Abstract
The epithelial Na+ channel (ENaC) plays an essential role in the regulation of whole body Na+ balance and blood pressure. The cell surface expression of this channel, a complex of three subunits (α, β and γENaC), has been shown to be regulated by hormones such as aldosterone and vasopressin and by intracellular signaling, including ubiquitylation and/or phosphorylation. However, the molecular mechanisms involving phosphorylation in the regulation of ENaC are unclear. Here we show by expression studies in Xenopus laevis oocytes that the aldosterone-induced Sgk1 kinase interacts with the ubiquitin protein ligase Nedd4-2 in a PY motif-dependent manner and phosphorylates Nedd4-2 on Ser444 and, to a lesser extent, Ser338. Such phosphorylation reduces the interaction between Nedd4-2 and ENaC, leading to elevated ENaC cell surface expression. These data show that phosphorylation of an enzyme involved in the ubiquitylation cascade (Nedd4-2) controls cell surface density of ENaC and propose a paradigm for the control of ion channels. Moreover, they suggest a novel and complete signaling cascade for aldosterone-dependent regulation of ENaC.
Keywords: aldosterone/ion channel/Na+ transport/phosphorylation/ubiquitylation
Introduction
Na+ homeostasis, which is crucial for the maintenance of blood volume and pressure, is primarily regulated in the kidney, particularly in the distal areas of the nephron including the cortical collecting duct (CCD). Specialized epithelial cells (principal cells) express the amiloride-sensitive epithelial Na+ channel (ENaC) at the apical side (Garty and Palmer, 1997), allowing entry of Na+ into the cell and a Na+,K+–ATPase, extruding Na+ in exchange for K+, at the basolateral side. The concerted action of these two membrane proteins results in transepithelial Na+ absorption from the urinary to the sanguine compartment. In this context, ENaC activity, the rate-limiting step of Na+ transport, is under complex hormonal regulation, including aldosterone, vasopressin and insulin. However, the molecular mechanisms of this regulation are poorly understood (Verrey et al., 2000).
ENaC comprises three homologous subunits (α, β and γ) that are each composed of two transmembrane domains, an extracellular loop and short N- and C-termini (Canessa et al., 1993, 1994). Significantly, each subunit contains a PY motif (xPPxY) in the C-terminal region, which we and others have shown to interact with the WW domains of the ubiquitin protein ligases Nedd4-1 and Nedd4-2 (Staub et al., 1996; Kanelis et al., 1998, 2001; Abriel et al., 1999; Harvey et al., 1999; Farr et al., 2000; Kamynina et al., 2001a,b; Snyder et al., 2001). Because ubiquitylation (the covalent attachment of ubiquitin polypeptides to target proteins) of plasma membrane proteins is recognized as a mechanism to target such proteins for internalization (reviewed in Hicke, 1997, 1999; Rotin et al., 2000), we postulated that Nedd4 isoforms may control cell surface expression of ENaC via a ubiquitylation-dependent mechanism (Staub et al., 1996). This model is supported by the findings that the α and γ ENaC subunits are subjected to ubiquitylation (which controls cell surface expression; Staub et al., 1997), and that Nedd4-2 and, to a weaker extent Nedd4-1, regulate ENaC activity (Dinudom et al., 1998; Goulet et al., 1998; Abriel et al., 1999; Harvey et al., 1999, 2001; Farr et al., 2000; Kamynina et al., 2001a,b; Snyder et al., 2001). However, it is not known whether such ubiquitylation is under physiological control. The importance of the ENaC PY motifs is corroborated by findings that Liddle’s syndrome, an inherited form of human hypertension (Liddle et al., 1963; Botero-Velez et al., 1994), is linked to mutations in ENaC that invariably cause either the deletion or the alteration of the β or γ PY motif (Shimkets et al., 1994; Hansson et al., 1995a,b; Tamura et al., 1996; Jeunemaitre et al., 1997; J.Inoue et al., 1998; T.Inoue et al., 1998; Gao et al., 2001; Yamashita et al., 2001). When channels containing Liddle mutations are expressed in heterologous cell systems (e.g. Xenopus laevis oocytes), higher amiloride-sensitive Na+ currents (a measure of ENaC activity) are observed (Schild et al., 1995, 1996; Snyder et al., 1995), which are due to increased cell surface expression, open probability (Firsov et al., 1996) and reduced Na+ feedback inhibition (Kellenberger et al., 1998).
Recently, it has been reported that the expression of Sgk1 kinase (serum- and glucocorticoid-regulated kinase; Webster et al., 1993), a member of the PKB/Akt family of serine/threonine kinases, is induced by aldosterone in cells of the CCD and, importantly, stimulates ENaC activity when co-expressed in Xenopus oocytes (Chen et al., 1999; Naray-Fejes-Toth et al., 1999). These findings suggested that Sgk1 may represent an important mediator for aldosterone-dependant ENaC regulation. It was further demonstrated in oocytes that Sgk1 increases the cell surface expression of ENaC (Alvarez de la Rosa et al., 1999; Loffing et al., 2001), may be regulated by insulin via PI-3 kinase-dependent phosphorylation and interacts in vitro with the C-termini of α and β ENaC (Wang et al., 2001). However, the target(s) of Sgk1 and the molecular mechanism(s) of its action on ENaC are not known.
In this study, we have determined that the Sgk1 sequence contains a PY motif and Nedd4-2 comprises two conserved consensus sites for phosphorylation by Sgk1 [RXRXX(S/T); Kobayashi and Cohen, 1999; Park et al., 1999], suggesting that (i) Sgk1 may interact with Nedd4-2 in a PY motif–WW domain-dependent mode and (ii) Nedd4-2 may be a target of Sgk1. Indeed, we found in Xenopus oocytes that Sgk1 phosphorylates Nedd4-2 in a PY motif-dependent manner, Nedd4-2 phosphorylation is required for Sgk1 action on cell surface density of ENaC and Sgk1 reduces the interaction between Nedd4-2 and ENaC. These data therefore show a novel mechanism of regulated ubiquitylation of an ion channel and suggest a complete signaling pathway between aldosterone and its main downstream target in the distal nephron, ENaC.
Results
Sgk1 stimulates phosphorylation of Nedd4-2
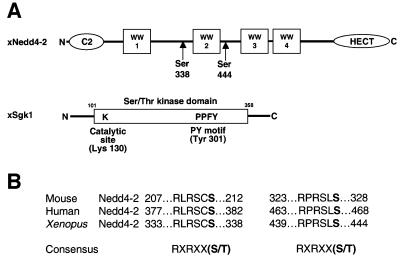
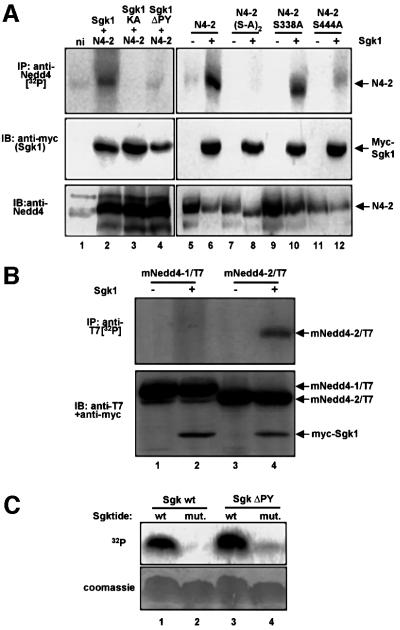
The recent reports that the aldosterone-inducible Sgk1 kinase controls ENaC activity (Chen et al., 1999; Naray-Fejes-Toth et al., 1999) and the identification of consensus phosphorylation sites for Sgk1 (Kobayashi and Cohen, 1999; Park et al., 1999) prompted us to look for potential phosphorylation sites in Nedd4-2, which is a regulator of ENaC. Indeed, we found two such conserved consensus motifs at Ser338 and Ser444 in Xenopus Nedd4-2 (Figure 1), suggesting that Nedd4-2 may be a target of Sgk1. To test this hypothesis, we expressed Nedd4-2 in Xenopus oocytes with or without c-myc-tagged Sgk1, incubated the oocytes with [32P]ortho–phosphate and immunoprecipitated Nedd4-2 from a cellular lysate. We found that Nedd4-2 was strongly phosphorylated when Sgk1 was co-expressed (Figure 2A, lanes 2 and 6, top). Weak phosphorylation, possibly due to endogenous Sgk1 activity, was observed when Sgk1 was omitted (lane 5) or when a catalytically inactive Sgk1 was used (lane 3; Sgk1-KA, K130A). We then mutated, either together or separately, the two putative phosphorylation sites on Nedd4-2. This led to complete inhibition of Nedd4-2 phosphorylation when both sites were mutated [Figure 2A, lane 8; N4-2(S-A)2, S338AS444A], weak reduction when Ser338 was mutated (lane 10; Nedd4-2-S338A) and a much stronger reduction when Ser444 was changed to Ala (lane 12; Nedd4-2-S444A). As mentioned, Sgk1 also contains a PY motif, which may serve as an interaction site with the Nedd4-2 WW domains. Indeed, mutation of the PY motif abrogated Nedd4-2 phosphorylation almost completely (Figure 2A, lane 4; Sgk1ΔPY), although the same mutant was still able to phosphorylate a synthetic peptide substrate (Sgktide; Park et al., 1999), demonstrating that mutation of the PY motif does not impair catalytic activity (Figure 2C). As shown in Figure 2B, Sgk1 did not efficiently phosphorylate the paralog Nedd4-1, which contains no consensus sequences for Sgk1-dependent phosphorylation and does not seem to be primarily involved in ENaC regulation (Kamynina et al., 2001a). Hence, Sgk1 induces phosphorylation of Nedd4-2 (but not Nedd4-1) via a PY motif-dependent mechanism, essentially on Ser444 and to a lesser extent on Ser338.
Fig. 1. Schematic view of Nedd4-2 and Sgk1. (A) Scheme of Xenopus Nedd4-2 with the consensus phosphorylation sites and Xenopus Sgk1 with the indication of the catalytic domain, the catalytically essential Lys130 and the PY motif. (B) Conserved consensus phosphorylation sites in mouse, human and Xenopus Nedd4-2.
Fig. 2. Sgk1 phosphorylates Nedd4-2, but not Nedd4-1. (A) Oocytes expressing either wild-type Nedd4-2 (N4-2) or the phosphorylation mutants Nedd4-2-S338A-S444A [N4-2(S-A)2], Nedd4-2-S338A, Nedd4-2-S444A and myc-Sgk1 [wild-type, catalytically inactive (KA, K130A) or PY motif-mutated (ΔPY, Y301A)] were incubated with [32P]ortho–phosphate and treated as follows: top, immunoprecipitation from lysates with anti-Nedd4-2 antibodies and autoradiography; middle, western blot on lysates with anti-myc antibody (recognizing Sgk1); bottom, western blot on lysates with anti-Nedd4-2 antibodies. (B) Mouse Nedd4-1 (mNedd4-1/T7) or Nedd4-2 (mNedd4-2/T7), both epitope-tagged with a T7 epitope (Novagen), were expressed with or without Sgk1 (as indicated) and phosphorylation was followed as described in (A), except that the Nedd4 proteins were immunoprecipitated with anti-T7 antibody (top). Expression of mNedd4-1, mNedd4-2 and Sgk1 was followed by western blot analysis on lysates, using both anti-T7 (Nedd4-1 or Nedd4-2) and anti-myc (Sgk1) antibodies. (C) Phosphorylation of a synthetic peptide substrate (Sgktide) by Sgk1. Wild-type and mutant myc-Sgk1 (lacking a functional PY motif) were expressed in Xenopus oocytes and immunoprecipitated with anti-myc antibodies. The immunoprecipitated kinases were assayed in a kinase assay with Sgktide or a mutant peptide lacking the phosphorylation site as described in Materials and methods. Phosphorylated peptides were then analyzed by separation on a tricine acrylamide gel followed by autoradiography. Both wild-type and mutant Sgk1 were able to phosphorylate Sgktide, but not its mutant (top). Bottom, Coomassie Blue staining.
Sgk1 regulates ENaC via Nedd4-2 phosphorylation
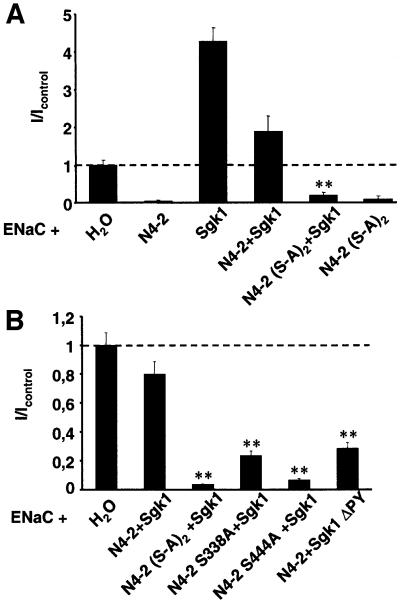
If phosphorylation of Nedd4-2 is the mechanism by which Sgk1 stimulates ENaC activity, mutation of the phosphorylation sites on Nedd4-2 would be expected to abrogate the stimulatory effect of Sgk1. To test this hypothesis, we expressed ENaC together with various Sgk1 and Nedd4-2 mutants in Xenopus oocytes (Figure 3). As reported previously, wild-type Nedd4-2 strongly reduced ENaC currents, whereas Sgk1 stimulated them (Figure 3A). When Sgk1 was expressed in excess over Nedd4-2, the inhibitory effect of Nedd4-2 on ENaC was reduced, leading to currents that were sometimes even higher than in the control oocytes (Figure 3A; N4-2 + Sgk1). However, Sgk1 did not increase ENaC activity when co-expressed with Nedd4-2 lacking both phosphorylation sites [Figure 3A, compare N4-2(S-A)2 with N4-2(S-A)2 + Sgk1]. In a separate experiment, we determined the importance of the individual phosphorylation sites for ENaC regulation. We found that when Ser338 alone was mutated (Figure 3B; Nedd4-2 S338A + Sgk1), Sgk1’s effect was markedly reduced, whereas when Ser444 was mutated, Sgk1’s effect was blunted (Nedd4-2 S444A + Sgk1). Thus, phosphorylation of Nedd4-2 on Ser444 is essential for the regulation of ENaC by Sgk1, while phosphorylation of Ser338 may also play a role. Finally, mutation of the Sgk1 PY motif reduced the ability of Sgk1 to interfere with Nedd4-2-dependent inhibition of ENaC (Figure 3B), consistent with an Sgk1–Nedd4-2 interaction involving the Sgk1 PY motif.
Fig. 3. Sgk1-dependent regulation of ENaC requires Nedd4-2 phosphorylation sites and the PY motifs on Sgk1. (A) Oocytes were injected with cRNA encoding Nedd4-2 or the Nedd4-2 phosphorylation mutant, Sgk1 cRNA and ENaC (as indicated). Amiloride-sensitive Na+ currents were measured and normalized to control oocytes (expressing only ENaC). n = 15 oocytes from three animals; **p <0.01 level of significance versus N4-2 + Sgk1. (B) Same as (A), but cRNA encoding either wild-type or mutant Sgk1 (Sgk1ΔPY), wild-type or phosphorylation site mutant Nedd4-2 and ENaC (as indicated) were injected, ** p <0.01 versus control. The observed variation of N4-2 + Sgk1 between (A) and (B) is due to batch-to-batch variation.
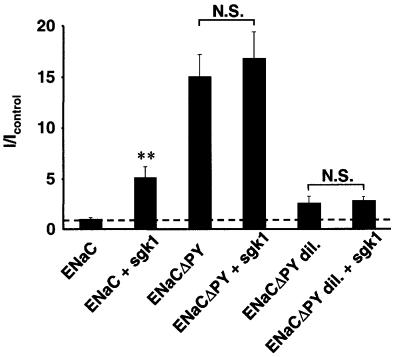
Because Nedd4-2 interacts not only via its WW domains with the PY motifs of Sgk1 but also with the PY motifs of ENaC, thereby exerting its effect on ENaC (Kamynina et al., 2001a), one would expect that removal or mutation of the ENaC PY motifs abrogates Sgk1-dependent ENaC stimulation. Indeed, we found that ENaC channels lacking all functional PY motifs were not stimulated by Sgk1 (Figure 4, ENaCΔPY). Removal of PY motifs resulted in enhanced activity of ENaC, due in part to the described additional effects of the PY motif on open probability (Firsov et al., 1996), raising the possibility that the system was unable to support additional Na+ transport, which may explain the lack of stimulation by Sgk1. However, Sgk1 remained without effect, even when 10 times less ENaCΔPY cRNA was injected, yielding low basal currents comparable to wild-type ENaC (Figure 4; compare ENaCΔPYdil with ENaCΔPYdil + Sgk1). Therefore, the PY motifs of ENaC are required for the stimulation of ENaC-related currents by Sgk1.
Fig. 4. The PY motifs of ENaC are required for stimulation of ENaC by Sgk1. Currents of oocytes expressing either wild-type ENaC or mutant ENaC lacking all PY motifs (ENaCΔPY), with and without Sgk1. ENaCΔPYdil containing 10 times less cRNA encoding ENaC was injected in order to get lower basal amiloride-sensitive currents. n = 18 oocytes from three animals; **p >0.01 versus ENaC.
Sgk1-dependent phosphorylation of Nedd4-2 controls cell surface expression of ENaC
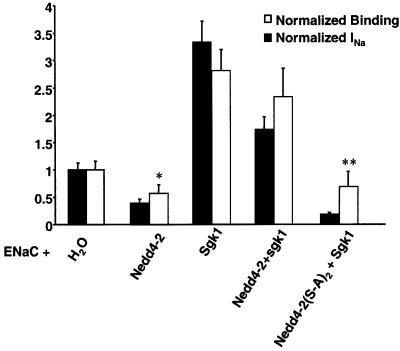
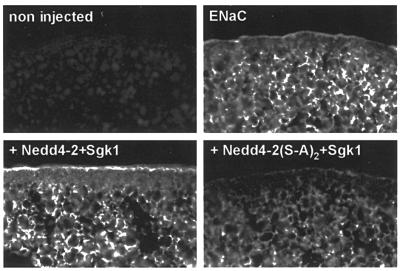
We then investigated whether Sgk1-dependent phosphorylation of Nedd4-2 affects cell surface density of ENaC. We expressed ENaC, which was FLAG-tagged at the extracellular loops of the β and γ subunits, together with Sgk1 and various forms of Nedd4-2. This allowed quantification of ENaC surface expression by binding of 125I-labeled FLAG antibodies (Firsov et al., 1996). As shown previously, both Nedd4-2 (negatively; Abriel et al., 1999) and Sgk1 (positively; Alvarez de la Rosa et al., 1999; Loffing et al., 2001) influenced amiloride-sensitive Na+ currents (Figure 5, filled bars) and antibody binding proportionally (non-filled bars), confirming that they mostly affect the expression of channels at the cell surface and not the intrinsic properties of ENaC. When Nedd4-2 and Sgk1 were co-expressed, channel density was high, which is compatible with the idea that Sgk1 interferes with Nedd4-2-dependent suppression of ENaC. In contrast, mutations of the phosphorylation sites on Nedd4-2 lead to a low number of ENaC channels even in the presence of Sgk1 [Nedd4-2(S-A)2 + Sgk1], corroborating that phosphorylation of Nedd4-2 is required for Sgk1-dependent control of ENaC levels at the plasma membrane. We further confirmed this effect by immunocytochemical detection of FLAG-tagged ENaC on cryosections of Xenopus oocytes (Figure 6). Consistent with the binding experiments, co-expression of ENaC with Nedd4-2 and Sgk1 (+ Nedd4-2 + Sgk1) increased ENaC cell surface abundance, whereas co-expression of ENaC with Sgk1 and the phosphorylation mutant Nedd4-2 [+Nedd4-2(S-A)2 + Sgk1] decreased it.
Fig. 5. Sgk1-dependent phosphorylation of Nedd4-2 controls ENaC cell surface expression. Oocytes were co-injected with cRNA encoding FLAG-tagged ENaC together with either H2O, wild-type or mutant Nedd4-2 lacking both phosphorylation sites [Nedd4(S-A)2] and Sgk1, as indicated. Amiloride-sensitive Na+ currents (filled bars) and binding of iodinated anti-FLAG antibodies (non-filled bars) to quantitate the number of channels at the cell surface were measured in the same oocytes, as described previously (Firsov et al., 1996; Abriel et al., 1999). Current and binding values were normalized to control values (ENaC + H2O). n = 18 oocytes from three animals; *p <0.05 versus control, **p <0.01 versus Nedd4-2 + Sgk1.
Fig. 6. Immunostaining of ENaC in oocytes expressing either wild-type or phosphorylation mutant Nedd4-2 and Sgk1. FLAG-tagged ENaC was followed by immunofluorescence with anti-FLAG antibodies on cryosections in either uninjected oocytes or oocytes expressing ENaC alone, ENaC plus wt-Nedd4-2 plus Sgk1 (+ Nedd4-2 + Sgk1) or ENaC plus Nedd4-2 S338A-S444A plus Sgk1 [+ Nedd4(S-A)2 + Sgk1].
Sgk1 interferes with the interaction between Nedd4-2 and ENaC
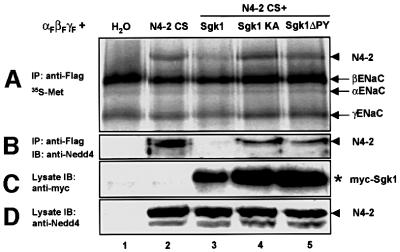
We wished to know whether Sgk1-dependent phosphorylation of Nedd4-2 influenced ENaC cell surface expression. Sgk1 may interfere either with Nedd4-2–ENaC interaction or with other parameters, such as enzymatic Nedd4-2 activity. To determine whether the Nedd4-2–ENaC interaction was regulated by Sgk1, we expressed FLAG-tagged ENaC subunits together with mutant Nedd4-2 (Nedd4-2-CS) and various Sgk1 mutants. We chose to use the catalytically inactive Nedd4-2-CS (Abriel et al., 1999) in order to avoid interference of ENaC ubiquitylation with the Nedd4-2–ENaC interaction. Cells were labeled overnight with [35S]methionine and immunoprecipitations with anti-FLAG antibodies (recognizing FLAG-tagged ENaC) were performed. The immunoprecipitated material was analyzed by SDS–PAGE and autoradiography or western blotting. As can be seen, all three ENaC subunits were immunoprecipitated (Figure 7A, arrows). When Nedd4-2-CS was co-expressed, an additional band was observed (Figure 7A, arrowhead), which was identified as the Nedd4-2 mutant protein using anti-Nedd4-2 antibodies (Figure 7B, arrowhead). When Sgk1 was added, the intensity of the Nedd4-2 band was reduced (Figure 7A, lane 3) and dropped below the detection limit by the anti-Nedd4-2 antibody (Figure 7B, lane 3). When either catalytically inactive Sgk1 (Sgk1-KA, lane 4) or Sgk1 lacking the PY motif (Sgk1-ΔPY, lane 5) were expressed, the quantity of co-immunoprecipitated Nedd4-2-CS was not affected. Therefore, these data suggest that Sgk1 interferes with the interaction of Nedd4-2 and ENaC in a phosphorylation- and PY motif-dependent manner. This reduced interaction is likely to be the cause of the observed stimulation of ENaC surface expression by Sgk1.
Fig. 7. Sgk1 interferes with ENaC–Nedd4-2 interaction. Oocytes were injected with cRNA encoding FLAG-tagged ENaC, Nedd4-2-CS and Sgk1, as indicated. After overnight incubation with [35S]methionine, membrane fractions were lysed and immunoprecipitations performed with anti-FLAG antibodies. (A) Immunoprecipitated material analyzed by SDS–PAGE autoradiography and (B) western blotting using an anti-Nedd4-2 antiserum. Lysates were analyzed by western blotting using (C) anti-c-myc (i.e. Sgk1) and (D) anti-Nedd4-2 antibodies.
Discussion
In this study, we demonstrate that Sgk1-dependent phosphorylation of Nedd4-2 regulates ENaC cell surface expression and suggest a novel mechanism for the hormonal regulation of ENaC by aldosterone. Phosphorylation events have long been recognized to play a role in the regulation of ENaC (Sariban-Sohraby et al., 1988; Ling and Eaton, 1989; Matsumoto et al., 1993; Shimkets et al., 1998; Blazer-Yost et al., 1999; Chigaev et al., 2001), but the molecular mechanisms (identity of the substrates, phosphorylation sites and involved kinases) remained vague. The recent discoveries that aldosterone-induced expression of Sgk1 kinase correlates with induction of transepithelial Na+ transport and that this kinase stimulates the ENaC activity at the cell membrane (Alvarez de la Rosa et al., 1999; Chen et al., 1999; Naray-Fejes-Toth et al., 1999; Loffing et al., 2001) pointed to Sgk1 as an important player in ENaC regulation, but the molecular mechanisms remained elusive.
The present data reveal the mechanism of Sgk1-dependent ENaC regulation. As indicated in Figure 1, Nedd4-2 contains two conserved consensus sites for phosphorylation by Sgk1 kinase [RXRXX(S/T); Kobayashi and Cohen, 1999; Park et al., 1999]. Indeed, we demonstrate by co-expression of Sgk1 and Nedd4-2 that primarily Ser444 and, to a lesser extent, Ser338 are phosphorylated by Sgk1 kinase and that this phosphorylation depends on intrinsic Sgk1 kinase activity (Figure 2A). In Xenopus oocytes, when both phosphorylation sites are mutated, no phosphorylation of Nedd4-2 is observed. Phosphorylation of Nedd4-2 by the overexpressed Sgk1 kinase in the oocytes appears to be specific, as the paralog Nedd4-1, which does not contain a consensus for Sgk1 phosphorylation, is not primarily involved in ENaC regulation and is not phosphorylated by Sgk1. In addition, co-expression of Sgk1 together with ENaC in Xenopus oocytes does not induce phosphorylation of ENaC subunits (P.J.Plant and O.Staub, unpublished observations), which is in accordance with other reports finding no evidence for ENaC phosphorylation (Alvarez de la Rosa et al., 1999; Chigaev et al., 2001).
Several lines of evidence suggest that Sgk1 and Nedd4-2 interact via the PY motif on Sgk1 and, consequently, on the WW domains on Nedd4-2: (i) Nedd4-2 phosphorylation is largely reduced when Sgk1 is mutated on the tyrosine of the PY motif, (ii) mutation of the Sgk1 PY motif leads to a reduced effect of Sgk1 on Nedd4-2-dependent inhibition of ENaC activity, (iii) the PY mutant of Sgk1 does not interfere with ENaC–Nedd4-2 interaction, (iv) the PY mutant retains catalytic activity toward a synthetic peptide substrate and (v) in a two-hybrid screen using Sgk1 as a bait, Nedd4-1, which contains highly similar WW domains to Nedd4-2, was pulled out as a weak interacting protein with Sgk1 (E.Kamynina and O.Staub, unpublished observations). The fact that we are unable to co-immunoprecipitate the two proteins when co-expressed in oocytes suggests that the interaction between Sgk1 and Nedd4-2 is of a transient nature or, alternatively, this interaction may be indirect.
We found that the PY motifs of ENaC are required for Sgk1 to stimulate ENaC activity, which would be expected if the effect of Sgk1 is via Nedd4-2. These results apparently differ from earlier published data in which additional effects of Liddle mutations and Sgk1 were observed (Alvarez de la Rosa et al., 1999; Shigaev et al., 2000). However, the differences may be explained by the fact that only one subunit (β) was mutated (Shigaev et al., 2000) and that both studies employed heterologous (mouse) Sgk1 in Xenopus oocytes (Alvarez de la Rosa et al., 1999; Shigaev et al., 2000). Alternatively, Sgk1 may act through other signaling pathways, in addition to phosphorylation of Nedd4-2.
Sgk1-dependent phosphorylation is likely to interfere with the ENaC–Nedd4-2 interaction, as suggested by the co-immunoprecipitation experiments, which show that less Nedd4-2 is co-immunoprecipitated with ENaC when Sgk1 is present. These effects depend on the catalytic activity of Sgk1 (the inactive SgkK130A does not interfere with ENaC–Nedd4-2 binding), which excludes the idea that the observed reduction of co-immunoprecipitated Nedd4-2 protein is simply due to a displacement of Nedd4-2 by Sgk1 via WW domain–PY motif interaction. Intriguingly, the phosphorylation sites are not localized in the vicinity of the WW domains (the interacting domains with ENaC). In fact, the predominantly used Ser444 is situated between WW domains two and three. Therefore, it is likely that phosphorylation at this site does not directly disturb interactions with ENaC, but instead induces a conformational change that renders the protein less competent for interaction with ENaC.
Cell surface expression of plasma membrane proteins is controlled by ubiquitylation (Bonifacino and Weissman, 1998; Hicke, 1999; Rotin et al., 2000), primarily by regulating the rate of endocytosis, although alternative mechanisms (i.e. control of sorting at the level of the Golgi apparatus) have been proposed (Helliwell et al., 2001). As a previous report suggested that Sgk1 does not affect the retrieval of ENaC from the plasma membrane (Alvarez de la Rosa et al., 1999), it will be of further interest to determine whether the Sgk1–Nedd4-2 effect is primarily on insertion, retrieval or both. Our data show by two different approaches that Sgk1-dependent phosphorylation of Nedd4-2 and, consequently, regulated ubiquitylation affects channel number at the cell surface. To our knowledge, this is the first reported case where phosphorylation of a ubiquitin protein ligase controls cell surface expression of an ion channel protein. In view of the increasing number of channels and membrane proteins containing PY motifs that are regulated by the family of Nedd4/Nedd4-like proteins [e.g. cardiac voltage-gated Na+ channel (Abriel et al., 2000) or chloride channel ClC-5 (Schwake et al., 2001)], it is possible that the proposed regulatory mechanism represents a common mode of channel regulation.
In conclusion, our data suggest that Sgk1 binds via its PY motif to the WW domains of Nedd4-2. This interaction leads to the phosphorylation of Ser444 and, to a lesser extent, Ser338, which in turn reduces the affinity of Nedd4-2 towards ENaC. Consequently, ENaC becomes less ubiquitylated, leading to the accumulation of ENaC channels at the cell surface. This accumulation may be due to either increased insertion of ENaC at the cell surface or reduced internalization, or both. This novel mode of regulation of ENaC by the aldosterone-induced Sgk1 kinase suggests, for the first time, an entire signaling pathway from aldosterone to ENaC, the major target of aldosterone regulation in the distal nephron.
Materials and methods
cDNA constructs
Xenopus Nedd4-2 constructs (Abriel et al., 1999) were mutated to Ala on Ser338 or Ser444 or both together. Mouse Nedd4-1 and Nedd4-2 (Kamynina et al., 2001a) were labeled with a T7 epitope (Novagen) at their C-terminus. Xenopus Sgk1 (Chen et al., 1999) was labeled with a c-myc epitope (AEEQKLISEEDL) at its N-terminus. To create the catalytically inactive mutant K130A, Lys130 was mutated to Ala in the c-myc construct and Tyr301 changed to Ala to create an Sgk1 construct lacking a functional PY motif (Sgk1-ΔPY). The following Xenopus ENaC constructs were used: wild-type α, β and γ xENaC as described (Puoti et al., 1995) or wild-type α, β and γ xENaC subunits each containing a FLAG epitope (DYKDDDDK). In α, the FLAG epitope replaced amino acids 133–140 (VQGWIPNN), in β it was inserted between Phe143 and Thr144 and in γ it replaced amino acids 140–147 (KRDVGVNV). xENaC subunits lacking PY motifs (ENaCΔPY) were generated by mutating Tyr599 in αxENaC to Ala, Arg583 in βxENaC to a stop codon and Tyr637 in γxENaC to Ala. cDNAs encoding rENaC subunits (Figures 3B, 5 and 6) were used as described (Canessa et al., 1994). All cDNAs were subcloned into the pSDeasy plasmid (Puoti et al., 1997).
Expression in Xenopus oocytes and electrophysiological measurements
Plasmids encoding ENaC, Sgk1 and Nedd4-2 proteins were linearized and transcribed, the cRNA injected into Xenopus oocytes and electrophysiological measurements performed after overnight incubation as described previously (Puoti et al., 1995; Abriel et al., 1999; Chen et al., 1999). The following quantities of cRNA were injected: ENaC, 3 ng for each subunit; Sgk1, 7 ng; Nedd4-2, 6.6 ng (Figures 2 and 7) and 1.5 ng (Figures 3, 5 and 6).
Cell surface binding assay and immunofluorescence studies
The binding assay and the immunofluorescence studies using anti-FLAG antibodies were performed as described (Firsov et al., 1996; Abriel et al., 1999) in modified Barth’s solution (MBS) containing 10 mM Na+.
Biochemical analysis of Xenopus oocytes
For the phosphorylation experiment, injected oocytes were kept overnight in MBS (Schild et al., 1996), labeled for 3 h in MBS (5 µl/oocyte) containing 4 µCi/µl of [32P]ortho–phosphate, washed three times with MBS and lysed in Triton X-100 homogenization buffer (20 µl/oocyte, 20 mM Tris–HCl pH 7.4, 100 mM NaCl, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml leupeptin, 10 µg/ml pepstatin A, 10 µg/ml aprotinin, 100 mM NaF, 10 mM Na pyrophosphate and 10 mM Na–ortho–vanadate). After two centrifugations at 4°C for 10 min at 20 000 g, the supernatant was recovered and immunoprecipitations with anti-Xenopus Nedd4-2 antibodies (Abriel et al., 1999) were performed. The immunoprecipitated material was analyzed by SDS–PAGE (7%) followed by autoradiography. Western blot analysis and the co-immunoprecipitation analysis were performed as described by Kamynina et al. (2001a), using either anti-c-myc (Santa Cruz Biotechnology), anti-FLAG (Sigma; M2) or anti-Xenopus Nedd4-2 antibodies (Abriel et al., 1999).
In vitro kinase assay
Oocytes injected with cRNA encoding myc-Sgk1 or myc-Sgk1 Y301A were kept overnight in MBS and lysed in Triton X-100 homogenization buffer without phosphatase inhibitors. After centrifugation at 4°C for 10 min at 20 000 g, the supernatant was recovered and immunoprecipitations of wild-type or mutant myc-Sgk1 with anti-myc antibodies were performed. The immune complexes were washed five times with Triton X-100 homogenization buffer without phosphatase inhibitors, followed by washing with kinase assay buffer [50 mM Tris–HCl pH 7.5 and 0.1% (v/v) 2-mercaptoethanol]. The kinase assay was carried out as described (Park et al., 1999). Briefly, 30 µl of beads containing the immunoprecipitated kinase were incubated in a final volume of 50 µl for 1 h at 30°C with 1 mM Sgktide (KKRNRRLSVA) or 1 mM of mutated Sgktide (KKRNRRLAVA) as peptide substrates, 10 mM MgCl2 and 100 µM [γ-32P]ATP (1000–2000 c.p.m./pmol). Phosphorylated peptides were separated on 16% tricine acrylamide gel and analyzed by autoradiography.
Acknowledgments
Acknowledgements
We thank Drs Bernard Rossier, Laurent Schild, Dmitri Firsov and Lukas Müller for critically reading the manuscript. The technical assistance of Ms Lea Kläusli is gratefully acknowledged. This work was supported by grants from the Swiss National Science Foundation, the Leenaards Foundation in Lausanne, Switzerland and the Human Frontier Science Program.
References
- Abriel H., Loffing,J., Rebhun,J.F., Pratt,J.H., Horisberger,J.-D., Rotin,D. and Staub,O. (1999) Defective regulation of the epithelial Na+ channel (ENaC) by Nedd4 in Liddle’s syndrome. J. Clin. Invest., 103, 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abriel H., Kamynina,E., Horisberger,J.-D. and Staub,O. (2000) Regulation of the cardiac voltage-gated Na+ channel (rH1) by the ubiquitin-protein ligase Nedd4. FEBS Lett., 466, 377–380. [DOI] [PubMed] [Google Scholar]
- Alvarez de la Rosa D., Zhang,P., Naray-Fejes-Toth,A., Fejes-Toth,G. and Canessa,C.M. (1999) The serum and glucocorticoid kinase sgk increases the abundance of epithelial sodium channels in the plasma membrane of Xenopus oocytes. J. Biol. Chem., 274, 37834–37839. [DOI] [PubMed] [Google Scholar]
- Blazer-Yost B.L., Paunescu,T.G., Helman,S.I., Lee,K.D. and Vlahos,C.J. (1999) Phosphoinositide 3-kinase is required for aldosterone-regulated sodium reabsorption. Am. J. Physiol., 277, C531–C536. [DOI] [PubMed] [Google Scholar]
- Bonifacino J.S. and Weissman,A.M. (1998) Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu. Rev. Cell. Dev. Biol., 14, 19–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botero-Velez M., Curtis,J.J. and Warnock,D.G. (1994) Brief report: Liddles’s syndrome revisited. N. Engl. J. Med., 330, 178–181. [DOI] [PubMed] [Google Scholar]
- Canessa C.M., Horisberger,J.-D. and Rossier,B.C. (1993) Epithelial sodium channel related to proteins involved in neurodegeneration. Nature, 361, 467–470. [DOI] [PubMed] [Google Scholar]
- Canessa C.M., Schild,L., Buell,G., Thorens,B., Gautschi,I., Horisberger,J.-D. and Rossier,B.C. (1994) Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature, 367, 463–467. [DOI] [PubMed] [Google Scholar]
- Chen S., Bhargava,A., Mastroberardino,L., Meijer,O.C., Wang,J., Buse,P., Firestone,G.L., Verrey,F. and Pearce,D. (1999) Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc. Natl Acad. Sci. USA, 96, 2514–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigaev A., Lu,G., Shi,H., Asher,C., Xu,R., Latter,H., Seger,R., Garty,H. and Reuveny,E. (2001) In vitro phosphorylation of COOH termini of the epithelial Na(+) channel and its effects on channel activity in Xenopus oocytes. Am. J. Physiol. Renal Physiol., 280, F1030–F1036. [DOI] [PubMed] [Google Scholar]
- Dinudom A., Harvey,B.J., Komwatana,P., Young,J.A., Kumar,S. and Cook,D.I. (1998) Nedd4 mediates control of an epithelial Na+ channel in salivary duct cells by cytosolic Na+. Proc. Natl Acad. Sci. USA, 95, 7169–7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr T.J., Coddington-Lawson,S.J., Snyder,P.M. and McDonald,F.J. (2000) Human Nedd4 interacts with the human epithelial Na+ channel: WW3 but not WW1 binds to Na+-channel subunits. Biochem. J., 345, 503–509. [PMC free article] [PubMed] [Google Scholar]
- Firsov D., Schild,L., Gautschi,I., Mérillat,A.-M., Schneeberger,E. and Rossier,B.C. (1996) Cell surface expression of the epithelial Na+ channel and a mutant causing Liddle syndrome: a quantitative approach. Proc. Natl Acad. Sci. USA, 93, 15370–15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P.J., Zhang,K.X., Zhu,D.L., He,X., Han,Z.Y., Zhan,Y.M. and Yang,L.W. (2001) Diagnosis of Liddle syndrome by genetic analysis of β and γ subunits of epithelial sodium channel—a report of five affected family members. J. Hypertens., 19, 885–889. [DOI] [PubMed] [Google Scholar]
- Garty H. and Palmer,L.G. (1997) Epithelial sodium channels: function, structure and regulation. Physiol. Rev., 77, 359–396. [DOI] [PubMed] [Google Scholar]
- Goulet C.C., Volk,K.A., Adams,C.M., Prince,L.S., Stokes,J.B. and Snyder,P.M. (1998) Inhibition of the epithelial Na+ channel by interaction of Nedd4 with a PY motif deleted in Liddle’s syndrome. J. Biol. Chem., 273, 30012–30017. [DOI] [PubMed] [Google Scholar]
- Hansson J.H. et al. (1995a) Hypertension caused by a truncated epithelial sodium channel γ subunit: genetic heterogeneity of Liddle syndrome. Nature Genet., 11, 76–82. [DOI] [PubMed] [Google Scholar]
- Hansson J.H., Schild,L., Lu,Y., Wilson,T.A., Gautschi,I., Shimkets,R.A., Nelson-Williams,C., Rossier,B.C. and Lifton,R.P. (1995b) A de novo missense mutation of the β subunit of the epithelial sodium channel causes hypertension and Liddle syndrome, identifying a proline-rich segment critical for regulation of channel activity. Proc. Natl Acad. Sci. USA, 92, 11495–11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey K.F., Dinudom,A., Komwatana,P., Jolliffe,C.N., Day,M.L., Parasivam,G., Cook,D.I. and Kumar,S. (1999) All three WW domains of murine Nedd4 are involved in the regulation of epithelial sodium channels by intracellular Na+. J. Biol. Chem., 274, 12525–12530. [DOI] [PubMed] [Google Scholar]
- Harvey K.F., Dinudom,A., Cook,D.I. and Kumar,S. (2001) The Nedd4-like protein KIAA0439 is a potential regulator of the epithelial sodium channel. J. Biol. Chem., 276, 8597–8601. [DOI] [PubMed] [Google Scholar]
- Helliwell S.B., Losko,S. and Kaiser,C.A. (2001) Components of a ubiquitin ligase complex specify polyubiquitination and intracellular trafficking of the general amino acid permease. J. Cell Biol., 153, 649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L. (1997) Ubiquitin-dependent internalization and down-regulation of plasma membrane proteins. FASEB J., 11, 1215–1226. [DOI] [PubMed] [Google Scholar]
- Hicke L. (1999) Gettin’ down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol., 9, 107–112. [DOI] [PubMed] [Google Scholar]
- Inoue J., Iwaoka,T., Tokunaga,H., Takamune,K., Naomi,S., Araki,M., Takahama,K., Yamaguchi,K. and Tomita,K. (1998) A family with Liddle’s syndrome caused by a new missense mutation in the β subunit of the epithelial sodium channel. J. Clin. Endocrinol. Metab., 83, 2210–2213. [DOI] [PubMed] [Google Scholar]
- Inoue T. et al. (1998) Identification of a single cytosine base insertion mutation at Arg597 of the β subunit of the human epithelial sodium channel in a family with Liddle’s disease. Eur. J. Endocrinol., 138, 691–697. [DOI] [PubMed] [Google Scholar]
- Jeunemaitre X., Bassilana,F., Persu,A., Dumont,C., Champigny,G., Lazdunski,M., Corvol,P. and Barbry,P. (1997) Genotype-phenotype analysis of a newly discovered family with Liddle’s syndrome. J. Hypertens., 15, 1091–1100. [DOI] [PubMed] [Google Scholar]
- Kamynina E., Debonneville,C., Bens,M., Vandewalle,A. and Staub,O. (2001a) A novel mouse Nedd4 protein suppresses the activity of the epithelial Na+ channel. FASEB J., 15, 204–214. [DOI] [PubMed] [Google Scholar]
- Kamynina E., Tauxe,C. and Staub,O. (2001b) Differential characteristics of two human Nedd4 proteins with respect to epithelial Na+ channel regulation. Am. J. Physiol. Renal Physiol., 281, F469–F477. [DOI] [PubMed] [Google Scholar]
- Kanelis V., Farrow,N.A., Kay,L.E., Rotin,D. and Forman-Kay,J.D. (1998) NMR studies of tandem WW domains of Nedd4 in complex with a PY motif-containing region of the epithelial sodium channel. Biochem. Cell Biol., 76, 341–350. [DOI] [PubMed] [Google Scholar]
- Kanelis V., Rotin,D. and Forman-Kay,J.D. (2001) Solution structure of a Nedd4 WW domain–ENaC peptide complex. Nature Struct. Biol., 8, 407–412. [DOI] [PubMed] [Google Scholar]
- Kellenberger S., Gautschi,I., Rossier,B.C. and Schild,L. (1998) Mutations causing Liddle-syndrome reduce sodium-dependent downregulation of the epithelial sodium channel in the Xenopus oocyte expression system. J. Clin. Invest., 101, 2741–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T. and Cohen,P. (1999) Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem. J., 339, 319–328. [PMC free article] [PubMed] [Google Scholar]
- Liddle G.W., Bledsoe,T. and Coppage,W.S.,Jr (1963) A familial renal disorder simulating primary aldosteronism but with negligible aldosterone secretion. Trans. Assoc. Am. Physicians, 76, 199–213. [Google Scholar]
- Ling B.N. and Eaton,D.C. (1989) Effects of luminal Na+ on single Na+ channels in A6 cells, a regulatory role for protein kinase C. Am. J. Physiol., 256, F1094–F1103. [DOI] [PubMed] [Google Scholar]
- Loffing J., Zecevic,M., Feraille,E.B.K., Asher,C., Rossier,B.C., Firestone,G.L., Pearce,D. and Verrey,F. (2001) Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am. J. Physiol. Renal Physiol., 280, F675–F682. [DOI] [PubMed] [Google Scholar]
- Matsumoto P.S., Ohara,A., Duchatelle,P. and Eaton,D.C. (1993) Tyrosine kinase regulates epithelial sodium transport in A6 cells. Am. J. Physiol., 264, C246–C250. [DOI] [PubMed] [Google Scholar]
- Naray-Fejes-Toth A., Canessa,C.M., Cleaveland,E.S., Aldrich,G. and Fejes-Toth,G. (1999) Sgk is an aldosterone-induced kinase in the renal collecting duct. J. Biol. Chem., 274, 16973–16978. [DOI] [PubMed] [Google Scholar]
- Park J., Leon,M.L.L., Buse,P., Maiyar,A.C., Firestone,G.L. and Hemmings,B.A. (1999) Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J., 18, 3024–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puoti A., May,A., Canessa,C.M., Horisberger,J.-D., Schild,L. and Rossier,B.C. (1995) The highly selective low-conductance epithelial Na channel of Xenopus laevis A6 kidney cells. Am. J. Physiol., 269, C188–C197. [DOI] [PubMed] [Google Scholar]
- Puoti A., May,A., Rossier,B.C. and Horisberger,J.-D. (1997) Novel isoforms of the α and γ subunits of the Xenopus epithelial Na+ channel provide information about the amiloride binding site and extracellular sodium sensing. Proc. Natl Acad. Sci. USA, 94, 5949–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D., Staub,O. and Haguenauer-Tsapis,R. (2000) Ubiquitination and endocytosis of plasma membrane proteins; role of Nedd4/Rsp5 family of ubiquitin-protein ligases. J. Membr. Biol., 176, 1–17. [DOI] [PubMed] [Google Scholar]
- Sariban-Sohraby S., Sorscher,E.J., Brenner,B.M. and Benos,D.J. (1988) Phosphorylation of a single subunit of the epithelial Na+ channel protein following vasopressin treatment of A6 cells. J. Biol. Chem., 263, 13875–13879. [PubMed] [Google Scholar]
- Schild L., Canessa,C.M., Shimkets,R.A., Warnock,D.G., Lifton,R.P. and Rossier,B.C. (1995) A mutation in the epithelial sodium channel causing Liddle’s disease increases channel activity in the Xenopus laevis oocyte expression system. Proc. Natl Acad. Sci. USA, 92, 5699–5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild L., Lu,Y., Gautschi,I., Schneeberger,E., Lifton,R.P. and Rossier,B.C. (1996) Identification of a PY motif in the epithelial Na+ channel subunits as a target sequence for mutations causing channel activation found in Liddle syndrome. EMBO J., 15, 2381–2387. [PMC free article] [PubMed] [Google Scholar]
- Schwake M., Friedrich,T. and Jentsch,T.J. (2001) An internalization signal in ClC-5, an endosomal Cl-channel mutated in dent’s disease. J. Biol. Chem., 276, 12049–12054. [DOI] [PubMed] [Google Scholar]
- Shigaev A., Asher,C., Latter,H., Garty,H. and Reuveny,E. (2000) Regulation of sgk by aldosterone and its effects on the epithelial Na(+) channel. Am. J. Physiol. Renal Physiol., 278, F613–F619. [DOI] [PubMed] [Google Scholar]
- Shimkets R.A. et al. (1994) Liddle’s syndrome: heritable human hypertension caused by mutations in the β subunit of the epithelial sodium channel. Cell, 79, 407–414. [DOI] [PubMed] [Google Scholar]
- Shimkets R.A., Lifton,R.P. and Canessa,C.M. (1998) In vivo phosphorylation of the epithelial sodium channel. Proc. Natl Acad. Sci. USA, 95, 3301–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder P.M., Price,M.P., McDonald,F.J., Adams,C.M., Volk,K.A., Zeiher,B.G., Stokes,J.B. and Welsh,M.J. (1995) Mechanism by which Liddle’s syndrome mutations increase activity of a human epithelial Na+ channel. Cell, 83, 969–978. [DOI] [PubMed] [Google Scholar]
- Snyder P.M., Olson,D.R., McDonald,F.J. and Bucher,D.B. (2001) Multiple WW domains, but not the C2 domain, are required for the inhibition of ENaC by human Nedd4. J. Biol. Chem., 276, 28321–28326. [DOI] [PubMed] [Google Scholar]
- Staub O., Dho,S., Henry,P.C., Correa,J., Ishikawa,T., McGlade,J. and Rotin,D. (1996) WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J., 15, 2371–2380. [PMC free article] [PubMed] [Google Scholar]
- Staub O., Gautschi,I., Ishikawa,T., Breitschopf,K., Ciechanover,A., Schild,L. and Rotin,D. (1997) Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J., 16, 6325–6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H., Schild,L., Enomoto,N., Matsui,N., Marumo,F., Rossier,B.C. and Sasaki,S. (1996) Liddle disease caused by a missense mutation of β subunit of the epithelial sodium channel gene. J. Clin. Invest., 97, 1780–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrey F., Hummler,E., Schild,L. and Rossier,B.C. (2000) Control of sodium transport by aldosterone. In Seldin,D.W. and Giebisch,G. (eds), The Kidney: Physiology and Pathophysiology. Lippincott, Williams and Wilkins, Philadelphia, PA, pp. 1441–1471.
- Wang J., Barbry,P., Maiyar,A.C., Rozansky,D.J., Bhargava,A., Leong,M., Firestone,G.L. and Pearce,D. (2001) SGK integrates insulin and mineralocorticoid regulation of epithelial sodium transport. Am. J. Physiol. Renal Physiol., 280, F303–F313. [DOI] [PubMed] [Google Scholar]
- Webster M.K., Goya,L., Ge,Y., Maiyar,A.C. and Firestone,G.L. (1993) Characterization of Sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol. Cell. Biol., 13, 2031–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y. et al. (2001) Two sporadic cases of Liddle’s syndrome caused by de novo ENaC mutations. Am. J. Kidney Dis., 37, 499–504. [PubMed] [Google Scholar]