Abstract
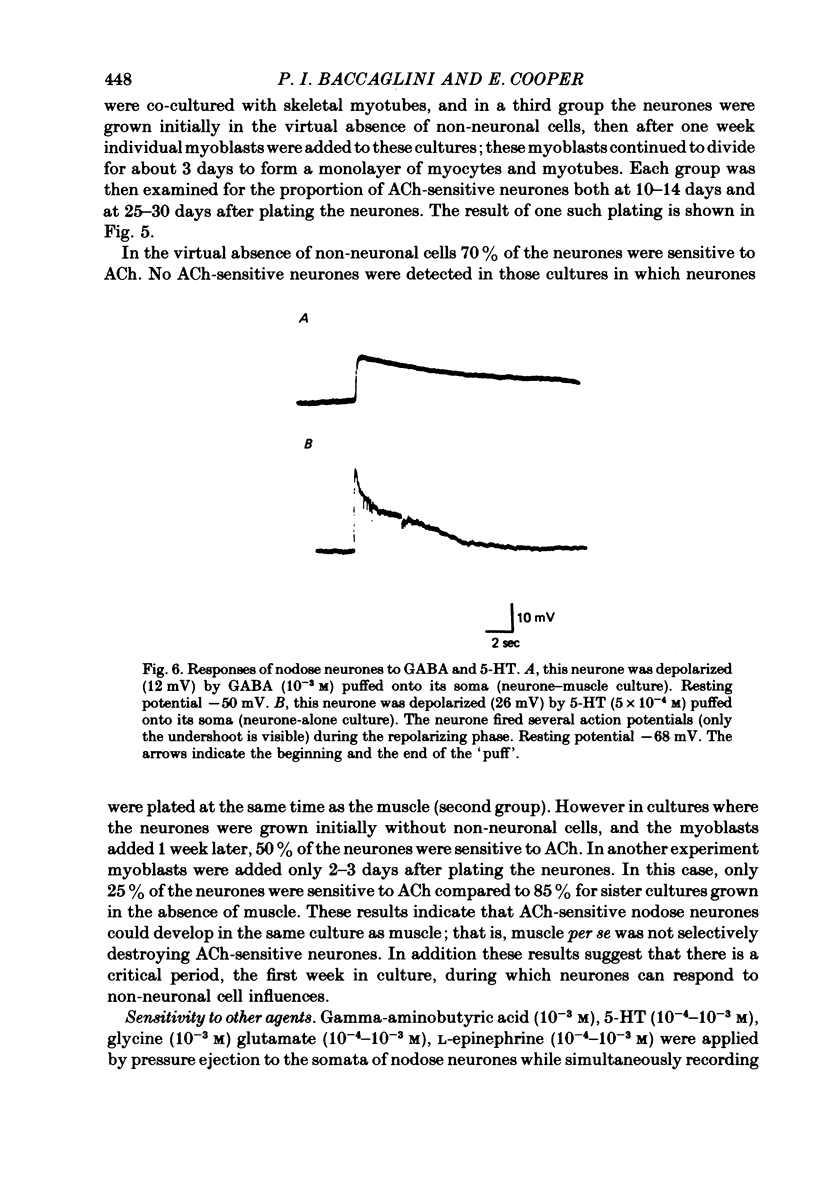
1. Nodose neurones dissociated from new-born rats were grown in culture in the absence or presence of cells from neonatal skeletal muscle or heart. 2. In cultures devoid of non-neuronal cells cholinergic interactions between the neurones were common. In the presence of non-neuronal cells such interactions were rare. 3. A decrease in the proportion of neurones responsive to ACh was primarily responsible for the reduced incidence of synaptic interactions. Non-neuronal cells influenced the expression of ACh receptors in developing nodose neurones in culture. 4. Most neurones appeared susceptible to the non-neuronal influence during the first week in culture. 5. Many nodose ganglion neurones, whether grown in the presence or absence of non-neuronal cells, were sensitive to gamma-aminobutyric acid and serotonin but were insensitive to glutamate, glycine and L-epinephrine.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMETT C. J., RITCHIE J. M. The action of acetylcholine on conduction in mammalian non-myelinated fibres and its prevention by an anticholinesterase. J Physiol. 1960 Jun;152:141–158. doi: 10.1113/jphysiol.1960.sp006476. [DOI] [PMC free article] [PubMed] [Google Scholar]
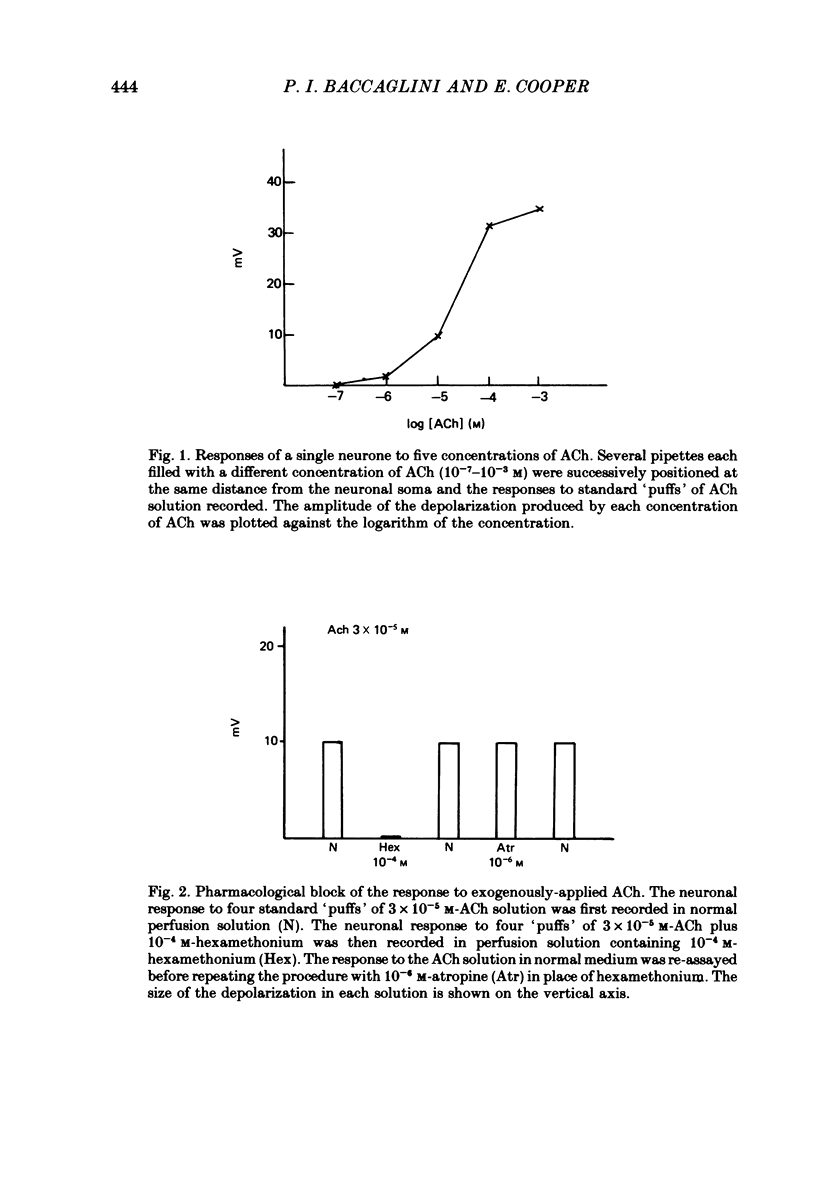
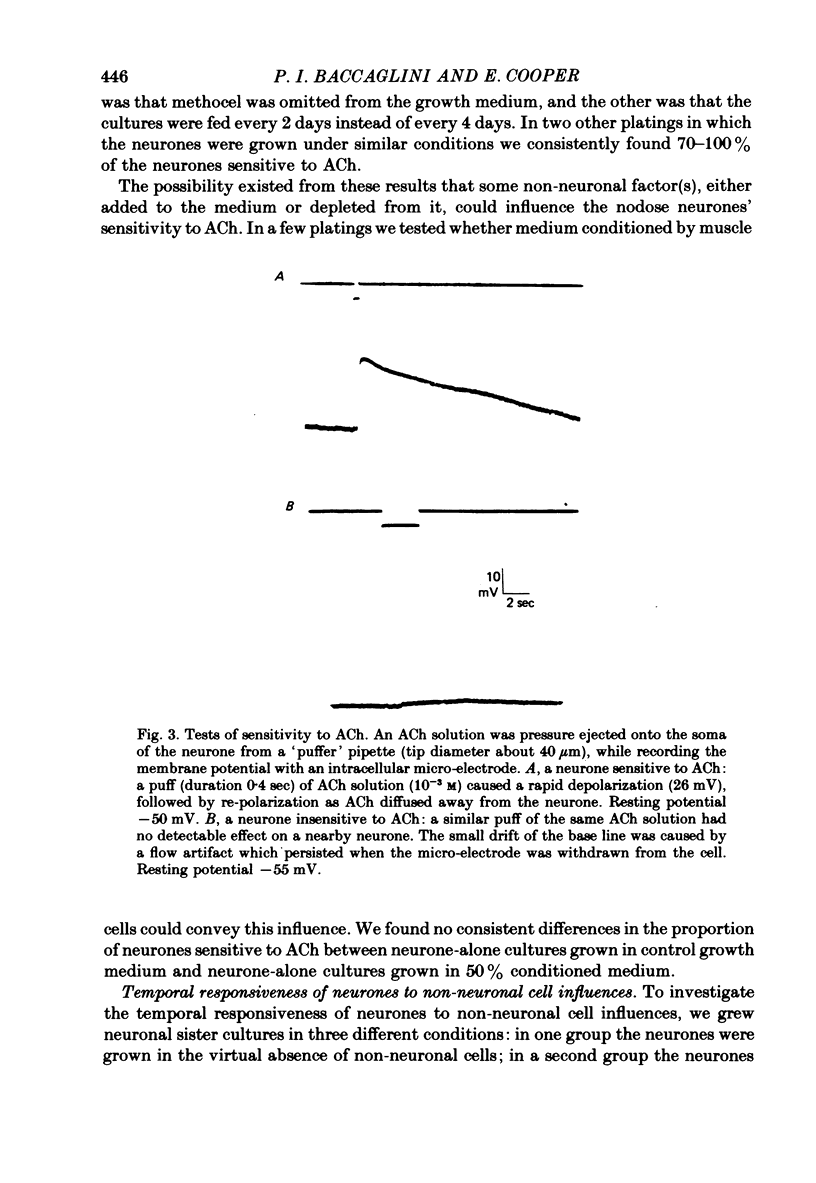
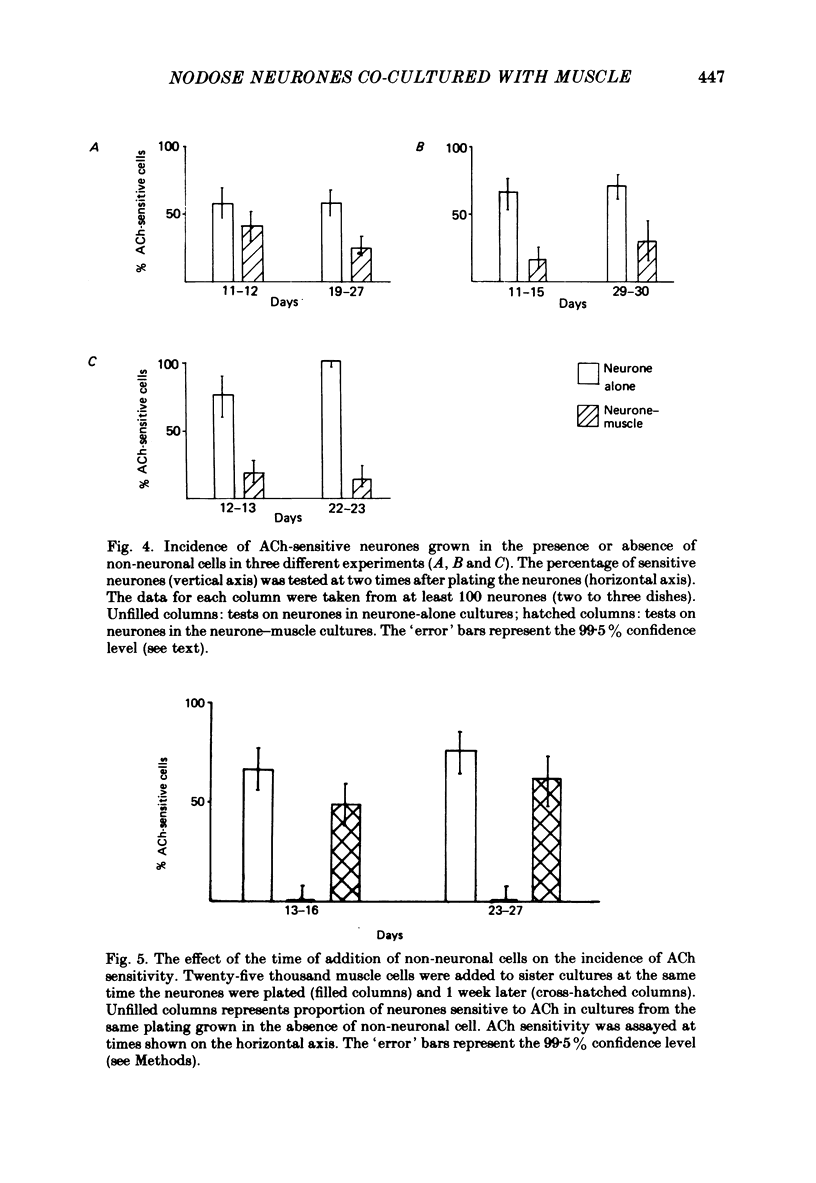
- Baccaglini P. I., Cooper E. Electrophysiological studies of new-born rat nodose neurones in cell culture. J Physiol. 1982 Mar;324:429–439. doi: 10.1113/jphysiol.1982.sp014122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W., Fischbach G. D. GABA conductance of chick spinal cord and dorsal root ganglion neurons in cell culture. J Neurophysiol. 1981 Apr;45(4):605–620. doi: 10.1152/jn.1981.45.4.605. [DOI] [PubMed] [Google Scholar]
- DIAMOND J. Observations on the excitation by acetylcholine and by pressure of sensory receptors in the cat's carotid sinus. J Physiol. 1955 Dec 29;130(3):513–532. doi: 10.1113/jphysiol.1955.sp005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat W. C. GABA-depolarization of a sensory ganglion: antagonism by picrotoxin and bicuculline. Brain Res. 1972 Mar 24;38(2):429–432. doi: 10.1016/0006-8993(72)90726-3. [DOI] [PubMed] [Google Scholar]
- Hawrot E. Cultured sympathetic neurons: effects of cell-derived and synthetic substrata on survival and development. Dev Biol. 1980 Jan;74(1):136–151. doi: 10.1016/0012-1606(80)90057-3. [DOI] [PubMed] [Google Scholar]
- Higashi H. 5-hydroxytryptamine receptors on visceral primary afferent neurones in the nodose ganglion of the rabbit. Nature. 1977 Jun 2;267(5610):448–450. doi: 10.1038/267448a0. [DOI] [PubMed] [Google Scholar]
- Landis S. C. Rat sympathetic neurons and cardiac myocytes developing in microcultures: correlation of the fine structure of endings with neurotransmitter function in single neurons. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4220–4224. doi: 10.1073/pnas.73.11.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H., Chun L. L. The induction of acetylcholine synthesis in primary cultures of dissociated rat sympathetic neurons. I. Effects of conditioned medium. Dev Biol. 1977 Apr;56(2):263–280. doi: 10.1016/0012-1606(77)90269-x. [DOI] [PubMed] [Google Scholar]


