Abstract
Binding to Golgi membranes of ADP ribosylation factor 1 (ARF1) is the first event in the initiation of COPI coat assembly. Based on binding studies, a proteinaceous receptor has been proposed to be critical for this process. We now report that p23, a member of the p24 family of Golgi-resident transmembrane proteins, is involved in ARF1 binding to membranes. Using a cross-link approach based on a photolabile peptide corresponding to the cytoplasmic domain of p23, the GDP form of ARF1 (ARF1-GDP) is shown to interact with p23 whereas ARF1-GTP has no detectable affinity to p23. The p23 binding is shown to localize specifically to a 22 amino acid C-terminal fragment of ARF1. While a monomeric form of a non-photolabile p23 peptide does not significantly inhibit formation of the cross-link product, the corresponding dimeric form does compete efficiently for this interaction. Consistently, the dimeric p23 peptide strongly inhibits ARF1 binding to native Golgi membranes suggesting that an oligomeric form of p23 acts as a receptor for ARF1 before nucleotide exchange takes place.
Keywords: ARF1/COPI-coat assembly/membrane binding/oligomeric membrane proteins/p24 proteins
Introduction
The small GTPase ARF1 is a structural coat component of Golgi-derived COPI vesicles (Serafini et al., 1991; Spang et al., 1998; Bremser et al., 1999) and mediates both coat assembly (Serafini et al., 1991; Donaldson et al., 1992a; Orci et al., 1993; Ostermann et al., 1993; Palmer et al., 1993) and disassembly (Tanigawa et al., 1993). Upon activation by a nucleotide exchange factor (Donaldson et al., 1992b; Helms and Rothman, 1992; Chardin et al., 1996; Peyroche et al., 1996; Franco et al., 1998; Togawa et al., 1999; Yamaji et al., 2000), cytosolic ARF1-GDP is converted into membrane-bound ARF1-GTP (Regazzi et al., 1991; Serafini et al., 1991; Haun et al., 1993; Helms et al., 1993; Randazzo et al., 1995; Goldberg, 1998), which, in turn, triggers membrane recruitment of coatomer (Donaldson et al., 1992a; Palmer et al., 1993; Teal et al., 1994). Direct interactions between ARF1 and coatomer have been demonstrated and shown to be GTP-dependent (Zhao et al., 1997, 1999). Additionally, coatomer was shown to bind to the cytoplasmic domains of some but not all members of the p24 family of type I transmembrane proteins (Sohn et al., 1996; Dominguez et al., 1998), suggesting that coatomer is recruited to Golgi membranes via a bivalent interaction. p24 proteins are localized to the early secretory pathway i.e. the intermediate compartment and the Golgi complex (Sohn et al., 1996; Rojo et al., 1997; Dominguez et al., 1998; Füllekrug et al., 1999) and are known to form oligomers (Belden and Barlowe, 1996; Füllekrug et al., 1999; Gommel et al., 1999; Marzioch et al., 1999; Wen and Greenwald, 1999). According to a liposome-based system utilizing chemically defined components, the soluble and membrane-associated factors described above are sufficient to drive COPI vesicle formation (Bremser et al., 1999). Con sistently, an oligomeric form of the cytoplasmic domain of p23 (Fligge et al., 2000; Weidler et al., 2000) causes a conformational change and, in the absence of membranes, subsequent aggregation of coatomer (Reinhard et al., 1999), suggesting that COPI coat polymerization is triggered through this interaction (Wieland and Harter, 1999).
Based on binding studies employing native Golgi-enriched membrane fractions, a proteinaceous ARF1 receptor was postulated to reside in Golgi membranes (Helms et al., 1993). While only ARF1-GTP is stably associated with the membrane (Regazzi et al., 1991; Serafini et al., 1991; Haun et al., 1993; Helms et al., 1993; Randazzo et al., 1995; Goldberg, 1998), two independent lines of evidence suggest that ARF1-GDP can associate with membranes as well. First, biochemical studies established that membrane recruitment of ARF1 must take place as a prerequisite for nucleotide exchange to proceed (Beraud-Dufour et al., 1999). Secondly, ARF1-mediated GTP hydrolysis has been shown to be required for efficient uptake by COPI vesicles of biosynthetic cargo (Nickel et al., 1998; Malsam et al., 1999; Pepperkok et al., 2000) and therefore multiple cycles of GDP to GTP exchange and GTP hydrolysis are likely to occur during early stages of coat assembly. Thus, a mechanism that prevents release of ARF1-GDP from the membrane would ensure efficient assembly of a pre-budding complex yet allow biosynthetic cargo to be taken up during formation of transport vesicles. Taken together, these findings raise the possibility that the relatively weak binding to Golgi membranes of ARF1-GDP observed (Serafini et al., 1991; Helms et al., 1993) might be both specific and physiologically relevant.
We now demonstrate that the cytoplasmic domain of p23 is in direct contact with ARF1-GDP during early stages of COPI coat assembly. A photo-cross-linking approach is used to show that p23 interacts with ARF1- GDP in a direct fashion. Domain mapping experiments revealed a 22 amino acid C-terminal fragment of ARF1 as the specific interaction site. Moreover, binding to native Golgi membranes of ARF1 in the presence of both GTP and GDP is inhibited by a dimeric form of the p23 C-tail peptide indicating that a p23 oligomer is involved in the recruitment step. These results establish specific binding to the Golgi of ARF1-GDP as the first step of ARF1 recruitment and identify the cytoplasmic domain of p23 as a receptor for ARF1-GDP.
Results
Direct interaction of ARF1-GDP with the cytoplasmic domain of p23
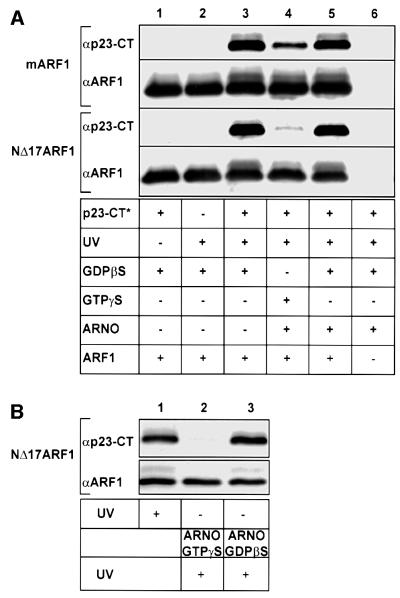
In order to probe a possible interaction between ARF1 and the cytoplasmic domain of p23, recombinant N-myristoylated human ARF1 (mARF1) was incubated with a peptide analogous to the cytoplasmic domain of p23 that contained a photolabile derivative of phenylalanine (trifluoro methyl-diazirino-phenylalanine, F*) in position 8 (p23- CT*; Table I). Following irradiation, samples were separated on 16.5% tricine SDS gels (Schägger and von Jagow, 1987), transferred to PVDF membranes and analyzed with antibodies directed against ARF1 or p23-CT (Figure 1). A cross-link product containing both ARF1 and the p23-CT was observed with a mobility consistent with the expected molecular mass of ∼22 kDa (Figure 1, lane 3). Cross-link product formation was found to depend on irradiation and the presence of p23-CT* (Figure 1, lanes 1 and 2). A truncated version of ARF1 lacking amino acids 1–17 (NΔ17ARF1; Kahn et al., 1992) was also capable of interacting with p23-CT (Figure 1, lane 3), demonstrating that the extreme N-terminus as well as the myristic acid moiety of full-length ARF1 are not directly involved in the interaction with p23.
Table I. Synthetic peptides used in this study.
| Peptide | Monomer | Dimer |
|---|---|---|
| p23-CT* | YLRRFF*KAKKLIE | |
| p23-CT | YLRRFFKAKKLIE | CYLRRFFKAKKLIE |
| | | ||
| CYLRRFFKAKKLIE | ||
| p24-CT | YLKRFFEVRRVV | CYLKRFFEVRRVV |
| | | ||
| CYLKRFFEVRRVV | ||
| p25-CT | HLKSFFEAKKLV | CHLKSFFEAKKLV |
| | | ||
| CHLKSFFEAKKLV | ||
| p26-CT | LLKSFFTEKRPISRAVHS | CLLKSFFTEKRPISRAVHS |
| | | ||
| CLLKSFFTEKRPISRAVHS | ||
| p27-CT | LLKSFFSDKRTTTTRVGS | CLLKSFFSDKRTTTTRVGS |
| | | ||
| CLLKSFFSDKRTTTTRVGS | ||
| Wbp1-CT | CSSVGKKLETFKKTN | CSSVGKKLETFKKTN |
| | | ||
| CSSVGKKLETFKKTN | ||
| KDEL-R-CT | CITKVLKGKKLSLPA | CITKVLKGKKLSLP |
| | | ||
| CITKVLKGKKLSLPA |
An asterisk indicates a photolabile residue.
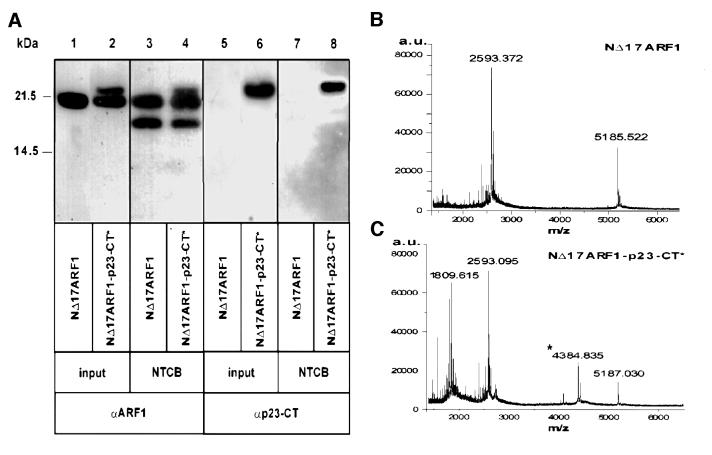
Fig. 1. Direct interaction of ARF1-GDP with the cytoplasmic domain of p23. (A) Nucleotide-dependent photo-cross-linking between a photolabile peptide corresponding to the cytoplasmic domain of p23 (50 µM p23-CT*; see Table I) with 2.5 µM of either recombinant myristoylated ARF1 (mARF1) or recombinant NΔ17ARF1 was conducted in the presence of 3 mM l-α-dimyristoyl-phosphatidyl-choline liposomes. Components were mixed as indicated in a total volume of 20 µl. Control conditions included omission of UV-irradiation (lane 1), omission of p23-CT* (lane 2) or omission of recombinant ARF1 (lane 6). In lanes 4 and 5, mARF1 or NΔ17ARF1 were pre-incubated with the nucleotide exchange factor ARNO (0.8 µM) in the presence of 50 µM GDPβS or GTPγS for 30 min at 37°C. Incorporation of GTP was monitored by the addition of trace amounts of [α-32P]GTP and found to be complete at 70 and 95% for mARF1 and NΔ17ARF1, respectively (data not shown). Proteins in lane 3 were pre-incubated with GDPβS in the absence of ARNO. Photo-cross-link reactions were conducted as described under Materials and methods and analyzed on 16.5 % tricine SDS gels (Schägger and von Jagow, 1987) followed by western blotting and immunodetection with antibodies against ARF1 (αARF1) and p23-CT (αp23-CT). (B) NΔ17ARF1 was incubated with p23-CT* as described in (A) in a final volume of 60 µl. The sample was split into three aliquots. One aliquot was irradiated (lane 1) and the two other aliquots were incubated with ARNO (0.8 µM), either in the presence of 50 µM GTPγS (lane 2) or GDPβS (lane 3). Following irradiation, the samples were analyzed as described above.
To investigate a potential nucleotide dependence of this interaction, mARF1 and NΔ17ARF1 were pre-incubated with either GDPβS or GTPγS in the presence or absence of the ARF-specific nucleotide exchange factor ARNO (Chardin et al., 1996; Goldberg, 1998) and liposomes for 30 min at 37°C followed by the addition of p23-CT*. The mixture was incubated for a further 60 min at 25°C followed by irradiation. Nucleotide exchange efficiency was monitored by analyzing incorporation of [α-32P]GTP into ARF1 employing a nitrocellulose filter trapping assay (Northup et al., 1982). Nucleotide exchange was 70% for mARF1 and 95% for NΔ17ARF1 (data not shown). Strikingly, for both mARF1 and NΔ17ARF1, cross-link product formation was found to depend on their GDP state (Figure 1, lane 3) rather than on their GTP state (Figure 1, lane 4). Cross-link product formation detected in the presence of GTPγS and ARNO is likely to be due to residual ARF1-GDP in the incubation mixture because it was directly correlated to the differing degrees of nucleotide exchange efficiency observed with mARF1 and NΔ17ARF1 (70 and 95%, respectively). The high efficiency of nucleotide exchange on NΔ17ARF1 prompted us to use this form of ARF1 for most of the subsequent studies.
Binding to p23-CT of ARF1-GDP dissociates upon nucleotide exchange
In order to analyze whether GDP-specific binding to p23-CT of NΔ17ARF1 can be dissociated by exchange of GDP to GTP on ARF1, a two-step experiment was performed. NΔ17ARF1 was incubated with p23-CT* under the conditions described above and the sample was split into three aliquots. One aliquot was irradiated and the two other aliquots were incubated with ARNO, either in the presence of GTPγS or GDPβS. These samples were irradiated as well and analyzed as described above. As depicted in Figure 1B, exchange of GDPβS by GTPγS results in an efficient release of p23-CT (lane 2), whereas p23-CT remains associated with ARF1 in the presence of ARNO and GDPβS (lane 3).
Analysis of specificity of the interaction between ARF1 and p23-CT
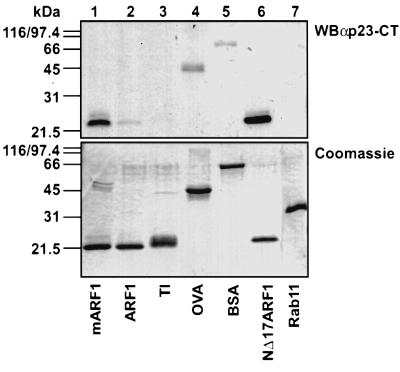
Specificity of the observed interaction was probed using a variety of unrelated proteins (Figure 2). In addition to myristoylated ARF1 (mARF1), non-myristoylated ARF1 (ARF1), trypsin inhibitor (TI), ovalbumin and bovine serum albumin (BSA) as well as the small GTP-binding protein rab11 were incubated together with p23-CT* and irradiated. Samples were subjected to gel electrophoresis and probed for cross-link product formation (Figure 2, top). Similar amounts of protein were confirmed by Coomassie Blue staining (Figure 2, bottom). Efficient cross-linking of p23-CT* to target proteins could only be observed with mARF1 (Figure 2, lane 1) and NΔ17ARF1 (Figure 2, lane 6). Using comparable amounts of protein, weak cross-linking could also be observed between p23-CT* and ovalbumin (Figure 2, lane 4). However, this was faint and regarded as unspecific since these proteins could not compete for the interaction between ARF1 and p23-CT (data not shown). Cross-link product formation between p23-CT* and either TI, BSA or the small GTPase rab11 were weak or not detectable at all (Figure 2, lanes 2, 3, 5 and 7). Surprisingly, non-myristoylated ARF1 did not show pronounced cross-link product formation.
Fig. 2. p23-CT* interacts specifically with mARF1. Comparable amounts (∼1 µg) of mARF1, ARF1, TI, ovalbumin (OVA), BSA, NΔ17ARF1 and rab11 were incubated in the absence of liposomes with the photolabile peptide p23-CT* (50 µM; see Table I) and irradiated as described in Materials and methods. Samples were analyzed on 16.5% tricine SDS gels (Schägger and von Jagow, 1987) and were further processed for either western blotting and immunodetection against p23-CT (WBαp23-CT, top) or Coomassie Blue staining (bottom).
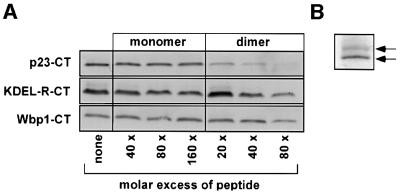
To further characterize the interaction between p23-CT* and ARF1-GDP, we conducted peptide competition experiments utilizing a pre-illuminated p23-CT*, p23-CT wild-type peptide as well as two control peptides corresponding to the cytoplasmic domains of two unrelated proteins, the Golgi-localized mammalian KDEL receptor and the ER-localized yeast oligosaccharyl transferase (Wbp1), respectively (see Table I). As expected, addition of excess amounts of pre-illuminated p23-CT* abolished cross-link formation (data not shown). Surprisingly, monomeric wild-type p23-CT did not interfere with cross-link formation. However, when p23-CT was included as a preformed dimer, cross-link product formation was almost completely abolished. By contrast, dimeric control peptides did not inhibit cross-link product formation to a significant extent (Figure 3A). Independent of this observation, an oligomeric nature of binding of p23-CT is indicated by the fact that, after prolonged gel electrophoresis, two species of ARF1 were detected with a migration consistent with one and two p23-CT molecules covalently bound, respectively (Figure 3B).
Fig. 3. An oligomeric form of p23-CT* interacts with ARF1-GDP.(A) Competition experiments were performed by incubating 12.5 µM p23-CT* (see Table I) and 2.5 µM mARF1 in the presence of excess amounts of wild-type p23-CT, the cytoplasmic domain of Wbp1 (Wbp1-CT) or the cytoplasmic domain of the KDEL receptor (KDEL-R-CT) in the absence of liposomes. See Table I for sequence information. Both monomeric and preformed dimers were used at the concentrations indicated. mARF1 was pre-incubated with candidate competitor peptides for 30 min at 25°C. Photo-cross-link reactions were conducted as described in Materials and methods. Cross-link products were analyzed on 16.5% tricine SDS gels (Schägger and von Jagow, 1987) followed by western blotting and immunodetection with an antibody directed against p23-CT. (B) Photo-cross-linking between p23-CT* and mARF1 was analyzed after prolonged gel electrophoresis as described above.
In summary, the interaction between p23-CT and ARF1 is highly specific and it appears that an oligomeric form of p23, possibly a tetramer (Fligge et al., 2000; Weidler et al., 2000), interacts with ARF1-GDP.
Interface between p23-CT and ARF1
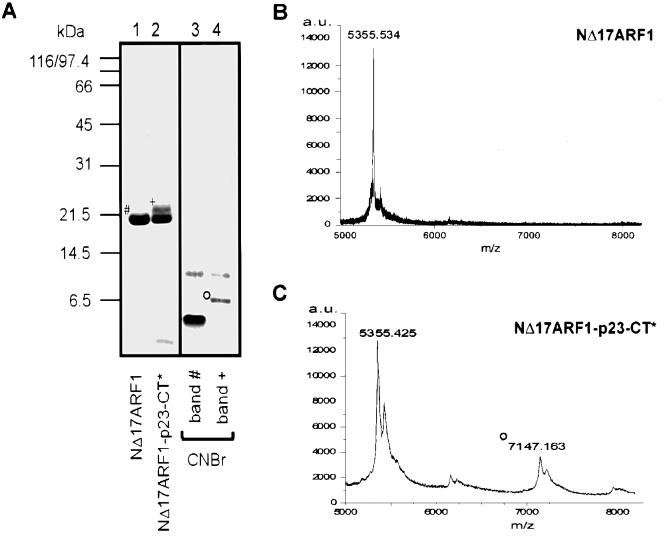
The binding site within ARF1 for p23-CT was analyzed by peptide mapping. To this end, NΔ17ARF1 was either incubated with p23-CT* followed by irradiation or left untreated as a control. Both samples were subjected to SDS–PAGE and analyzed by Coomassie Blue staining. Cross-linked and starting material (Figure 4A, band + and band #) were reisolated from the gel and treated with CNBr, a reagent that cleaves peptide bonds specifically on the C-terminal side of methionine residues. Since p23-CT* lacks methionine, CNBr-mediated cleavage should only occur within ARF1, leaving the cross-linked p23-CT* intact. Comparable amounts of the cleavage products were subsequently subjected to SDS–PAGE and analyzed by Coomassie Blue staining. Based on sequence analysis, three larger polypeptides were predicted to result from CNBr cleavage: a fragment consisting of residues 23–108 (9812 Da), a fragment consisting of residues 111–134 (2637 Da) and a C-terminal fragment of NΔ17ARF1 consisting of residues 135–181 (5356 Da; numbers according to full-length ARF1). The two larger fragments were observed by SDS–PAGE and Coomassie Blue staining (Figure 4A). While the largest fragment (residues 23–108) was observed in both samples, the C-terminal fragment was not observed in the irradiated sample. Instead, a new fragment with an apparent molecular mass of ∼6.5 kDa was detected by SDS–PAGE and Coomassie Blue staining (Figure 4, band °). This fragment was isolated from an SDS gel and analyzed by microsequencing. Parallel peptide sequences of the first 12 amino acid residues of the predicted C-terminal fragment and of p23-CT* (residue 1–5) were detected. Aliquots of CNBr-treated cross-linked and mock-treated NΔ17ARF1 were analyzed by MALDI-TOF. Both a molecular mass corresponding to residues 135–181 of ARF1 (Figure 4B and C) and the corresponding fragment cross-linked to p23-CT* (Figure 4C) were detected. Additionally, a molecular mass corresponding to residues 111–134 was detected; consistently, a cross-link product of this fragment to p23-CT* was absent from the mass spectra (data not shown).
Fig. 4. Interface between NΔ17ARF1 and p23-CT*. (A) 25 nmol NΔ17ARF1 was incubated with 50 nmol of p23-CT* in the presence of GDPβS and irradiated as described in Materials and methods. Samples of starting material and cross-linked NΔ17ARF1 were separated by SDS–PAGE with subsequent Coomassie Blue staining (lanes 1 and 2). Bands # and + were dissected from various lanes of an SDS gel and cleaved with CNBr as described in Materials and methods. Cleavage products were analyzed by SDS–PAGE and the band positive for a cross-link product (band °) was analyzed by Edman microsequencing. (B) NΔ17ARF1 was cleaved with CNBr, purified and analyzed by MALDI-TOF as described in Materials and methods. The peak at 5355.5 Da corresponds to the molecular mass of residues 135–181 of NΔ17ARF1. (C) NΔ17ARF1 was cross-linked to p23-CT*, cleaved with CNBr, purified and analyzed by MALDI-TOF as decribed in Materials and methods. The molecular mass indicated by ° corresponds to the cross-linked C-terminal fragment (residues 135–181) of NΔ17ARF1.
In order to analyze the specificity of the interaction of p23-CT with the C-terminal part of ARF1 by an independent method and to further narrow the site of interaction, NΔ17ARF1 was cross-linked to p23-CT* and subsequently fragmented using 2-nitro-5-thiocyanobenzoic acid (NTCB), a reagent that cleaves peptide bonds specifically at the N-terminal side of cysteine residues. Based on sequence analysis, two fragments of the protein corresponding to the molecular masses of 18 413 Da (N-terminal fragment) and 2594 Da (C-terminal fragment, residues 159–181) were expected. Although quantitative fragmentation could not be obtained, both a fragment corresponding to residues 159–181 of ARF1 and a fragment corresponding to these residues cross-linked to p23-CT* were observed by MALDI-TOF (Figure 5B and C). Additionally a fragment of 5185 ± 2 Da corresponding to a dimer of residues 159–191 was detected (Figure 5B and C). Due to limitations of the mass resolution of MALDI-TOF in the high molecular range, the N-terminal cleavage product of ARF1 was analyzed by SDS–PAGE with subsequent western blotting using antibodies directed against ARF1 and p23-CT (Figure 5A). As a result, a fragment with an apparent molecular weight of 18 kDa as well as residual full-length NΔ17ARF1 was detected using an antibody directed against ARF1 (Figure 5A, lanes 3 and 4). Strikingly, a cross-link product between p23-CT* and the 18 kDa N-terminal fragment was not detectable with an antibody directed against p23-CT (Figure 5A, lane 8). By contrast, a cross-link product between full-length NΔ17ARF1 and p23-CT* was clearly observed (Figure 5A, lanes 6 and 8). Taken together, fragmentation of NΔ17ARF1 cross-linked to p23-CT* by both CNBr and NTCB demonstrates that the cytoplasmic domain of p23 interacts specifically with the 22 amino acid C-terminal fragment of ARF1.
Fig. 5. Interface between NΔ17ARF1 and p23-CT*. (A) Samples of 8.3 nmol of NΔ17ARF1, either mock-treated (lanes 1, 3, 5 and 7) or cross-linked to p23-CT* (lanes 2, 4, 6 and 8), were cleaved with NTCB as described in Materials and methods. Samples of starting material (lanes 1, 2, 5 and 6) and NTCB-cleaved material (lanes 3, 4, 7 and 8) were separated by SDS–PAGE with subsequent western blotting using antibodies directed against ARF1 (lanes 1–4) or p23-CT (lanes 5–8). (B) NΔ17ARF1 was cleaved with NTCB, purified and analyzed by MALDI-TOF as decribed in Materials and methods. The peak at 2593.4 Da corresponds to the molecular mass of residues 159–181 of NΔ17ARF1, while 5185.5 Da represents a dimer of this fragment. (C) NΔ17ARF1 was cross-linked to p23-CT*, cleaved with NTCB, purified and analyzed by MALDI-TOF as decribed in Materials and methods. The molecular mass indicated by an asterisk corresponds to the cross-linked C-terminal fragment (residues 159–181) of NΔ17ARF1. The peak at 1809.6 Da corresponds to the molecular mass of p23-CT* cross-linked to H2O, while 5187.0 Da represents a dimer of the C-terminal fragment.
Dimers of p23-CT inhibit ARF1-GDP recruitment to Golgi membranes
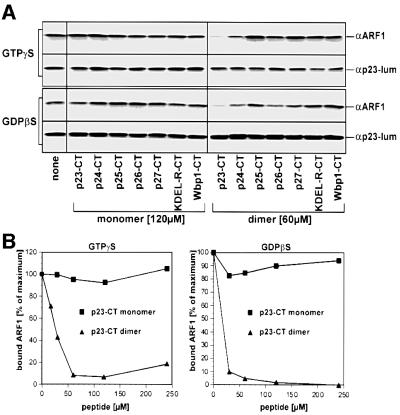
In order to determine whether the interaction between p23-CT and ARF1-GDP plays a role in recruitment to Golgi membranes of ARF1, we conducted ARF1-binding studies in the presence of various peptides, included as monomers and dimers (see Table I). GTPγS-dependent recruitment to membranes of ARF1 was almost completely abolished in the presence of preformed dimers of p23-CT (Figure 6A, top). Titration experiments revealed that half-maximal inhibition of ARF1 recruitment to membranes occurs at a p23-CT dimer concentration of ∼25 µM (Figure 6B). Consistent with the cross-linking experiments, p23-CT monomers did not inhibit ARF1 binding, suggesting that the p23–ARF1 interaction is indeed based on an oligomeric form of p23. While the addition of dimeric p24-CT peptide also caused a slight reduction of ARF1 binding to membranes, this was less effective. All other dimeric p24 family CT peptides as well as the control peptides (see above) did not interfere with ARF1 binding to Golgi membranes to an appreciable extent, regardless of whether they were added as monomers or dimers (Figure 6A).
Fig. 6. Dimeric p23-CT inhibits recruitment to Golgi membranes of ARF1-GDP. Binding studies were performed in a final volume of 50 µl using mARF1 (0.4 µM) and rat liver Golgi membranes (9.0 µg) in the presence of excess amounts of peptides corresponding to the cytoplasmic domains of mammalian p24 proteins as well as control proteins (p23-CT, p24-CT, p25-CT, p26-CT, p27-CT, Wbp1-CT and KDEL-R-CT; for sequence information see Table I). Incubations contained either GTPγS or GDPβS as indicated. Total ARF1 binding to membranes was typically enhanced 3- to 5-fold in the presence of GTPγS (data not shown). In the presence of GDPβS, the amount of mARF1 was increased (0.8 µM) and exposition times were elongated in order to obtain signal intensities comparable to ARF1 binding experiments in the presence of GTPγS. For additional details see Materials and methods. (A) GTPγS- and GDPβS-dependent mARF1 binding was analyzed in the presence of monomeric (120 µM) and dimeric (60 µM) peptides, respectively. After incubation the membranes were collected by centrifugation through a 15% (w/v) sucrose cushion followed by analysis of the membrane pellet employing SDS–PAGE and western blotting for immunodetection of ARF1 (αARF1; Palmer et al., 1993). In order to normalize for recovery of Golgi membranes, each experimental condition was analyzed for the amount of endogenous p23 as a Golgi marker utilizing an antibody directed against the lumenal part of p23 (αp23-lum; Sohn et al., 1996). (B) Concentration dependence of competition in the presence of either GDPβS or GTPγS. Peptides were added at the concentrations indicated. Determination of membrane-bound ARF1 was performed as described in (A).
Strikingly, p23-CT dimer was capable of almost fully inhibiting ARF1 binding to membranes measured in the presence of both GTPγS and GDPβS (Figure 6), suggesting that p23-CT dimers inhibit the earliest possible step of ARF1 recruitment to membranes.
In order to test the formal possibility that p23-CT dimers may compromise membrane integrity, protease protection experiments were performed. As expected for an undamaged membrane, the lumenal domain of p23 was found to be protected against proteinase K, irrespective of the presence or absence of p23-CT dimers (data not shown). As a control, complete digestion was observed in the presence of detergent. Consistent with the observation that membrane recovery in the presence of p23-CT dimers was normal compared with control conditions, as shown by analyzing the western blot with antibodies against the Golgi protein p23 (Figure 6A), protease protection experiments confirm that p23-CT dimers do not interfere with membrane integrity. Therefore, it can be excluded that inhibition by p23-CT dimers of ARF1 binding to the Golgi is due to a general damaging of the membrane.
Taken together, these findings directly establish that GDP-dependent binding to membranes of ARF1 is not an unspecific process but rather is likely to represent the first step of the overall process of ARF1 recruitment to membranes.
Discussion
Previous studies suggested the existence of a proteinaceous ARF1 receptor based on saturable binding to Golgi membranes of ARF1-GTP (Helms et al., 1993). However, such a receptor has not since been characterized at the molecular level, which, in the light of the current study, appears to be due to the fact that the putative receptor was assumed to be specific for ARF1-GTP.
In this study we present evidence that p23 is involved in early stages of ARF1 recruitment to Golgi membranes. Employing a photo-cross-linking approach, a direct and specific interaction of the cytoplasmic domain of p23 with myristoylated ARF1 is demonstrated. This interaction is specific for ARF1-GDP and dissociated when GDP was replaced by GTP. The binding interface between ARF1 and the cytoplasmic domain of p23 lies within the 22 amino acid C-terminal of ARF1 as analyzed by peptide mapping. Interestingly, this region of the protein is believed to be in close proximity to the membrane surface upon ARF1 binding (Goldberg, 1998). Structural data on the conformation of ARF1-GDP and ARF1-GTP show that the N-terminal helix that lies in parallel to the C-terminal helix of ARF1 is displaced upon GDP to GTP exchange. The displacement is mediated by the λ3-loop that occupies a position in ARF1-GTP, in which the N-terminus of ARF1-GDP is otherwise accommodated. This conformational change might affect the C-terminal helix of ARF1 in a way to allow efficient interaction with the cytoplasmic domain of p23 only if the protein is in its GDP-bound state.
Exposure of unrelated proteins (such as ovalbumin, TI and rab11) to the photolabile p23-CT peptide did not result in the formation of appreciable amounts of cross-link products, demonstrating specificity at the level of the target protein. To assess specificity at the level of the photolabile peptide, competition studies were performed. Excess amounts of non-photolabile peptides that correspond to the cytoplasmic domain of p23 as well as two control peptides analogous to the cytoplasmic domains of the KDEL receptor and Wbp1 were not able to compete for the interaction when added as monomers. However, preformed p23-CT dimers and pre-illuminated p23-CT* efficiently inhibited cross-link product formation. By contrast, the control peptides did not compete for cross-link product formation, irrespective of whether they were added as monomers or preformed dimers. These results suggest that it may be an oligomeric form of the photolabile peptide that interacts with ARF1-GDP, although it was not included in the incubation mixture as a preformed dimer. Indeed, peptides analogous to the cytoplasmic domains of p24 proteins have been shown to spontaneously associate in solution, forming dimers and tetramers with various degrees of stability (Fligge et al., 2000). Thus, it appears possible that the photolabile derivative of the p23-CT peptide forms an oligomer even more readily than the p23-CT wild-type peptide and, therefore, cross-link product formation is competed poorly by p23-CT monomers. In any case, the efficient competition of dimeric p23-CT peptide demonstrates that an oligomerized form of these protein domains is the principle of binding to ARF1-GDP. Future studies need to focus on the analysis of native Golgi membranes with respect to the exact determination of the oligomeric status of p23 bound to ARF1-GDP.
To investigate the physiological significance of the interaction observed between ARF1-GDP and the p23 cytoplasmic tail peptide, we conducted ARF1 binding studies utilizing native Golgi membranes (Helms et al., 1993). Both p24 family cytoplasmic tail peptides and control peptides (see above) were analyzed in competition experiments as monomers and preformed dimers. Consistent with the cross-linking studies, none of the monomeric peptides efficiently inhibited binding to Golgi membranes of either ARF1-GDP or ARF1-GTP. How ever, the dimeric p23-CT peptide significantly affected binding of both ARF1-GDP and ARF1-GTP, reaching half-maximal inhibition at a concentration of ∼25 µM. While the addition of dimeric p24-CT peptide also caused a slight reduction of ARF1 binding to membranes, this was less effective. All other dimeric p24 family CT peptides as well as the control peptides did not have any appreciable impact on ARF1 binding to Golgi membranes. Therefore, we conclude that an oligomeric form of p23 is directly involved in the recruitment to Golgi membranes of ARF1-GDP.
Our data are in good agreement with in vivo results of Majoul and co-workers (2001). Studying protein interactions in living cells, they observed fluorescence resonance energy transfer (FRET) between spectrally shifted mutants of green fluorescent protein, namely p23-CFP and ARF1-YFP, by multifocal multiphoton microscopy and bulk-cell spectrofluorimetry. To address whether this interaction was nucleotide specific, they analyzed the interaction between p23 and ARF1-Q71L, an ARF1 mutant that exists mainly in the GTP-bound form. While FRET between p23-CFP and ARF1-YFP was detected, FRET between p23-CFP and ARF1-Q71L-YFP was negligible and thus indicates, in line with our findings, that p23 binds preferentially to ARF1 in its GDP-bound form.
In addition, the data presented here are consistent with earlier findings of Antonny and co-workers who demonstrated that ARF1-GDP must be recruited to the membrane as a pre-requisite for nucleotide exchange (Beraud-Dufour et al., 1999). In fact, binding of myristoylated ARF1-GDP to membrane phospholipids has been described (Franco et al., 1995). However, as shown in this study, binding of ARF1-GDP to purified Golgi membranes is virtually abolished in the presence of a peptide resembling a dimeric form of p23, indicating that an interaction between ARF1 and p23 at the membrane is necessary for efficient recruitment of the GTPase. This process is likely to promote contact between ARF1 and lipids that facilitates activation by a nucleotide exchange factor (Franco et al., 1996; Antonny et al., 1997; Paris et al., 1997). Finally, GDP to GTP exchange results in a conformational switch in ARF1 (Amor et al., 1994; Greasley et al., 1995; Randazzo et al., 1995; Goldberg, 1998) that leads to dissociation from p23.
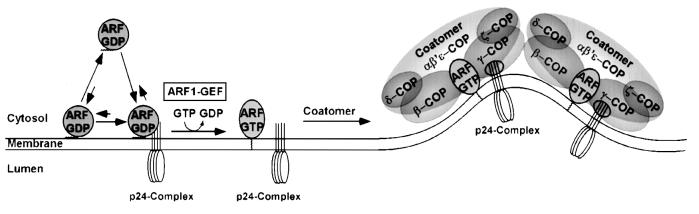
A model to illustrate early steps of ARF1 recruitment to membranes is depicted in Figure 7. Based on binding studies of myristoylated ARF1-GDP to membrane phospholipids by Franco et al. (1995), an equilibrium between soluble and membrane-bound ARF1-GDP was suggested. Data presented in this study suggest that this equilibrium is shifted towards the membrane-bound state upon binding of ARF1 to p23. Based on the observation that myristoylation of full-length ARF1-GDP is a requirement for the interaction with p23, myristic acid is either directly involved in the interaction or may positively modulate the binding site for p23. The first possibility can be excluded because an N-terminal truncation mutant of ARF1 (NΔ17ARF1; Kahn et al., 1992) is capable of interacting with p23 in a strictly GDP-dependent fashion despite lacking a myristic acid residue. The second possibility is supported by the observation that, following nucleotide exchange and conformational rearrangements that cause myristic acid to interact with the lipid bilayer, ARF1-GTP no longer binds to p23. This suggests that nucleotide exchange and ARF1 release from p23 are concerted events. As a consequence, the membrane becomes primed for coatomer recruitment by both membrane-associated ARF1-GTP (which interacts with β- and γ-COP; Zhao et al., 1997, 1999) and, in close proximity, by accessibility of p23 cytoplasmic tails (which interact with γ-COP; Harter and Wieland, 1998).
Fig. 7. A model for the recruitment to Golgi membranes of ARF1. Soluble ARF1-GDP binds to membrane phospholipids at low affinity. Upon binding to a p23 oligomer this interaction is stabilized. If, subsequently, a nucleotide exchange factor acts on ARF1-GDP, the resulting ARF1-GTP is released from p23 and two binding sites for coatomer are generated in close proximity: membrane-bound ARF1-GTP and a p23 oligomer. For further details, see Discussion.
Since coatomer cannot bind to Golgi membranes without the preceding recruitment of ARF1-GTP, it is also possible that p23-mediated ARF1-GDP binding and subsequent nucleotide exchange modulates the oligomeric status of the complex of p24 proteins in order to generate coatomer binding sites (Wieland and Harter, 1999). Although highly speculative at this time, p23 homo-oligomers might be generated from hetero-oligomers of p23 with other family members, driven by cycles of nucleotide exchange and ARF1-dependent GTP-hydrolysis. In addition, ARF1-mediated GTP hydrolysis has been shown to be required for efficient uptake by COPI vesicles of biosynthetic cargo (Malsam et al., 1999; Pepperkok et al., 2000) and, therefore, ARF1-GDP is continuously produced during coat assembly. Thus, a mechanism to prevent release from membranes of ARF1-GDP during early stages of coat recruitment would enhance assembly of a pre-budding complex that eventually leads to the formation of a COPI-coated transport vesicle.
Materials and methods
Preparation of recombinant proteins and Golgi membranes
Recombinant N-myristoylated human ARF1 (mARF1) was prepared to near homogeneity based on a protocol by Franco et al. (1995) and modified as described (Nickel and Wieland, 2001). Typically, ∼75% of the total ARF1 population was found to contain myristic acid. Non-myristoylated human ARF1 (ARF1) and NΔ17ARF1, containing an N-terminal His6-tag (ARF1 lacking amino acids 1–17; Kahn et al., 1992), were prepared (Helms et al., 1993; Goldberg, 1998). Recombinant ARNO (Chardin et al., 1996), containing an N-terminal His6-tag was over-expressed in Escherichia coli and purified to near homogeneity by Ni-NTA agarose chromatography according to Mossessova et al. (1998). rab11 was a kind gift of Birte Sönnichsen (EMBL, Heidelberg, Germany). TI, ovalbumin and BSA were purchased from Sigma (Deideshofen, Germany). Rabbit liver Golgi membranes were prepared as described by Tabas and Kornfeld (1979).
Peptide synthesis
Synthetic peptides used in this study are listed in Table I. Peptides referred to as p23-CT, p24-CT, p25-CT, p26-CT, p27-CT, Wbp1-CT and KDEL-CT, respectively, were designed corresponding to the C-terminal cytoplasmic sequences (-CT) of the human p24 family members p23 (hp24δ), p24 (hp24β), p25 (hp24α), p26 (hp24γ4), p27 (hp24γ3; Sohn et al., 1996; Dominguez et al., 1998), yeast Wbp1 (te Heesen et al., 1992) and the human KDEL receptor (Lewis and Pelham, 1990). A photolabile analogue of p23-CT, referred to as p23-CT*, was synthesized by replacing the natural F at position 8 by the photoreactive analogue F* (Photo probes, Sins, Switzerland) as described by Harter and Wieland (1998). Peptides were prepared by automated solid-phase synthesis using the Fmoc strategy and purified by high-performance liquid chromatography (HPLC). The disulfide-bridged dimeric peptides were prepared by oxidation of cysteines introduced at the N-terminus, in aqueous 20% dimethylsulfoxide for 48 h at room temperature (RT). Subsequently, the dimers were isolated by HPLC and characterized by mass spectrometry. Stock solutions (2 mM) were prepared in H2O, divided into aliquots and stored at –20°C immediately after preparation.
Photo-cross-linking experiments
In a typical photo-cross-linking assay, 2.5 µM of either recombinant mARF1, NΔ17ARF1 or control proteins (as indicated in the legend to Figure 2) were incubated with 50 µM of the photolabile peptide p23-CT* in 25 mM HEPES–KOH pH 7.2, 20 mM KCl, 2.5 mM magnesium acetate (buffer A) and 50 µM GDPβS in a total volume of 20 µl for 1 h at 25°C. 3 mM l-α-dimyristoyl-phosphatidylcholine liposomes were included if nucleotide dependence of the cross-link product was analyzed. To prevent ARF1 binding to the tube walls, incubations were performed in 1.5 ml silanized tubes. For competition experiments, recombinant mARF1 was pre-incubated for 30 min at 25°C with monomeric or dimeric peptides at the concentrations indicated. p23-CT* was then added to a final concentration of 12.5 µM and incubated for 1 h at 25°C. Photo-activation was performed on ice by illumination at λ = 365 nm for 2 min at a distance of 12 cm (4.6 W/cm2). Samples were analyzed by SDS–PAGE on 16.5% tricine gels (Schägger and von Jagow, 1987) followed by western blotting and immunodetection with antibodies directed against ARF1 (Palmer et al., 1993) and p23-CT (Sohn et al., 1996).
Analysis of nucleotide dependence of cross-link product formation
A reaction mixture of 20 µl containing 2.5 µM of either mARF1 or NΔ17ARF1, 0.8 µM ARNO, 3 mM l-α-dimyristoyl-phosphatidylcholine liposomes, ovalbumin (1 mg/ml), 50 µM GTPγS (or GDPβS in controls) in buffer A was pre-incubated for 30 min at 37°C in order to load GTPγS onto mARF1 and NΔ17ARF1, respectively. p23-CT* (final concentration 50µM) was added and incubated for 1 h at 25°C followed by irradiation as described above. Using these conditions, incorporation of GTP (measured by inclusion of [α32P]GTP; Chardin et al., 1996) into mARF1 and NΔ17ARF1 was complete at 70 and 95%, respectively.
Analysis of the interface between NΔ17ARF1 and p23-CT*
Fragmentation by treatment with cyanogen bromide (CNBr). NΔ17ARF1 (83 µM) was incubated with p23-CT* (166 µM) in a total volume of 300 µl. The sample was incubated for 1 h at 25°C, divided in 60 µl aliquots and irradiated for 5 min as mentioned above. Cross-linked material was separated from non-cross-linked material by 12% SDS–PAGE, Coomassie Blue stained and dissected from the gel. After two wash steps (each 20 min at RT) with 50% acetonitrile in 150 mM NH4HCO3 pH 8.9, the gel pieces were dehydrated in 60% acetonitrile for 20 min at RT. The samples were then lyophilized for 10 min and subsequently incubated in 10 mg CNBr/ml in 70 % TFA for 16 h in the dark. Extraction of the cleavage products from gel pieces was accomplished by three subsequent incubations with 60% acetonitrile, 0.1% TFA alternately with H2O, each for 20 min at RT. The combined extracts were lyophilized and subsequently analyzed both by MALDI-TOF (according to standard protocols) and SDS–PAGE on a 10% Bis-Tris NuPAGE gel with MES running buffer (Novex) followed by Coomassie Blue staining and microsequencing (Eckerskorn and Lottspeich, 1989).
Fragmentation by treatment with 2-nitro-5-thiocyanobenzoic acid. Samples according to 8.3 nmol of NΔ17ARF1, either mock-treated or cross-linked to p23-CT* as described above, were incubated with 5 mM NTCB in 50 mM Tris–HCl pH 8.0 and 8 M urea overnight at RT in a total volume of 400 µl. Subsequently the pH was shifted to 9.0 and the samples were further incubated 72 h at RT. Aliquots of the samples were either analyzed on a 10% NuPAGE gel with MES running buffer followed by western blotting with antibodies directed against p23-CT and ARF1 or analyzed by MALDI-TOF. For the latter analysis, the samples were purified using successive C4- and C18-Zip-Tip (Millipore) purification according to the manufacturer’s protocols.
ARF1 binding assay
In a typical ARF1 binding assay, mARF1 (0.4 µM in the presence of GTPγS and 0.8 µM in the presence of GDPβS) was incubated in a total volume of 50 µl for 20 min at 37°C in silanized tubes with rabbit liver Golgi membranes (9.0 µg) in buffer A, ovalbumin (1.6 mg/ml), 0.2 M sucrose and 25 µM GTPγS or 50 µM GDPβS, respectively. The binding reaction was terminated by transferring the incubation to ice. The reaction mixture was loaded onto a 165 µl cushion of 15% sucrose (w/v) in buffer A in a 1.5 ml BSA-coated tube and centrifuged for 1 h in a microfuge at 14 000 r.p.m. (4°C). The supernatant was removed and the membrane pellet was separated by SDS–PAGE followed by western blotting and immunodetection with antibodies directed against ARF1 (Palmer et al., 1993) and p23-CT (Sohn et al., 1996). Recovery of Golgi membranes was normalized by detection of endogenous p23, employing an antibody directed against the lumenal part of p23 (Sohn et al., 1996). Binding of mARF1 was analyzed in the presence of monomeric and dimeric forms of peptides analogous to the cytoplasmic domains of p24 proteins as well as control proteins (Wpb1 and KDEL receptor, respectively) at the concentrations indicated in the legend to Figure 6.
Acknowledgments
Acknowledgements
We thank B.Sönnichsen and M.Zerial for a kind gift of recombinant rab11 and P.Ihrig for MALDI-TOF analysis. This work was supported by grants from the German Research Council (DFG): SFB 352, C6 to W.N. and F.T.W., the Heidelberg Graduate Student Program Molecular Cell Biology to D.U.G. and a grant from the Human Frontiers Science Program Organization (HSFPO) to F.T.W.
References
- Amor J.C., Harrison,D.H., Kahn,R.A. and Ringe,D. (1994) Structure of the human ADP-ribosylation factor 1 complexed with GDP. Nature, 372, 704–708. [DOI] [PubMed] [Google Scholar]
- Antonny B., Beraud-Dufour,S., Chardin,P. and Chabre,M. (1997) N-terminal hydrophobic residues of the G protein ADP ribosylation factor 1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry, 36, 4675–4684. [DOI] [PubMed] [Google Scholar]
- Belden W.J. and Barlowe,C. (1996) Erv25p, a component of COPII-coated vesicles, forms a complex with Emp24p that is required for efficient endoplasmic reticulum to Golgi transport. J. Biol. Chem., 271, 26939–26949. [DOI] [PubMed] [Google Scholar]
- Beraud-Dufour S., Paris,S., Chabre,M. and Antonny,B. (1999) Dual interaction of ADP ribosylation factor 1 with Sec7 domain and with lipid membranes during catalysis of guanine nucleotide exchange. J. Biol. Chem., 274, 37629–37636. [DOI] [PubMed] [Google Scholar]
- Bremser M., Nickel,W., Schweikert,M., Ravazzola,M., Amherdt,M., Hughes,C.A., Söllner,T.H., Rothman,J.E. and Wieland,F.T. (1999) Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell, 96, 495–506. [DOI] [PubMed] [Google Scholar]
- Chardin P., Paris,S., Antonny,B., Robineau,S., Beraud-Dufour,S., Jackson,C.L. and Chabre,M. (1996) A human exchange factor for ARF contains Sec7- and pleckstrin-homology domains. Nature, 384, 481–484. [DOI] [PubMed] [Google Scholar]
- Dominguez M., Dejgaard,K., Fullekrug,J., Dahan,S., Fazel,A., Paccaud,J.P., Thomas,D.Y., Bergeron,J.J. and Nilsson,T. (1998) gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J. Cell Biol., 140, 751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J.G., Cassel,D., Kahn,R.A. and Klausner,R.D. (1992a) ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein β-COP to Golgi membranes. Proc. Natl Acad. Sci. USA, 89, 6408–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J.G., Finazzi,D. and Klausner,R.D. (1992b) Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature, 360, 350–352. [DOI] [PubMed] [Google Scholar]
- Eckerskorn C. and Lottspeich,F. (1989) Internal amino acid sequence analysis of proteins separated by gel electrophoresis after tryptic digestion in polyacrylamide matrix. Chromatographia, 28, 92–94. [Google Scholar]
- Fligge T.A., Reinhard,C., Harter,C., Wieland,F.T. and Przybylski,M. (2000) Oligomerization of peptides analogous to the cytoplasmic domains of coatomer receptors revealed by mass spectrometry. Biochemistry, 39, 8491–8496. [DOI] [PubMed] [Google Scholar]
- Franco M., Chardin,P., Chabre,M. and Paris,S. (1995) Myristoylation of ADP-ribosylation factor 1 facilitates nucleotide exchange at physiological Mg2+ levels. J. Biol. Chem., 270, 1337–1341. [DOI] [PubMed] [Google Scholar]
- Franco M., Chardin,P., Chabre,M. and Paris,S. (1996) Myristoylation-facilitated binding of the G protein ARF1GDP to membrane phospholipids is required for its activation by a soluble nucleotide exchange factor. J. Biol. Chem., 271, 1573–1578. [DOI] [PubMed] [Google Scholar]
- Franco M., Boretto,J., Robineau,S., Monier,S., Goud,B., Chardin,P. and Chavrier,P. (1998) ARNO3, a Sec7-domain guanine nucleotide exchange factor for ADP ribosylation factor 1, is involved in the control of Golgi structure and function. Proc. Natl Acad. Sci. USA, 95, 9926–9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füllekrug J., Suganuma,T., Tang,B.L., Hong,W., Storrie,B. and Nilsson,T. (1999) Localization and recycling of gp27 (hp24γ3): complex formation with other p24 family members. Mol. Biol. Cell, 10, 1939–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. (1998) Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell, 95, 237–248. [DOI] [PubMed] [Google Scholar]
- Gommel D., Orci,L., Emig,E.M., Hannah,M.J., Ravazzola,M., Nickel,W., Helms,J.B., Wieland,F.T. and Sohn,K. (1999) p24 and p23, the major transmembrane proteins of COPI-coated transport vesicles, form hetero-oligomeric complexes and cycle between the organelles of the early secretory pathway. FEBS Lett., 447, 179–185. [DOI] [PubMed] [Google Scholar]
- Greasley S.E., Jhoti,H., Teahan,C., Solari,R., Fensome,A., Thomas,G.M., Cockcroft,S. and Bax,B. (1995) The structure of rat ADP-ribosylation factor-1 (ARF-1) complexed to GDP determined from two different crystal forms. Nature Struct. Biol., 2, 797–806. [DOI] [PubMed] [Google Scholar]
- Harter C. and Wieland,F.T. (1998) A single binding site for dilysine retrieval motifs and p23 within the γ subunit of coatomer. Proc. Natl Acad. Sci. USA, 95, 11649–11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun R.S., Tsai,S.C., Adamik,R., Moss,J. and Vaughan,M. (1993) Effect of myristoylation on GTP-dependent binding of ADP-ribosylation factor to Golgi. J. Biol. Chem., 268, 7064–7068. [PubMed] [Google Scholar]
- Helms J.B. and Rothman,J.E. (1992) Inhibition by Brefeldin A of a Golgi membrane enzyme that catalyses exchange of a guanine nucleotide bound to ARF. Nature, 360, 352–354. [DOI] [PubMed] [Google Scholar]
- Helms J.B., Palmer,D.J. and Rothman,J.E. (1993) Two distinct populations of ARF bound to Golgi membranes. J. Cell Biol., 121, 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R.A. et al. (1992) The amino terminus of ADP-ribosylation factor (ARF) is a critical determinant of ARF activities and is a potent and specific inhibitor of protein transport. J. Biol. Chem., 267, 13039–13046. [PubMed] [Google Scholar]
- Lewis M.J. and Pelham,H.R. (1990) A human homologue of the yeast HDEL receptor. Nature, 348, 162–163. [DOI] [PubMed] [Google Scholar]
- Majoul I., Straub,M., Hell,S.W., Duden,R. and Söling,H.-D. (2001) KDEL-cargo regulates interactions between proteins involved in COPI vesicle traffic: measurements in living cells using FRET. Dev. Cell, 1, 139–153. [DOI] [PubMed] [Google Scholar]
- Malsam J., Gommel,D., Wieland,F.T. and Nickel,W. (1999) A role for ADP ribosylation factor in the control of cargo uptake during COPI-coated vesicle biogenesis. FEBS Lett., 462, 267–272. [DOI] [PubMed] [Google Scholar]
- Marzioch M., Henthorn,D.C., Herrmann,J.M., Wilson,R., Thomas,D.Y., Bergeron,J.J., Solari,R.C. and Rowley,A. (1999) Erp1p and Erp2p, partners for Emp24p and Erv25p in a yeast p24 complex. Mol. Biol. Cell, 10, 1923–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossessova E., Gulbis,J.M. and Goldberg,J. (1998) Structure of the guanine nucleotide exchange factor Sec7 domain of human ARNO and analysis of the interaction with ARF GTPase. Cell, 92, 415–423. [DOI] [PubMed] [Google Scholar]
- Nickel W. and Wieland,F.T. (2001) Receptor-dependent formation of COPI-coated vesicles from chemically defined donor liposomes. Methods Enzymol., 329, 388–404. [DOI] [PubMed] [Google Scholar]
- Nickel W., Malsam,J., Gorgas,K., Ravazzola,M., Jenne,N., Helms,J.B. and Wieland,F.T. (1998) Uptake by COPI-coated vesicles of both anterograde and retrograde cargo is inhibited by GTPγS in vitro. J. Cell Sci., 111, 3081–3090. [DOI] [PubMed] [Google Scholar]
- Northup J.K., Smigel,M.D. and Gilman,A.G. (1982) The guanine nucleotide activating site of the regulatory component of adenylate cyclase. Identification by ligand binding. J. Biol. Chem., 257, 11416–11423. [PubMed] [Google Scholar]
- Orci L., Palmer,D.J., Amherdt,M. and Rothman,J.E. (1993) Coated vesicle assembly in the Golgi requires only coatomer and ARF proteins from the cytosol. Nature, 364, 732–734. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Orci,L., Tani,K., Amherdt,M., Ravazzola,M., Elazar,Z. and Rothman,J.E. (1993) Stepwise assembly of functionally active transport vesicles. Cell, 75, 1015–1025. [DOI] [PubMed] [Google Scholar]
- Palmer D.J., Helms,J.B., Beckers,C.J., Orci,L. and Rothman,J.E. (1993) Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J. Biol. Chem., 268, 12083–12089. [PubMed] [Google Scholar]
- Paris S., Beraud-Dufour,S., Robineau,S., Bigay,J., Antonny,B., Chabre,M. and Chardin,P. (1997) Role of protein-phospholipid interactions in the activation of ARF1 by the guanine nucleotide exchange factor ARNO. J. Biol. Chem., 272, 22221–22226. [DOI] [PubMed] [Google Scholar]
- Pepperkok R., Whitney,J.A., Gomez,M. and Kreis,T.E. (2000) COPI vesicles accumulating in the presence of a GTP restricted ARF1 mutant are depleted of anterograde and retrograde cargo. J. Cell Sci., 113, 135–144. [DOI] [PubMed] [Google Scholar]
- Peyroche A., Paris,S. and Jackson,C.L. (1996) Nucleotide exchange on ARF mediated by yeast Gea1 protein. Nature, 384, 479–481. [DOI] [PubMed] [Google Scholar]
- Randazzo P.A., Terui,T., Sturch,S., Fales,H.M., Ferrige,A.G. and Kahn,R.A. (1995) The myristoylated amino terminus of ADP-ribosylation factor 1 is a phospholipid- and GTP-sensitive switch. J. Biol. Chem., 270, 14809–14815. [DOI] [PubMed] [Google Scholar]
- Regazzi R., Ullrich,S., Kahn,R.A. and Wollheim,C.B. (1991) Redistribution of ADP-ribosylation factor during stimulation of permeabilized cells with GTP analogues. Biochem. J., 275, 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard C., Harter,C., Bremser,M., Brugger,B., Sohn,K., Helms,J.B. and Wieland,F. (1999) Receptor-induced polymerization of coatomer. Proc. Natl Acad. Sci. USA, 96, 1224–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo M., Pepperkok,R., Emery,G., Kellner,R., Stang,E., Parton,R.G. and Gruenberg,J. (1997) Involvement of the transmembrane protein p23 in biosynthetic protein transport. J. Cell Biol., 139, 1119–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H. and von Jagow,G. (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem., 166, 368–379. [DOI] [PubMed] [Google Scholar]
- Serafini T., Orci,L., Amherdt,M., Brunner,M., Kahn,R.A. and Rothman,J.E. (1991) ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell, 67, 239–53. [DOI] [PubMed] [Google Scholar]
- Sohn K., Orci,L., Ravazzola,M., Amherdt,M., Bremser,M., Lottspeich,F., Fiedler,K., Helms,J.B. and Wieland,F.T. (1996) A major membrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding. J. Cell Biol., 135, 1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A., Matsuoka,K., Hamamoto,S., Schekman,R. and Orci,L. (1998) Coatomer, arf1p and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes. Proc. Natl Acad. Sci. USA, 95, 11199–11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. and Kornfeld,S. (1979) Purification and characterization of a rat liver Golgi α-mannosidase capable of processing asparagine-linked oligosaccharides. J. Biol. Chem., 254, 11655–11663. [PubMed] [Google Scholar]
- Tanigawa G., Orci,L., Amherdt,M., Ravazzola,M., Helms,J.B. and Rothman,J.E. (1993) Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J. Cell Biol., 123, 1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teal S.B., Hsu,V.W., Peters,P.J., Klausner,R.D. and Donaldson,J.G. (1994) An activating mutation in ARF1 stabilizes coatomer binding to Golgi membranes. J. Biol. Chem., 269, 3135–3138. [PubMed] [Google Scholar]
- te Heesen S., Janetzky,B., Lehle,L. and Aebi,M. (1992) The yeast WBP1 is essential for oligosaccharyl transferase activity in vivo and in vitro. EMBO J., 11, 2071–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togawa A., Morinaga,N., Ogasawara,M., Moss,J. and Vaughan,M. (1999) Purification and cloning of a brefeldin A-inhibited guanine nucleotide-exchange protein for ADP-ribosylation factors. J. Biol. Chem., 274, 12308–12315. [DOI] [PubMed] [Google Scholar]
- Weidler M., Reinhard,C., Friedrich,G., Wieland,F.T. and Rösch,P. (2000) Structure of the cytoplasmic domain of p23 in solution: implications for the formation of COPI vesicles. Biochem. Biophys. Res. Commun., 271, 401–408. [DOI] [PubMed] [Google Scholar]
- Wen C. and Greenwald,I. (1999) p24 proteins and quality control of LIN-12 and GLP-1 trafficking in Caenorhabditis elegans. J. Cell Biol., 145, 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland F. and Harter,C. (1999) Mechanisms of vesicle formation: insights from the COP system. Curr. Opin. Cell Biol., 11, 440–446. [DOI] [PubMed] [Google Scholar]
- Yamaji R., Adamik,R., Takeda,K., Togawa,A., Pacheco-Rodriguez,G., Ferrans,V.J., Moss,J. and Vaughan,M. (2000) Identification and localization of two brefeldin A-inhibited guanine nucleotide-exchange proteins for ADP-ribosylation factors in a macromolecular complex. Proc. Natl Acad. Sci. USA, 97, 2567–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Helms,J.B., Brügger,B., Harter,C., Martoglio,B., Graf,R., Brunner,J. and Wieland,F.T. (1997) Direct and GTP-dependent interaction of ADP ribosylation factor 1 with coatomer subunit β. Proc. Natl Acad. Sci. USA, 94, 4418–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Helms,J.B., Brunner,J. and Wieland,F.T. (1999) GTP-dependent binding of ADP-ribosylation factor to coatomer in close proximity to the binding site for dilysine retrieval motifs and p23. J. Biol. Chem., 274, 14198–14203. [DOI] [PubMed] [Google Scholar]