Abstract
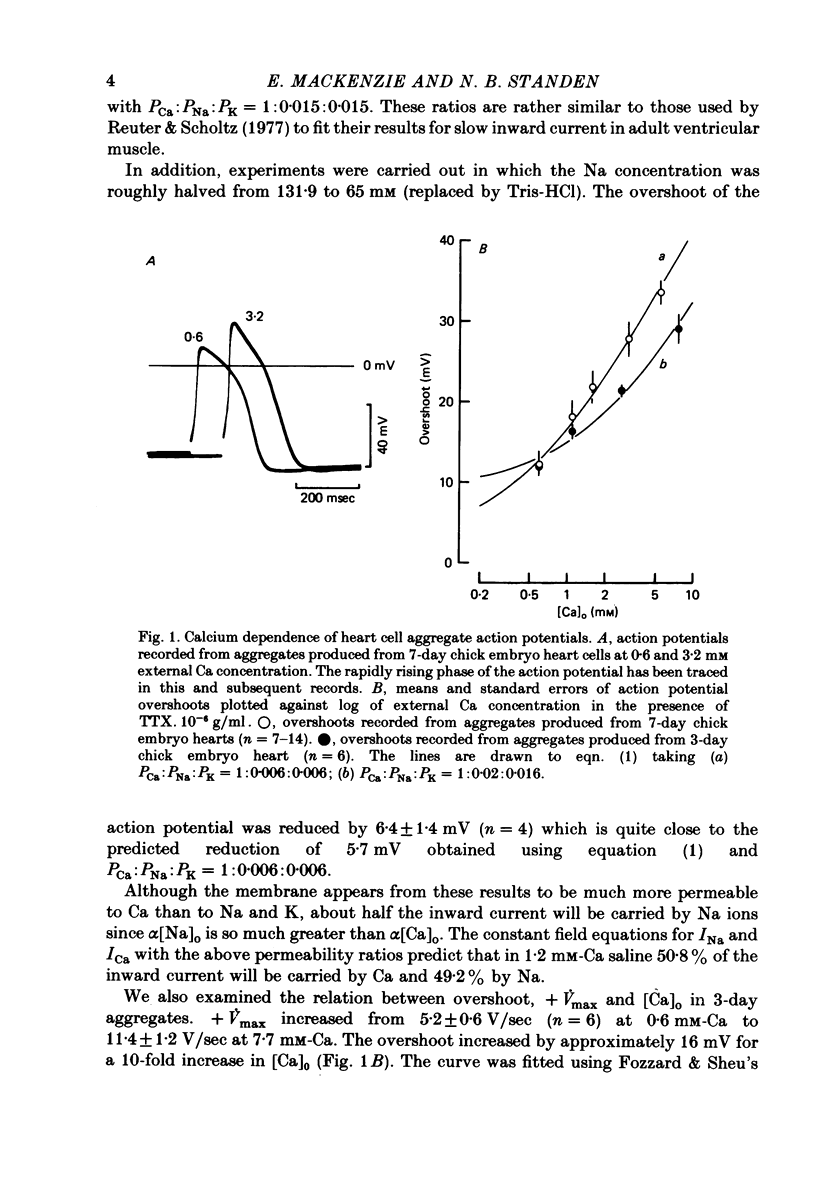
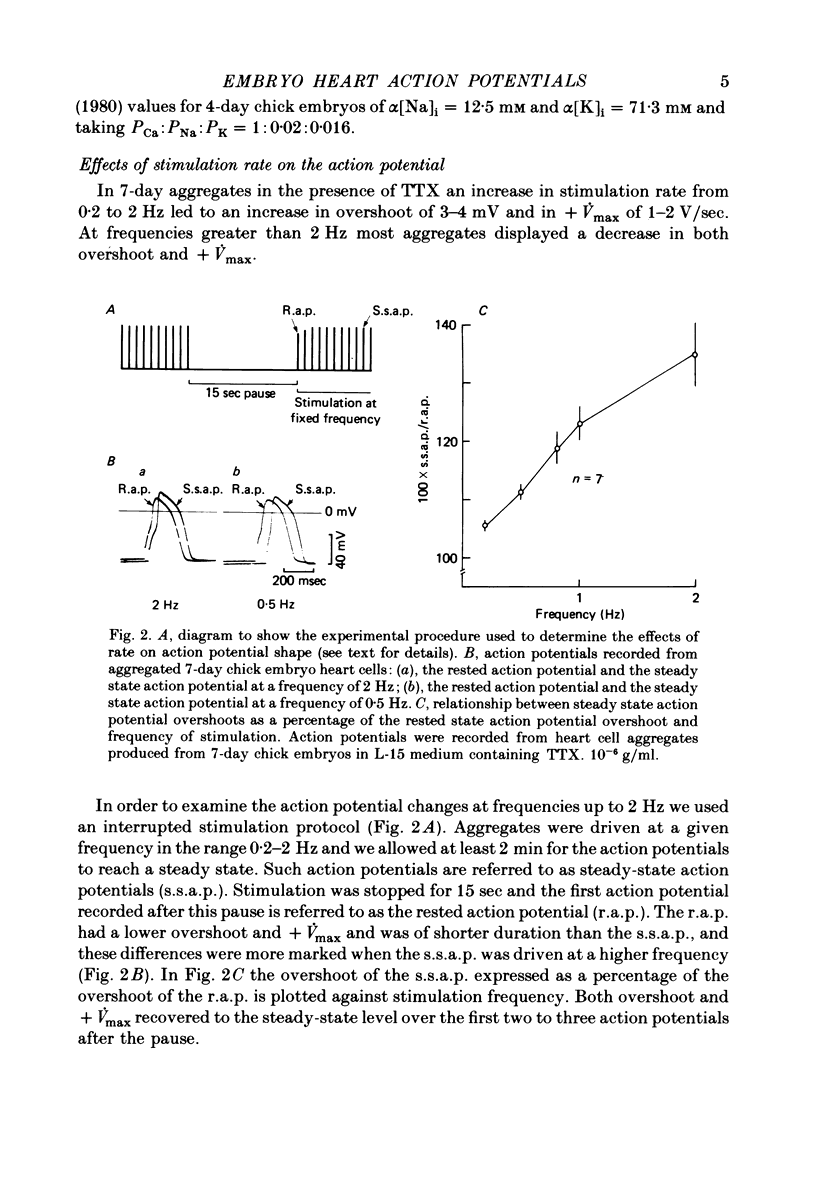
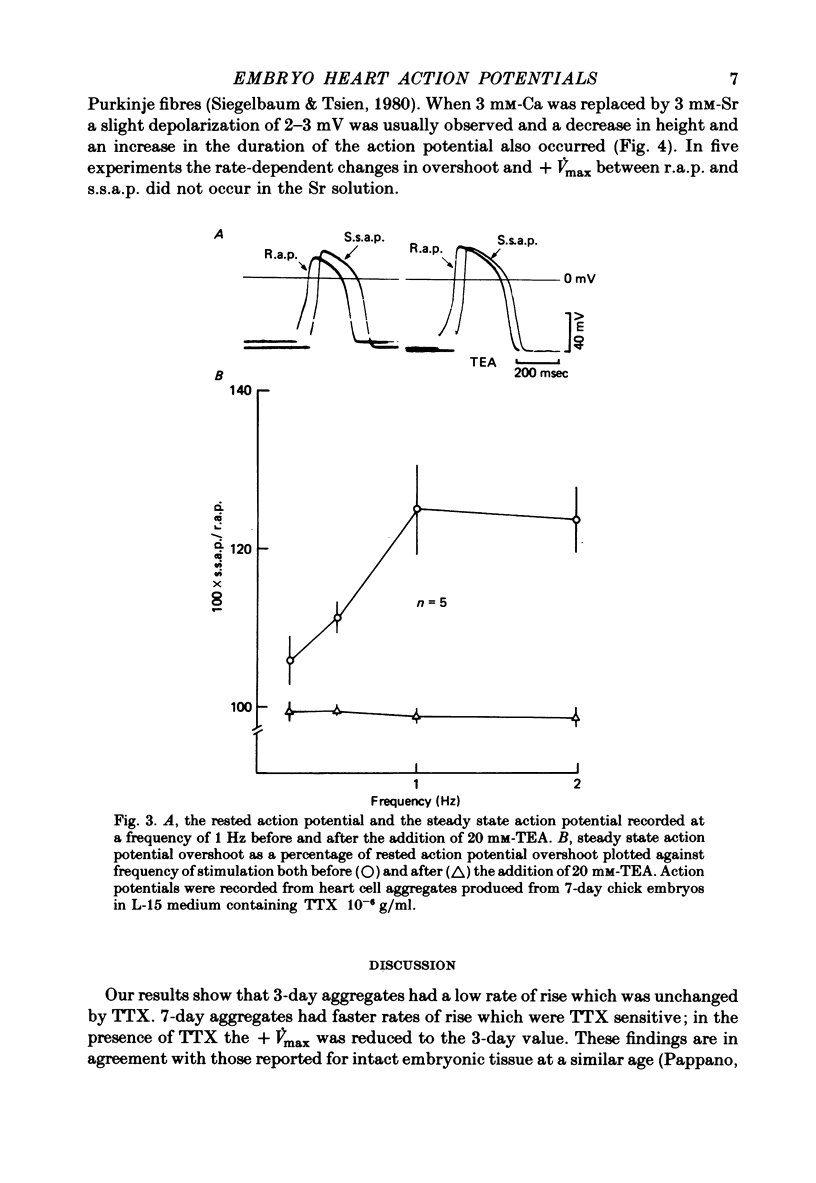
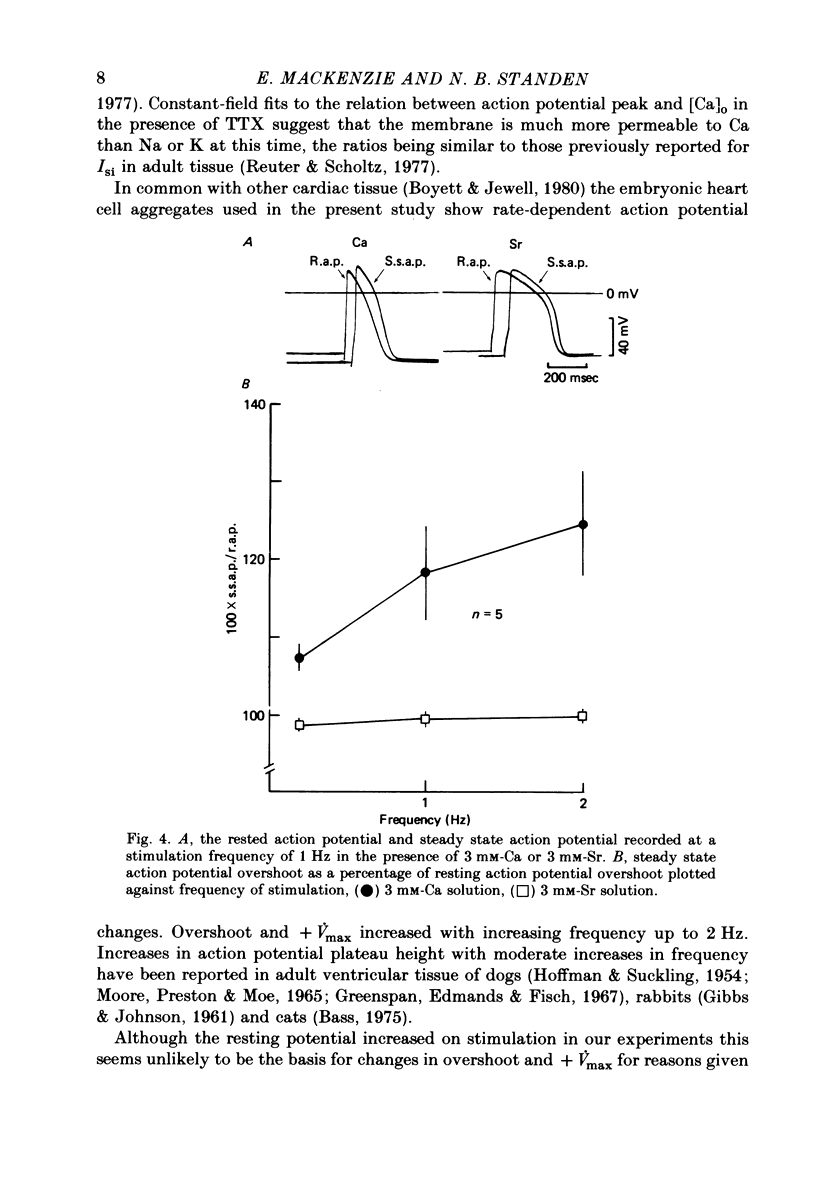
1. Action potentials were recorded from aggregates of heart cells prepared from 3- to 7-day chick embryos. At 3 days the maximum rate of rise (+ Vmax) was insensitive to TTX; at 7 days it was considerably reduced by TTX. 2. In the presence of TTX the action potential overshoot was dependent on [Ca]0; the results may be fitted using constant field theory and assuming that the membrane is over a hundred times more permeable to Ca than to Na or K. 3. An increase in stimulation rate in the range 0.2-2 Hz led to an increase in both overshoot and + Vmax. This effect was not seen after addition of 20 mM-tetraethylammonium ions, nor when Sr was substituted for Ca in the external medium. We suggest that these rate-dependent changes may result from partial inactivation of an outward K current.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass B. G. Restitution of the action potential in cat papillary muscle. Am J Physiol. 1975 Jun;228(6):1717–1724. doi: 10.1152/ajplegacy.1975.228.6.1717. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Boyett M. R., Jewell B. R. Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart. Prog Biophys Mol Biol. 1980;36(1):1–52. doi: 10.1016/0079-6107(81)90003-1. [DOI] [PubMed] [Google Scholar]
- CRANEFIELD P. F., HOFFMAN B. F. Electrophysiology of single cardiac cells. Physiol Rev. 1958 Jan;38(1):41–76. doi: 10.1152/physrev.1958.38.1.41. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Repolarisation and frequency in cardiac cells. J Physiol (Paris) 1977;73(7):903–923. [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Sodium permeability in toad nerve and in squid nerve. J Physiol. 1960 Jun;152:159–166. doi: 10.1113/jphysiol.1960.sp006477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard H. A., Sheu S. S. Intracellular potassium and sodium activities of chick ventricular muscle during embryonic development. J Physiol. 1980 Sep;306:579–586. doi: 10.1113/jphysiol.1980.sp013416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBS C. L., JOHNSON E. A. Effect of changes in frequency of stimulation upon rabbit ventricular action potential. Circ Res. 1961 Jan;9:165–170. doi: 10.1161/01.res.9.1.165. [DOI] [PubMed] [Google Scholar]
- Gibbons W. R., Fozzard H. A. Slow inward current and contraction of sheep cardiac Purkinje fibers. J Gen Physiol. 1975 Mar;65(3):367–384. doi: 10.1085/jgp.65.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski W., Lüttgau H. C., Schulze J. J. The effects of isoprenaline and a new beta-sympathomimetic amine upon spontaneous activity, diastolic depolarization and plateau height in cardiac Purkinje fibres. Br J Pharmacol. 1978 Jul;63(3):427–434. doi: 10.1111/j.1476-5381.1978.tb07794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan K., Edmonds R. E., Fisch C. The relation of contractile enhancement to action potential change in canine myocardium. Circ Res. 1967 Mar;20(3):311–320. doi: 10.1161/01.res.20.3.311. [DOI] [PubMed] [Google Scholar]
- HOFFMAN B. F., SUCKLING E. E. Effect of heart rate on cardiac membrane potentials and the unipolar electrogram. Am J Physiol. 1954 Oct;179(1):123–130. doi: 10.1152/ajplegacy.1954.179.1.123. [DOI] [PubMed] [Google Scholar]
- Hiraoka M., Sano T. Role of slow inward current in the genesis of ventricular arrhythmia. Jpn Circ J. 1976 Dec;40(12):1419–1427. doi: 10.1253/jcj.40.1419. [DOI] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. Influence of chloride, potassium, and tetraethylammonium on the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol. 1979 Feb;73(2):117–138. doi: 10.1085/jgp.73.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Haastert H. P., Krause H. Evidence of non-specificity of the Ca channel in mammalian myocardial fibre membranes. Substitution of Ca by Sr, Ba or Mg as charge carriers. Pflugers Arch. 1973 Aug 17;342(2):125–136. doi: 10.1007/BF00587843. [DOI] [PubMed] [Google Scholar]
- MOORE E. N., PRESTON J. B., MOE G. K. DURATIONS OF TRANSMEMBRANE ACTION POTENTIALS AND FUNCTIONAL REFRACTORY PERIODS OF CANINE FALSE TENDON AND VENTRICULAR MYOCARDIUM: COMPARISONS IN SINGLE FIBERS. Circ Res. 1965 Sep;17:259–273. doi: 10.1161/01.res.17.3.259. [DOI] [PubMed] [Google Scholar]
- MOSCONA A. Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interactions in vitro. Exp Cell Res. 1961 Jan;22:455–475. doi: 10.1016/0014-4827(61)90122-7. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Sachs H. G. Electrical activity in embryonic heart cell aggregates. Developmental aspects. Pflugers Arch. 1975;354(2):151–164. doi: 10.1007/BF00579945. [DOI] [PubMed] [Google Scholar]
- Meves H., Vogel W. Calcium inward currents in internally perfused giant axons. J Physiol. 1973 Nov;235(1):225–265. doi: 10.1113/jphysiol.1973.sp010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan R. D., DeHaan R. L. Voltage clamp analysis of embryonic heart cell aggregates. J Gen Physiol. 1979 Feb;73(2):175–198. doi: 10.1085/jgp.73.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan R. D., Pooler J. P., DeHaan R. L. Ultraviolet-induced alterations of beat rate and electrical properties of embryonic chick heart cell aggregates. J Gen Physiol. 1976 Jan;67(1):27–44. doi: 10.1085/jgp.67.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S., Shimoni Y. Voltage-dependent potentiation of the slow inward current in frog atrium. J Physiol. 1981 Jan;310:77–95. doi: 10.1113/jphysiol.1981.sp013538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappano A. J. Ontogenetic development of autonomic neuroeffector transmission and transmitter reactivity in embryonic and fetal hearts. Pharmacol Rev. 1977 Mar;29(1):3–33. [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenobu K., Sperelakis N. Calcium current channels induced by catecholamines in chick embryonic hearts whose fast sodium channels are blocked by tetrodotoxin or elevated potassium. Circ Res. 1972 Dec;31(6):932–952. doi: 10.1161/01.res.31.6.932. [DOI] [PubMed] [Google Scholar]
- Shigenobu K., Sperelakis N. Development of sensitivity to tetrodotoxin of chick embryonic hearts with age. J Mol Cell Cardiol. 1971 Dec;3(3):271–286. doi: 10.1016/0022-2828(71)90046-0. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalle M. Electrogenic suppression of automaticity in sheep and dog purkinje fibers. Circ Res. 1970 Sep;27(3):361–377. doi: 10.1161/01.res.27.3.361. [DOI] [PubMed] [Google Scholar]
- Vereecke J., Carmeliet E. Sr action potentials in cardiac Purkyne fibres. I. Evidence for a regenerative increase in Sr conductance. Pflugers Arch. 1971;322(1):60–72. doi: 10.1007/BF00586665. [DOI] [PubMed] [Google Scholar]
- Vitek M., Trautwein W. Slow inward current and action potential in cardiac Purkinje fibres. The effect of Mn plus,plus-ions. Pflugers Arch. 1971;323(3):204–218. doi: 10.1007/BF00586384. [DOI] [PubMed] [Google Scholar]
- Weingart R., Kass R. S., Tsien R. W. Is digitalis inotropy associated with enhanced slow inward calcium current? Nature. 1978 Jun 1;273(5661):389–392. doi: 10.1038/273389a0. [DOI] [PubMed] [Google Scholar]


