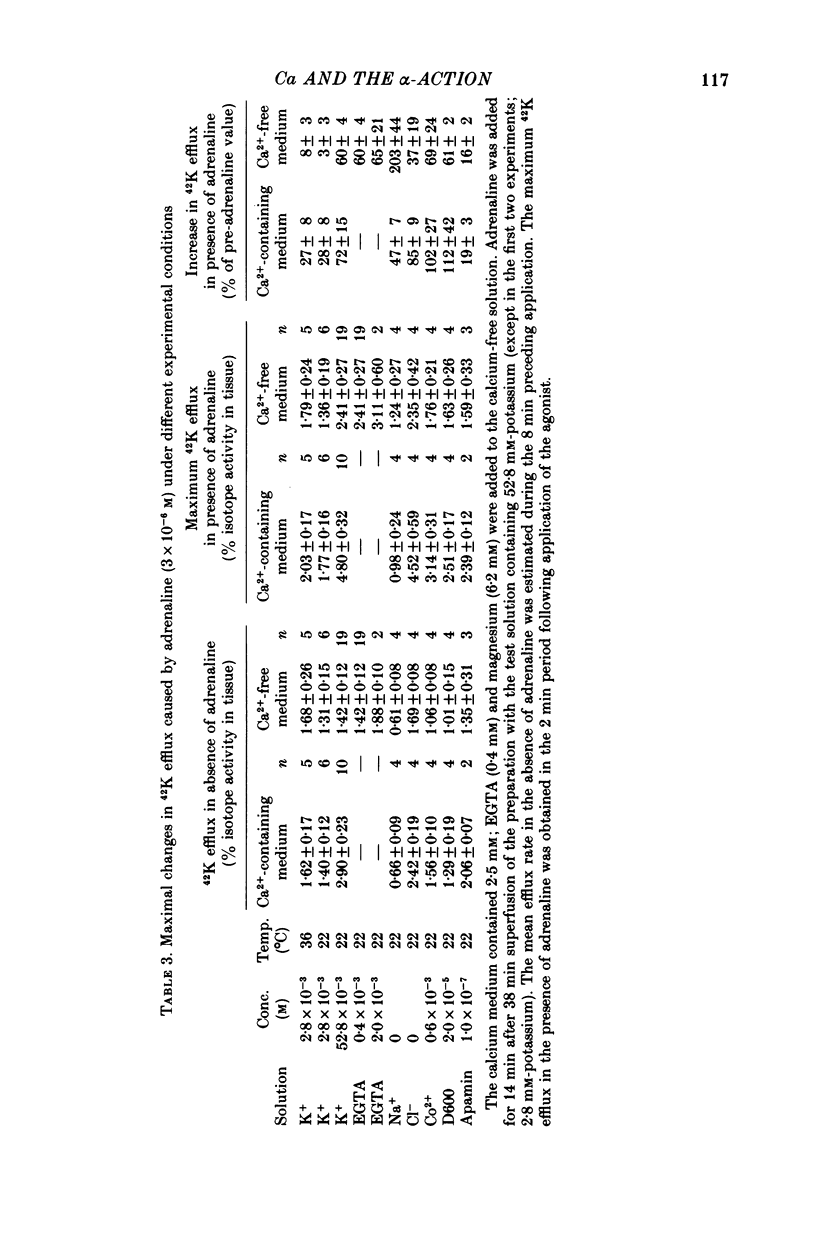
3. Both the sustained and the transient hyperpolarization were associated with an increase in 42K efflux which had a similar time course. 42K flux measurements were made in depolarized tissue (52·8 mm-potassium), in which the effect was consistent and more pronounced than in polarized muscle (2·8 mm-potassium).
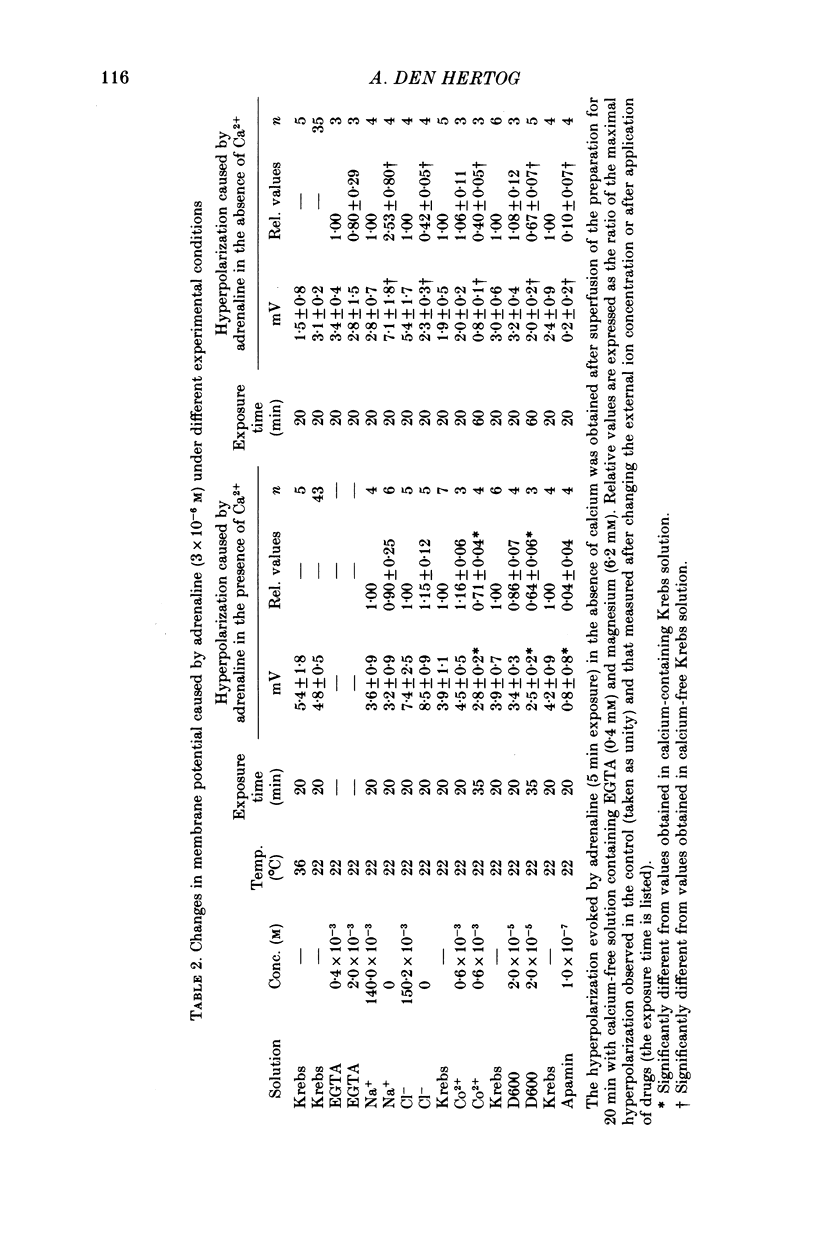
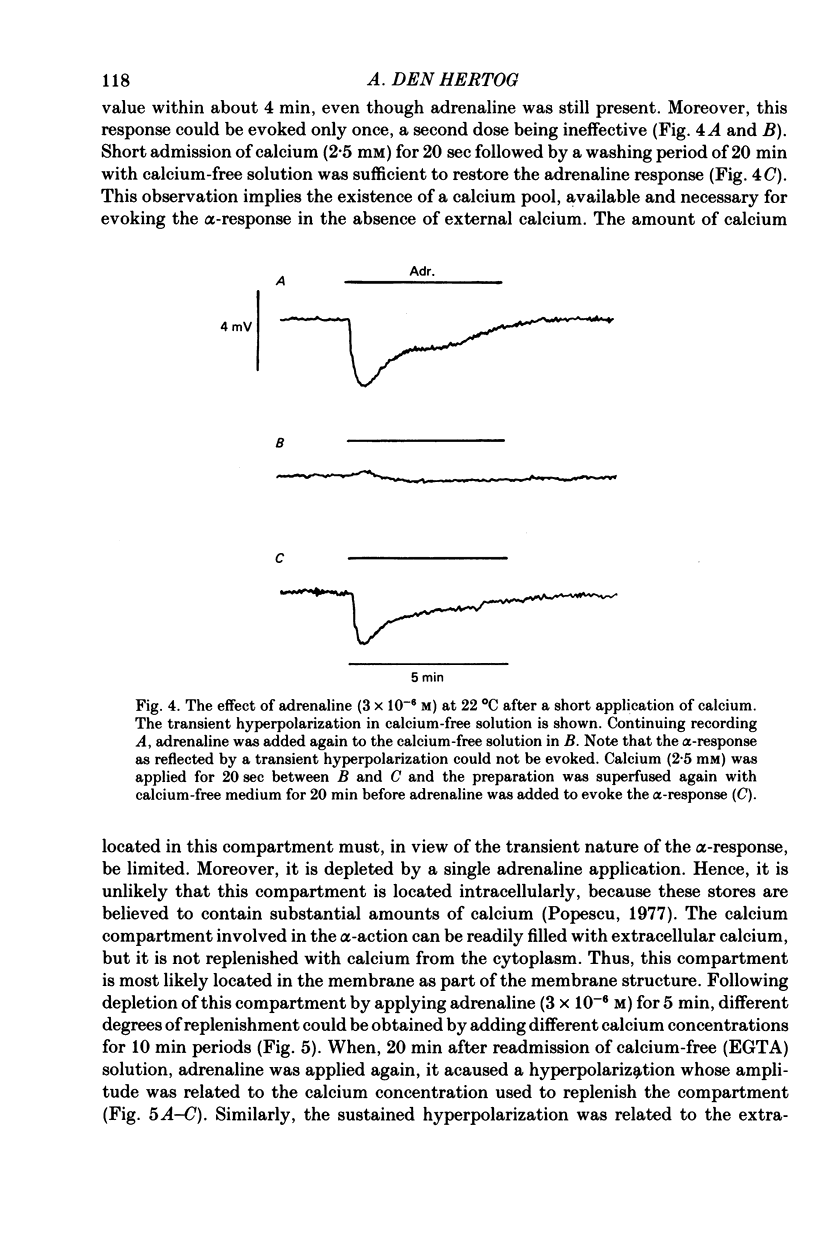
4. The transient hyperpolarization which is resistant to calcium removal and EGTA (0·1-2·0 mm) could be evoked only once but, following a short exposure to calcium (2·5 mm) for 20 sec and readmission of calcium-free medium, it was restored.
5. The sustained and the transient hyperpolarization and the increase in 42K efflux were abolished by the α-antagonist phentolamine (10-5 m); their amplitude was dependent on the adrenaline concentration in the range 10-7 to 3 × 10-6 m, and both responses persisted in the absence of sodium or chloride.
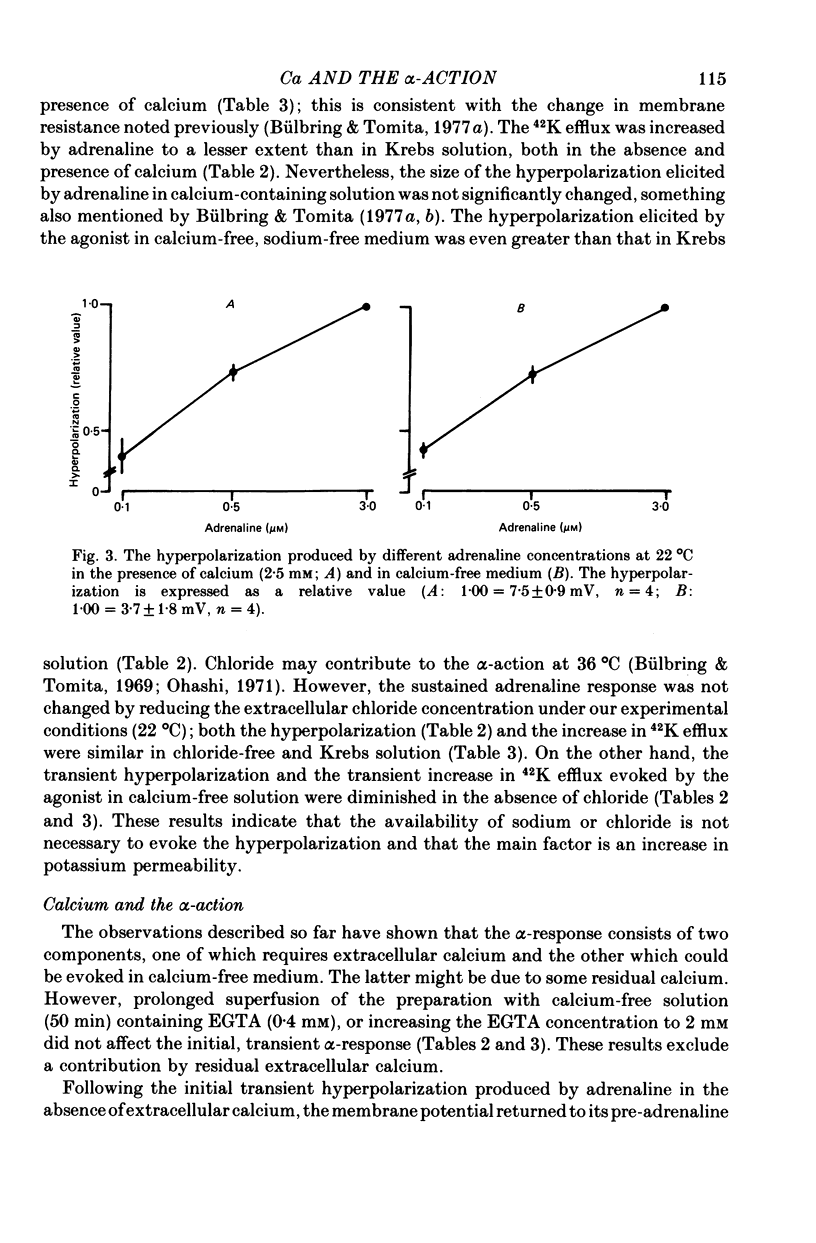
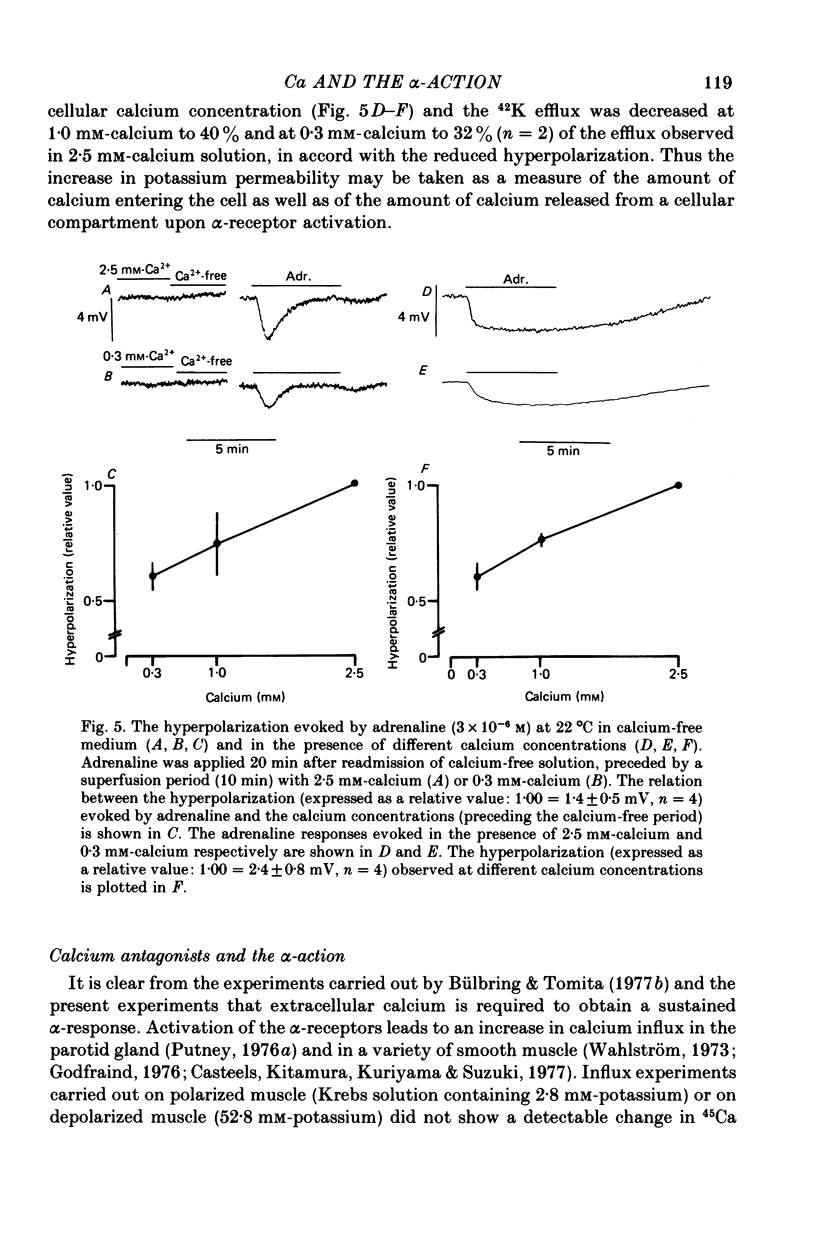
6. The hyperpolarization and the increase in 42K efflux were greater at higher external calcium concentrations (0·3-2·5 mm).
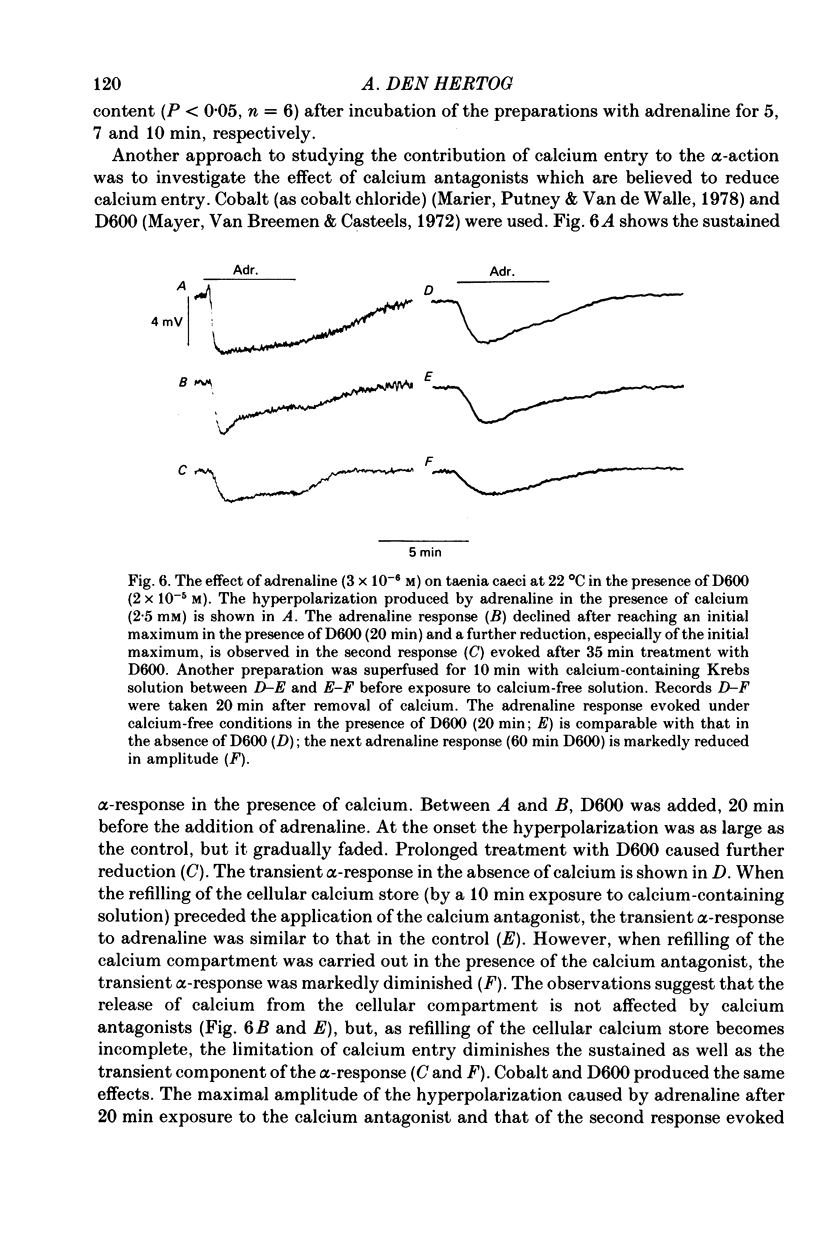
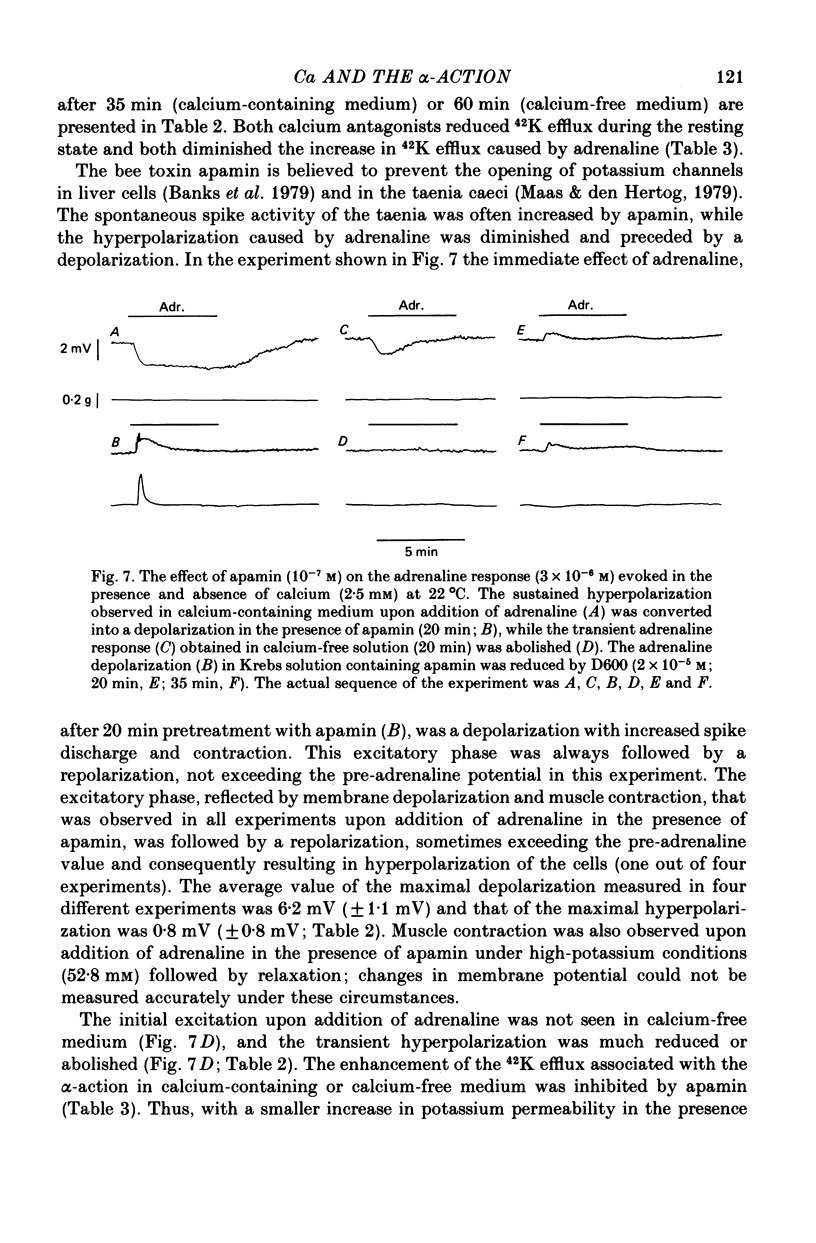
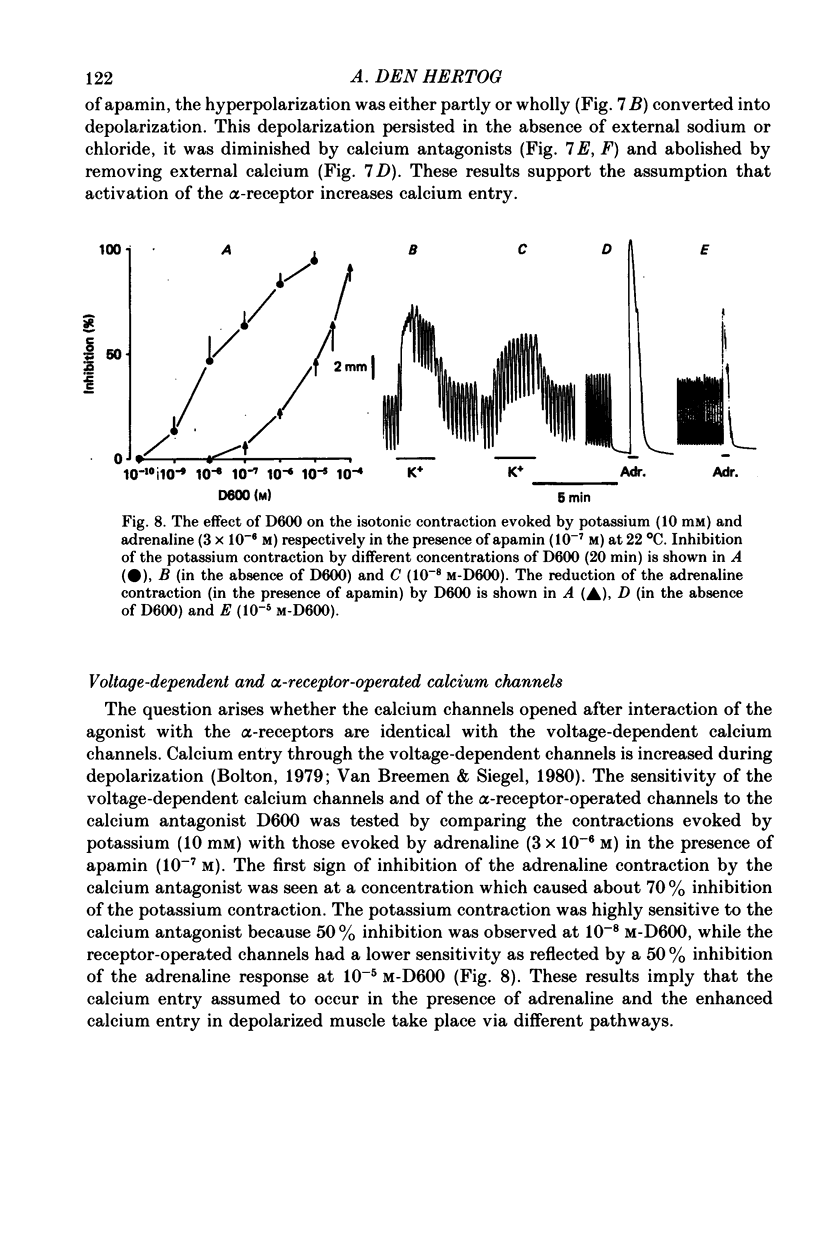
7. Cobalt (0·6 mm), D600 (2·5 × 10-5 m) and the bee toxin apamin (10-7 m) reduced the α-response.
8. In the presence of apamin, in calcium-containing solution, the sustained hyperpolarization caused by adrenaline was preceded by, or converted to, depolarization, spike discharge and contraction.
9. The depolarizing effect of adrenaline in the presence of apamin persisted in sodium-free or chloride-free medium, but was blocked in the absence of calcium and diminished by cobalt and D600.
10. It is concluded that the α-response of guinea-pig taenia caeci consists of two components, both involving calcium. First, the activation of α-receptors increases calcium entry, which leads to the opening of potassium channels, a sustained hyperpolarization and inhibition of muscle activity. Secondly, in the absence of external calcium, a transient hyperpolarization is revealed, presumably due to the release of bound calcium from a limited cellular store which can be replenished by addition of external calcium, and this leads to an increase in potassium permeability.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bozler E. Role of calcium in initiation of activity of smooth muscle. Am J Physiol. 1969 Mar;216(3):671–674. doi: 10.1152/ajplegacy.1969.216.3.671. [DOI] [PubMed] [Google Scholar]
- Brading A. F. A constant flow apparatus for measuring radioactive ion effluxes from guinea-pig taenia coli. J Physiol. 1967 Sep;192(2):15P–16P. [PubMed] [Google Scholar]
- Brading A. F. Calcium-induced increase in membrane permeability in the guinea-pig taenia coli: evidence for involvement of a sodium-calcium exchange mechanism. J Physiol. 1978 Feb;275:65–84. doi: 10.1113/jphysiol.1978.sp012178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Calcium requirement for the alpha-action of catecholamines on guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1977 Jun 15;197(1128):271–284. doi: 10.1098/rspb.1977.0070. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Effects of Ca removal on the smooth muscle of the guinea-pig taenia coli. J Physiol. 1970 Sep;210(1):217–232. doi: 10.1113/jphysiol.1970.sp009205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Increase of membrane conductance by adrenaline in the smooth muscle of guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):89–102. doi: 10.1098/rspb.1969.0013. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. The alpha-action of catecholamines on the guinea-pig taenia coli in K-free and Na-free solution and in the presence of ouabain. Proc R Soc Lond B Biol Sci. 1977 Jun 15;197(1128):255–269. doi: 10.1098/rspb.1977.0069. [DOI] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. Excitation-contraction coupling in the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):63–79. doi: 10.1113/jphysiol.1977.sp011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T. Calcium exchange in vascular smooth muscle, action of noradrenaline and lanthanum. J Physiol. 1976 Aug;260(1):21–35. doi: 10.1113/jphysiol.1976.sp011501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haylett D. G. Effects of sympathomimetic amines on 45Ca efflux from liver slices. Br J Pharmacol. 1976 May;57(1):158–160. doi: 10.1111/j.1476-5381.1976.tb07668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson D. H., Morton I. K. The effect of noradrenaline on the permeability of depolarized intestinal smooth muscle to inorganic ions. J Physiol. 1967 Feb;188(3):373–386. doi: 10.1113/jphysiol.1967.sp008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge W. R. Ca concentration and flux in Ca-deprived arteries. J Physiol. 1972 Jul;224(1):35–59. doi: 10.1113/jphysiol.1972.sp009880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge W. R. Mechanical response with reversed electrical response to noradrenaline by Ca-deprived arterial smooth muscle. J Physiol. 1972 Jul;224(1):21–34. doi: 10.1113/jphysiol.1972.sp009879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas A. J., Den Hertog A. The effect of apamin on the smooth muscle cells of the guinea-pig taenia coli. Eur J Pharmacol. 1979 Sep 15;58(2):151–156. doi: 10.1016/0014-2999(79)90006-2. [DOI] [PubMed] [Google Scholar]
- Marier S. H., Putney J. W., Jr, Van de Walle C. M. Control of calcium channels by membrane receptors in the rat parotid gland. J Physiol. 1978 Jun;279:141–151. doi: 10.1113/jphysiol.1978.sp012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C. J., van Breemen C., Casteels T. The action of lanthanum and D600 on the calcium exchange in the smooth muscle cells of the guinea-pig Taenia coli. Pflugers Arch. 1972;337(4):333–350. doi: 10.1007/BF00586650. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi H. The relative contribution of K and Cl to the total increase of membrane conductance produced by adrenaline on the smooth muscle of guinea-pig Taenia coli. J Physiol. 1971 Jan;212(2):561–575. doi: 10.1113/jphysiol.1971.sp009342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr Biphasic modulation of potassium release in rat parotid gland by carbachol and phenylephrine. J Pharmacol Exp Ther. 1976 Aug;198(2):375–384. [PubMed] [Google Scholar]
- Putney J. W., Jr Muscarinic, alpha-adrenergic and peptide receptors regulate the same calcium influx sites in the parotid gland. J Physiol. 1977 Jun;268(1):139–149. doi: 10.1113/jphysiol.1977.sp011851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr Stimulation of 45Ca influx in rat parotid gland by carbachol. J Pharmacol Exp Ther. 1976 Dec;199(3):526–537. [PubMed] [Google Scholar]
- Shuba M. F., Klevets M. Iu. Ionnii mekhanizm gal'mivnoi di adrenalinu ga noradrenalinu na gladkom'iazovi klitini. Fiziol Zh. 1967 Jan-Feb;13(1):3–11. [PubMed] [Google Scholar]
- Whittam R. Control of membrane permeability to potassium in red blood cells. Nature. 1968 Aug 10;219(5154):610–610. doi: 10.1038/219610a0. [DOI] [PubMed] [Google Scholar]


