Abstract
Adult T cell leukemia/lymphoma (ATLL) has been characterized as one of the most aggressive human neoplasias and its incidence is thought to be caused by both genetic and epigenetic alterations to the host cellular genes of T cells infected with human T cell leukemia virus type I (HTLV-I). A multilobulated nuclear appearance is an important diagnostic marker of ATLL, and we have now identified that the molecular mechanisms underlying these formations occur through microtubule rearrangement via phosphatidylinositol 3-kinase (PI3-kinase) activation by AILIM/ICOS signaling. We also show that PTEN and/or SHIP-1, which are PIP3 inositol phosphatases that inhibit the activation of downstream effectors of the PI3-kinase cascade, are disrupted in both ATLL neoplasias and in multilobulated nuclei-forming Jurkat cells. This down-regulation of PTEN was found to be essential for the formation of ATLL-type nuclear lobules. Furthermore, PI3-kinase and PTEN activities were observed to be closely associated with cellular proliferation. Thus, our results suggest that alteration of PI3-kinase signaling cascades, as a result of the down-regulation of inositol phosphatases, induces ATLL-type multilobulated nuclear formation and is also associated with the cellular proliferation of malignant T cell leukemias/lymphomas.
Keywords: activation-inducible lymphocyte immunomediatory molecule/inducible costimulator, human T cell leukemia virus type I, multilobulated nuclei, PTEN, SHIP
Adult T cell leukemia/lymphoma (ATLL) is one of the most aggressive neoplastic diseases and is caused by human T cell leukemia virus type I infection (HTLV-I; refs. 1–3). Although ≈20 million people worldwide are thought to be infected with HTLV-I, ATLL is regarded as a relatively rare occurrence in HTLV-I carriers (2–5%) requiring a long latent period of 40–60 years (4–7). Malignant T cells in acute-type ATLL patients show nuclear polymorphisms, and these typical multilobulated appearances (“flower-like” nuclei) have been shown to be a specific morphological feature of malignant T cells (8). These polymorphisms are observed in >50% of patients with aggressive acute-type ATLL and are an important clinical diagnostic marker of this disease (6–9). Previous studies have also suggested that genetic and epigenetic alterations to host cellular genes in HTLV-I-infected T cells are necessary for the development of ATLL (3, 4). However, it is still unknown why ATLL occurs rarely in HTLV-I carriers and the molecular mechanisms underlying the manifestation of nuclear polymorphisms have yet to be fully elucidated.
Malignant T cells in acute-type ATLL patients are activated and induced to proliferate in the lymph nodes (4), which undergo swelling and enter the peripheral blood stream. Therefore, it is plausible that antigenic molecules that are presented in the lymph nodes in ATLL have an important role in the activation of malignant T cells in vivo. During the immune response, T cells are optimally activated in secondary lymphoid tissues, such as the lymph nodes, and a second costimulatory signal, CD28, is necessary for the activation of naïve T cells upon antigen recognition via the T cell receptor/CD3 complex (10, 11). T cell activation also induces the activation-inducible lymphocyte immunomediatory molecule (AILIM)/inducible costimulator (ICOS; refs. 12 and 13). AILIM/ICOS-mediated signals contribute mainly to the regulation of activated T cells and to memory/effector T cell functions via the phosphatidylinositol-3-kinase (PI3-kinase) cascade (14, 15). Recently, we have demonstrated that AILIM/ICOS signaling in the absence of T cell receptor (TCR)/CD3 stimulation has a distinct biological role in T cell migration and in the polarization of activated T cells through cytoskeletal rearrangements (16).
PI3-kinase is well known to play an essential role in T cell activation and cell motility in not only cells of the immune system but also in other cell types through cytoskeleton rearrangements (17, 18). The activation of PI3-kinase induces the production of phosphatidylinositol-3,4,5-trisphosphate (PIP3) from phosphatidylinositol-4,5-bisphosphate (PIP2), which then binds to the PH domains of intracellular signaling molecules and thus has essential roles in the rearrangement of the cytoskeleton (17–20). In addition, inositol phosphatases, including the phosphatase and tensin homolog deleted on chromosome 10 (PTEN) tumor suppressor and Src homology 2 domain containing inositol polyphosphate phosphatase (SHIP), have been implicated in the degradation of PIP3 and shown to antagonize the PI3-kinase/Akt signaling pathway (21–26).
Here, we show that ATLL-type multilobulated nuclei are produced through a distinct rearrangement of microtubules via PI3-kinase cascade that has been activated by both AILIM/ICOS and cell surface molecules. We also demonstrate that the formation of ATLL-type multilobulated nuclei requires the alteration of PI3-kinase/Akt cascade activation via the down-regulation of PTEN and/or SHIP-1. We propose a molecular mechanism for ATLL-type multilobulated nucleus formation and for the development of ATLL.
Materials and Methods
Cell Culture and Treatment. Highly purified T cells (>97%) were isolated from patients diagnosed as having chronic-type ATLL and from healthy adult volunteers upon informed consent as described previously (27). Hut102, Jurkat, SupT1, and H9 cells were maintained in RPMI medium 1640 supplemented with 10% FCS. The PI3-kinase inhibitor, LY294002 (Promega), the microtubule polymerization inhibitor, nocodazol (Sigma), and depolymerization inhibitor, taxol (paclitaxel; Sigma), were used for analysis of microtubule rearrangement. Treatments were performed on precultures for 30 min at 37°C before stimulation under various conditions.
Fluorescence Microscopy. Cells (3 × 104 per well) were stimulated with various mAbs that were precoated at the concentration of 2 μg per well in eight-well chamber slides (Becton Dickinson). Cells were then fixed, permeabilized, and stained with FITC-conjugated anti-α-tubulin (clone DM1, Sigma), rhodamine-conjugated phalloidin (Molecular Probes) and Hoechst dye 33258 (Sigma), as described (16). Cells were analyzed by a fluorescent microscope using an Axiovert S100 (Carl Zeiss). Image acquisition from the Zeiss inscribe was made with a cooled charged-coupled device camera using quantix (Photometrix, Victoria, Australia), and the images were processed with metamorph software (Molecular Devices, Sunnyvale, CA). Cells were also observed by using confocal laser scanning microscopy (LSM-510META, Carl Zeiss). The images acquired by confocal microscopy were then processed with Imaris 4 software (Carl Zeiss). Different numbers and levels of multilobulated nuclear formations were determined in at least 100 cells, in five independent experiments, and the average and the standard error were calculated as the frequency of these formations.
Western Blot Analysis. Cells were lysed as described (27) and the lysates were subjected to electrophoresis on SDS/PAGE and transferred to Immobilon-P membrane (Millipore, Billerica, MA). The blotted membranes were blocked and subsequently incubated with anti-PTEN polyclonal antibody (anti-PTEN pAb; Cell Signaling Technologies), anti-SHIP-1 pAb (Upstate Biotechnologies, Lake Placid, NY), anti-Akt/PKB pAb (Cell Signaling Technologies) or anti-phospho-Akt/PKB pAb (Cell Signaling Technologies) according to the manufacturer's instructions. After washing, bound primary Abs were visualized with secondary horseradish peroxidase-conjugated F(ab′)2 fragment of IgG (ICN/Cappel, Aurora OH) and visualized by using enhanced chemiluminescence (ECL) (Amersham Pharmacia).
Results
The Involvement of AILIM/ICOS Signaling in the Multilobulated Nucleus Formation. We first investigated whether the induction of costimulatory molecules is involved in typical multilobulated nuclear formations in peripheral T cells isolated from ATLL patients. Chronic-type ATLL-derived T cells, which show relatively low levels of these nuclear polymorphisms, were tested in this experiment via a multilobulated nucleus formation assay because acute-type ATLL-derived T cells have been shown to have already formed these abnormal nuclei in vivo (6–9). The clinical characteristics of the T cell isolates from 11 chronic-type ATLL individuals are summarized in Table 1 and Supporting Text, which are published as supporting information on the PNAS web site.
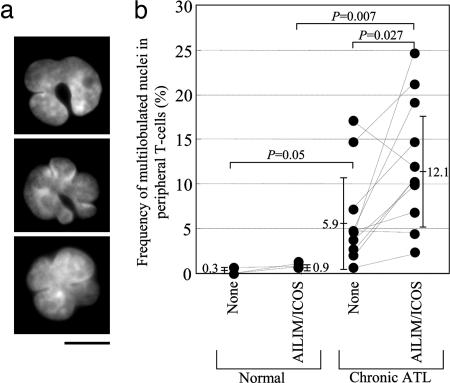
In T cells isolated from chronic-type ATLL patients, multilobulated nucleus formation was significantly induced by AILIM/ICOS signaling, but not CD28 signaling (data not shown), in the absence of CD3 stimulation (Fig. 1). Fig. 1a shows a typical multilobulated (“flower-like”) nucleus induced by AILIM/ICOS signaling in chronic-type ATLL patients. In such T cells, the nuclear shape is altered such that it forms several deep cavities and results in a multilobulated nucleus with typically three to six small lobules. The frequencies of the appearance of ATLL-type multilobulated nuclei after AILIM/ICOS signaling in our chronic-ATLL patient group are shown in Fig. 1b and were found to be significantly increased when compared to unstimulated T cells isolated from chronic-type ATLL patients (5.90 ± 5.14% to 12.10 ± 6.86%, P = 0.027) but not when compared to healthy donors.
Fig. 1.
AILIM/ICOS signaling induces multilobulated nucleus formation in T cells isolated from chronic ATLL patients. (a) A typical multilobulated nucleus from chronic ATLL-derived T cells stimulated with AILIM/ICOS signaling. T cells isolated from chronic ATLL patients were stimulated by AILIM/ICOS mAb (clone SA12)-precoated slides for 4 h and analyzed by Hoechst dye 33258 as described in Supporting Text. (Scale bar, 10 μm.) (b) T cells isolated from healthy donors and chronic ATLL patients were stimulated with AILIM/ICOS mAb. Frequencies of multilobulated nucleus formation were determined. Mean ± SEM and P values are indicated in the graph.
Induction of ATLL-Type Multilobulated Nucleus Formation in AILIM/ICOS-Expressing Jurkat Cells. We transduced a cDNA encoding AILIM/ICOS into the human T cell leukemia cell lines, such as Hut102, H9, SupT1, and Jurkat, in which the expression of AILIM/ICOS could not be detected, and isolated stably expressing clones. Among these AILIM/ICOS-expressing leukemia cells, only AILIM/ICOS-Jurkat cells formed typical ATLL-type multilobulated nuclei after stimulation by exogenous AILIM/ICOS signaling (Fig. 6a, Table 2, and Supporting Text, which are published as supporting information on the PNAS web site). Quantitative evaluation of ATLL-type multilobulated nuclear formations in AILIM/ICOS-Jurkat cells is shown in Fig. 6b and Supporting Text.
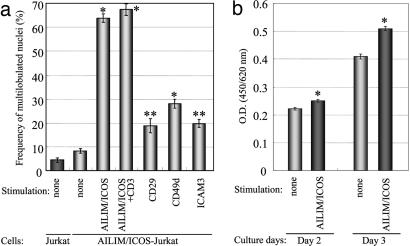
In AILIM/ICOS-expressing Jurkat cells, ATLL-type multilobulated nucleus formation was dramatically induced with a maximal induction of 63.3 ± 2.2% at 4–8 h after AILIM/ICOS stimulation, followed by a gradual decrease in the frequency of altered nuclei within 48 h (Fig. 2a and Fig. 7 a and b, which is published as supporting information on the PNAS web site). However, these frequencies were not enhanced with a combination of CD3 stimulation (Fig. 2a). Although the cell adhesion molecules CD29, CD49d, and ICAM-3 are expressed at high levels on the cell surface of AILIM/ICOS-Jurkat cells (Fig. 6c) and the nonstimulated frequencies of multilobulated nuclear formations were significantly higher in these cells than in the controls, these frequencies were at relatively low levels when compared to AILIM/ICOS signaling (Fig. 2a).
Fig. 2.
AILIM/ICOS signaling specifically induces ATLL-type multilobulated nucleus formation in AILIM/ICOS-expressing Jurkat cells. (a) AILIM/ICOS-Jurkat cells were stimulated by AILIM/ICOS signaling and by anti-AILIM/ICOS, CD29, CD49d, or ICAM3 mAbs precoated on slides for 4 h. Values are represented as the mean ± SEM. *, P < 0.0001 compared with control; **, P < 0.0005 compared with control. (b) AILIM/ICOS-Jurkat cells were stimulated by anti-AILIM/ICOS mAb precoated at 500 ng per well on 96-well type plates for 2 or 3 days. Cell proliferation was measured with WST-8 reagent by using a Cell Counting kit (Dojindo Labs, Tokyo) according to the manufacturer's instruction. The cells were cultured for 2 or 3 days, and WST-8 reagent was added for the last 2 h of the culture. Values are represented as the mean ± SEM. *, P < 0.01 compared with control.
We further investigated whether AILIM/ICOS signaling affects cell growth in AILIM/ICOS-Jurkat cells. We also evaluated cell growth in AILIM/ICOS-Jurkat cells after AILIM/ICOS stimulation by using WST-8 reagent. The stimulation significantly induced the proliferation of AILIM/ICOS-Jurkat cells (Fig. 2b). These results indicate that AILIM/ICOS signaling induces both ATLL-type multilobulated nucleus formation and cellular growth in AILIM/ICOS-Jurkat cells.
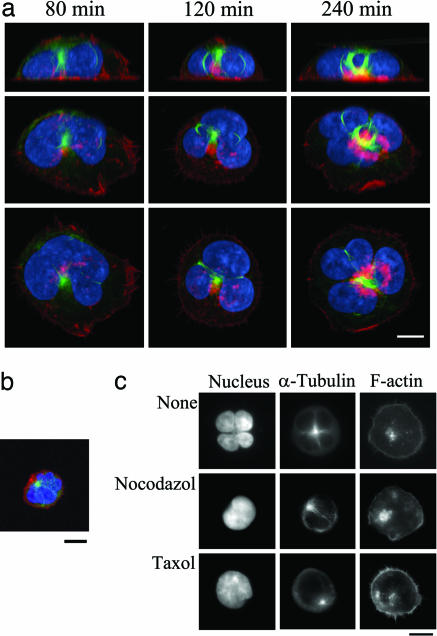
The Involvement of Microtubule Rearrangement in ATLL-Type Multilobulated Nucleus Formation. We next performed a time course analysis of nuclear morphological changes and cytoskeletal rearrangements of actin and microtubules during AILIM/ICOS-mediated ATLL-type multilobulated nucleus formation (Fig. 7a and Supporting Text). We also investigated microtubule reorganization during ATLL-type multilobulated nucleus formation by using 3D analyses with confocal microscopy. Our subsequent findings clearly demonstrated that microtubules were bundled and localized within the small cavities between the small nuclear lobules by the 3D analyses with confocal microscopy (Fig. 3a). Surprisingly, these bundled microtubules that were present between the lobules formed a loop structure, which then appeared to contract the nuclei and thus induce the formation of small lobules (Fig. 3a Top). The mean values ± SEM., which were calculated from the independent image analysis of five individual nuclei, of the circumference of the microtubule loops at 40, 120, and 240 min after stimulation were found to be 25.2 ± 2.46, 17.8 ± 1.32, and 8.92 ± 1.86 nm, respectively. Ring-like organizations of microtubules were also observed in T cells isolated from one acute-type ATLL patient (Fig. 3b). All of the morphological features of ATLL nuclei and the ring-like microtubule formations were also representative of AILIM/ICOS-Jurkat cells cocultured with CHO cells expressing B7h, the native ligand of AILIM/ICOS (Fig. 7c).
Fig. 3.
Microtubule rearrangement is involved in ATLL-type multilobulated nucleus formation by AILIM/ICOS signaling. (a) After AILIM/ICOS stimulation for 80–240 min, the cells were stained with FITC-conjugated anti-α-tubulin mAb (green), rhodamine-phalloidin (red), and Hoechst dye 33258 (blue) as described in Materials and Methods and then analyzed by confocal laser microscopy by using an LSM510-META; the images were processed with imaris 4 software. (Top) x–z sliced sections. (Middle and Bottom) Three-dimensional views at 45° and 90° from the x–z projections, respectively. (Scale bar, 10 μm.) (b) Analyses of the nuclear morphology and microtubule filaments of multilobulated nuclei of T cells isolated from an acute-type ATLL patient. The cells were stained and analyzed as described above. (Scale bar, 10 μm.) (c) Representative images of AILIM/ICOS-Jurkat cells treated with either 100 nM nocodazol or taxol are shown. (Scale bar, 10 μm.)
We next examined the effects of the microtubule polymerization and depolymerization inhibitors nocodazol and taxol on these multilobulated nuclear formations. Pretreatment with these agents did not affect filamentous actin formation but inhibited the AILIM/ICOS-mediated nuclear transformations and prevented formation of the bundled microtubule structures (Fig. 3c and Fig. 8, which is published as supporting information on the PNAS web site). These findings indicate that microtubule rearrangements including the contraction of looped microtubules in fact regulate the ATLL-type multilobulated nucleus formation that is induced by AILIM/ICOS stimulation.
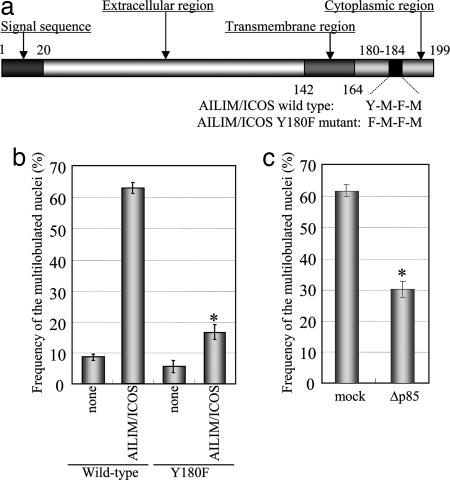
The Role of PI3-Kinase in ATLL-Type Multilobulated Nucleus Formation. In the intracellular region of AILIM/ICOS, PI3-kinase binds to the YMFM sequence between residues 180–183 (Fig. 4a and refs. 12 and 13) and the activation of PI3-kinase is essential for T cell activation of AILIM/ICOS-mediated signaling (27). The Y180F mutation resulted in a dramatic decrease in the frequency of nuclear abnormalities when compared to the wild-type AILIM/ICOS-expressing Jurkat cells (Fig. 4b). The PI3-kinase inhibitor, LY294002, also significantly inhibited multilobulated nucleus formation in AILIM/ICOS-Jurkat cells in a dose-dependent manner and this inhibition by LY294002 was also observed in peripheral T cells isolated from chronic-type ATLL patients (Fig. 9, which is published as supporting information on the PNAS web site). Moreover, transduction of a dominant-negative p85 subunit (Δp85), which lacks residues 479–513 of the p85α subunit of PI3-kinase (28), also significantly inhibited ATLL-type multilobulated nucleus formation by AILIM/ICOS stimulation (Fig. 4c). These results indicate that the PI3-kinase cascade induced by AILIM/ICOS signaling is essential for ATLL-type multilobulated nucleus formation.
Fig. 4.
PI3-kinase is essential for ATLL-type multilobulated nucleus formation induced by AILIM/ICOS signaling. (a) Schematic diagram of the AILIM/ICOS domain structure with wild-type and Y180F mutant sequences of AILIM/ICOS, from amino acids 180 to 183 indicated at the bottom. (b) Expression vectors containing wild-type and Y180F mutant AILIM/ICOS cDNA were transfected into Jurkat cells, and stable clones were isolated. Clones expressing wild-type and Y180F mutants of AILIM/ICOS were then stimulated by AILIM/ICOS, and the frequencies of multilobulated nucleus formation were analyzed and calculated as the mean ± SEM. *, P < 0.0001 compared with control. (c) Wild-type AILIM/ICOS-expressing Jurkat cells were transfected with SR α-Δp85 and cultured for 1 day. Live cells were purified by using a Dead Cell Removal kit (Miltenyi Biotec) and then stimulated by ligation with anti-AILIM/ICOS mAb. After 4 h of stimulation, the effects of exogenous gene expression on the frequency of multilobulated nucleus formation were analyzed. Values are again given as the mean ± SEM. *, P < 0.0001 compared with mock transfected cells.
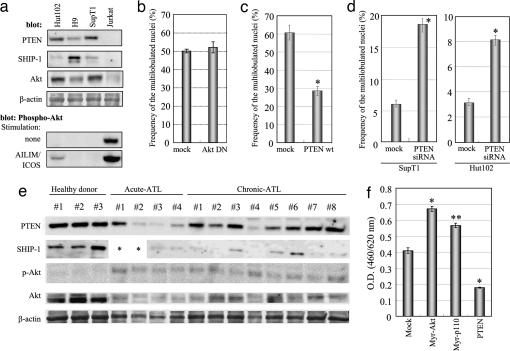
Alteration of the PI3-Kinase Pathway by Inositol Phosphatase Disruption Has an Essential Role in ATLL-Type Multilobulated Nucleus Formation. The inositol phosphatases PTEN and SHIP-1 are antagonists of the PI3-kinase cascade via their PIP3 phosphatase activity and their activation regulates the PI3-kinase/Akt signaling pathway (21–26). We next examined the expression profiles of these phosphatases. PTEN and SHIP-1 expression was not detectable in AILIM/ICOS-expressing Jurkat cells, in which multilobulated nuclei are induced. However, we could detect these phosphatases in Hut102, H9, or SupT1 cells, in which these ATLL-type nuclear formations cannot not be induced (Fig. 5a). We also found that the levels of phosphorylated-Akt (p-Akt) inversely correlate with the expression levels of PTEN and SHIP-1 and that p-Akt is markedly induced by AILIM/ICOS signaling (Fig. 5a). These results were consistent with previous reports (25).
Fig. 5.
Overactivation of PI3-kinase is essential for ATLL-type multilobulated nucleus formation and can be observed in T cells isolated from ATLL patients.(a) Western blot analysis showing the expression of PTEN, SHIP-1, total Akt, β-actin, and phosphorylated-Akt (p-Akt) in various human leukemia cell lines. Phosphorylated-Akt was detected with or without AILIM/ICOS signaling. (b) Wild-type AILIM/ICOS-expressing Jurkat cells were transfected with the dominant-negative form of Akt (Akt DN), and then the effects of exogenous gene expression on the frequency of multilobulated nucleus formation were analyzed as described in Fig. 4c. Values are given as the mean ± SEM. *, P < 0.0001 compared with mock transfected cells. (c) AILIM/ICOS expressing Jurkat cells were transfected with wild-type PTEN and an EYFP-C1 plasmid control and cultured for 1 day. The cells were then purified and subjected to multilobulated nucleus formation analysis as described in Fig. 4c, and values are given as the means ± SEM. *, P < 0.0001 compared with mock transfected cells. (d) A PTEN-specific knockdown was performed by the expression of hairpin siRNA. A PTEN–RNAi plasmid was transfected into AILIM/ICOS-SupT1 and AILIM/ICOS-Hut102 cells, and stable transformants were subjected to the ATLL-type multilobulated formation analysis. *, P < 0.0001 compared with mock transfected cells. (e) Western blot analysis showing the expression of PTEN, SHIP-1, phosphorylated-Akt (p-Akt), total Akt, and β-actin in T cells isolated from healthy donors and from ATLL patients as indicated. *, Not determined. (f) Expression vectors containing myristoylated Akt (Myr-Akt), myristoylated PI3-kinase p110 subunit (Myr-p110), and PTEN cDNAs were transfected into AILIM/ICOS-Jurkat cells, and cell proliferation was measured with WST-8 reagent by using a Cell Counting kit (Dojindo Labs) according to the manufacturer's instruction. The cells were cultured for 3 days, and WST-8 reagent was added for the last 4 h of the culture. Values are represented as the mean ± SEM. *, P < 0.0001 compared with control; **, P < 0.0005 compared with control.
We next examined whether the activation of the Akt cascade effects the nuclear formations induced by AILIM/ICOS signaling in AILIM/ICOS-Jurkat cells (Fig. 5b). The transduction of a dominant-negative form of Akt (Akt DN), in which both Thr-308 and Ser-473 are substituted by alanine, did not inhibit ATLL-type multilobulated nucleus formation by AILIM/ICOS stimulation (Fig. 5b). This finding indicates that the activation of the downstream effectors of Akt signaling pathway is not essential for ATLL-type nuclear formation.
To examine the role of the alteration of PI3-kinase activity on the nuclear formations in ATLL, a wild-type PTEN-expressing vector was introduced into AILIM/ICOS-Jurkat cells and was found to significantly reduce multilobulated nuclear development (Fig. 5c). In contrast, in stably expressing AILIM/ICOS-SupT1 and Hut102 cells, multilobulated nuclei were produced at relatively low levels compared to AILIM/ICOS-Jurkat cells (Fig. 5d and Table 2). We examined the down-regulation of PTEN by siRNA in AILIM/ICOS-expressing SupT1 and Hut 102 cells (Fig. 10, which is published as supporting information on the PNAS web site) and observed that ATLL-type multilobulated nucleus formation in PTEN disrupted AILIM/ICOS-expressing SupT1 and Hut 102 cells was significantly induced to levels of 18.5% and 8.2%, respectively (Fig. 5d). These data indicate that down-regulation of PTEN expression is essential for nuclear transformation in ATLL.
We next analyzed the expression of PTEN and SHIP-1 in peripheral T cells isolated from acute and chronic-type ATLL patients (Table 3, which is published as supporting information on the PNAS web site). The expression of PTEN and SHIP-1, but not p-Akt, was clearly detectable in peripheral T cells isolated from healthy donors (Fig. 5e). In contrast, the expression of PTEN and SHIP-1 in peripheral T cells isolated from acute and chronic-type ATLL patients were significantly decreased and these cells displayed an inverse up-regulation of p-Akt (Fig. 5e and Fig. 11, which is published as supporting information on the PNAS web site).
The Role of the ATLL Multilobulated Nucleus Formation-Associated Genes, PI3-Kinase and PTEN, in Cellular Proliferation. Finally, we examined the effects of the PI3-kinase and PTEN genes, which are associated with ATLL-type multilobulated nucleus formation, upon cellular proliferation in AILIM/ICOS-Jurkat cells. The proliferation of AILIM/ICOS-Jurkat cells was found to be significantly induced by the expression of the constitutively active myristoylated forms of Akt (Myr-Akt) and the PI3-kinase p110 subunit (Myr-p110) in the absence of AILIM/ICOS signaling (Fig. 5f). In contrast, the transduction of wild-type PTEN in these cells strongly reduced their proliferation. These results suggest that the alteration of the PI3-kinase pathway, caused by the disruption of inositol phosphatases, is associated with not only ATLL-type multilobulated nucleus formation but also with the incidence and development of ATLL.
Discussion
Here, we have provided evidence that ATLL-type multilobulated nucleus formation, which is thought to be an important clinical marker of ATLL and to be closely related to the progression and incidence of this cancer, is caused by microtubule constriction via the overactivation of the PI3-kinase cascade. This altered PI3-kinase activity is induced by the down-regulation of the inositol phosphatases, PTEN and/or SHIP-1, after AILIM/ICOS signaling in both peripheral T cells isolated from chronic ATLL patients and in AILIM/ICOS-Jurkat cells. Our results also show that PI3-kinase and PTEN are closely associated with the cellular proliferative capacity.
The PI3-kinase cascade is well known as a regulator of the activation, proliferation, and cytokine production of T cells (17, 18). The major functions of the inositol phosphatases, PTEN and SHIP-1, rely on their phosphatase activity and subsequent antagonism of the PI3-kinase cascade. In addition, the activation levels of the PI3-kinase cascade are also regulated by the expression levels of PTEN and SHIP (21–26). Loss of function of these inositol phosphatases results in the accumulation of PIP3 and the subsequent activation of its downstream effectors, such as the PI3-kinase/Akt cascade (21–26). Jurkat cells, which is the only T cell leukemia cell line that can induce multilobulated nuclear formations after AILIM/ICOS signaling, are deficient in both PTEN and SHIP-1 expression and show a highly inverse up-regulation of p-Akt, as reported (25, 29). Significant decreases in inositol phosphatase levels were also observed in ATLL patient-derived T cells, which resulted in an inverse up-regulation of p-Akt (Figs. 5e and 11). We showed that the alteration in PI3-kinase cascade activity, but not in the activation of Akt, is indeed essential for these multilobulated formations under conditions where inositol phosphatase expression is disrupted.
The PI3-kinase cascade is also thought to play a role in the development of ATLL. We showed in our current study that AILIM/ICOS signaling, and the subsequent alteration of PI3-kinase activity, contributes to the growth rate of AILIM/ICOS-Jurkat cells. Previous studies have demonstrated that the selective suppression of PTEN in T cells by using conditional knockout mice (tPTENflox/-mice), leads to an increase in the levels of mature CD4+ T cells in the periphery (21–23, 30). The subsequent onset of malignant T cell lymphomas, which were classified as CD4+ and not CD8+ T cell lymphomas, could be observed from 10 weeks in these PTEN knockouts, and all of the animals died within 17 weeks (30). Furthermore, transgenic mice expressing an active form of PI3-kinase in their T cells, derived from a thymic lymphoma (p65), developed an infiltrating lymphoproliferative disorder with an increased number of T cells exhibiting a memory CD4+ cell phenotype and a reduced apoptotic response (31). Most ATLL malignant T cells are also classified as CD4+ (5–7). Restoration of SHIP activity in a human leukemia cell line, which has lost expression of endogenous SHIP, down-regulates the constitutively active PI3-kinase/Akt/GSK-3beta signaling pathways and leads to an increased transit time through the G1 phase of the cell cycle (26). Previous reports have also demonstrated that transgenic mice expressing constitutively active myristoylated Akt in T cells had an accumulation of CD4+ T cells and displayed an increased incidence of lymphoma (32). Taken together, these observations suggest that the irregular overactivation of the PI3-kinase cascade, including the disruption of inositol phosphatases such as PTEN and SHIP-1, has an essential role both in the ATLL-specific nuclear phenotype and in the development of ATLL in patients.
Infection of HTLV-I is thought to be essential for the incidence of ATLL (1–3), but in the present study, HTLV-I-negative Jurkat cells also formed ATLL-type multilobulated nuclei. However, it was previously reported that some patients diagnosed with ATLL had typical clinicohematological, morphological, and immunophenotypic characteristics of this cancer but showed no integration of HTLV-I in their leukemic cells and presented with HTLV-I-negative sera (33, 34). Furthermore, previous studies have demonstrated that genetic and epigenetic alterations in host cellular genes are necessary for the development of malignant T cells in ATLL (3, 4). These observations suggested the existence of cellular oncogenesis in HTLV-I-negative ATLL patients and that the phenotype of Jurkat cells is closely related both to in vivo leukomogenesis and to the development of ATLL by infection with HTLV-I.
Further studies of the mechanisms underlying both the down-regulation of inositol phosphatases during the development and progression of ATLL, and the downstream effectors of microtubule rearrangement by PIP3, produced by the active PI3-kinase, will provide further important insights into the mechanisms underlying the incidence and progression of ATLL.
Supplementary Material
Acknowledgments
We thank Dr. M. Matsuoka (Kyoto University, Kyoto) for providing Hut102 cells; Dr. W. Ogawa (Kobe University, Kobe, Japan) for providing SRα-Δp85 plasmid; Dr. Y. Nishi (Nagahama Institute of Bio-Science and Technology, Nagahama, Shiga, Japan) for encouragement; Dr. M. Sugai (Kyoto University) for critical reading of our manuscript; and Mr. K. Koga (Carl Zeiss) for technical support of our microscopic observations. This work was partially supported by “Academic Frontier” Project for Private Universities: matching fund subsidy from Ministry of Education, Culture, Sports and Technology 2003-2007 (to T.T.).
Author contributions: K.M. and T.T. designed research; R.-i.F., A.H., A.U., Y.N., R.F., and K.I. performed research; A.U., K.T., M.S., and M.H. contributed new reagents/analytic tools; K.I., K.O., K.M., and T.T. analyzed data; and T.T. wrote the paper.
Abbreviations: ATLL, adult T cell leukemia/lymphoma; HTLV-I, human T cell leukemia virus type I; AILIM, activation-inducible lymphocyte immunomediatory molecule; ICOS, inducible costimulator; PI3-kinase; phosphatidylinositol-3-kinase; PIP3; phosphatidylinositol-3,4,5-trisphosphate.
References
- 1.Uchiyama, T., Yodoi, J., Sagawa, K., Takatsuki, K. & Uchino, H. (1977) Blood 50, 481-492. [PubMed] [Google Scholar]
- 2.Yoshida, M., Miyoshi, I. & Hinuma, Y. (1982) Proc. Natl. Acad. Sci. USA 79, 2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okamoto, T., Ohno, Y., Tsugane, S., Watanabe, S., Shimoyama, M., Tajima, K., Miwa, M. & Shimotohno, K. (1989) Jpn. J. Cancer Res. 80, 191-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasunaga, J. & Matsuoka, M. (2003) Int. J. Hematol. 78, 312-320. [DOI] [PubMed] [Google Scholar]
- 5.Tajima, K. (1990) Int. J. Cancer 45, 237-243. [DOI] [PubMed] [Google Scholar]
- 6.Takatsuki, K., Ymaguchi, K. & Matsuoka, M. (1994) in Adult T-Cell Leukaemia, ed. Takatsuki, K. (Oxford Univ. Press, New York), pp. 1-27.
- 7.Shimoyama, M. (1991) Br. J. Haematol. 79, 428-437. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi, M., Jaffe, E. S. & Ralfkiaer, E. (2001) in Pathology and Genetics of Tumors of Haemopoietic and Lymphoid Tissues, eds. Jaffe, E. S., Harris, N. L., Stein, H. & Vardiman, J. W. (IARC Press, Lyon), pp. 200-203.
- 9.Shimoyama, M., Minato, K., Tobinai, K., Nagai, M., Setoya, T., Takenaka, T., Ishihara, K., Watanabe, S., Hoshino, H., Miwa, M., et al. (1983) Jpn. J. Clin. Oncol. 13, 165-187. [PubMed] [Google Scholar]
- 10.von Andrian, U. H. & Mackay, C. R. (2000) N. Engl. J. Med. 343, 1020-1034. [DOI] [PubMed] [Google Scholar]
- 11.Chambers, C. A. & Allison, J. P. (1999) Curr. Opin. Cell Biol. 11, 203-210. [DOI] [PubMed] [Google Scholar]
- 12.Hutloff, A., Dittrich, A. M., Beier, K. C., Eljaschewitsch, B., Kraft, R., Anagnostopoulos, I. & Kroczek, R. A. (1999) Nature 397, 263-266. [DOI] [PubMed] [Google Scholar]
- 13.Tezuka, K., Tsuji, T., Hirano, D., Tamatani, T., Sakamaki, K., Kobayashi, Y. & Kamada, M. (2000) Biochem. Biophys. Res. Commun. 276, 335-345. [DOI] [PubMed] [Google Scholar]
- 14.Rudd, C. E. & Schneider, H. (2003) Nat. Rev. Immunol. 3, 544-556. [DOI] [PubMed] [Google Scholar]
- 15.Liang, L. & Sha, W. C. (2002) Curr. Opin. Immunol. 14, 384-390. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto, N., Nukada, Y., Tezuka, K., Ohashi, K., Mizuno, K. & Tsuji, T. (2004) Int. Immunol. 16, 1515-1522. [DOI] [PubMed] [Google Scholar]
- 17.Koyasu, S. (2003) Nat. Immunol. 4, 313-319. [DOI] [PubMed] [Google Scholar]
- 18.Rickert, P., Weiner, O. D., Wang, F., Bourne, H. R. & Servant, G. (2000) Trends Cell Biol. 10, 466-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu, J., Wang, F., van Keymeulen, A., Herzmark, P., Straight, S., Kelly, K., Takuwa, Y., Sugimoto, N., Mitchison, T. & Bourne, H. R. (2003) Cell 114, 201-214. [DOI] [PubMed] [Google Scholar]
- 20.Chung, C. Y., Potikyan, G. & Firtel, R.A. (2001) Mol. Cell 7, 937-947. [DOI] [PubMed] [Google Scholar]
- 21.Waite, K. A. & Eng, C. (2002) Am. J. Hum. Genome 70, 829-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goberdhan, D. C. I. & Wilson, C. (2003) Hum. Mol. Genet. 12, 239-248. [DOI] [PubMed] [Google Scholar]
- 23.Kishimoto, H., Hamada, K., Saunders, M., Backman, S., Sasaki, T., Nakano, T., Mak, T. W. & Suzuki, A. (2003) Cell Struct. Funct. 28, 11-21. [DOI] [PubMed] [Google Scholar]
- 24.Rohrschneider, L. R., Fuller, J. F., Wolf, I., Liu, Y. & Lucas, D. M. (2000) Genes Dev. 14, 505-520. [PubMed] [Google Scholar]
- 25.Freeburn, R. W., Wright, K. L., Burgess, S. J., Astoul, E., Cantrell, D. A. & Ward, S. G. (2002) J. Immunol. 169, 5441-5450. [DOI] [PubMed] [Google Scholar]
- 26.Horn, S., Endl, E., Fehse, B., Weck, M. M., Mayr, G. W. & Jucker, M. (2004) Leukemia 18, 1839-1849. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto, N., Tezuka, K., Kato, M., Abe, R. & Tsuji, T. (2003) Biochem. Biophys. Res. Commun. 310, 691-702. [DOI] [PubMed] [Google Scholar]
- 28.Hara, K., Yonezawa, K., Sakaue, H., Ando, A., Kotani, K., Kitamura, T., Kitamura, Y., Ueda, H., Stephens, L., Jackson, T. R., et al. (1994) Proc. Natl. Acad. Sci. USA 91, 7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu, Z., Stokoe, D., Kane, L. P. & Weiss, A. (2002) Cell Growth Differ. 13, 285-296. [PubMed] [Google Scholar]
- 30.Suzuki, A., Yamaguchi, M. T., Ohteki, T., Sasaki, T., Kaisho, T., Kimura, Y., Yoshida, R., Wakeham, A., Higuchi, T., Fukumoto, M., et al. (2001) Immunity 14, 523-534. [DOI] [PubMed] [Google Scholar]
- 31.Borlado, L. R., Redondo, C., Alvarez, B., Jimenez, C., Criado, L. M., Flores, J., Marcos, M. A., Martinez-A, C, Balomenos, D. & Carrera, A. C. (2000) FASEB J. 14, 895-903. [DOI] [PubMed] [Google Scholar]
- 32.Rathmell, J. C., Elstrom, R. L., Cinalli, R. M. & Thompson, C. B. (2003) Eur. J. Immunol. 33, 2223-2232. [DOI] [PubMed] [Google Scholar]
- 33.Shimoyama, M., Kagami, Y., Shimotohno, K., Miwa, M., Minato, K., Tobinai, K., Suemasu, K. & Sugimura, T. (1986) Proc. Natl. Acad. Sci. USA 83, 4524-4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimoyama, M., Minato, K., Tobinai, K., Nagai, M., Setoya, T., Watanabe, S., Hoshino, H., Miwa, M., Nagoshi, H., Ichiki, N., et al. (1983) Jpn. J. Clin. Oncol. 13, 245-256. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.