Abstract
1. The mechanical resistance of the human forearm to imposed sinusoidal movements has been determined. By means of a visual monitor, subjects maintained a steady force (typically 100 N) by flexing the elbow so as to pull with the wrist against an isometric force transducer. This was mounted upon a stretcher which displaced the forearm sinusoidally at frequencies of 7-11 Hz with a peak-to-peak amplitude of movement of about 1 mm. The average mechanical resistance over 10-40 sec of stretching was analysed into its vector components at the fundamental of the stretching frequency. Observations were made of both the normal resistance and that obtained while applying continuous vibration at 100 Hz to the tendon of either the biceps (agonist) or triceps (antagonist).
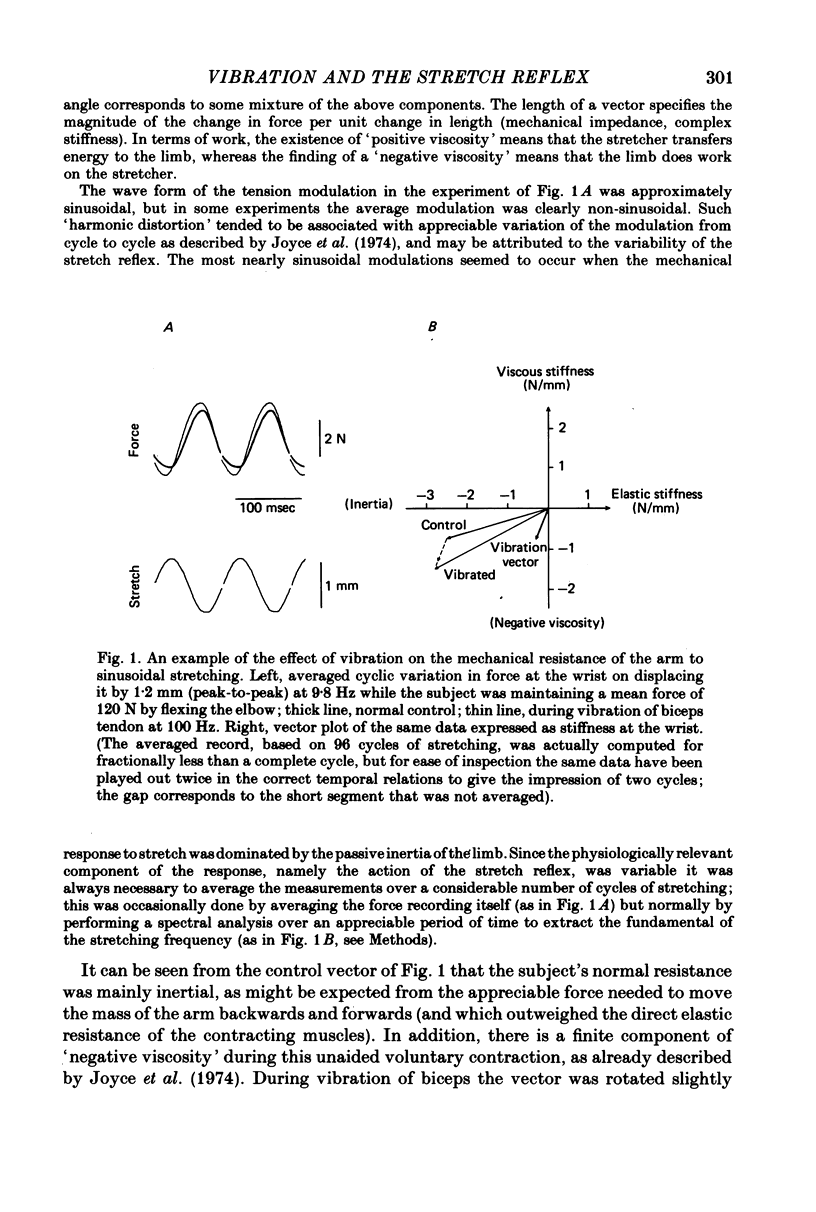
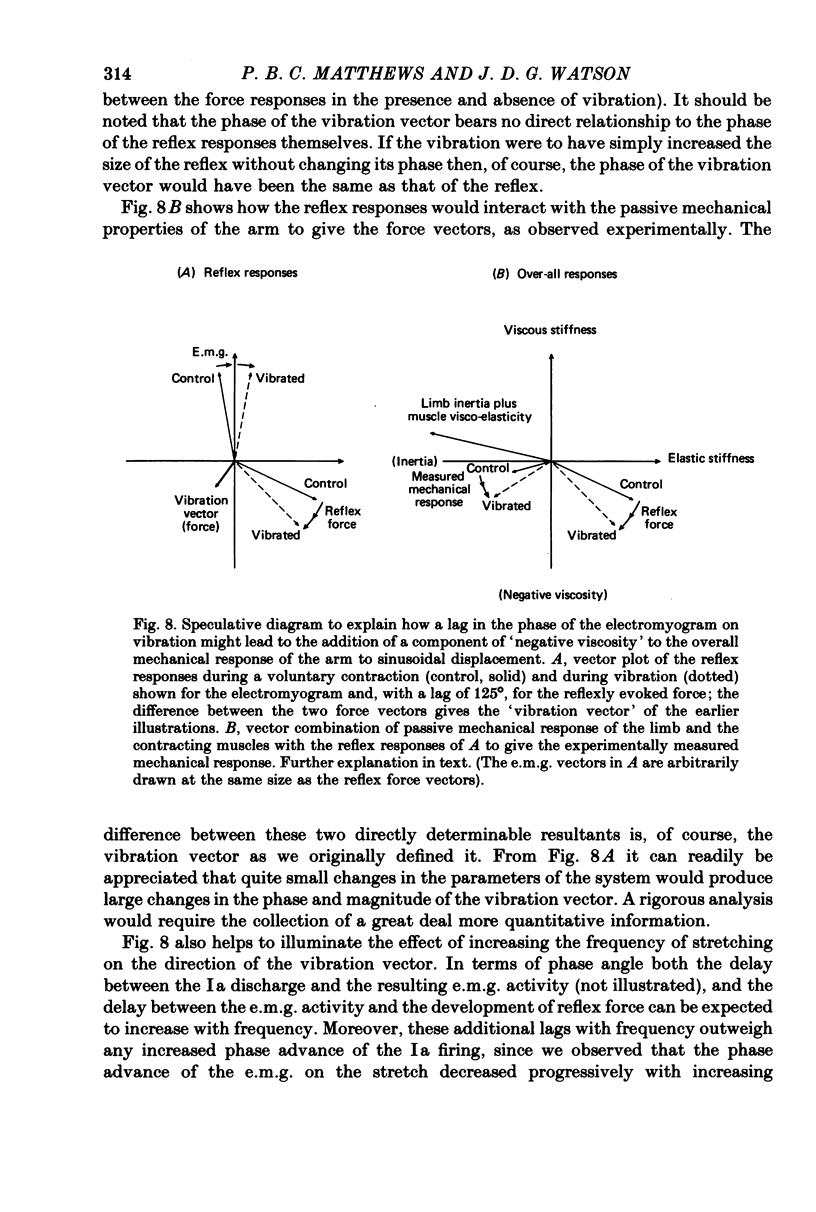
2. In confirmation of Joyce, Rack & Ross (1974), at frequencies around 10 Hz the normal (unvibrated) response sometimes showed a component of `negative viscosity' (force increasing during muscle shortening), rather than the simple `positive viscosity' attributable to muscle visco-elasticity; this effect is attributable to the stretch reflex being appropriately delayed and of sufficient magnitude to over-ride the inherent properties of muscle. Vibration of either agonist or antagonist usually increased the extent of the `negative viscosity' (negative quadrature component of force), as well as changing the `elastic' stiffness of the arm (in-phase component of force).
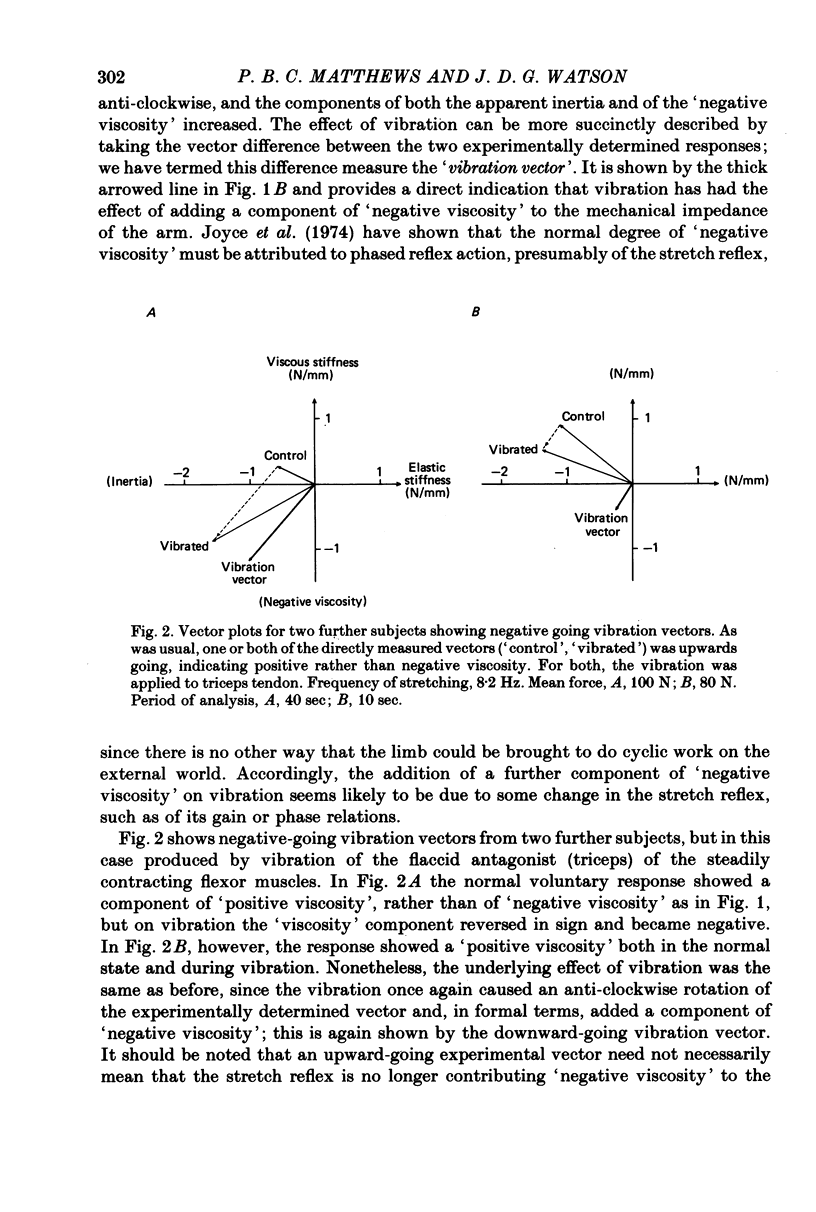
3. More commonly, the component of viscosity was initially positive. It was then normally reduced by vibration; that is, the vibration had (in formal terms) again added a component of negative viscosity.
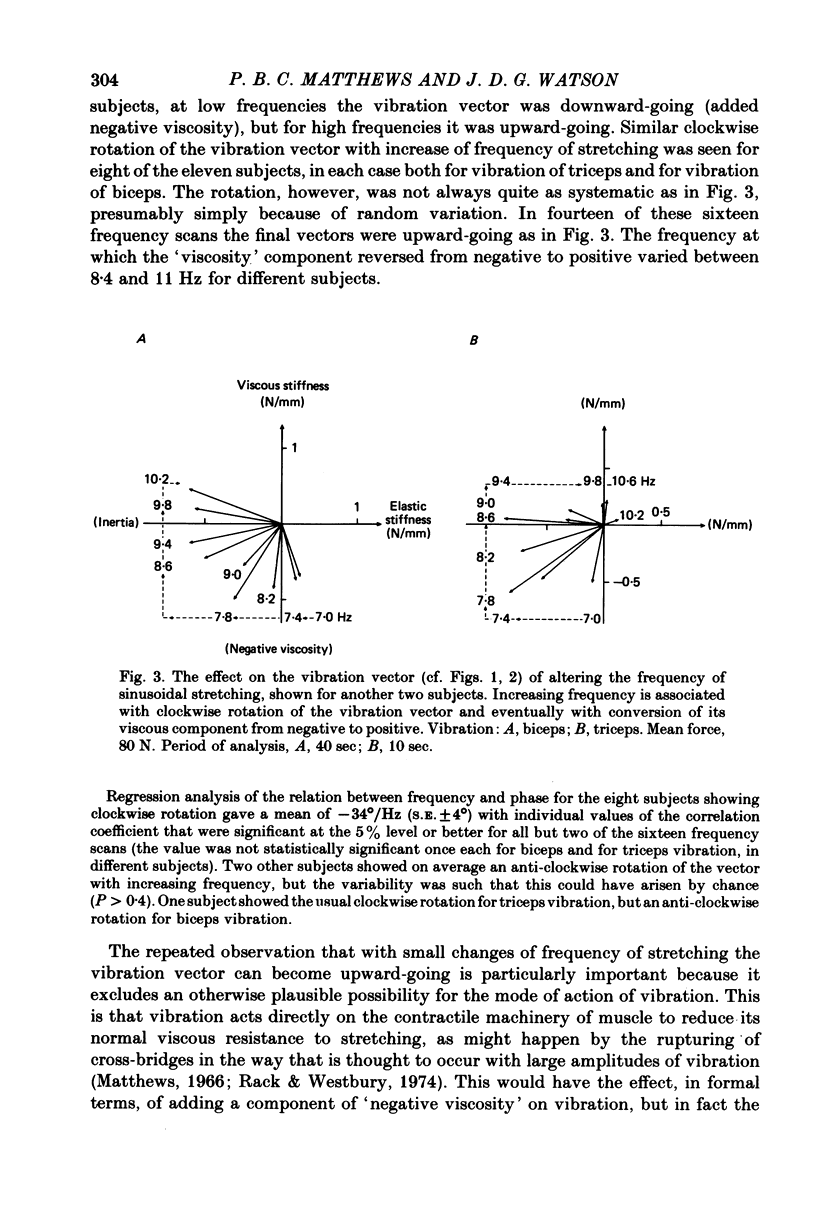
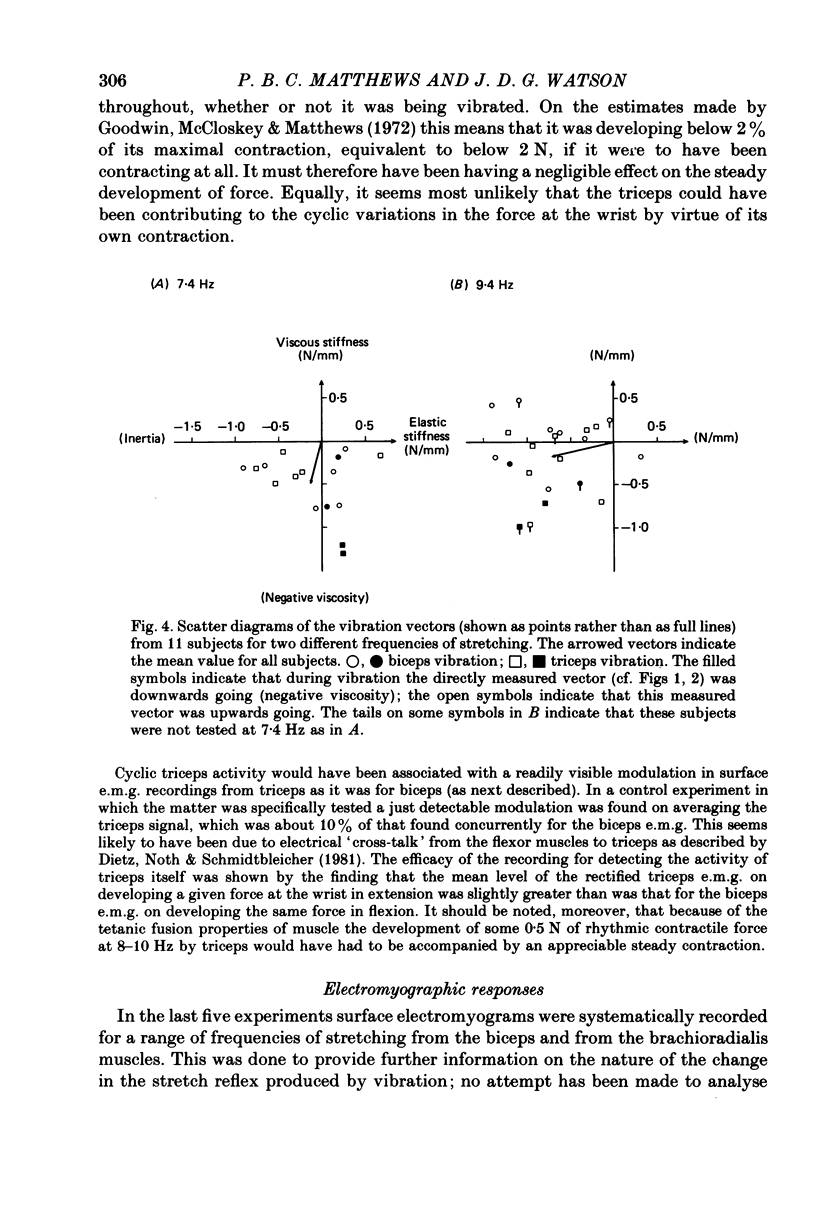
4. The vibration did not produce these effects by acting directly upon the contractile system of muscle to reduce its `visco-elasticity'. On increasing the frequency of stretching the effect of vibration systematically shifted from being the addition of a negative viscosity, as above, to being the addition of a positive viscosity. These effects may all be attributed to an action of vibration on the stretch reflex, with the precise action of the reflex determined by the relation between the cycle time and the delays round the reflex pathway.
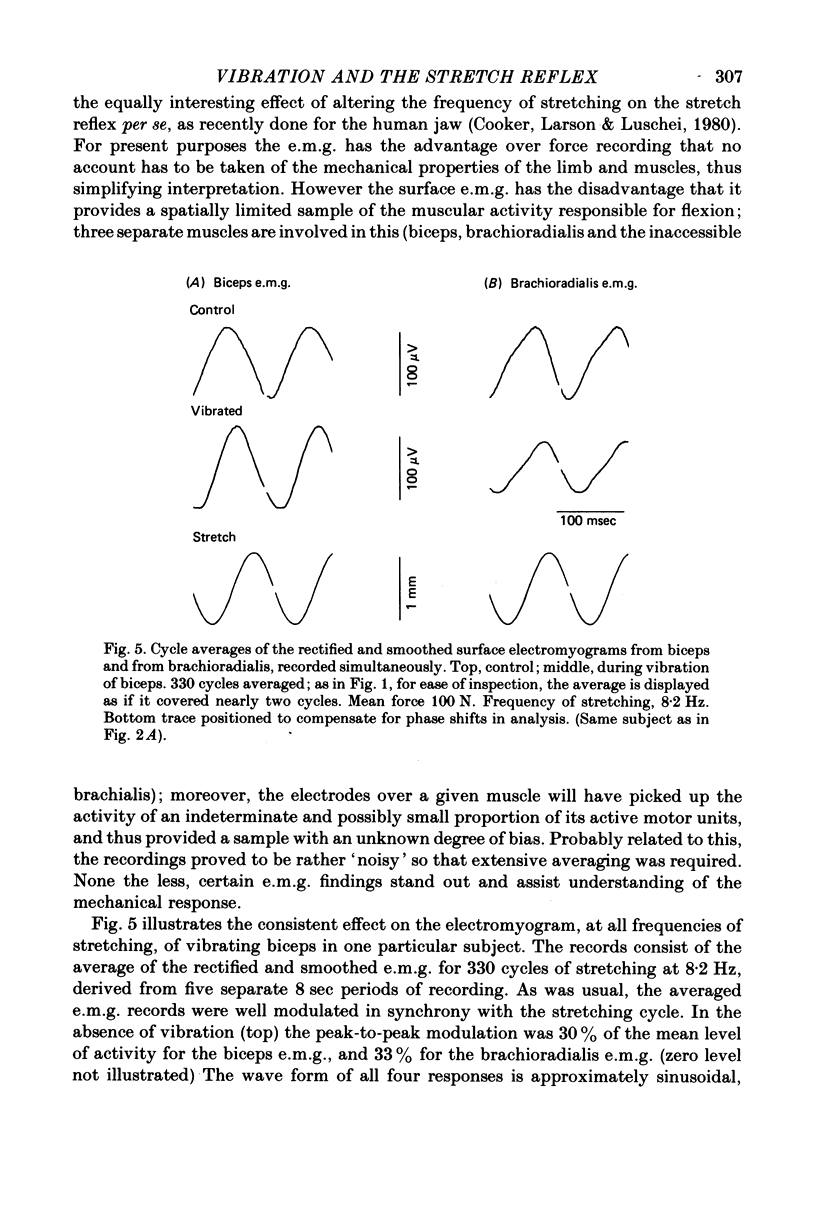
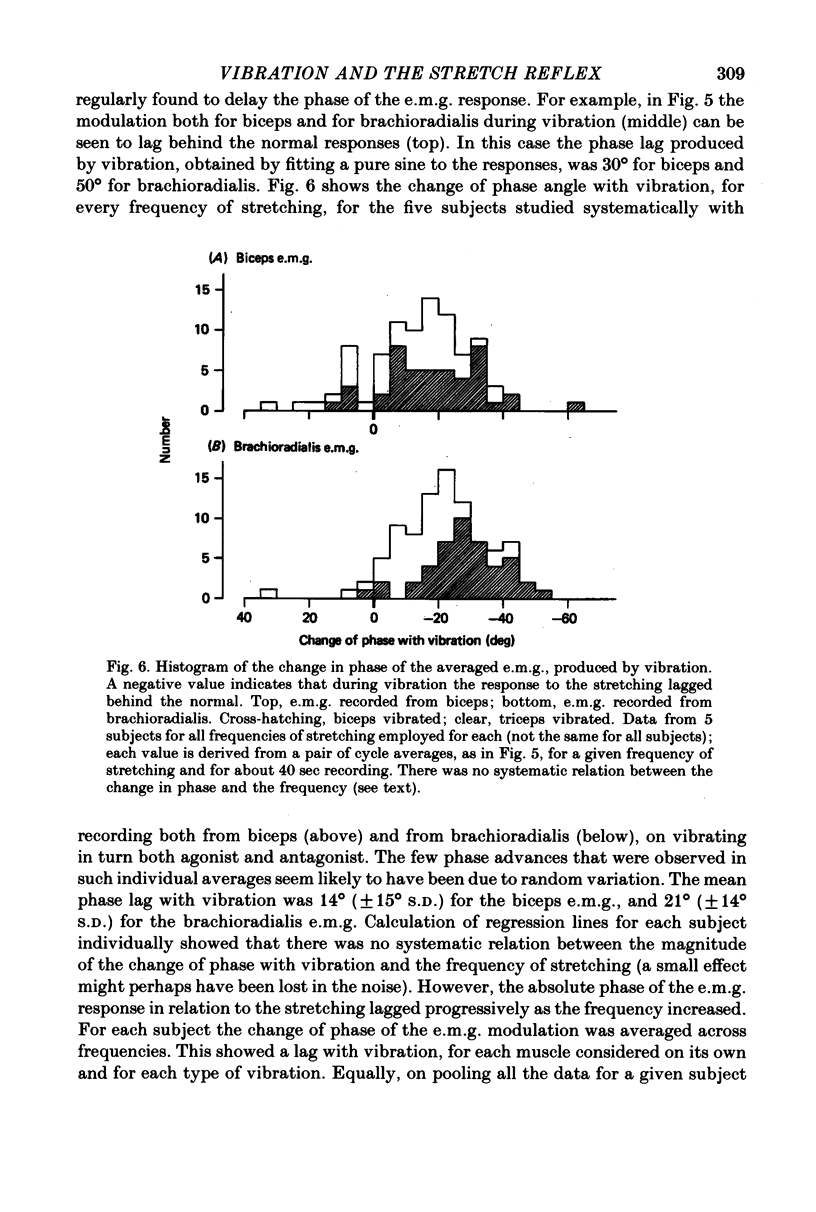
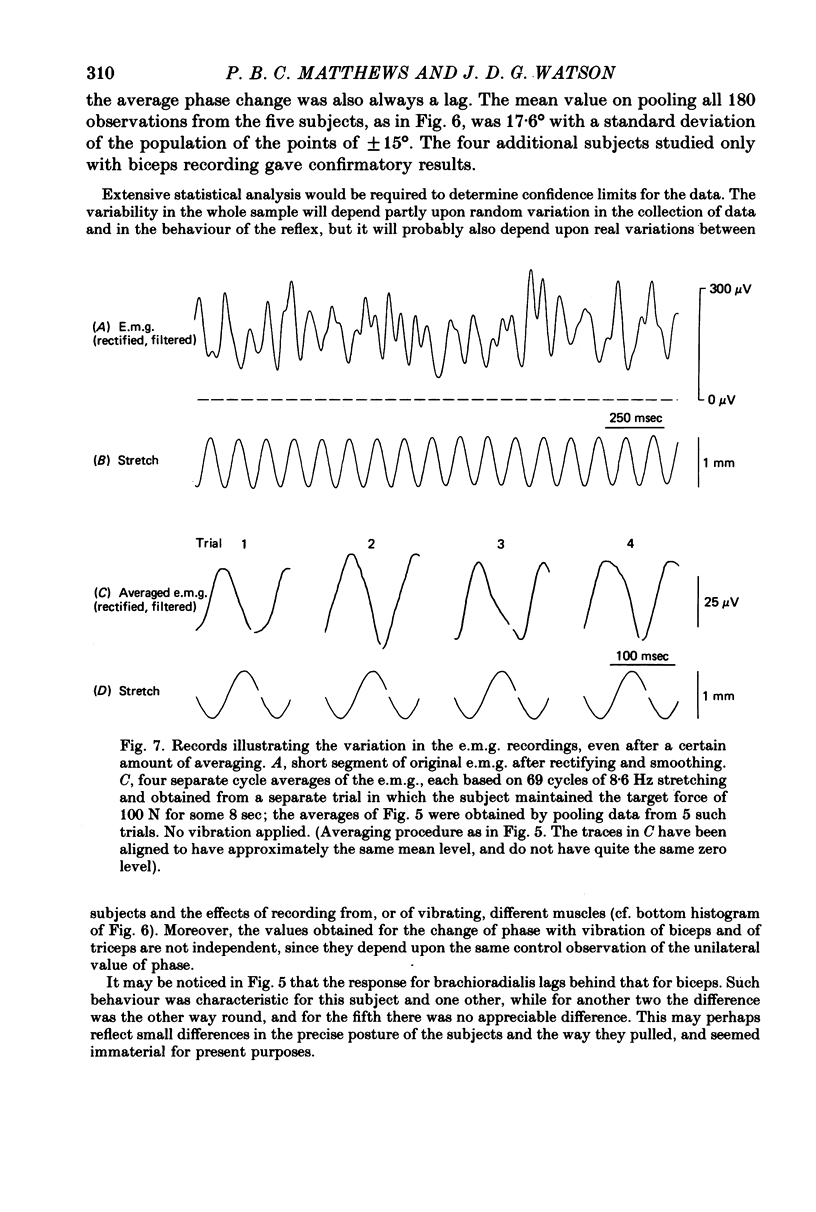
5. In some experiments the activity of the flexor muscles was sampled by surface electromyograms from biceps and from brachioradialis; these were rectified, smoothed and averaged. For biceps, the absolute depth of e.m.g. modulation in relation to the cycle of stretching was sometimes, but not always, increased by vibration; but for brachioradialis the modulation was always reduced. Thus vibration cannot invariably produce its effects on the mechanical resistance of the arm by increasing the size (gain) of the stretch reflex. However, in all subjects the phase of the electromyographic modulation of both muscles was significantly delayed during vibration, whether of biceps or of triceps. In comparison with the normal, vibration introduced a phase lag on average of 18°. In qualitative terms, this can be shown to explain the typical augmentation of `negative viscosity'.
6. The findings are discussed in relation to the genesis of tremor and to the reflex regulation of muscle contraction. They support the classical idea that afferent activity from the antagonist is as crucially implicated as that from the agonist.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal G. C., Gottlieb G. L. Oscillation of the human ankle joint in response to applied sinusoidal torque on the foot. J Physiol. 1977 Jun;268(1):151–176. doi: 10.1113/jphysiol.1977.sp011852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooker H. S., Larson C. R., Luschei E. S. Evidence that the human jaw stretch reflex increases the resistance of the mandible to small displacements. J Physiol. 1980 Nov;308:61–78. doi: 10.1113/jphysiol.1980.sp013462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussons P. D., Matthews P. B., Muir R. B. Enhancement by agonist or antagonist muscle vibration of tremor at the elastically loaded human elbow. J Physiol. 1980 May;302:443–461. doi: 10.1113/jphysiol.1980.sp013255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V., Noth J., Schmidtbleicher D. Interaction between pre-activity and stretch reflex in human triceps brachii during landing from forward falls. J Physiol. 1981 Feb;311:113–125. doi: 10.1113/jphysiol.1981.sp013576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. M., McCloskey D. I., Matthews P. B. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain. 1972;95(4):705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Jansen J. K., Rack P. M. The reflex response to sinusoidal stretching of soleus in the decerebrate cat. J Physiol. 1966 Mar;183(1):15–36. doi: 10.1113/jphysiol.1966.sp007849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M., Ross H. F. The forces generated at the human elbow joint in response to imposed sinusoidal movements of the forearm. J Physiol. 1974 Jul;240(2):351–374. doi: 10.1113/jphysiol.1974.sp010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M. The effects of load and force on tremor at the normal human elbow joint. J Physiol. 1974 Jul;240(2):375–396. doi: 10.1113/jphysiol.1974.sp010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Muir R. B. Comparison of electromyogram spectra with force spectra during human elbow tremor. J Physiol. 1980 May;302:427–441. doi: 10.1113/jphysiol.1980.sp013254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Muir R. B., Watson J. D. Effect of vibration on the mechanical resistance of the human forearm to small imposed sinusoidal movements [proceedings]. J Physiol. 1979 Nov;296:16P–17P. [PubMed] [Google Scholar]
- Matthews P. B. The reflex excitation of the soleus muscle of the decerebrate cat caused by vibbration applied to its tendon. J Physiol. 1966 May;184(2):450–472. doi: 10.1113/jphysiol.1966.sp007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Watson J. D. Action of vibration on the response of cat muscle spindle Ia afferents to low frequency sinusoidal stretching. J Physiol. 1981 Aug;317:365–381. doi: 10.1113/jphysiol.1981.sp013830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack P. M., Ross H. F., Walters D. K. Interactions between the stretch reflex and a 'repeat tendency' of the motoneurone pool in the human [proceedings]. J Physiol. 1979 May;290(2):21P–22P. [PubMed] [Google Scholar]
- Rack P. M., Westbury D. R. The short range stiffness of active mammalian muscle and its effect on mechanical properties. J Physiol. 1974 Jul;240(2):331–350. doi: 10.1113/jphysiol.1974.sp010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo A. B., Hagbarth K. E., Torebjörk H. E., Wallin B. G. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979 Oct;59(4):919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Westbury D. R. The response of alpha-motoneurones of the cat to sinusoidal movements of the muscles they innervate. Brain Res. 1971 Jan 8;25(1):75–86. doi: 10.1016/0006-8993(71)90568-3. [DOI] [PubMed] [Google Scholar]


