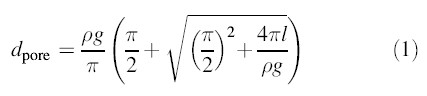Abstract
We report the molecular cloning and characterization of MscMJLR, a second type of mechanosensitive (MS) channel found in the archaeon Methanococcus jannaschii. MscMJLR is structurally very similar to MscMJ, the MS channel of M.jannaschii that was identified and cloned first by using the TM1 domain of Escherichia coli MscL as a genetic probe. Although it shares 44% amino acid sequence identity and similar cation selectivity with MscMJ, MscMJLR exhibits other major functional differences. The conductance of MscMJLR of ∼2 nS is approximately 7-fold larger than the conductance of MscMJ and rectifies with voltage. The channel requires ∼18 kT for activation, which is three times the amount of energy required to activate MscMJ, but is comparable to the activation energy of Eco-MscL. Our study indicates that a multiplicity of conductance-wise and energetically well-tuned MS channels in microbial cell membranes may provide for cell survival by the sequential opening of the channels upon challenge with different osmotic cues.
Keywords: Archaea/MS channels/MscL/MscMJ/patch–clamp
Introduction
Mechanosensitive (MS) channels have been discovered in organisms of different phylogenetic origin including bacteria, fungi, plants and mammalian cells (Sachs, 1988; Morris, 1990; Martinac, 1993; Sackin, 1995; Sachs and Morris 1998). MS channels are thought to form the molecular basis of mechanosensory transduction events underlying touch, hearing, balance and osmosensation in higher organisms, as well as osmoregulation in bacteria (García-Añovernos and Corey, 1997; Sachs and Morris, 1998). Since they are present in species belonging to all three domains of the phylogenetic tree (Woese, 1994; Pace, 1997), MS channels probably evolved along with the first life forms on Earth (Martinac, 1999).
Three types of MS channel were identified in Escherichia coli, and based on their conductance were named MscM (M for mini), MscS (S for small) and MscL (L for large) (Berrier et al., 1996). All three types of channel were found to respond to membrane tension originating within the lipid bilayer consistent with the bilayer model of MS channel gating by mechanical force (Martinac et al., 1990; Hamill and McBride, 1997). Identification and cloning of the 17 kDa MscL protein (Sukharev et al., 1994) was instrumental in advancing our understanding of the molecular principles underlying the MS class of ion channels. Functional MscL homologues have been identified to date in many Gram-positive and Gram-negative bacteria (Moe et al., 1998). Furthermore, cloning of two other genes of E.coli, yggB and kefA, which encode the membrane proteins associated with the MscS activity (Levina et al., 1999), enabled testing of the physiological role of MS channels in bacterial osmoregulation. Based on the sequence similarity, many microorganisms were found to harbour multiple homologues of YggB or KefA (Levina et al., 1999; Kloda and Martinac, 2001a). Presumably, the multiplicity of MS channels enables microbial cells to ‘fine-tune’ their cellular responses to challenges originating from different osmotic cues in their living environments.
Until recently nothing was known of MS channels in Archaea, the third domain of the phylogenetic tree (Woese, 1994; Pace, 1997), which includes prokaryotic inhabitants of hot springs, highly alkaline or acid waters, waters of high salinity, as well as high-pressure environments found on the sea bottom (Barinaga, 1994; Brock et al., 1994). To date, MS channels have been identified in halophilic Haloferax volcanii, acidophilic Thermoplasma acidophilum, and barophilic and methanogenic Methano coccus jannaschii (Le Dain et al., 1998; Kloda and Martinac, 2001a,b,c). A recent study reported the molecular cloning of MscMJ, the first MS channel of M.jannaschii, which was identified by using the TM1 domain of Eco-MscL as a genetic probe. The study further revealed that prokaryotic (i.e. bacterial and archaeal) MS channels belonged to a channel family originating from a common ancestral MscL-like protein (Kloda and Martinac, 2001a).
In this study we report that the MscMJLR protein, which shares 44% sequence identity with MscMJ, forms a distinct type of MS channel present in the M.jannaschii cell envelope.
Results
Structural similarities and differences between MscMJLR and MscMJ
A pairwise alignment of the amino acid sequence of MJ1143 and its homologue MscMJ (Kloda and Martinac, 2001a) revealed a high degree of sequence conservation (Figure 1). The global sequence alignment identified 44.2% identity between the two protein sequences. The C-termini of both homologues were found to contain an identical cluster of charged residues KIKEE, which shares a great similarity to charged clusters also found within the C-termini of two bacterial MS channels: MscL (RKKEE) and MscS (RIKRE), as well as the mammalian mechanogated potassium channel TREK-1 (KKTKEE) (Blount et al., 1996; Häse et al., 1997; Patel et al., 1998; Kloda and Martinac, 2001a).
Fig. 1. Sequence alignment of MscMJLR and MscMJ. The alignment revealed 44% sequence identity. Identical residues are marked with asterisks. The highly conserved C-terminal cluster of charged residues is boxed.
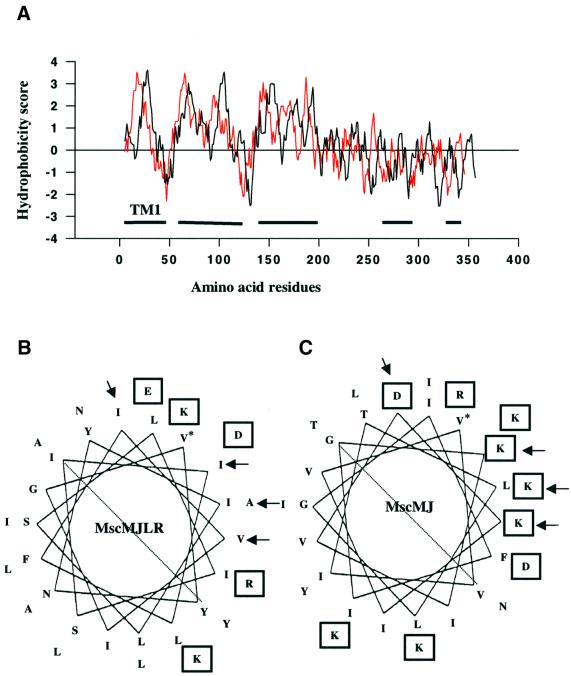
The MJ1143 protein has a predicted molecular weight of 40 kDa and isoelectric point (pI) value (the pH at which a protein has a net zero charge) of 8.8. Its size is very similar to the molecular weight of ∼37 kDa of the MscMJ protein (Kloda and Martinac, 2001a) as well as for MscA1, the MS channel of H.volcanii (Le Dain et al., 1998). Hydropathy plot analysis showed that the profile of MscMJLR was nearly identical to the profile of MscMJ, such that both plots could be superimposed with a high level of accuracy (Figure 2A). Similar to MscMJ, three main hydrophobic regions revealed by the hydropathy plot were found to contain sequences with a high probability of adopting helical secondary structures as predicted by PSIPRED (see Materials and methods). Two shorter helical regions were identified within the hydrophilic C-termini of both proteins, with one of the helices harbouring the highly conserved cluster of charged residues.
Fig. 2. Structural similarities and differences of the two MS channels of M.jannaschii. (A) Hydropathy profile of MscMJLR (black line) and MscMJ (red line) and predicted helical regions (black bars). Positive numbers indicate relative hydrophobicity of the protein profile. (B and C) Helical wheel representation of the putative TM1 domains of MscMJLR and MscMJ, respectively. Charged residues are boxed and the start of each helix is marked with an asterisk. Arrows indicate positions at which hydrophobic residues within the TM1 helix of MscMJLR are replaced with hydrophilic (charged) residues in the MscMJ TM1 helix.
Helical wheel analysis showed that the first hydrophobic region of MscMJLR, assigned as a putative transmembrane TM1 domain, is amphipathic in character and thus may line the channel pore. Although its amphipathic character is similar to the TM1 domain of MscMJ, the TM1 domain of MscMJLR differs in the number of charged groups on the hydrophilic side of the helix (Figure 2B and C). In MscMJ, this side of the amphipathic helix contains two negatively charged aspartic acid residues separated by five positively charged residues (four lysines and one arginine). In contrast, the corresponding region of the MscMJLR helix harbours only four charged residues. Negatively charged glutamic acid and aspartic acid together with a positively charged lysine form a charged cluster separated by several hydrophobic residues from the fourth charged residue, a positively charged arginine (Figure 2B). Thus, the more hydrophobic TM1 domain of MscMJLR resembles more the TM1 helix of MscL (Chang et al., 1998) than the TM1 helix of MscMJ.
According to the hydropathy plot the second and the third helical hydrophobic domains of MscMJLR and MscMJ are represented by two hydrophobic peaks each (Figure 2A). Consequently, both MscMJLR and MscMJ possibly contain five hydrophobic membrane-spanning segments instead of three. Indeed, the transmembrane region detection program TMHMM (see Materials and methods) predicted five TM helices for both proteins (Figure 3A and B). To further compare the secondary structure of MscMJLR and MscMJ we examined both sequences for coiled-coil regions (COILS, see Materials and methods) (Figure 3C). Unlike the sequence of MscMJ, which did not show coiled-coil regions, the sequence of MscMJLR revealed two regions with a high probability of adopting coiled-coil conformation. The first coiled-coil region (approximately residues 130–150) corresponds to the proximal part of the third helical region (Figure 2A). This segment bridges the TM3 and TM4 hypothetical membrane spanning domains and includes a proximal part of TM4 (Figure 3A). Interestingly, a similar coiled-coil region was also identified within the sequence of Eco-MscL (approximately residues 93–110) (Figure 3D), which corresponds to an interface between a distal part of TM2 and proximal part of C-helix. The second coiled-coil region of MscMJLR can be mapped to the distal part of the hydrophilic C-terminal helix (approximately residues 270–290) (Figures 2A and 3C). A similar high probability coiled-coil region was identified within the sequence of MscL (approximately residues 115–135) (Figure 3D) that also corresponds to the distal part of the C-helix.
Fig. 3. (A and B) Number of transmembrane regions predicted for MscMJLR and MscMJ, respectively, obtained by the TMHMM detection program (see Materials and methods). Five TM helices were predicted for both proteins. (C) Prediction of the coiled-coil regions for MscMJLR and MscMJ. Two regions with a high probability of adopting coiled-coil conformation were identified in MscMJLR, whereas there was no such conformation in the corresponding sequence of MscMJ. (D) Prediction of the coiled-coil regions for Eco-MscL revealed two regions with coiled-coil conformation similar to MscMJLR.
MS channel activity of MJ1143 reconstituted into azolectin liposomes
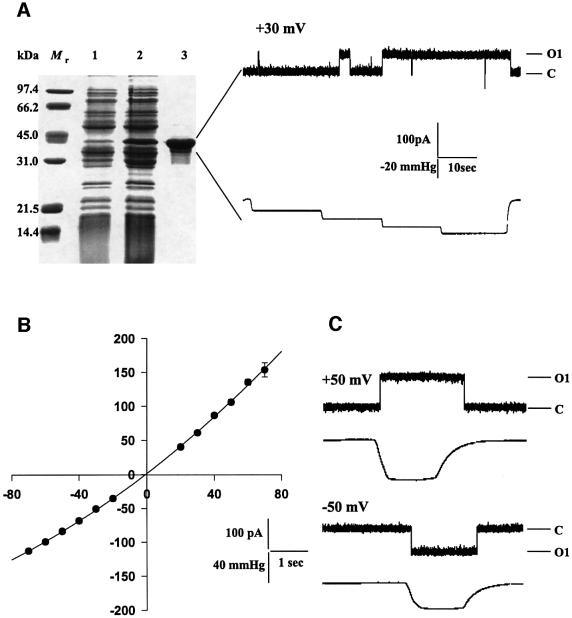
To determine whether mj1143 encodes an MS channel, the entire open reading frame was cloned into an expression vector and expressed in E.coli as a His6-tagged recombinant protein followed by single step purification on a Ni-NTA column. SDS–PAGE analysis (Figure 4A, left) showed that the recombinant protein ran as an ∼40 kDa band, which corresponds to its predicted size.
Fig. 4. Activity of the recombinant MscMJLR. (A, left) SDS–PAGE of the Ni-NTA purified His6 MscMJLR protein expressed in E.coli. Lane 1, total E.coli proteins before induction with IPTG; lane 2, total E.coli proteins after induction with IPTG; lane 3, purified and concentrated MscMJLR protein. (A, right) A current trace and the corresponding pressure trace of the liposome reconstituted MscMJLR activated by suction applied to the patch pipette at a pipette potential of +30 mV. C, closed state of channel; O1, open state of channel. (B, left) Current–voltage relationship for MscMJLR in a symmetric recording buffer, containing 200 mM KCl, 40 mM MgCl2 and 5 mM HEPES pH 7.2, rectifies with voltage. The I–V plot was fitted by a second order polynomial function. Each data point is presented as a mean ± SE (n ≥ 5). Except for the data point obtained at +70 mV error bars are smaller than the filled circles. (C) Current traces of MscMJ showing difference in the size of the single-channel current recorded at +50 and –50 mV pipette potential. The negative pressure applied to the patch pipette was 55 and 35 mmHg, respectively. C, closed state of channel; O1, open state of channel.
Upon reconstitution into azolectin liposomes and application of negative pressure to the patch pipette, the purified recombinant protein behaved as an MS channel with large conductance, which rectified with voltage (Figure 4A, right, and B and C). No channel activity was recorded in control experiments in which E.coli cells were transformed with an empty plasmid. By analogy with MscMJ (Kloda and Martinac, 2001a), we renamed MJ1143 as MscMJLR for a mechanosensitive channel of M.jannaschii of large conductance and rectifying.
MscMJLR is characterized by long openings (Figure 4A, right, and C), similar to MscA2, the MS channel of H.volcanii (Le Dain et al., 1998) and MscMJ, the MS channel of M.jannaschii (Kloda and Martinac, 2001a). Generally, the negative pressure required to activate MscMJLR was between 25 and 100 mmHg. MscMJLR was similar to MscMJ in that it was blocked by 0.1 mM gadolinium, but unlike MscMJ, the MscMJLR channel was not affected by amphipaths (data not shown).
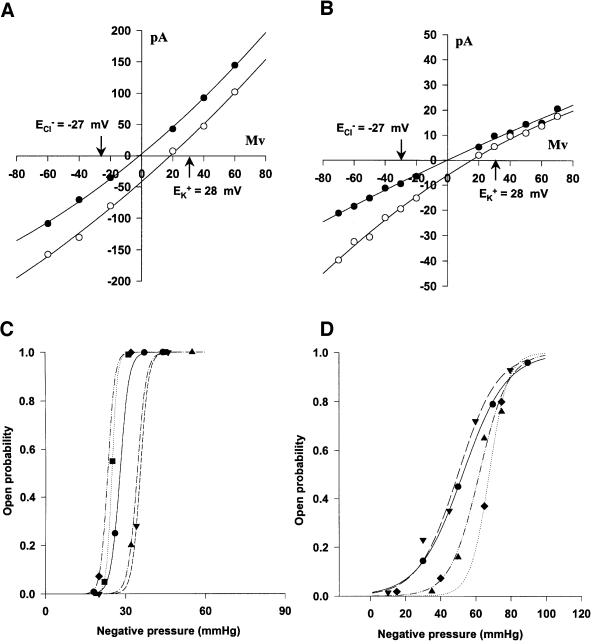
The conductance of MscMJLR estimated from the current–voltage plot (Figure 4B) was ∼2 nS, significantly larger than the 270 pS of MscMJ. In contrast to MscMJ (Kloda and Martinac, 2001a), MscMJLR exhibited rectification in the symmetric recording solution (Figures 4B and 5A and figure legends). For example, at –40 mV the channel conductance was 1.70 ± 0.03 nS (mean ± SE, n = 7) and at +40 mV it was 2.20 ± 0.05 nS (mean ± SE, n = 6). Unlike MscMJ, which is essentially non-rectifying in a symmetric buffer containing 200 mM KCl (Kloda and Martinac, 2001a), MscMJLR showed outward rectification (in reference to the pipette interior) at positive pipette potentials under identical conditions. The difference in the rectification pattern appeared to be more significant upon exchange of the bath solution to 600 mM KCl. Under these conditions MscMJ showed inward rectification at negative pipette potentials whereas MscMJLR continued to show outward rectification (Figure 5A and B). A similar pattern in rectifying behaviour was also reported for the two MS channels of H.volcanii, MscA1 and MscA2 (Le Dain et al., 1998).
Fig. 5. Comparison of conductive and MS properties of the two MS channels of M.jannaschii. Current–voltage relationship for (A) MscMJLR and (B) MscMJ obtained in a symmetric recording solution of 200 mM KCl, 5 mM MgCl2, 5 mM HEPES pH 7.2 (closed symbols) and after replacing the bath solution with a 3-fold gradient of 600 mM KCl, 5 mM MgCl2 and 5 mM HEPES pH 7.2 (open symbols). The reversal potential was obtained for the same patch by fitting a second order polynomial curve to the current–voltage plots. The calculated reversal potentials for potassium EK (+28 mV) and chloride ECl (–27 mV) are marked by arrows. The open probability of (C) MscMJLR (n = 5) and (D) MscMJ (n = 4) plotted versus negative pressure applied to the patch pipette was fitted to the Boltzmann distribution function of the form NPo = NPomax [1 + exp α(p1/2 – p)], where NPo and NPomax are the open probability and the maximum open probability, respectively, N is the number of channels in a liposome patch, α is the sensitivity to pressure corresponding to the slope of the linear fit of the function ln [NPo/(NPomax – NPo)], p is the negative pressure applied to the patch pipette and p1/2 is the negative pressure at which Po = 0.5. For a summary of the Boltzmann characteristics of MscMJLR and MscMJ compared with other prokaryotic MS channels see Table I. (B) and (D) were adapted from Kloda and Martinac (2001a).
Furthermore, unlike MscMJ (Kloda and Martinac, 2001a), the MscMJLR open channel kinetics did not appear to be significantly affected over the voltage range of ±70 mV (Figure 4C).
Estimating the size of the MscMJLR conducting pore
Assuming a channel with a cylindrical pore of uniform diameter, we calculated the diameter of the MscMJLR pore based on a channel conductance of 2 nS using the formula derived by Hille (1968):
where the resistivity of the recording solution ρ was measured as 49.7 Ωcm and the bilayer thickness (l) was assumed to be 35 Å (Cruickshank et al., 1997). The channel pore, estimated to be ∼27 Å, is ∼3-fold that estimated for MscMJ (i.e. 9 Å) (Kloda and Martinac, 2001a). The difference in the conductance and the pore size of MscMJLR and MscMJ parallels their 3-fold difference in free energy of activation (ΔG0) (see below).
Ionic selectivity
The selectivity of MscMJLR for potassium over chloride ions (Figure 5A and B) was measured by determining the shift in reversal potential when exchanging 200 mM KCl in the bath solution with 600 mM KCl. The reversal potential Erev of MscMJLR shifted by 17.6 ± 1.2 mV (mean ± SE, n = 4) towards the equilibrium potential for potassium. A similar shift of Erev ≈ 18 mV was reported for MscMJ (Kloda and Martinac, 2001a). Thus, the permeability ratio of MscMJLR for potassium versus chloride PK/PCl ≈ 5, similar to the permeability ratio of MscMJ (PK/PCl ≈ 6) (Kloda and Martinac, 2001a).
Mechanosensitive properties of MscMJLR
The MscMJLR channel open probability plotted against negative pipette pressure could be fitted to a Boltzmann distribution function (Figure 5C). The estimated sensitivity of MscMJLR to pressure 1/α was 1.7 ± 0.2 mmHg per e-fold change in the channel open probability (i.e. Po/Pc) (mean ± SE, n = 5). The pressure at which the channel open probability Po = 0.5 was calculated to be p1/2 = 29.2 ± 2.0 mmHg (mean ± SE, n = 5). Differences in both Boltzmann parameters p1/2 and α reflect in the free energy ΔG0 necessary for the channel activation, since the product ΓMSC = αp1/2 = ΔG0/kT (see Materials and methods). We compared the Boltzmann parameters of MscMJLR and its homologue MscMJ with other known prokaryotic MS channels (Table I). As summarized in Table I, ΔG0 ≈ 18 kT was required for the MscMJLR activation, which is ∼3-fold greater than ΔG0 ≈ 5 kT estimated for MscMJ (Kloda and Martinac, 2001a). However, it is very similar to ΔG0 ≈ 14–19 kT and 15 kT, which can be calculated for MscL of E.coli and MscA1 of H.volcanii, respectively (Häse et al., 1995; Gu et al., 1998; Le Dain et al., 1998). Similarly, the sensitivity to pressure of MscMJLR of 1/α = 1.7, although similar to MscA2 of H.volcanii of 1/α = 1.5, was found to be very different from the MscMJ sensitivity to pressure of 1/α = 11 mmHg.
Table I. Summary of the Boltzmann characteristics and conductance for the known prokaryotic MS channels.
| MS channel | p1/2 (mmHg) | 1/α (mmHg) | ΔG0 (kT) | Conductance (nS) | Reference |
|---|---|---|---|---|---|
| MscL | 75 | 4.4–5 | 14–19 | 3.3–3.8 | Häse et al. (1995); |
| Sukharev et al. (1999); | |||||
| Kloda and Martinac (2001b,c) | |||||
| MscS | 36 | 5 | 7 | 0.97 (+ve) | Martinac et al. (1987); |
| 0.65 (–ve) | B.Martinac (unpublished) | ||||
| Kloda and Martinac (2001c) | |||||
| MscA1 | 34 | 2.3 | 15 | 0.38 (+ve) | Le Dain et al. (1998) |
| 0.68 (–ve) | |||||
| MscA2 | 43 | 1.5 | 29 | 0.85 (+ve) | Le Dain et al. (1998) |
| 0.49 (–ve) | |||||
| MscMJ | 57 | 11 | 5 | 0.27 | Kloda and Martinac (2001a) |
| MscMJLR | 29.2 | 1.7 | 18 | 2.2 (+ve) | this study |
| 1.7 (–ve) | |||||
| MscTA | 78 | 2.4 | 35 | 2.8 | Kloda and Martinac (2001b,c) |
The values of the parameters shown were compiled from original research papers and from this study.
Discussion
We have identified and cloned the mscMJLR gene encoding a second type of MS protein of the archaeon M.jannaschii. MscMJLR belongs to a new family of prokaryotic MS channels that may have originated from a common MscL-like ancestor via gene duplication and diversification (Kloda and Martinac, 2001a). Interestingly, despite their high level of sequence identity, MscMJLR was found to be functionally different from MscMJ. Although both MS channels are cation selective with permeability ratios of PK/PCl ≈ 5 and 6 for MscMJLR and MscMJ, respectively, the MscMJLR channel has conductance of ∼2.0 nS, which is ∼7-fold greater than MscMJ. A difference in conductance may reflect a difference in the pore size, assuming both channels are cylinders of uniform diameter (Hille, 1968). The larger pore diameter the larger the change in the area, ΔA, between open and closed channel conformation. Since the sensitivity to pressure, 1/α, is inversely proportional to ΔA (see Materials and methods), it follows that the larger the ΔA, the higher channel sensitivity to membrane tension (Hamill and Martinac, 2001). Indeed a 6-fold difference in 1/α between MscMJ and MscMJLR seems to correspond well to a 7-fold difference in the channel conductance, despite the limitations of Hille’s model (Hille, 1968) (Table I).
The free energy of activation required for the activation of MscMJLR (ΓMSC ≈ 18 kT) was found to be similar to the energy necessary to activate Eco-MscL (14–19 kT), but different to the energy required for activation of its homologue MscMJ (5 kT) (Table I). The difference between ΓMSC of MscMJ and MscMJLR may originate from the number of charged groups and overall hydrophobicity within the critical region of the putative TM1 transmembrane domains lining the pore and forming the gate of the two MS channels. The TM1 helix of MscMJLR is quite different from the TM1 helix of MscMJ, which is highly amphipathic and contains many charged residues on one side of the helix (Figure 2B and C). If by analogy with MscL, whose first membrane-spanning domain TM1 is considered to be the helix important for the opening of the channel pore by membrane tension (Yoshimura et al., 1999; Ajouz et al., 2000; Hamill and Martinac, 2001), we assume that the TM1 helix also lines the MscMJLR pore and participates in the channel opening, it is tempting to speculate that its more hydrophobic character may be the reason for the observed 3-fold difference in energy of activation, compared with that of its homologue MscMJ (Table I). This notion seems to be supported by the fact that single substitutions of residues located on the same side of the TM1 domain of MscL (G22, V21, V23, G26, G30) resulted in major changes in the MscL sensitivity to membrane tension (Ou et al., 1998; Yoshimura et al., 1999). Most mutants with hydrophilic substitutions displayed a slow or no growth phenotype caused by solute loss and gated at membrane tension that was lower compared with wild-type MscL. According to the three-dimensional (3D) MscL crystal structure, these critical residues can be mapped to the channel gate (Chang et al., 1998; Spencer et al., 1999). Indeed, hydrophobic interactions have been suggested to play an important role in generating a gating force in MscL (Yoshimura et al., 1999; Hamill and Martinac, 2001; Sukharev et al., 2001). Therefore, the more hydrophobic character of the TM1 helix of MscMJLR and MscL may account for the similarity in the free energy of activation, ΔG0, calculated for these two MS channels. By analogy, the 3-fold lower ΔG0 calculated for MscMJ should correspond to the more hydrophilic character of its TM1 domain. This finding suggests that the TM1 helix of bacterial as well as archaeal MS channels is a critical domain for the gating of this family of MS channels.
The similarity in energy requirements between MscMJLR and MscL may also reflect other structural similarities between the two proteins. Secondary structure analysis revealed two high probability coiled-coil regions within the sequence of MscMJLR as well as of MscL but none within the sequence of MscMJ (Figure 3C and D). The first coiled-coil region in both MscMJLR and MscL seems to be restricted to the transmembrane domains, the hypothetical TM4 in the case of MscMJLR and the TM2 domain of MscL. The second coiled-coil region is embedded within the hydrophilic C-terminus in both proteins. C-termini have been proposed to stabilize the closed configuration of the MscL channel (Batiza et al., 1999; Hamill and Martinac, 2001). In fact, many MS channels and their homologues, including prokaryotic MscL, YggB, MscMJ and MscMJLR (Moe et al., 1998; Levina et al., 1999; Kloda and Martinac, 2001a) and mammalian MS channel TREK-1 (Patel et al., 1998), possess a cluster of charged residues in their C-terminal domain. The deletion of the charged cluster from the MscL sequence was found to abolish the channel activity (Blount et al., 1996; Häse et al., 1997). Preservation of the charged structural motif in MS ion channels from all three domains of life possibly implies its as yet unknown functional importance.
Coiled-coil domains are structural elements found in membrane proteins, such as glycophorin (Lodish et al., 1995) and the light driven proton pump, bacteriorhodopsin (Henderson and Unwin, 1975). Several classes of ion channels, such as the α5 segment of Bacillus thuringiensis δ-endotoxin (Gazit et al., 1994), nicotinic acetylcholine receptor family (Kerr et al., 1994) and influenza A virus M2 channel (Sansom et al., 1997), have been modelled as coiled coils by molecular dynamic simulation studies. Furthermore, according to crystallographic studies, coiled coils were proposed to be necessary for the formation of the pentameric structure of a cartilage oligomeric protein (COMP), a putative ion channel (Malashkevich et al., 1996). Similarly, coiled-coil regions identified in MscL and MscMJLR may contribute to the oligomerization of these channels. The 3D crystal structure of MscL revealed a pentameric channel. Although the C-terminal cytoplasmic helices are not shown as coiled coils, they appear to be in close contact (Chang et al., 1998) in agreement with the idea that the C-termini may stabilize the oligomeric structure of the channel (Oakley et al., 1999; Hamill and Martinac, 2001). Given the very low pH (3.6–3.8) of the MscL crystallization conditions, the glutamic acid residue E104 (pKa = 4.25), located within the C-terminus, had to be protonated to stabilize the pentameric structure of MscL. Consequently, at physiological pH the glutamate residues become negatively charged by losing protons, making the structure of MscL less stable. Thus, a compensation mechanism, such as the presence of coiled coils, may serve as a stabilizing factor in favour of oligomerization of both MscL and MscMJLR.
The comparative approach presented in this study indicates that the two homologous types of archaeal MS channels, in spite of their 44% amino acid sequence identity and similar cation selectivity, show significant differences in conductance and free energy of activation (Table I). Similarly to E.coli MscL, MscS and MscM (Berrier et al., 1996) or H.volcanii MscA1 and MscA2 (Le Dain et al., 1998), MscMJ and MscMJLR of M.jannaschii may also present a set of energetically and conductance-wise well-tuned MS channels functioning as ‘safety-valves’ in osmotically challenged prokaryotic cells.
Materials and methods
Chemicals
Bacto-tryptone and yeast extract were from Oxoid (Hampshire, UK). The following reagents were purchased from Sigma (St Louis, MO): octylglucoside, HEPES, SDS, phosphatidylcholine, isopropyl-1-thio-β-d-galactopyranoside (IPTG), ampicillin and imidazole. Chlor amphenicol and kanamycin were purchased from Boehringer Mannheim (Germany) and Calbiochem-Novachem (La Jolla, CA), respectively. All other chemicals were analytical grade.
Bacterial strains and culture conditions
Escherichia coli cells harbouring the AMJ FE41 clone encoding the MJ1143 protein were obtained from ATCC (Rockville, MD). Escherichia coli strain M15 (pREP4::kan) (Qiagen) was used as a host for recombinant plasmid harbouring the MJ1143 gene. Escherichia coli strain MJF465 (ΔmscL::Cm, ΔyggB, ΔkefA::kan) or MJF431 [ΔkefA::kan, ΔyggB, ΔyjeP (Levina et al., 1999)] was used for protein expression and electrophysiology. Strains were grown at 37°C in Luria-Bertani broth (LB) containing (10 g/l bacto-tryptone, 5 g/l yeast extract, 5 g/l NaCl) supplemented with 100 µg/ml ampicillin, 20 µg/ml chloramphenicol or 25 µg/ml kanamycin, according to the selection requirements. The gene expression was induced with IPTG once the cell culture had reached mid-log phase (OD600 ≈ 0.6).
Cloning, protein purification and liposome reconstitution
The entire open reading frame of mj1143 was amplified by PCR using the AMJ FE41 clone as a template and cloned into the pQE-30 expression vector (Qiagen) as a BamHI–SmaI fragment in-frame with His6 using standard cloning procedures (Sambrook et al., 1989). The MJ1143 recombinant protein was purified as previously described (Sukharev et al., 1999; Kloda and Martinac, 2001a,b). The MJ1143 protein was reconstituted into liposomes according to the methods described previously for MS channels in E.coli, H.volcanii and M.jannaschii (Delcour et al., 1989; Sukharev et al., 1993; Häse et al., 1995; Le Dain et al., 1998; Kloda and Martinac, 2001a).
Electrophysiological recording
Single channel currents were filtered at 2 kHz, digitized at 5 kHz and analysed using pCLAMP6 data acquisition and analysis software (Axon Instruments, Foster City, CA). Current recordings were viewed with the Axoscope for Windows program (Axon Instruments). Current amplitudes were determined by measuring the difference between the cursor aligned at peak and baseline currents. Channel conductance was estimated from the current voltage plots. Suction applied to the patch–clamp pipette was measured with the piezoelectric pressure transducer (Omega Engineering, Stamford, CT).
Estimate of the free energy of activation of the MJ1143 channel
To estimate the free energy of activation of the MJ1143 channel protein, we used the same method that was previously described for MscMJ (Kloda and Martinac, 2001a). Briefly, it can be shown that by using a two-state Boltzmann model of gating an MS channel by mechanical force, with the change of area tΔA being the dominant energy term (Sukharev et al., 1999), the free energy ΔG is a linear function of membrane tension, t, i.e. ΔG = tΔA – ΔG0, where ΔG0 is the difference in free energy between the closed and open conformations of the channel in the absence of the externally applied membrane tension, ΔA is the difference in membrane area occupied by an open and closed channel at a given membrane tension, and tΔA is the work required for an MS channel to gate at the open probability of 0 < Po < 1. Since the expression for the Boltzmann function describing the open probability of a single MS channel is given by:
Po/(1 – Po) = exp [α (p – p1/2)] = exp [(tΔA – ΔG0)/kT],
where α is the channel sensitivity to pressure and p1/2 is the pressure at which Po = 0.5, it follows, by using Laplace’s law
t – t1/2 = (p – p1/2) (r/2),
where r is the radius of curvature of the membrane patch under external negative pressure p applied to the patch pipette, that the free energy difference ΔG = 0 (i.e. p = p1/2 and t = t1/2) when the channel open probability Po = 0.5. Consequently, t1/2 = ΔG0/ΔA and p1/2 = 2ΔG0/rΔA, whereas α = rΔA/2kT. It follows that the expression for the free energy of activation of the MS channel in our study is given by:
ΓMSC = αp1/2 = ΔG0/kT.
Sequence alignment and secondary structure prediction
The sequences of the MscMJ and MJ1143 proteins were aligned with the SIM Alignment Tool for Protein Sequences. The hydropathy plot analysis was performed with PROTSCALE using the Kyte and Doolittle algorithm. Helical regions and transmembrane domains were detected with the PSIPRED and TMHMM programs, respectively, while the COILS program was used for detection of coiled-coil regions. The molecular weight and pI values were calculated with the COMPUTE pI/Mw tool. All programs are available at the EXPASY Molecular Biology Server (http:\\expasy.proteome.org.au).
Acknowledgments
Acknowledgements
We are indebted to Dr Owen Hamill from the Department of Physiology and Biophysics, University of Texas, Medical Branch for critical comments on the revised manuscript. We would also like to thank Dr Ian Booth from the Department of Molecular and Cell Biology, University of Aberdeen for providing the MJF465 and MJF431 strains of E.coli. We thank Mr Jay Steer and Mr Ke Nguyen from the Department of Pharmacology (UWA) for computer assistance. This study was supported by the grant A09941004 from the Australian Research Council.
References
- Ajouz B., Berrier,C., Besnard,M., Martinac,B. and Ghazi,A. (2000) Contributions of the different extramembraneous domains of the mechanosensitive ion channel MscL to its response to membrane tension. J. Biol. Chem., 275, 1015–1022. [DOI] [PubMed] [Google Scholar]
- Barinaga M. (1994) Molecular evolution. Archaea and eukaryotes grow closer. Science, 264, 1251. [DOI] [PubMed] [Google Scholar]
- Batiza A.F., Rayment,I. and Kung,C. (1999) Channel gate! Tension, leak and disclosure. Struct. Fold. Des., 7, R99–R103. [DOI] [PubMed] [Google Scholar]
- Berrier C., Besnard,M., Ajouz,B., Coulombe,A. and Ghazi,A. (1996) Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J. Membr. Biol., 151, 175–187. [DOI] [PubMed] [Google Scholar]
- Blount P., Sukharev,S., Moe,P., Nagle,S. and Kung,C. (1996) Towards an understanding of the structural and functional properties of MscL, a mechanosensitive channel in bacteria. Biol. Cell, 87, 1–8. [PubMed] [Google Scholar]
- Brock T.D., Madigan,M.T., Martinko,J.M. and Parker,J. (1994) Biology of Microorganisms. Prentice Hall, Upper Saddle River, NJ.
- Chang G., Spencer,R., Lee,A., Barclay,M. and Rees,D. (1998) Structure of the MscL homologue from Mycobacterium tuberculosis: A gated mechanosensitive ion channel. Science, 282, 2220–2226. [DOI] [PubMed] [Google Scholar]
- Cruickshank C., Minchin,R., Le Dain,A. and Martinac,B. (1997) Estimation of the pore size of the large-conductance mechano sensitive ion channel of Escherichia coli.Biophys. J., 73, 1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcour A.H., Martinac,B., Adler,J. and Kung,C. (1989) Modified reconstitution method used in patch–clamp studies of Escherichia coli ion channels. Biophys. J., 56, 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Añovernos J. and Corey,D.P. (1997) The molecules of mechanosensation. Annu. Rev. Neurosci., 20, 567–594. [DOI] [PubMed] [Google Scholar]
- Gazit E., Bach,D., Kerr,I.D., Sansom,M.S., Chejanovsky,N. and Shai,Y. (1994) The α5 segment of Bacillus thuringiensis δ-endotoxin: in vitro activity, ion channel formation and molecular modeling. Biochem. J., 304, 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L., Liu,W. and Martinac,B. (1998) Electromechanical coupling model of gating the large mechanosensitive ion channel (MscL) of Escherichia coli by mechanical force. Biophys. J., 74, 2889–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O.P. and Martinac,B. (2001) Molecular basis of mechano transduction in living cells. Physiol. Rev., 81, in press. [DOI] [PubMed] [Google Scholar]
- Hamill O.P. and McBride,D.W.,Jr (1997) Induced membrane hypo/hypermechanosensitivity: a limitation of patch clamp recording. Annu. Rev. Physiol., 59, 621–631. [DOI] [PubMed] [Google Scholar]
- Häse C.C., Le Dain,A.C. and Martinac,B. (1995) Purification and functional reconstitution of the recombinant large mechanosensitive ion channel (MscL) of Escherichia coli.J. Biol. Chem., 270, 18329–18334. [DOI] [PubMed] [Google Scholar]
- Häse C.C., Le Dain,A.C. and Martinac,B. (1997) Molecular dissection of the large mechanosensitive ion channel (MscL) of Escherichia coli: mutants with altered channel gating and pressure sensitivity. J. Membr. Biol., 157, 17–25. [DOI] [PubMed] [Google Scholar]
- Henderson R. and Unwin,P.N.T. (1975) Three-dimensional model of purple membrane obtained by electron microscopy. Nature, 257, 28–32. [DOI] [PubMed] [Google Scholar]
- Hille B. (1968) Pharmacological modification of the sodium channels of frog nerve. J. Gen. Physiol., 51, 199–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I.D., Sankararamakrishnan,R., Smart,O.S. and Sansom,M.S. (1994) Parallel helix bundles and ion channels: molecular modeling via simulated annealing and restrained molecular dynamics. Biophys. J., 67, 1501–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloda A. and Martinac,B. (2001a) Molecular identification of a mechanosensitive channel in Archaea. Biophys. J., 80, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloda A. and Martinac,B. (2001b) A novel mechanosensitive ion channel in the thermophilic cell-wall-less archaeon Thermoplasma volcanium. Cell Biochem. Biophys., in press. [DOI] [PubMed] [Google Scholar]
- Kloda A. and Martinac,B. (2001c) Mechanosensitive channels in Archaea. Cell Biochem. Biophys., in press. [DOI] [PubMed] [Google Scholar]
- Le Dain A.C., Saint,N., Kloda,A., Ghazi,A. and Martinac,B. (1998) Mechanosensitive ion channels of the archaeon Haloferax volcanii.J. Biol. Chem., 273, 12116–12119. [DOI] [PubMed] [Google Scholar]
- Levina N., Totemeyer,S., Stokes,N.R., Louis,P., Jones,M.A. and Booth,I.R. (1999) Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J., 18, 1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H., Baltimore,D., Berk,A., Zipursky,S.L., Matsudaira,P. and Darnell,J. (1995) Molecular Cell Biology, 3rd edn. Scientific American Books, New York, NY, p. 607.
- Malashkevich V.N., Kammerer,R.A., Efimov,V.P., Schulthess,T. and Engel,J. (1996) The crystal structure of a five-stranded coiled coil in COMP: a prototype ion channel? Science, 274, 761–765. [DOI] [PubMed] [Google Scholar]
- Martinac B. (1993) Mechanosensitive ion channels: biophysics and physiology. In Jackson,M.B. (ed.), Thermodynamics of Membrane Receptors and Channels. CRC Press, Boca Raton, FL, pp. 327–351.
- Martinac B. (1999) Mechanosensitive ion channels—universal biological transducers of mechanical stimuli. Australian Biochemist, 8, 6–10. [Google Scholar]
- Martinac B., Buechner,M., Delcour,A.H., Adler,J. and Kung,C. (1987) Pressure-sensitive ion channel in Escherichia coli.Proc. Natl Acad. Sci. USA, 84, 2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B., Adler,J. and Kung,C. (1990) Mechanosensitive ion channels of E.coli activated by amphipaths. Nature, 348, 261–263. [DOI] [PubMed] [Google Scholar]
- Moe P.C., Blount,P. and Kung,C. (1998) Functional and structural conservation in the mechanosensitive channel MscL implicate elements crucial for mechanosensation. Mol. Microbiol., 28, 583–592. [DOI] [PubMed] [Google Scholar]
- Morris C.E. (1990) Mechanosensitive ion channels. J. Membr. Biol., 113, 93–107. [DOI] [PubMed] [Google Scholar]
- Oakley A., Martinac,B. and Wilce,M. (1999) Structure and function of the bacterial mechanosensitive channel of large conductance. Protein Sci., 8, 1915–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X., Blount,P., Hoffman R.J. and Kung,C. (1998) One face of the transmembrane helix is crucial for mechanosensitive channel gating. Proc. Natl Acad. Sci. USA, 95, 11471–11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace N.R. (1997) A molecular view of microbial diversity and the biosphere. Science, 276, 734–740. [DOI] [PubMed] [Google Scholar]
- Patel A., Honoré,E., Maingret,F., Lesage,F., Fink,M., Duprat,F. and Lazdunski,M. (1998) A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J., 17, 4283–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F. (1988) Mechanical transduction in biological systems. CRC Crit. Rev. Biomed. Eng., 16, 141–169. [PubMed] [Google Scholar]
- Sachs F. and Morris,C.E. (1998) Mechanosensitive ion channels in nonspecialized cells. Rev. Physiol. Biochem. Pharmacol., 132, 1–77. [DOI] [PubMed] [Google Scholar]
- Sackin H. (1995) Mechanosensitive channels. Annu. Rev. Physiol., 57, 333–353. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sansom M.S., Kerr,I.D., Smith,G.R. and Son,H.S. (1997) The influenza A virus M2 channel: a molecular modeling and simulation study. Virology, 233, 163–173. [DOI] [PubMed] [Google Scholar]
- Sokabe M., Hasegawa,N. and Yamamori,K. (1993) Blockers and activators for stretch-activated ion channels of chick skeletal muscle. NY Acad. Sci., 707, 417–420. [DOI] [PubMed] [Google Scholar]
- Spencer R.H., Chang,G. and Rees,D.C. (1999) Feeling the pressure: structural insights into a gated mechanosensitive channel. Curr. Opin. Struct. Biol., 9, 448–454. [DOI] [PubMed] [Google Scholar]
- Sukharev S.I., Martinac,B., Arshavsky,V.Y. and Kung,C. (1993) Two types of mechanosenitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys. J., 65, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S.I., Blount,P., Martinac,B., Blattner,F.R. and Kung,C. (1994) A large mechanosensitive channel in E.coli encoded by mscL alone. Nature, 368, 265–268. [DOI] [PubMed] [Google Scholar]
- Sukharev S.I., Sigurdson,W.J., Kung,C. and Sachs,F. (1999) Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J. Gen. Physiol., 113, 525–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S.I., Batanzos,M., Chiang,C.-S. and Guy,H.R. (2001) The gating mechanism of the large mechanosensitive channel MscL. Nature, 409, 720–724. [DOI] [PubMed] [Google Scholar]
- Woese C.R. (1994) There must be a prokaryote somewhere: microbiology’s search for itself. Microbiol. Rev., 58, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K., Batiza,A., Schroeder,M., Blount,P. and Kung,C. (1999) Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophys. J., 77, 1960–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]