Abstract
Background
Traditional medicinal plants are central to healthcare, nutrition, and cultural practices in rural Ethiopia, yet ethnobotanical knowledge is underdocumented and increasingly threatened. This study aimed to document medicinal plant diversity, usage, preference, and conservation status in Menz Keya Gebreal District, North Shewa Zone, to inform sustainable management and pharmacological research.
Methods
Data were collected from 80 informants using semi-structured interviews, guided field walks, focus group discussions, and field observations. Quantitative ethnobotanical analyses included Informant Consensus Factor (ICF), Fidelity Level (FL), Relative Frequency of Citation (RFC), Relative Popularity Level (RPL), Rank Order Priority (ROP), Cultural Value Index (CVI), paired and preference ranking, and direct matrix ranking. Similarity with other Ethiopian districts was assessed using Jaccard’s and Rahman’s indices. Statistical analyses, including t-tests, ANOVA, correlation, and regression, were conducted using R to evaluate variation in knowledge across demographic groups.
Results
A total of 121 medicinal plant species from 61 families were documented, with Asteraceae, Fabaceae, and Euphorbiaceae being the most represented. Leaves were the most frequently used plant part, and oral administration was the predominant route of remedy preparation. High ICF values were observed for skin (0.87) and digestive disorders (0.82). Hagenia abyssinica (Bruce) J.F.Gmel., Ocimum lamiifolium Hochst. ex Benth., and Echinops kebericho Mesfin exhibited high FL, RFC, and ROP values, while Clutia abyssinica Jaub. & Spach and Euphorbia abyssinica J.F.Gmel.were prioritized for hepatitis treatment. Major threats to medicinal plants included agricultural expansion, overharvesting, and firewood collection. Ethnobotanical knowledge varied significantly by informant groups (P < 0.05). RSI and JSI revealed both shared and unique knowledge patterns across regions. Knowledge transfer occurred primarily within families, while sacred groves, home gardens, and cultural practices contributed to in situ conservation.
Conclusion
Menz Keya Gebreal District harbors rich medicinal plant diversity and traditional knowledge, but anthropogenic pressures threaten their persistence. Integrating community-based conservation, sustainable harvesting, pharmacological validation, and youth-focused knowledge preservation is essential to safeguard this ethnobotanical heritage.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-025-05158-5.
Keywords: Conservation, Ethiopia, Ethnobotany, Medicinal plants, Menz keya gebreal, Traditional knowledge
Background
Traditional medicinal knowledge remains central to primary healthcare in many parts of the world, particularly in rural and underserved areas where biomedical infrastructure is limited [1–3]. Ethnobotany the study of human–plant relationships within cultural and ecological contexts offers critical insights into healing practices, biodiversity use, and cultural heritage [4–6]. Recognizing its importance, the World Health Organization has emphasized the integration of traditional medicine into national health systems [7].
Ethiopia, home to over 6,000 higher plant species across diverse agro-ecological zones, is one of Africa’s richest centers of ethnomedicinal practice [8, 9]. In remote districts such as Menz Keya Gebreal (North Shewa Zone), traditional medicine remains a primary healthcare strategy, relying on species such as Ocimum lamiifolium Hochst. ex Benth., Ruta chalepensis L., Croton macrostachyus Hochst. ex Delile, and Hagenia abyssinica (Bruce) J.F. Gmel. However, this orally transmitted knowledge is increasingly threatened by deforestation, agricultural expansion, climate variability, sociocultural change, and weakening intergenerational transfer [10–14]. As in other biodiversity-rich countries, the erosion of ethnobotanical knowledge coincides with global shifts toward integrating traditional medicine into sustainable healthcare frameworks [15–18]. Growing reliance on biomedical care and formal education further disrupts oral traditions, diminishing interest among younger generations [19, 20].
While countries such as India, Brazil, and China have established policies integrating traditional medicine and promoting key species like Withania somnifera (L.) Dunal and Ocimum sanctum L [5, 21]., Ethiopia’s institutional frameworks remain underdeveloped [22, 23]. Local initiatives, including community medicinal gardens and sustainable harvesting projects, exist [24, 25], but coordinated policy development and research investment are urgently needed to ensure long-term sustainability. Documenting indigenous knowledge held by healers and elder informants supports intergenerational transmission, strengthens community resilience, and contributes to holistic public health strategies [26–28].
Despite Ethiopia’s rich cultural and ecological diversity, systematic ethnobotanical studies remain fragmented, and knowledge from many districts including Menz Keya Gebreal remains undocumented. This gap threatens not only cultural heritage but also biodiversity conservation and the potential for novel pharmacological discoveries.
Accordingly, this study aims to: (i) document medicinal plant species and their therapeutic applications in Menz Keya Gebreal; (ii) analyze variations in knowledge across informant groups using the Botanical Ethnoknowledge Index (BEI); (iii) identify common ailments and their remedies; (iv) compare findings with prior Ethiopian and regional studies; and (v) highlight culturally significant species through quantitative ethnobotanical measures. We hypothesize that demographic factors particularly age, gender, and education influence ethnobotanical knowledge, with older, male, and less formally educated individuals retaining deeper knowledge than their younger or more educated counterparts. We further anticipate signs of knowledge decline among youth due to modernization and lack of documentation, while conservation awareness and sustainable practices are more prevalent among elders and traditional healers.
Addressing these objectives is significant for supporting intergenerational knowledge transfer, informing culturally appropriate health strategies, and promoting biodiversity conservation. Situating this work within regional and global ethnobotanical initiatives also strengthens Ethiopia’s role in safeguarding traditional knowledge while aligning with international efforts to conserve medicinal plant resources and integrate ethnomedicine into sustainable healthcare systems.
Materials and methods
Description of the study area
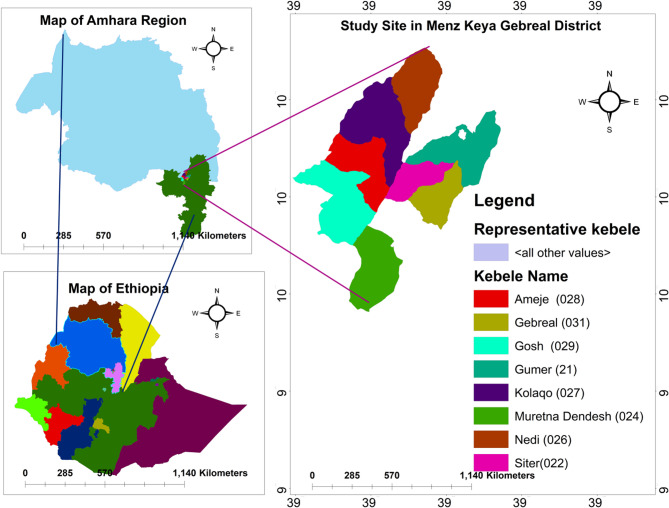
The study was conducted in Menz Keya Gebreal District, one of five administrative districts in the Menz region of Amhara Regional State, Ethiopia (Fig. 1). The district, part of North Shewa Zone, was reorganized in 1991 from the former Gera Midirna Keya Gebreal District. It covers about 595 km² and consists of 13 kebeles (12 rural, 1 urban), with Zemero as its administrative center. Geographically, it lies between 10°00′–10°21′N and 39°12′–39°30′E, at altitudes of 1,400–2,960 m a.s.l. The terrain is predominantly mountainous, with considerable climatic variability. It borders Jamma (South Wollo Zone) to the north, Merhabiete to the west, Menz Gera Midir to the east, and Menz Lalo Midir and Moretna Jiru to the south. The Jamma and Qechene Rivers, part of the Blue Nile watershed, form natural boundaries [29].
Fig. 1.
Map of the study area (Generated by ArcGis 10.4.1)
The district is culturally conservative and predominantly Ethiopian Orthodox Christian. According to the 2007 CSA census, its population was 46,219, of which 5.7% were urban residents. Agriculture is the main livelihood, practiced through mixed farming of crops and livestock. Agroecological zones range from lowland (kolla) to highland (dega), supporting crops such as teff (Eragrostis tef (Zuccagni) Trotter), barley (Hordeum vulgare L.), wheat (Triticum spp.), chickpeas (Cicer arietinum), and Niger seed (Guizotia abyssinica). Productivity is affected by seasonal variability, especially erratic Belg rains and frost at higher altitudes. Cash crops including coffee (Coffea Arabica L.), chat (Catha edulis Forsk), pumpkin (Cucurbita pepo L.), and Rhamnus prinoides (“Gesho”) are cultivated near irrigation sources, while cotton, fruits, and vegetables are grown in smaller areas, mostly for market sale. Livestock such as cattle, sheep, goats, donkeys, horses, and mules are central to the local economy, with sheep valued for meat and hides. Due to fuelwood scarcity, dried cow dung (kubet) is commonly used as household fuel [29].
Traditional medicine remains important, with healers (Ye-bahel medihanit awaki) treating conditions such as fractures and dislocations. Handicrafts including weaving, pottery, basketry, and metalwork supplement household income and are traded in local markets held on Saturdays, Mondays, and Tuesdays.
Infrastructure, however, is limited. Roads are underdeveloped though improving through regional projects. Healthcare services remain inadequate, with only four health centers serving the 13 kebeles. Common health problems include dyspepsia, pneumonia, helminthiasis, typhoid, scabies, injuries, hypertension, dental issues, and amebiasis, particularly in rural areas where modern healthcare is costly and inaccessible. A recently opened traditional medicine shop provides some additional support.
Social cohesion in the district is reinforced by strong religious institutions, customary governance, and local dialects. Dispute resolution is commonly facilitated by the Yegobez Aleqa (council of elders), reflecting the enduring significance of traditional institutions in community life.
Climate of the study area
Menz Keya Gebreal encompasses three agro-climatic zones shaped by elevation and temperature: highland (Dega), which dominates the district, followed by mid-altitude (Weyna Dega) and lowland (Kolla) areas. Much of the district lies within the cool, wind-exposed Shewa highlands [29]. Its topography is characterized by elevated plateaus, basalt cliffs, and deep river gorges, creating a rugged landscape that limits mobility and accessibility.
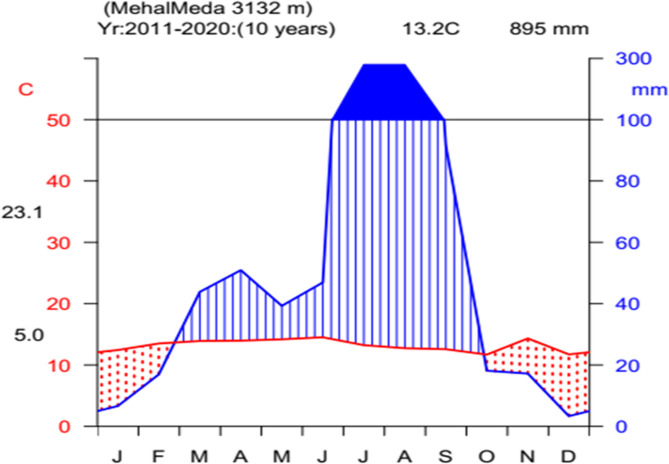
Rainfall follows a bimodal pattern. The main rainy season (Kiremt) extends from June to September, with peak precipitation in July and August. The short rainy season (Belg), occurring from March to April, is less reliable, particularly in mid- and low-altitude zones where onset is often delayed and rainfall insufficient. Meteorological data from Kombucha station (2011–2020) show a mean annual rainfall of 894.7 mm. August is the wettest month, averaging 280.2 mm, while December is the driest, with only 33.5 mm (Fig. 2).
Fig. 2.
Climadiagram of the study area. (source: NMSA, 2020)
Temperature patterns reflect the district’s high elevation. The mean monthly maximum is 21.4 °C, typically in June, while the lowest mean minimum of 2.5 °C occurs in December (Fig. 2). These climatic conditions directly influence agricultural productivity and water availability in the district.
Reconnaissance survey, study site and informant selection
This ethnobotanical study was conducted in Menz Keya Gebreal District, North Shewa Zone, Amhara Regional State, Ethiopia, to document and analyze traditional knowledge of medicinal plants. Both qualitative and quantitative methods were applied to capture data on plant use, knowledge variation among informants, cultural significance, and conservation concerns.
A reconnaissance survey was undertaken from September 10–17, 2021, to familiarize the research team with the district’s biophysical and socio-cultural context, identify suitable study sites, and establishes rapport with local stakeholders. During this phase, meetings were held with community elders, traditional healers, and local leaders to locate areas of intensive medicinal plant use and to assess biodiversity hotspots and resource accessibility. Data collection followed between October 8, 2021, and March 7, 2022.
Study sites were purposively selected, focusing on kebeles with rich vegetation and strong traditions of medicinal plant use. Prior studies, health professionals, elders, and traditional practitioners informed this process. Eight kebeles Amija, Gebreal, Gumer, Kolaqo, Mureyna Dendesh, Nedi, Siter, and Gosh were chosen, representing 61.5% of the district’s 13 kebeles (Table 1). These predominantly rural sites maintain strong oral traditions of ethnobotanical knowledge.
Table 1.
Sampled study sites: altitude, coordinates, agro-ecology, number of households, and sociodemographic characteristics of informants
| Name of study sites | Altitude | GPS Coordinates | Gender | Ethnicity | Age categories | Language | Occupation | NH | AE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Latitude (N, S) | Longitude (E, W) | M | F | 20–30 | 31–50 | 51–85 | |||||||
| Amija | 2684 m | 10°12’10"N | 39°19’19"E | 7 | 3 | Am | 2 | 4 | 4 | Amc | Far, Mer, HW, Stu, Tch | 684 | Highland |
| Gebreal | 2712 m | 10°09’00"N | 39°24’22"E | 6 | 4 | Am | 2 | 3 | 5 | Amc | Far, Mer, HW, Stu, Tch | 763 | Highland |
| Gumer | 2770 m | 10°12’41"N | 39°25’46"E | 7 | 3 | Am | 2 | 4 | 4 | Amc | Far, Mer, HW, Stu, Tch | 698 | Highland |
| Kolaqo | 2095 m | 10°16’46"N | 39°21’02"E | 6 | 4 | Am | 2 | 3 | 5 | Amc | Far, Mer, HW, Stu, Tch | 682 | Highland |
| Mureyna dendesh | 2640 m | 10°04’40"N | 39°21’00"E | 8 | 2 | Am | 1 | 4 | 5 | Amc | Far, Mer, HW, Stu, Tch | 656 | Highland |
| Nedi | 2255 m | 10°17’11"N | 39°21’58"E | 6 | 4 | Am | 2 | 2 | 6 | Amc | Far, Mer, HW, Stu, Tch | 545 | Highland |
| Siter | 1915 m | 10°10’23"N | 39°21’55"E | 7 | 3 | Am | 2 | 3 | 5 | Amc | Far, Mer, HW, Stu, Tch | 453 | Mid-highland |
| Gosh | 1748 m | 10°07’15"N | 39°19’50"E | 6 | 4 | Am | 2 | 2 | 6 | Amc | Far, Mer, HW, Stu, Tch | 645 | Mid-highland |
| Total | 53 | 27 | 15 | 25 | 40 | 5126 | |||||||
Key: Am Amhara, Amc Amharic, M Male, F Female, AE Agro-ecology, NH Number of households, Far Farmer, Mer Merchant, HW House wife, Stu Student, Tch Teacher
Informant selection combined purposive and systematic random sampling. In total, 80 participants (53 males, 27 females) aged 20–85 were included, with 10 informants drawn from each kebele. Of these, 60 general informants were randomly selected, while 20 key informants primarily traditional healers were purposively chosen based on community recommendations. Informants were further grouped into three age categories: young adults (20–30 years), middle-aged (31–50 years), and elderly (51–85 years), to explore generational differences in knowledge transmission. Selection emphasized participants’ willingness, experience with medicinal plants, and recognition within the community. Criteria were refined in collaboration with local leaders to ensure the inclusion of individuals with authentic and valuable knowledge.
Ethnobotanical data collection
Ethnobotanical data were collected through semi-structured interviews, guided field walks, direct observation, and focus group discussions (FGDs) to document traditional knowledge on medicinal plants in Menz Keya Gebreal District. These methods provided complementary perspectives on plant identification, preparation, application, and conservation.
Semi-structured interviews
Interviews formed the primary data source and were conducted using a pre-tested checklist of 31 open-ended questions. Initially prepared in English and translated into Amharic, the checklist covered demographic details (age, sex, address) and ethnobotanical information, including vernacular plant names, treated ailments (human, animal, or both), plant parts used, sources of collection (wild or cultivated), abundance, preparation methods, duration of treatment, routes of administration, conservation practices, and seasonal availability. This approach allowed flexible probing of each informant’s experiences [30, 31]. Plant collection was authorized by landowners and the district administrative and agricultural offices, ensuring compliance with ethical guidelines.
Guided field walks and observations
Field visits with local guides and key informants enabled in situ observation of medicinal plants. Transect walks were used to verify species cited during interviews and to document growth habits, ecological distribution, abundance, harvested parts, preparation methods, therapeutic applications, and indigenous conservation measures [32, 33].
Focus group discussions
FGDs were organized in each study kebele with 5–8 participants, including traditional healers, elders, religious leaders, and local administrators. Discussions addressed preparation techniques, dosage forms, additive ingredients, side effects, administration routes, and systems of knowledge transmission. A structured checklist, translated into Amharic, guided the sessions. These group interactions enriched the dataset by validating individual reports, capturing community perspectives, and highlighting consensus or divergence in knowledge [34].
Voucher specimen collection, identification, and herbarium Preparation
Medicinal plant specimens were collected from both wild and cultivated habitats with the assistance of local herbalists and agricultural experts. Two specimens per species were taken, each labeled with a collection number and collector’s name. Specimens were pressed between blotting papers, oriented to capture all morphological features, and air-dried under sunlight, with regular inspections to prevent insect damage [30].
Preliminary identification was carried out at the Mini Herbarium, Debre Berhan University, and further taxonomic verification was performed using the Flora of Ethiopia and Eritrea and standard taxonomic keys. Final confirmation was conducted at the National Herbarium (ETH), Addis Ababa University, through comparison with authenticated herbarium specimens and published descriptions [31, 35]. Additional references included field guides on Ethiopian trees and shrubs as well as multiple online taxonomic databases, such as Plants of the World Online (Kew), PlantNet, Flora Finder, USDA Plants Database, African Plant Database, the World Checklist of Selected Plant Families, and JSTOR Global Plants for authoritative nomenclature verification [36].
The formal identification of plant material was carried out by Dr. Abiyou Tilahun. Voucher specimens of all documented species were deposited in the Mini Herbarium, Department of Biology, Debre Berhan University, under the deposition number DBU002023, for future reference and taxonomic research.
Quantitative ethnobotanical analysis
To assess the reliability, depth, and community consensus surrounding traditional medicinal plant knowledge, a range of established quantitative ethnobotanical indices were applied. These included the Botanical Ethnoknowledge Index (BEI), Relative Frequency of Citation (RFC), Relative Popularity Level (RPL), Rank-Order Priority (ROP), Fidelity Level (FL), Cultural Value Index, and Informant Consensus Factor (ICF). Additionally, participatory ranking techniques such as preference ranking, direct matrix ranking, and paired comparison were employed to capture local perceptions and prioritization of plant species. Similarity analyses were also conducted using Rahman’s Similarity Index (RSI) and the Jaccard Similarity Index to compare ethnobotanical patterns within and across study sites.
Botanical ethnoknowledge index (BEI)
The Botanical Ethnoknowledge Index (BEI) is designed to quantify the complex interplay of factors influencing ethnobotanical knowledge within a specific group and enables comparative analysis across groups sharing similar ecological and floristic settings [37]. The index incorporates several key variables: the total number of plant species reported by all members of a group, the mean number of species reported per participant, the mean number of citations per species, the number of participants in the group, and the total number of species reported across all groups in the study. BEI values range from just above 0 to a maximum of 2. A higher BEI indicates a greater depth of ethnobotanical knowledge within the group. Although values between 1 and 2 are theoretically possible, they are relatively rare and typically reflect distinct or highly specialized knowledge compared to other groups.
The BEI is calculated using the following formula:
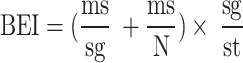 |
Where, BEI: Botanical Ethnoknowledge Index. ms: mean number of species reported per participant in a particular group. Sg: total number of species reported by all participants in a particular group. mc: mean number of citations per species in a particular group. N: number of participants in the particular group. St: total number of species reported by all compared groups in the study. The above-presented index is designed to compare groups with similar sample sizes [37]. However, in order to overcome this limitation when comparing groups with diferent sample sizes, the index value can be relatively corrected by multiplying it with a coefcient as follows:
 |
Where, F: correcting factor. N mean: mean number of participants among all compared groups. √N min: square root of the number of participants in the smallest group.
Calculation of the relative frequency of citation (RFC)
The relative frequency of citation (RFC) was calculated for each species to highlight its local importance. RFC was given by the following formula [38]:
 |
Where; FC = the number of informants citing the use of the species and N = the total number of respondents participating in the survey.
Relative popularity level (RPL)
The Relative Popularity Level (RPL) is a metric used in ethnobotanical studies to evaluate how commonly a medicinal plant is known or used among informants. It helps distinguish between popular and less popular medicinal species [38].
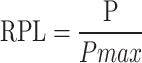 |
Where: P = Number of informants who cited the plant species for medicinal use, Pmax= Number of informants who cited the most popular plant species (i.e., the highest frequency among all species). The Relative Popularity Level (RPL) value ranges between 0 and 1 and provides a comparative measure of a plant species cultural popularity among informants. An RPL value of 1 indicates the most popular plant, cited by the highest number of informants. In contrast, values less than 1 represent less popular species, cited by fewer individuals. This index is useful for distinguishing widely recognized medicinal plants from those with more limited traditional use.
Fidelity level (FL)
The Fidelity Level (FL) quantifies the percentage of informants who consistently report the use of a specific plant for treating a particular ailment. It reflects the degree of specificity and reliability associated with the therapeutic application of a given species. A higher FL value indicates greater agreement among informants regarding a plant’s use, suggesting its cultural and medicinal prominence within the community [32]. The FL was calculated using the formula:
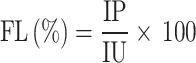 |
Where: FL = Fidelity Level (expressed as a percentage), Ip = Number of informants who independently cited a species for a specific ailment, Iu = Total number of informants who mentioned the species for any use.
This index enabled the identification of species with high therapeutic specificity and potential pharmacological interest.
Calculation of the rank-order priority (ROP)
The Rank-Order Priority (ROP) is an ethnobotanical index that combines both the Relative Popularity Level (RPL) and Fidelity Level (FL) to help prioritize medicinal plants for further pharmacological investigation [38].
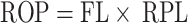 |
Cultural value index (CVI)
The Cultural Value Index (CVI) is an ethnobotanical measure used to assess the cultural significance of a plant species based on its use diversity, frequency of citation, and the number of use categories [39]. The cultural value index was calculated using the formula:
 |
Where: FC = Number of informants citing the species, Ni = Total number of informants, Nu = Number of use reports for the species, Nc = Total number of use categories.
Informant consensus factor (ICF)
The Informant Consensus Factor (ICF) was used to evaluate the level of agreement among informants regarding the use of medicinal plants for specific categories of ailments. A high ICF value implies that a few species are widely used to treat a particular condition, which may indicate both the cultural salience and potential effectiveness of those plants [33, 40]. In contrast, a low ICF suggests a lack of consensus and potentially random or individualized plant usage. The ICF was computed using the following formula:
 |
Where: Nur = Number of use-reports for a particular illness category, Nt = Number of species used for that illness category. Values range from 0 to 1, with values closer to 1 denoting a higher consensus among informants.
Preference ranking
Preference ranking was utilized to determine the relative importance and perceived efficacy of selected medicinal plant species in treating specific ailments. Following the approach of [33], ten key informants were asked to rank five medicinal plants based on their effectiveness against intestinal parasites. Rankings ranged from 1 (least effective) to 5 (most effective). The cumulative scores were then used to determine the overall ranking of each species, highlighting those considered most efficacious by the local community [34]. This method provided crucial insights into community priorities and treatment preferences.
Direct matrix ranking
To assess the multipurpose utility of medicinal plant species beyond their therapeutic applications, direct matrix ranking was conducted. Five frequently cited tree species were evaluated by ten key informants across seven use categories: medicinal value, construction material, firewood, charcoal production, furniture, agricultural tools, and food. Informants assigned scores from 1 (least important) to 5 (most important) for each use category. The cumulative scores helped determine which species were most valued for their multifunctional roles, guiding conservation strategies accordingly [33, 34]. This approach also highlighted the broader socio-economic contributions of medicinal plants within rural livelihoods.
Paired comparison
To explore the relative preference of informants regarding specific medicinal plant species or uses, paired comparison analysis was employed. All possible pairs of selected items were generated using the formula:
 |
Where n is the number of items being compared and each informant was asked to select the more preferred item from each pair. The number of times each item was chosen was tallied to determine its overall ranking [34]. This method facilitated a nuanced understanding of community values and priorities concerning medicinal plant use. Together, these quantitative techniques provided robust insights into the local ethnopharmacological landscape, guiding both research prioritization and sustainable resource management.
Jaccard similarity index
To evaluate the degree of overlap in medicinal plant knowledge between different groups or regions, the Jaccard Similarity Index (JSI) was applied. The JSI quantifies the proportion of shared species between two datasets and is especially useful in comparing knowledge among traditional healers, elders, and general community members, or between distinct geographic regions [2, 41]. The index was computed using the following formula:
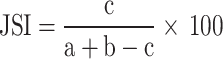 |
Where: JSI = Jaccard Similarity Index (as a percentage), a = Number of species recorded in the comparison area, b = Number of species recorded in the current study area, c = Number of species common to both areas. The JSI ranges from 0 to 100%, where 0% indicates no similarity and 100% indicates complete similarity. This approach provides insights into how traditional medicinal knowledge is shared or differs across communities and environments.
Rahman’s similarity index (RSI)
Rahman’s Similarity Index (RSI) was employed to assess the cultural congruence of medicinal plant usage across different study areas. Unlike JSI, RSI emphasizes not just shared species but also the similarity in their therapeutic applications, offering a more nuanced view of ethnobotanical overlap [41]. The RSI was calculated as follows:
 |
Where: Na = Number of unique species in area A (previous study area), Nb = Number of unique species in area B (current study area), Nc = Number of species common to both areas, Nd = Number of species used for the same ailments in both areas. This index ranges from 0% to 100%, with higher values indicating greater cultural similarity in medicinal plant knowledge and usage between study areas.
Data analysis
A mixed-methods approach integrating both qualitative and quantitative analyses was employed to interpret the ethnobotanical data. Qualitative information obtained through semi-structured interviews and field observations was analyzed thematically to identify patterns in plant use and cultural perceptions. Quantitative data were processed using descriptive statistics (mean,) and ethnobotanical indices, notably the Botanical Ethnoknowledge Index (BEI). Statistical analyses were performed using R software (version 4.4.3). The Shapiro–Wilk test assessed data normality, while analysis of variance (ANOVA) evaluated differences among age groups. Pearson correlation and linear regression were used to explore the relationship between age and the number of medicinal plants reported [20, 21]. This integrated analytical framework facilitated a comprehensive understanding of traditional plant knowledge, its demographic drivers, and regional patterns.
Ethical considerations
This research was conducted in accordance with established ethical guidelines for ethnobotanical studies. Prior informed consent was obtained from all participants after explaining the study’s objectives, methods, and voluntary nature. Confidentiality was maintained throughout, and personal identifiers were omitted from the dataset. Ethical approval was granted by the appropriate local authorities and community leaders. The research team ensured cultural sensitivity, particularly when documenting knowledge about sacred or ritually significant plants. The study adhered to international ethical frameworks, including the Code of Ethics of the International Society of Ethnobiology (ISE), to protect indigenous knowledge and promote its sustainable and respectful use.
Results and discussion
Sociodemographic attributes of informants
A total of 80 informants participated in this study, comprising 53 males (66.2%) and 27 females (33.8%). General community members accounted for 75% (n = 60), while 25% (n = 20) were key informants recognized for their specialized ethnomedicinal knowledge and long-standing experience. Participants ranged in age from 20 to 85 years, with half (50%, n = 40) belonging to the 51–85 age group, followed by 31.3% (n = 25) in the 31–50 group. Educationally, 73.7% (n = 59) were illiterate and 26.3% (n = 21) had some formal education (Table 1).
These sociodemographic patterns mirror findings from other Ethiopian ethnobotanical studies, where knowledge is predominantly concentrated among older, often illiterate men who serve as primary custodians through oral transmission and experiential learning [2, 20, 26, 42]. Similar trends have been observed in Kenya and Nigeria, where elder men often retain the most comprehensive ethnomedicinal knowledge due to their roles as community leaders and healers [39, 43].
The lower representation of women reflects cultural norms that limit their participation in formal knowledge sharing, despite their central role in home-based healthcare and informal healing practices [16, 17, 22]. Comparable gender dynamics are reported in South Asia and Latin America, where women hold significant medicinal plant knowledge that is less visible in formal ethnobotanical research [44, 45].
The predominance of elderly informants with limited formal education highlights the oral, undocumented nature of indigenous knowledge, typically transmitted through storytelling, apprenticeship, and family networks [2, 10]. This reliance on oral transmission makes the knowledge vulnerable to erosion amid sociocultural change. Consequently, systematic documentation is critical. Ethiopian studies recommend incorporating visual and oral methods into ethnomedicine education and conservation initiatives to overcome literacy barriers [20, 21, 46], while international research emphasizes gender-inclusive approaches and youth engagement to strengthen intergenerational transfer and sustain traditional practices [12, 13].
Cultural naming of medicinal plants in the study area
In Menz Keya Gebreal District, the naming of medicinal plants reflects deep cultural connections, often based on therapeutic properties, morphological traits, or social significance. All documented species had local names derived from indigenous languages, frequently describing a plant’s function or distinctive characteristics such as stem color, leaf shape, aroma, or taste. For instance, Becium polystachya is called Nech Anfar (“white stem”) for its pale stalk, Rosmarinus officinalis is Siga Metibesha, referencing its culinary use as a meat seasoning, and Agave sisalana is Yeset Qest, linked to Ensirt, a traditional cotton-processing tool, highlighting its cultural importance. Other examples include Rumex nepalensis (Yewusha Milas, “dog’s tongue”) for its leaf shape and Ruta chalepensis (Tena Adam, “Adam’s health”) for its general healing properties.
These findings align with research from Ethiopian regions such as Kaffa, Sheka, and Gamo-Gofa, where plant names similarly encode functional, morphological, and symbolic information, reflecting shared ethno-linguistic patterns and cultural heritage [20, 36, 47, 48]. Comparable practices are reported across Africa, China, India, and Latin America, where vernacular names serve as repositories of indigenous knowledge and cultural identity [1, 3, 43, 44]. Locally embedded names function not only as taxonomic labels but also as mnemonic devices, teaching tools, and central elements of oral knowledge transmission in largely unwritten ethnomedicinal traditions [47–49]. These names often endure beyond precise botanical identification, helping preserve ethnobotanical knowledge despite environmental and cultural changes.
However, assigning identical or similar names to different species with overlapping uses can lead to confusion or misapplication of remedies, an issue documented in Ethiopian districts such as Metema and Quara, as well as in traditional communities worldwide [2, 3, 6, 50].
The cultural naming system has important implications. First, vernacular names constitute vital intangible cultural heritage that should be safeguarded alongside medicinal plant conservation. Documenting both scientific and local names supports cultural continuity, educational initiatives, and cross-disciplinary research. Second, these names provide valuable leads for pharmacological studies by highlighting species with notable therapeutic roles based on traditional classification and use [2, 6, 23]. Finally, integrating cultural naming traditions into conservation policies, community healthcare planning, and sustainable development strategies can strengthen respect for indigenous knowledge systems, promote biodiversity preservation, and enhance the role of traditional medicine in rural livelihoods [24, 25, 34].
Diversity and distribution of medicinal plants in Menz Keya Gebreal District
A total of 121 medicinal plant species were documented in Menz Keya Gebreal District, representing 105 genera and 61 families. This richness reflects substantial phytomedicinal diversity and underscores the district’s exceptional ethnobotanical heritage. Compared with nearby Ethiopian districts reporting 73–85 species [8, 42, 51], the diversity in Menz Keya Gebreal is relatively high. Globally, documented medicinal plant diversity varies, ranging from 42 to 55 species in Tanzania and China [50, 52] to 122–168 species in other Ethiopian regions [3, 20, 22], reflecting differences in biodiversity, ecological conditions, and cultural ethnopharmacological practices.
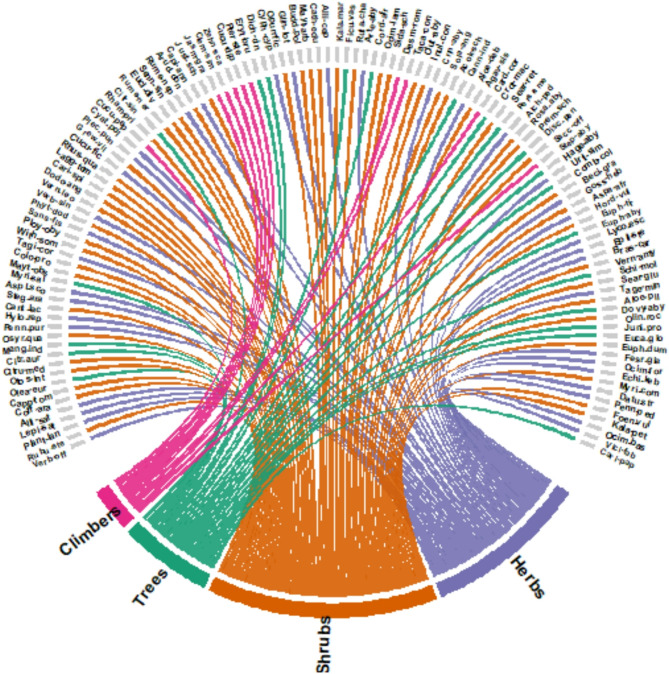
Among plant families, Asteraceae, Euphorbiaceae, and Fabaceae were the most represented, each with seven species (5.8%), followed by Poaceae and Solanaceae with six species each (4.9%), and Anacardiaceae with five species (4.1%) (Table 2). The prominence of Asteraceae aligns with other Ethiopian and global ethnobotanical studies, where Asteraceae and Fabaceae are widely cited for their ecological adaptability and pharmacological importance [6, 21, 53, 54]. These families are commonly used for anti-inflammatory, antimicrobial, and gastrointestinal remedies, reflecting both local therapeutic priorities and sophisticated botanical knowledge.
Table 2.
Lists of medicinal plants used to treat different ailments in the study area
| Scientific Name | Short code | Family | Local Name | GF | HB | PU | CP | Disease treated | Mode of preparation | RA | VN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acokanthera schimperi (A.DC.) Schweinf. | Acok-sch | Apocynaceae | Mirez | Sh | W | Rt | D | Ayne wog for animal | The roots of Machid Seber, Shenbeko, Chifirg, Jibra Kestencha, Gumaro, Gizewa, Yemidir Embuay, Ameraro, Koshim, Mirez, Zert Embuay, and Kitkta are pounded and then burned. As the animal inhales the smoke, the harmful spirit is believed to be driven away. | Nasal | MY075 |
| Agave sisalana Perrine | Agav-sis | Asparagaceae | Sete Qacha | Sh | W | Lf | F | Kimajir | The leaf-like structures of Qacha are crushed, pounded, and soaked in a small amount of water. This liquid is then used to wash the infected area of the animal. | Dermal | MY078 |
| Alchemilla pedata Hochst. ex A.Rich. | Alch-ped | Rosaceae | yemidir koso | H | W | Wp | F | Ymirech | All parts are pounded, soaked for three days, and the resulting liquid is sprinkled onto the infected areas of the baby. | Dermal | MY083 |
| Allium cepa L. | Alli-cep | Amaryllidaceae | Key Shinkurt | H | HG/MK | Bu | F | Hypertension | The harvested fruit of Key Shinkurt is eaten together with fresh meat. | Oral | MY060 |
| Allium sativum L. | Alli-sat | Amaryllidaceae | Nech shinkurt | H | HG/MK | Bu | D | Eczema | The fruit is first crushed and dried. Once fully dried, it is ground into a fine powder. This powder is then mixed with butter and applied directly to the affected area. | Dermal | MY005 |
| Eye disease | The fruit of nech shinkurt, the leaf of dedho, and cotton fruit are pounded together. The mixture is then squeezed to extract the juice, which is carefully applied to the affected area of the eye. | Nasal | |||||||||
| Aloe debrana Christian | Aloe-deb | Asphodelaceae | Wonde ret | H | W | Lf | F | Athlete foot | Heat the inner moist parts and apply them to the infected areas. | Dermal | MY077 |
| Rt | F | Venom of scorpion | When a person is bitten by a scorpion, they eat the fruit as a remedy. | Oral | |||||||
| Aloe pulcherrima M.G.Gilbert & Sebsebe | Aloe-pil | Asphodelaceae | Sete Eret | Sh | W | Lf | F | Cancroid | The residue is mixed with suet and applied directly to the affected areas. | Dermal | MY105 |
| Rt | D | Evil eye | The roots of Etse Sabe’e, Gizewa, Kestenicha, Yemidir Embuay, Zert Embuay, Jibira, Ahiya Joro Agam, and Sete Ret are pounded together. The dried mixture is then ground into powder and used as incense smoke for a person believed to be afflicted by the evil eye spirit. | Dermal | |||||||
| Artemisia abyssinica Sch.Bip. ex Oliv. & Hiern | Arte-aby | Asteraceae | Chiqugn | H | W/MK | Wp | F/D | Evil sprit | The whole plants of Chiqugn, Tiena Adam, and Nech Shinkutr are crushed together, and the mixture is then inhaled. | Nasal | MY064 |
| Arundo donax L. | Arud-don | Poaceae | Shenbeko | Sh | HG/W | Rt | D | Evil eye | The moist roots of Shenbeko Kentefa, Wuyign, Gumaro, Dediho, and Gizewa are cut into very small pieces and dried. These are then mixed with the leaves of Keskeso and the whole parts of Chikugn. Finally, the dried mixture is powdered and smoked over a fire. | Nasal | MY044 |
| St | F/D | Bone fracture | The stem of Arundo donax is crushed into equal-sized pieces and then used to wrap the broken bone. This is kept in place for three days and the process is repeated until the bone heals and connects properly. | Dermal | |||||||
| Asparagus africanus Lam. | Aspa-afr | Asparagaceae | Kestenicha | Sh | W | Lf | F | Herpes | The leaves are finely chopped and pounded, then left to macerate for three days. Finally, the preparation is either applied as a cream to the affected area or consumed as a drink until recovery. | Dermal/Oral | MY094 |
| Rt | D | Evil eye | The roots of Etse Sabe’e, Gizewa, Kestenicha, Yemidir Embuay, Zert Embuay, Jibira, Ahiya Joro Agam, and Sete Ret are pounded together. After drying, the mixture is ground into a powder and smoked for a person believed to be afflicted by an evil eye spirit. | Nasal | |||||||
| Asplenium aethiopicum (Burm.f.) Bech. | Aspl-aet | Aspleniaceae | Goliba | H | W | Rt | F | Diarrhea | The leaves are pounded and crushed, then mixed with water and salt. This mixture is given to drink to those suffering from diarrhea. | Oral | MY018 |
| Becium grandiflorum (Lam.) Pic.Serm. | Beci-gra | Lamiaceae | Muatish | Sh | W | Rt | D | Abdominal pain | The root is powdered, mixed with water, and then filtered. Finally, a cup of the filtered liquid is taken every morning for five days. | Oral | MY092 |
| Brassica carinata A.Braun | Bras-car | Brassicaceae | Habesha Gomen | H | HG/MK | Lf | F | Fibril illness | It is cooked, and the water is then drunk to help recover from a febrile illness. | Oral | MY100 |
| Buddleja polystachya Fresen. | Budd-pol | Scrophulariaceae | Anfar | Sh | HG/W | Lf | F | Leech | The crushed leaves are mixed with water, and the resulting liquid is given to the infected animal to drink. | Oral | MY057 |
| Calotropis procera (Aiton) W.T.Aiton | Colo-pro | Apocynaceae | Kinbo | Sh | W | Lx | F | Kunchir | Apply cream to the infected areas. | Dermal | MY021 |
| Lx | F | Wound | The collected latex from Calotropis procera is applied as a cream on wounds until they heal. | Dermal | |||||||
| Canna indica L. | Cann-ind | Cannaceae | Koba | T | HG | Rt | F | Stomachache | The root is crushed and mixed with water, then drunk. | Oral | MY076 |
| Capparis tomentosa Lam. | Capp-tom | Capparaceae | Gumero | Sh | W | Rt | D | Evil eye | The fresh root is chopped into very small pieces and dried. Once fully dried, it is ground into a powder, which is then used for smoking. | Oral/Nasal | MY007 |
| Capsicum annuum L. | Capi-ann | Solanaceae | Berbere | H | HG/MK | Fr | F | Hepatitis | The fruits of Berbere and Nech Shinkurt, leaves of Tenbelel, and lemon juice are mixed and soaked together. After soaking, the mixture is filtered, and the person drinks one glass of the filtered medicine daily for three days. Finally, they consume one glass of honey. | Oral | MY045 |
| Cardiospermum corindum L. | Card-cor | Sapindaceae | Semeg | Cl | W | Lf | F | Leech | The leaves are pounded into a powder, which is then sniffed. | Nasal | MY079 |
| Lf | F | Abscess | The fresh leaves are chewed with milk, and then the resulting mixture is applied to the infected areas for six days. | Dermal | |||||||
| Rt | F/D | Infertility | The root of Semeg is pounded, then squeezed and filtered. The filtered liquid is consumed as a drink. | Oral | |||||||
| Wp | F | Abscess | The whole parts of Semeg, bark of Seghed, leaves and whole parts of Dediho, and leaves of Jib Mirkuz are mixed and massaged with milk, then applied to the body experiencing trembling and bitterness. The infected areas are then smeared with this mixture for six days. | Dermal | |||||||
| Carica papaya L. | Cari-pap | Caricaceae | Papaya | T | HG/MK | Fr | F | Gastric | The fruits of papaya, Qimmo, Woyira, the juice from Eret, and the leaves of Sunsel are mixed and ground together. The mixture is taken daily before meals. | Oral | MY121 |
| Lf | D | Malaria | The collected papaya leaves are crushed and dried, then boiled like tea. One cup is taken daily for seven days. | Oral | |||||||
| Carissa spinarum L. | Cari-spi | Apocynaceae | Agam | Sh | W | Lf | F | Snake bite | The leaves are finely cut into small pieces and pounded. The prepared mixture is then consumed until full recovery. | Oral | MY030 |
| Rt | D | Ayne Tila | The fresh root is cut into very small pieces and then dried. Once dried, it is ground into powder, which can be either consumed as a drink or smoked for treatment. | Oral | |||||||
| Rt | D | Evil eye | The roots of Etse Sabe’e, Gizewa, Kestenicha, Yemidir Embuay, Zert Embuay, Jibira, Ahiya Joro Agam, and Sete Ret are pounded together. After drying, the mixture is powdered and smoked to treat individuals believed to be affected by an evil eye spirit. | Nasal | |||||||
| Catha edulis (Vahl) Forssk. ex Endl. | Cath-edu | Celasteraceae | Kenbet(chat) | Sh | HG | Lf | F | Wound | The dried and powdered leaves of chat are combined with salt, lemon juice, and ladybird honey. First, the affected areas of the body are coated with white honey, then the prepared mixture is applied and gently massaged onto the infected parts until healing occurs. | Dermal |
MY/ 059 |
| Cenchrus purpureus (Schumach.) Morrone | Cenc-pur | Poaceae | Gosh mika | H | W | Rt | D | Kunchir | The root is pounded, dried, and ground into powder. Then, the powdered Goshmika is mixed with black teff dough and applied as a cream to the infected areas until recovery. | Dermal | MY014 |
| Citrus × aurantium L. | Citr-aur | Rutaceae | Lomi | T | W/HG | Lx | D | Kunchir | The fruit of yejib shinkurt, root of shimbrut, sindedo, goshmika, and the entire tenadam plant are pounded and dried, then ground into powder. Finally, this powder is applied by smearing it on the infected area. | Dermal | MY011 |
| Fr | Eye disease | The fruit of nech shinkurt, the leaf of dedho, and the fruit of cotton are pounded together, then squeezed to extract the juice. This pure liquid is finally applied to the infected parts of the eye. | Optical | ||||||||
| Citrus × sinensis (L.) Osbeck | Citr-sin | Rutaceae | Birtukan | T | W/HG | Fr | Gastric | Eat the fruit of birtucan directly before the daily meal. | Oral | MY039 | |
| Citrus medica L. | Citru-med | Rutaceae | Trngo | Sh | W/HG | Ba | F | Apetite | The bark of tiringo is eaten directly as a remedy. | Oral | MY010 |
| Fr | Liver | The fruit of the plant is eaten directly. | Oral | ||||||||
| Clematis simensis Fresen. | Clem-sim | Ranunculaceae | Azo Hareg | Cl | W | Rt | D | Wart | The dried root is ground into powder and mixed with barley batter. This mixture is then applied to all the infected areas. | Dermal | MY048 |
| Clutia abyssinica Jaub. & Spach | Clut-aby | Peraceae | Fiyele feji | Sh | W | Rt | D | Rabies | The roots of Ensilal, Fiyele Feji, Lutt, Woinagift, Gizewa, and Yemidir Enbuay are pounded together. After drying, the mixture is kneaded with teff flour and then formed into bread, which the sick person consumes. | Oral | MY071 |
| Lf | F | Jaundice | The collected leaves of Fiyelefeji are pounded and then squeezed with tella. The filtered liquid is then consumed as a drink. | Oral | |||||||
| Coffea arabica L. | Coff-ara | Rubiaceae | Buna | Sh | W/HG | Sd | D | Headache | Powdered coffee is steamed and then mixed with lemon before use. | Oral | MY006 |
| Sd | D | Diarrhea | To treat diarrhea, coffee (buna) is pounded and mixed with honey before consumption. | Oral | |||||||
| Combretum collinum Fresen. | Comb-col | Combretaceae | Abalo | T | W | Lf | D | Skin Rash | The leaves of Abalo and Kitikta are pounded and dried. Then, they are roasted and mashed before being kneaded into a paste, which is applied to the diseased areas of the body. | Dermal | MY091 |
| Cordia africana Lam. | Cord-afr | Boraginaceae | Wanza | T | HG | Fr | D | Wound | The fruits of Wanza and Injiry are pounded, dried, and ground into a powder. This powder is then sprinkled onto the infected areas. | Dermal | MY065 |
| Crinum abyssinicum Hochst. ex A.Rich. | Crin-aby | Amaryllidaceae | Yejib Shinkurt | H | W | Fr | D | Ayne Tila | The fresh root is cut into small pieces and dried. Once dry, the root is ground into powder, which is then smoked. | Nasal | MY073 |
| Croton macrostachyus Hochst. ex Delile | Crot-mac | Euphorbiaceae | Bisana | T | W | Ba | D | Eczema | The bark is crushed and dried. Once dried, it is further ground into a powder. Finally, the powdered bark is mixed with butter and applied to the infected area. | Dermal | MY080 |
| Lf | D | Wart | The dried leaves are ground into a powder and mixed with barley batter to form a paste. This paste is then applied to all the infected areas. | Dermal | |||||||
| Cucumis dipsaceus Ehrenb. ex Spach | Cucu-dip | Cucurbitaceae | Buhie hareg | Cl | W | Lf | F | Fibril illness | The fresh leaves of Haregresa are crushed, squeezed, and the liquid is filtered. Then, the liquid is mixed with sugar and consumed as a drink. | Oral | MY050 |
| Lf | F | Fibril illness | The fresh leaves of Haregresa are crushed, squeezed, and the fluid filtered. Then, this fluid is simply applied as a cream on the body. | Dermal | |||||||
| Cucumis ficifolius A.Rich. | Cucu-fic | Cucurbitaceae | yemidir Embuay | H | HG | Rt | D | Ayne Tila | The fresh root is cut into small pieces and dried. Once dry, it is powdered, and the medicine is either taken orally or inhaled as smoke. | Oral | MY033 |
| Rt | Rabies | The roots of Ensilal, Fiyele Feji, Lutt, Woinagift, Gizewa, and Yemidir Enbuay are pounded together. The resulting dry mixture is then kneaded with teff flour, and the sick person consumes it as bread. | Oral | ||||||||
| Cucurbita pepo L. | Cucu-pep | Cucurbitaceae | Duba | Cl | HG/MK | Fr | F | Abdominal disease | The cooked fruit is eaten by women experiencing the disorder locally known as marat. | Oral | MY037 |
| Cyathula polycephala Baker | Cyat-pol | Amaranthaceae | Chegogot | H | W | Lf | F | Fibril illness | The pounded leaves are squeezed, and the extracted juice is mixed with tea or coffee before being consumed. | Oral | MY036 |
| Cyphostemma cyphopetalum (Fresen.) Desc. ex Wild & R.B.Drumm. | Cyph-cyp | Vitaceae | Gindosh | H | W | Rt | F | Body abscission | The root is crushed into a fine powder, then mixed with a small amount of water and consumed as a drink. | Oral | MY054 |
| Datura stramonium L. | Datu-str | Solanaceae | Astenagr | H | W | Lf | F | Fowel pest | A mixture of Astenagir, Kebercho, garlic, Ahiya Joro, and Woina Gift is pounded and squeezed. The extracted juice is then given to animals suffering from fowl pest. | Oral | MY115 |
| Desmodium ramosissimum G.Don | Desm-rom | Fabaceae | Dirie | Cl | W | Lf | F | Girsha | The leaves are pounded and crushed, then soaked in water. The resulting mixture is applied as a cream to any affected area of the sick person. | Dermal | MY068 |
| Dichrostachys cinerea (L.) Wight & Arn. | Dich-cin | Fabaceae | Ader | T | W | Lf | F | Wart | The dried leaves are crushed, ground into a fine powder, and mixed with barley batter to make a paste. This paste is then applied to the infected areas. | Dermal | MY053 |
| Lf | F | Common cold | The leaves of Ader are steamed, and the resulting vapour is used to fumigate the person affected. | Nasal | |||||||
| Discopodium penninervium Hochst. | Disc-pen | Solanaceae | Ameraro | T | W | Rt | D | Evil eye | The roots of Ameraro, Koshim, and Kitkta are pounded and burned. As the animal inhales the smoke, the harmful spirit is believed to be driven away. | Nasal | MY086 |
| Dodonaea angustifolia L.f. | Dodo-ang | Sapindaceae | kitikta | Sh | W | Lf | D | Plague | The leaves are pounded and dried, then the resulting powder is smoked to treat the plague symptoms. | Nasal | MY029 |
| Lf | F | Fracture | The leaves are pounded and then applied directly to the injured area or tied in place. | Dermal | |||||||
| Lf | D | Skin rash | The leaves of Abalo and Kitikta are pounded and dried, then roasted and mashed. Finally, the resulting mixture is kneaded and applied as a coating to the affected areas of the body. | Dermal | |||||||
| Dovyalis abyssinica (A.Rich.) Warb. | Dovy-aby | Salicaceae | Koshim | T | W | Rt | D | Ayne wog for animal | The roots of Koshim, Ameraro, and Ktkta are first ground and dried, then ground again into a fine powder. Finally, the powder is burned to produce smoke for use in treatment. | Nasal | MY106 |
| Dracaena fischeri Baker | Drac-fis | Dracaenaceae | Wondie-kacha | H | W | Rt | D | Eczema | The root is crushed and dried. After burning, it is powdered, then mixed with butter and finally applied to the infected area. | Dermal | MY025 |
| Echinops kebericho Mesfin | Echi-keb | Asteraceae | Kebercho | H | W/MK | Rt | D | Evil Eye | The root is cut into very small pieces and burned to produce smoke, which is used to fumigate a person suffering from Buda. | Nasal | MY113 |
| Epilobium stereophyllum Fresen. | Epil-ste | Onagraceae | Bega sergie | H | W | Lf | F | Sirey | The leaves of Bega Sergie are pounded and mixed with a small amount of water, then filtered. Finally, a cup of the filtered liquid is drunk before meals for seven days. | Oral | MY099 |
| Erythrina brucei Schweinf. | Eryt-bru | Fabaceae | Gurgo | T | W | Fr | F | Wart | The dried fruit is ground into a powder and mixed with a barley batter to form a paste. This paste is then applied directly to the affected areas. | Dermal | MY052 |
| Eucalyptus globulus Labill. | Euca-glo | Myrtaceae | Nech Baherzaf | T | W/HG | Lf | F | Fibril illness | The steam from the boiled leaves of Nech Baherzaf is used to fumigate the water. | Nasal | MY109 |
| Euclea divinorum Hiern | Eucl-div | Ebenaceae | Dediho | Sh | W | Lf | F | Abscess | The fresh leaves are chewed together with milk, and then the infected areas are smeared with the mixture for six consecutive days. | Dermal | MY041 |
| Rt | D | Ayne Tila | The fresh root is cut into small pieces and dried. Once dried, the root is ground into powder, which is then used for smoking. | Nasal | |||||||
| Lf | F | Eye disease | The fruit of Nesch Shinkurt, the leaves of Dedho, and the fruit of cotton are pounded together. After squeezing, the pure fluid is applied to the infected parts of the eye. | Nasal | |||||||
| Lf | F | Abscess | The whole parts of Tibtibo, bark of Seghed, leaves of Dediho, whole parts of Semeg, and leaves of Jib Mirkuz are massaged with milk and then applied to areas affected by trembling and bitterness. This treatment is repeated by smearing the infected parts for six days. | Dermal | |||||||
| Euphorbia abyssinica J.F.Gmel. | Euph-aby | Euphorbiaceae | Kulkual | T | W | Lf | F | Wound | The leaves of Kulkual are mixed with salt, lemon juice, and ladybird honey. First, the affected areas of the body are coated with white honey, then the prepared medicine is applied to the infected parts repeatedly until they improve. | Dermal | MY097 |
| Euphorbia dumalis S.Carter | Euph-dum | Euphorbiaceae | Anterfa | Sh | W | Lf | D | Haemorrhoid | The entire Anterfa plant and the root of Endohahila are pounded and dried, then ground into a fine powder. This powder is mixed with white powder and finally blended with Vaseline before being applied to the skin. | Dermal | MY110 |
| Euphorbia tirucalli L. | Euph-tir | Euphorbiaceae | Kinchib | Sh | W | Lx | F | Kunchir | Apply the liquid obtained from cutting the stem as a cream to the affected areas. | Dermal | MY096 |
| Festuca glauca Vill. | Fesr-gla | Poaceae | Guassa | H | W | Lf | D | Eczema | The leaves are chopped into small pieces, crushed, and dried. Once dried, they are ground into a fine powder. Finally, the powdered leaves are mixed with butter and applied to the infected area. | Dermal | MY111 |
| Ficus vasta Forssk. | Ficu-vas | Moraceae | Warka | T | W | Ba | D | Eczema | The bark is crushed and dried. Once dry, it is crushed further into a powder, which is then mixed with butter and applied to the infected area. | Dermal | MY062 |
| Lx | F | Wound | The milky white substance from Warka is mixed with salt, lemon juice, and ladybird honey. First, the affected areas of the body are coated with white honey, then the prepared mixture is applied and gently massaged onto the infected parts until healing is achieved. | Dermal | |||||||
| Foeniculum vulgare Mill. | Foen-vul | Apiaceae | Ensilal | Sh | W | Lf | F | Stomachache | The leaves of Ensilal and Timatim are pounded together, and the squeezed juice is consumed as a drink. | Oral | MY117 |
| Glinus lotoides L. | Glin-lot | Molluginaceae | Metere | H | W | Wp | D | Tape warm | All the dried parts of the Metere climber are ground into powder and mixed with powdered barley or Baher Kel. This mixture is then given to a person infected with Kosso to eat. | Oral | MY056 |
| Gossypium herbaceum L. | Goss-heb | Malvaceae | Tit | H | HG/MK | Sd | D | Eye disease | The fruit of Nesch Shinkurt, leaves of Dedho, and cotton seeds are pounded together and then squeezed. The pure liquid extracted is applied to the infected parts of the eye. | Dermal | MY093 |
| Sd | D | Ear | The cotton seeds are boiled and filtered. The filtered liquid is then mixed with Tej and left to ferment for about four days. Finally, the mixture is dropped into the ear. | Ear | |||||||
| Grewia villosa Willd. | Grew-vil | Malvaceae | Lenkuata | Sh | W | Br | F | Retained placenta | The bark is pounded and squeezed, and the extracted pure juice is then drunk by the person suffering from the mentioned ailment. | Oral | MY034 |
| Gymnosporia obscura (A.Rich.) Loes. | Gymn-obs | Celastraceae | Kombel | Sh | W | Lf | D | Ayne Tila | The fresh leaves are cut into very small pieces and then dried. The dried leaves are powdered, and the medicine can be either taken orally or the powder smoked. | Oral | MY020 |
| Hagenia abyssinica (Bruce) J.F.Gmel. | Hage-aby | Rosaceae | Kosso | T | W | Fr | D | Tape warm | The dried fruit of the Kosso tree is powdered and mixed with barley flour. This mixture is then eaten by a person infected with Kosso. | Oral | MY089 |
| Hordeum vulgare L. | Hord-vul | Poaceae | Gebs | H | HG/MK | Lf | F | Dandruff | The pounded leaves of Gebs are squeezed, and the extracted liquid is applied to areas affected by dandruff. | Dermal | MY095 |
| Hylodesmum repandum (Vahl) H.Ohashi & R.R.Mill | Hylo-rep | Fabaceae | Moider | H | W | Rt | F | Wound | The dry, powdered root of Moider is mixed with salt, lemon juice, and ladybird honey. First, the affected areas are coated with white honey, then the prepared mixture is applied on the infected parts and left until healing occurs. | Dermal | MY015 |
| Inula confertiflora A.Rich. | Inul-con | Asteraceae | Woyinagift | Sh | W | Rt | D | Rabies | The roots of Ensilal, Fiyele Feji, Lutt, Woinagift, Gizewa, and Yemidir Enbuay are pounded together. After drying, the mixture is kneaded with teff flour and formed into bread, which the sick person then eats. | Oral | MY072 |
| Jasminum grandiflorum L. | Jasm-gra | Oleaceae | Tenbelel | Cl | W | Lf | F | Snake bite | The leaves are cut into very small pieces and pounded. Then, the preparation is taken as a drink until recovery. | Oral | MY047 |
| Kunchir | The root is pounded, dried, and ground into powder. This powder is then mixed with black teff dough. Finally, the mixture is applied as a cream on the infected areas until healing occurs. | Dermal | |||||||||
| Juniperus procera Hochst. ex Endl. | Juni-pro | Cuppressaceae | Tsid | T | W | Lf | F | Stomach aches | The leaves are ground into a powder and mixed with water. | Oral | MY108 |
| Justicia schimperiana (Hochst. ex Nees) | Just-sch | Acanthaceae | Sensel | Sh | HG/W | Lf | F | Gastric | The leaves of Sensel, fruits of Woyira, Papaya, and Kimmo, along with fluid from Eret, are mixed and ground together. This mixture is then taken daily before meals. | Oral | MY046 |
| Kalanchoe marmorata Baker | Kala-mar | Crassulaceae | Yezinjero kita | Sh | W | Lf | F | Fire burn | The collected leaves of Yezinjero Kita are ground into a paste and applied by tying it onto the injured area. | Dermal | MY061 |
| Kalanchoe petitiana A.Rich. | Kala-pet | Crassulaceae | Endohehila | Sh | W | Lf | F | Wound/lesion | The leaves of Endohehila are heated on embers, then the warm leaves are placed on the wounded area. | Dermal | MY118 |
| Rt | F | Uvula illness | The root is pounded and squeezed to extract the juice. The droplets are then placed in the nose until the infected person sneezes. | Nasal | |||||||
| Keetia lactescens (Hiern) Bridson | Keet-lac | Rubiaceae | Seghed | Sh | W | Br | F | Abscess | The entire parts of Semeg, bark of Seghed, leaves and whole parts of Dediho, along with leaves of Jib Mirkuz, are mixed and massaged with milk, then applied to trembling areas with bitterness. Afterward, the infected parts are smeared with this mixture for six days. | Dermal | MY016 |
| Laggera tomentosa (A.Rich.) Sch.Bip. ex Oliv. & Hiern | Lagg-tom | Asteraceae | keskeso | Sh | W | Rt | D | Evil eye | The fresh root is cut into very small pieces and then dried. After drying, the root is powdered, and the powder is smoked. | Oral | MY031 |
| Lepidium sativum L. | Lepi-sat | Brassicaceae | Feto | H | HG/MK | Wp | D | Malaria | The powdered and crushed leaves of Feto are mixed with water and taken orally as a remedy. | Oral | MY004 |
| Lobelia giberroa Hemsl. | Campanulaceae | Jibira | Sh | W | Rt | D | Evel eye | The roots of Etse Sabe’e, Gizewa, Kestenicha, Yemidir Embuay, Zert Embuay, Jibira, Ahiya Joro Agam, and Sete Ret are pounded together. Once dried, the mixture is ground into a powder and smoked for a person believed to be affected by an evil eye spirit. | Nasal | MY070 | |
| Lycopersicon esculentum Mill. | Lyco-esc | Solanaceae | Timatim | H | HG/MK | Lf | F | Stomach | The leaves are pounded and squeezed, and the extracted liquid is then drunk. | Oral | MY098 |
| Fr | F | Leech for animal | The fruit is pounded, mixed with water, and then drunk. | Oral | |||||||
| Mangifera indica L. | Mang-ind | Anacardiaceae | Mango | T | W/HG | Fr | F | Gastric | The ripe mango fruit is eaten regularly by the sick person before their daily meals. | Oral | MY012 |
| Maytenus arbutifolia (Hochst. ex A.Rich.) R.Wilczek | Mayt-arb | Celasteraceae | Atat | Sh | W | Lf | F | Herpes | The leaves are finely chopped and then pounded. The mixture is left to macerate for three days before being either applied as a cream to the affected areas or consumed as a drink until recovery. | Dermal/Oral | MY058 |
| Morella salicifolia (Hochst. ex A.Rich.) Verdc. | More-sal | Myricaceae | Shinet | T | W | Lf | F | Leech | The pounded leaves are mixed with salt and water, then the mixture is used to wash the infected area. | Oral | MY019 |
| Myrtus communis L. | Myri-com | Myrtaceae | Ades | Sh | W | Lf | F | Wart | The dried leaves are pounded, ground, and mixed with barley batter. The mixture is then applied to the infected areas. | Dermal | MY114 |
| Ocimum basilicum L. | Ocim-bas | Lamiaceae | Besobila | H | HG/MK | Lf | D | Headache | The leaves of Besobila are dried and powdered to be brewed as a tea, which helps promote a quick recovery. | Oral | MY119 |
| Ocimum forskaolii Benth. | Ocim-for | Lamiaceae | Nana | H | HG | Lf | F | Stress | The leaves of Nana are collected and pounded, then boiled with tea. The tea is consumed after meals. | Oral | MY112 |
| Ocimum lamiifolium Hochst. ex Benth. | Ocim-lam | Lamiaceae | Dama kessie | Sh | W/HG | Lf | F | Fibril illness | The leaves of Dama Kessie are cut, squeezed, and the liquid is filtered. This liquid can either be consumed with tea or applied topically to any affected area. | Dermal/Oral | MY066 |
| Olea europaea subsp. cuspidata (Wall. ex G.Don) Cif. | Olea-eur | Oleaceae | Woira | T | W/HG | Lf | F | Herpes | The leaves are finely chopped and pounded, then soaked (macerated) for three days. Afterward, the resulting mixture is applied as a cream to the affected area of the body. | Dermal | MY008 |
| Fr | Gastric | The fruits of kimmo, woyira, and papaya are combined with the juice from *eret* and the leaves of sunsel. This mixture is ground together, and the resulting preparation is taken daily on an empty stomach. | Oral | ||||||||
| Olinia rochetiana A.Juss. | Olin-roc | Oliniaceae | Tifie | Sh | W | Lf | F | Eye disease | The seven leaves of Tfie are cut, then squeezed and filtered. The extract is then applied directly into the infected eye. | Dermal | MY107 |
| Opuntia ficus-indica (L.) Mill. | Opun-fic | Cactaceae | Baher Qulqual | Sh | W | Fruit | F | Wound | The fruits of Baher Kulkual are mixed with salt, lemon juice, and ladybird honey. First, the affected areas of the body are coated with white honey. Then, the prepared mixture is applied and gently massaged into the infected areas repeatedly until healing occurs. | Dermal | MY055 |
| Osyris quadripartita Salzm. ex Decne. | Osyr-qua | Santalaceae | Keret | Sh | W | Lf | F | Herpes | The leaves are cut into very small pieces and pounded, then macerated for three days. Afterward, the preparation is either applied as a cream to the affected area or consumed as a drink until recovery. | Dermal/Oral | MY013 |
| Rt | Wart | The dried root is ground into powder and mixed with barley batter. This mixture is then applied by smearing it onto all the infected areas. | Dermal | ||||||||
| Otostegia integrifolia Benth. | Otos-int | Lamiaceae | Tinzhut | Sh | W | Rt | F | Snake bite | The root is cut into very small pieces and pounded, then the preparation is consumed regularly until recovery. | Oral | MY009 |
| Lf | Malaria | The powdered and crushed leaves of the plant are taken orally for a duration of seven days. | Oral | ||||||||
| Pennisetum pedicellatum Trin. | Penn-ped | poaceae | Sindedo | H | W | Rt | D | Kunchir | The roots of Sindedo, Goshmika, Shimbrut, and the bulbs of Yejibshinkurt are pounded together and dried. The powdered mixture is then kneaded with Lumen tint and applied as a cream to the infected area. | Dermal | MY116 |
| Persea americana Mill. | Pers-ame | Lauraceae | Avokado | T | W/HG | Fr | F | Madat | Apply cream to the infected area, then wash it with soap after 30 to 60 min. | Dermal | MY082 |
| Fr | F | Dandruff | The infected areas are coated with fresh avocado fruit, and the cream is washed off after 12 h. | Dermal | |||||||
| Phytolacca dodecandra L’Hér. | Phyt-dod | Phytolaccaceae | Mekan endod | Sh | W/HG | Rt | D | Jaundice | The dry root is pounded, then mixed with honey, and a spoonful of this mixture is taken daily before meals. | Oral | MY026 |
| Plantago lanceolata L. | Plant-lan | Plantaginaceae | Worteb | H | W | Lf | D | Wound | The leaves are pounded, dried, and ground into a powder. This powder is then sprinkled or applied to the infected areas. | Dermal | MY003 |
| Plectranthus punctatus (L.f.) L’Hér. | Plec-pun | Lamiaceae | Tibtibo | H | W | Lf | F | Herpes | The leaves are cut into very small pieces and pounded. They are then macerated for three days, after which the resulting preparation is either applied as a cream to the affected area or taken orally until recovery. | Dermal/Oral | MY035 |
| Wp | F | Abscess | The entire parts of Tibtibo, the bark of Seghed, leaves of Dediho, whole parts of Semeg, and leaves of Jib Mirkuz are mixed and massaged with milk. This mixture is then applied to areas experiencing trembling and bitterness. Finally, the infected parts are smeared with the preparation daily for six days. | Dermal | |||||||
| Polygala abyssinica R.Br. ex Fresen. | Ploy-oby | Polygalaceae | Etse- libona | Sh | W | Rt | F | Snake Bite | The root is cut into very small pieces and pounded. The resulting preparation is then drunk regularly until full recovery. | Oral | MY024 |
| Rt | D | Ayne Tila | The fresh root is cut into small pieces and dried. Once dried, it is ground into powder, which can be either ingested as medicine or smoked. | Oral/Nasal | |||||||
| Premna schimperi Engl. | Prem-sch | Lamiaceae | Chocho | Sh | W | Lf | F | Choq | The leaves of Chocho are pounded and mixed with a small amount of warm water. The resulting mixture is then used to wash the infected parts of the animal until it fully recovers. | Dermal | MY085 |
| Pterolobium stellatum (Forssk.) Brenan | Pter-ste | Fabaceae | kentefa | Cl | W | Rt | Evil eye | The fresh roots of Kentefa, Wuyign, Gumaro, Dediho, Shenbeko, and Gizewa are finely chopped and thoroughly dried. Alongside these, the dried leaves of Keskeso and the entire Chikugn plant are also prepared. Once all the ingredients are dried, they are ground into a powder, mixed together, and then burned to produce smoke for use. | Nasal | MY051 | |
| Rhamnus prinoides L’Hér. | Rham-pri | Rhamnaceae | Gesho | Sh | HG/MK | Fr | D | Itch | The fruit is pounded and mixed with butter, then applied to moisten the infected areas. | Dermal | MY038 |
| Rhus quartiniana A.Rich. | Rhus-qua | Anacardiaceae | Chakima | Sh | HG/W | Lf | F | Scorpion | The crushed leaves are bundled and applied to the area where the scorpion has stung. | Dermal | MY032 |
| Rosa abyssinica R.Br. ex Lindl. | Rosa-aby | Rosaceae | Kega | Sh | W | Lf | F | Body swelling | The leaves of Kega, Agam, Woira, and Kenbet are pounded and then squeezed. The resulting liquid is either drunk or applied as a compress to the affected areas. | Dermal/Oral | MY084 |
| Rubus steudneri Schweinf. | Rubu-ste | Rosaceae | Enjori | Sh | W | Fr | D | Wound | The fruit of Enjori is first pounded, dried, and then ground into a fine powder. This powder is then applied directly to the affected areas. | Dermal | MY002 |
| Rumex nepalensis Spreng. | Rume-nep | polygonaceae | Tult | H | W | Rt | D | Rabbis | The roots of Ensilal, Fiyele Feji, Lutt, Woinagift, Gizewa, and Yemidir Enbuay are pounded together. The dried mixture is then kneaded with teff flour, and the resulting dough is consumed by the sick person as bread. | Oral | MY043 |
| Rumex nervosus Vahl | Rume-ner | Polygonaceae | Embuacho | Sh | W | Lf | F | Wound | The leaves of Embuacho are mixed with salt, lemon juice, and ladybird honey. First, the affected body parts are coated with white honey, then the locally prepared medicine is applied repeatedly to the infected areas until healing occurs. | Dermal | MY040 |
| Ruta chalepensis L. | Ruta-cha | Rutaceae | Tenaadam | Sh | HG/MK | Wp | D | Kunchir | The root is crushed, dried, and ground into a powder. This powder is then mixed with black teff dough to form a paste, which is applied as a cream to the infected areas until healing occurs. | Dermal | MY063 |
| Wp | F | Evil sprit | The entire parts of Chiqugn, Tiena Adam, and Nech Shinkutr are crushed together, and the resulting substance is then sniffed. | Nasal | |||||||
| Saccharum officinarum L. | Sacc-off | Poaceae | Shenkora | Sh | HG/MK | St | F | Gastric | The stem of Shenkora is eaten directly before meals. | Oral | MY087 |
| Schinus molle L. | Schi-mol | Anacardiaceae | Kundo Berbere | T | W/HG | Lf | D | Plague | The leaves are pounded and dried, then smoked during a plague outbreak. | Nasal | MY102 |
| Fr | D | Evil eye | The dried fruit is burned in front of the person believed to be affected by an evil eye spirit. | Nasal | |||||||
| Searsia glutinosa (Hochst. ex A.Rich.) Moffett | Sear-glu | Anacardiaceae | Qimmo | Sh | W | Fr | D | Gastric | The fruits of Kimmo, Woyira, Papaya, the juice of Eret, and leaves of Sunsel are mixed and ground together. This mixture is then taken daily before meals. | Oral | MY103 |
| Searsia retinorrhoea (Steud. ex Oliv.) Moffett | Sear-ret | Anacardiaceae | Tilem | Sh | W | Lf | F | Liver infection | The leaves are dried, crushed, and boiled. The resulting liquid is then filtered through a clean cloth and consumed as a drink. | Oral | MY081 |
| Senna singueana (Delile) Lock | Senn- sin | Fabaceae | Gufa | Sh | W | Lf | F | Herpes | The leaves are cut into very small pieces and pounded. They are then macerated for three days before being used either as a cream applied to the affected area or taken orally until recovery. | Dermal/Oral | MY042 |
| Sida schimperiana Hochst. ex A.Rich. | Sida-sch | Malvaceae | Chifrig | Sh | W | Rt | D | Evil eye | The roots of Etse Sabe’e, Gizewa, Kestenicha, Yemidir Embuay, Zert Embuay, Jibira, Ahiya Joro Agam, Chifrig, and Sete Ret are pounded together. After drying, the mixture is ground into a powder and smoked for a person believed to be affected by an evil eye spirit. | Nasal | MY067 |
| Solanum anguivi Lam. | Sola-ang | Solanaceae | Zert Embuay | Sh | W | Rt | D | Evil eye | The roots of Etse Sabe’e, Gizewa, Kestenicha, Yemidir Embuay, Zert Embuay, Jibira, Ahiya Joro Agam, and Sete Ret are pounded together. After drying, the mixture is ground into a powder and smoked for a person believed to be affected by an evil eye spirit. | Nasal | MY074 |
| Steganotaenia araliacea Hochst. | Steg-ara | Apiaceae | Jib mirkuz | H | W | Lf | F | Abscess | Leaves of Yejib Mirkuz, Dediho, and the whole parts of Tibtibo, Semeg, along with the bark of Seged, are pounded and soaked. Then, the resulting mixture is applied to the infected areas and smeared for six days. | Dermal | MY017 |
| Stephania abyssinica (Quart.-Dill. & A.Rich.) Walp. | Step-aby | Menispermaceae | Etse Iyesus | Cl | W | Rt | D | Ayne tila | The roots of Etse-Eyesus, Aleblabit, Yemidir Embuay, Qestenicha, Sete-Eret, and Chifirg are ground, dried, and milled. The resulting powder is then mixed with white honey and eaten before any other food. | Oral | MY088 |
| Rt | F | Body swelling | The root of Etse-Iyesus is ground and squeezed, and the extracted liquid is applied as a cream to the infected area. | Dermal | |||||||
| Tacazzea conferta N.E.Br. | Taca-con | Apocynaceae | Shimbrut Hareg | Cl | W | Lf | F | Snake Bite | The leaves are finely chopped and pounded, then consumed as a drink until recovery. | Oral | MY069 |
| Tagetes minuta L. | Tage-min | Asteraceae | Gimie | H | W | Lf | F | Moybagegn for animal | The leaves of Gimie are first crushed and squeezed to extract their juice. This extract is then diluted with water and given to infected animals as a treatment through drenching. | Oral | MY104 |
| Tragia brevipes Pax. | Tagi-bre | Euphorbiaceae | Alebilabit | H | W | Rt | D | Ayne tila | The fresh root is cut into small pieces and dried. Once dried, it is ground into powder, which is then either taken orally or smoked for medicinal purposes. | Oral/Nasal | MY022 |
| Urtica simensis Hochst. ex A.Rich. | Urti-sim | Urticaceae | Sama | H | W | Lf | F | Gastric | The leaves of Sama are gathered and cooked like wot (stew), then eaten plain with injera. | Oral | MY090 |
| Lf | F | Gonorrhea | The leaves of Sama and the bark of Bisana are pounded and powdered, then mixed with water and filtered. Finally, a cup of the filtered liquid is taken every morning for five days. | Oral | |||||||
| Verbascum sinaiticum Benth. | Verb-sin | Scrophulariaceae | Ahiya joro/Ketetina | H | W | Rt | F | Snake bite | The root is cut into very small pieces and pounded, then the resulting preparation is consumed until recovery. | Oral | MY027 |
| Lf | Wound | The dried and powdered leaves of ketetina were mixed with salt, lemon juice, and ladybird honey. First, the affected areas were coated with white honey, then the locally prepared mixture was applied and left on the infected parts until healing occurred. | Dermal | ||||||||
| Rt | Jaundice | The roots of ketetina along with the roots and leaves of atuch were pounded together, then crushed into smaller pieces, and finally brewed into tea for the patient to drink. | Oral | ||||||||
| Verbena officinalis L. | Verb-off | Verbenaceae | Atuch | H | W | Rt/Lf | F | Jaundice | The root of ketetina is combined with either the root or leaves of atuch, and the mixture is pounded together. After being thoroughly crushed, it is prepared like tea, which the sick person then drinks. | Oral | MY001 |
| Vernonia amygidalia Del. | Vern-amy | Asteraceae | Girawa | Sh | W/HG | Lf | F | Dandruff | The leaves are pounded and squeezed to extract the liquid, which is then applied to the infected areas of the body. | Dermal | MY101 |
| Lf | F | Athlet foot | The leaves of Girawa are ground, and the extracted liquid is applied to the foot. | Dermal | |||||||
| Vernonia leopoldi (Sch.Bip. ex Walp.) Vatke | Vern-leo | Asteraceae | Wuyign | Sh | W | Lf | F | Herpes | The leaves were finely chopped into small pieces and pounded. They were then soaked for three days before the resulting mixture was applied as a cream to the affected area until the person fully recovered. | Dermal | MY028 |
| Rt | D | Haemorrhoid | The cut root of wuyign is first heated over a fire until it burns, then it is applied directly to the hemorrhoid to cauterize the area. | Dermal | |||||||
| Vicia faba L. | Vici-fab | Fabaceae | Bakela | H | HG/MK | Sd | D | Diarrhea | The seeds of Bakela are soaked in water and then perforated. Afterwards, they are worn around the neck as conchoidal or mollusk-like ornaments. | Dermal | MY120 |
| Withania somnifera (L.) Dunal | With-som | Solanaceae | Gizewa | Sh | W/HG | Rt | D | Ayne tila | The fresh root is cut into small pieces and dried. After drying, the root is ground into powder, which is then either consumed as a drink or inhaled by smoking. | Oral/Nasal | MY023 |
| Rt | D | Rabies | The roots of Ensilal, Fiyele Feji, Lutt, Woinagift, Gizewa, and Yemidir Enbuay are pounded together. The resulting mixture is dried, then kneaded with teff flour, and finally consumed by the patient in the form of bread. | Nasal | |||||||
| Zehneria scabra (L.f.) Sond. | Zehn-sca | Cucurbitaceae | Etse- Sabeq | Cl | W | Rt | D | Evil eye | The roots of Etse Sabe’e, Gizewa, Kestenicha, Yemidir Embuay, Zert Embuay, Jibira, Ahiya Joro Agam, and Sete Ret are pounded together. The dried mixture is then powdered and smoked for a person suffering from an evil eye spirit. | Nasal | MY049 |
Key: GF Growth form, HB Habitat, CP condition of preparation, PU part used, RA route of administration, VN Voucher number, T tree, H herb, Sh shrub, Cl climber, W Wild, HG Homegarden, MK Market, F fresh, D dry, F/D fresh and dry, L Leaf, R root, St stem, Ba bark, Fl flower, Fr fruit, Sd seed, Bu bulb, Lx latex, Wp whole plant
The district’s varied topography and microclimates likely support this floristic diversity, consistent with patterns observed in other Ethiopian highland regions where environmental heterogeneity fosters medicinal plant richness [46, 55–57]. This diversity has several implications. First, it highlights the urgent need for conservation strategies targeting endemic, overharvested, or slow-regenerating species, a concern echoed in other regions of Ethiopia [58, 59]. Second, the documented species provide a foundation for pharmacological research to validate traditional remedies and explore novel bioactive compounds [59, 60]. Finally, preserving both plant diversity and indigenous knowledge is crucial. Integrating traditional expertise into healthcare and biodiversity policies, alongside promoting sustainable harvesting and cultivation of key species, can enhance community health, environmental resilience, and the continuity of cultural heritage in Menz Keya Gebreal District.
Growth forms of medicinal plants
In Menz Keya Gebreal District, shrubs are the dominant growth form among medicinal plants, with 52 species (42.9%) used to treat various ailments (Table 2). Herbs follow with 38 species (31.4%), trees with 21 species (17.3%), and climbers are the least represented at 10 species (8.2%) (Fig. 3).
Fig. 3.
The habit of medicinal plant in Menz keya district
The predominance of shrubs is consistent with ethnopharmacological studies across Ethiopia, including southwestern and northern regions, where shrubs often constitute the largest proportion of medicinal plants [20, 61–63]. Ecologically, shrubs’ widespread distribution, resilience to environmental stress, and perennial life cycle make them accessible and sustainable sources of medicinal materials [33, 36, 58]. Their multi-part utility providing leaves, bark, roots, and fruits further enhances their value in traditional remedies. Culturally, shrubs and herbs are preferred over trees and climbers due to ease of harvesting and minimal ecological disturbance, reflecting practical knowledge and conservation ethics [21, 64].
In contrast, some studies in southwestern Ethiopia and other African regions report a higher reliance on herbs, sometimes exceeding 50% of medicinal flora [8, 22, 47, 50, 59]. This variation likely reflects ecological differences, with herbs thriving in disturbed or cultivated areas, and local cultural preferences shaped by disease prevalence and plant availability [60]. The lower use of trees and climbers in Menz Keya Gebreal may relate to longer regeneration times and restricted accessibility, a trend also observed in ethnobotanical surveys in Kenya and Uganda [65, 66].
The dominance of shrubs and herbs carries important conservation implications. Heavy dependence on these growth forms may lead to overharvesting, especially where cultivation or sustainable management practices are limited [2, 17, 51]. Given their extensive use, shrubs and herbs should be prioritized for phytochemical and pharmacological research to validate traditional applications and explore novel bioactive compounds [67, 68]. Continued ethnobotanical documentation is essential to capture the interactions among environmental factors, cultural practices, and health needs influencing plant use, supporting both biodiversity conservation and integration of traditional medicine into national healthcare frameworks.
Sources of medicinal plants
In Menz Keya Gebreal District, the majority of medicinal plants (84 species, 69.4%) were collected from the wild, while 20 species (16.5%) were cultivated in homegardens, and 17 species (14.1%) were obtained from both wild and cultivated habitats. This pattern highlights a strong reliance on natural ecosystems as the primary source of medicinal plants, consistent with studies from other Ethiopian districts and internationally, where 60–80% of medicinal plants are harvested from wild habitats [7, 21, 50, 69].
Wild-harvested plants are often considered more potent and authentic, and their abundant diversity and accessibility make them a practical choice, particularly where land for cultivation is limited. However, unregulated collection of slow-growing, endemic, or high-demand species poses significant sustainability risks, threatening both biodiversity and traditional healthcare systems [20, 41, 62, 70]. The relatively low proportion of cultivated species underscores an opportunity for homegarden and community-based cultivation. Domestication of frequently used or vulnerable plants can reduce pressure on wild populations, while agroforestry approaches integrating medicinal plants with food crops have proven effective in Ethiopia, China, India, and Kenya [5, 6, 22, 39, 58]. Integrating indigenous knowledge with conservation education and participatory management can further strengthen local stewardship and promote sustainable harvesting.
Medicinal plant parts used
Leaves were the most commonly utilized plant part, representing 40 species (33.1%), followed by roots (29 species, 24.0%) and fruits (17 species, 14.1%). Less frequently used parts included the whole plant (8 species, 6.6%), latex (4 species, 3.3%), and seeds, stems, or bark (each 2 species, 1.6%) (Fig. 4). Preference for leaves is consistent with Ethiopian studies in Bita, Yeki, and Metema, as well as research from Kenya, Tanzania, India, and Nepal, reflecting accessibility, ease of harvest, and rapid regeneration [8, 21, 39, 49, 50, 54, 60, 71].
Fig. 4.
Plant part used in menz keya district
Roots and bark, while pharmacologically important, are associated with more destructive harvesting that can threaten plant survival, particularly in slow-growing or endemic species [3, 42, 52, 63]. The lower use of these parts in Menz Keya Gebreal suggests a culturally mediated emphasis on sustainable practices. Fruits and latex, though less frequently used, provide bioactive compounds but require careful harvesting to avoid impairing regeneration and seed dispersal [36, 47, 72].
From a conservation perspective, the predominance of leaf use is encouraging. Educational programs should reinforce sustainable practices and promote cultivation or rotational harvesting for species where roots or bark are essential. Exploring the medicinal potential of less commonly used parts such as seeds and stems may diversify resource use, reduce pressure on vulnerable organs, and reveal novel bioactive compounds, supporting both sustainability and pharmacological innovation [48, 73–75].
Methods of medicinal plant preparation
In Menz Keya Gebreal District, local communities employ diverse preparation methods adapted to the type of ailment and route of administration. Pounding was the most common technique, used in 29 cases (23.9%), followed by crushing (23 cases, 19%) and powdering (18 cases, 14.9%). Less frequent methods included roasting and mixing, each cited once (0.8%). The predominance of pounding and crushing reflects practical considerations these methods release active phytochemicals efficiently without requiring specialized equipment and are particularly suited for topical applications, often mixed with carriers like water or oils. Powdering supports oral administration and facilitates preservation for longer-term use [22, 47, 64, 76].
Similar preparation patterns are documented in Ethiopia and internationally. In districts such as Bita, Metema, and Ensaro, pounding and crushing dominate due to efficacy and accessibility, while studies from Kenya, Tanzania, India, and Nepal report comparable reliance on pounding, crushing, powdering, and decoction in rural contexts [5, 39, 50, 71]. The rare use of roasting and mixing may indicate specialized treatments or perceived enhancement of therapeutic potency, consistent with findings from Ethiopian and Asian ethnomedicinal literature [76–78].
Documenting preparation methods is critical for preserving traditional knowledge threatened by modernization, standardizing herbal remedies for integration into formal healthcare, and guiding pharmacological research that replicates indigenous extraction techniques for bioactivity evaluation [22, 64, 67, 68].
Routes and methods of administration
Medicinal plants in Menz Keya Gebreal are administered via multiple routes. Topical application was most prevalent (45 cases, 37.2%), followed by oral administration (42 cases, 34.7%) and nasal routes (15 cases, 12.4%). Corresponding methods included creaming (37, 30.6%), drinking (33, 27.3%), eating (24, 19.8%), and smoking (18, 14.9%). Topical preparations, especially creaming, are primarily used for skin-related conditions, aligning with findings from Quarit, Guraferda, and Ensaro districts, as well as studies across Africa and South Asia [8, 11, 39, 58, 66]. Oral administration is mainly employed for systemic ailments such as digestive disorders and fevers, while nasal administration and smoking target respiratory and head-related conditions [20, 67, 78, 79].
These administration practices demonstrate sophisticated indigenous understanding of therapeutic needs, aligning application routes with disease localization and pharmacological rationale. Topical delivery facilitates localized healing, oral ingestion targets systemic conditions, and nasal or smoke-based methods provide rapid access to respiratory pathways [80, 81].
From a public health perspective, documenting these practices is essential for integrating traditional medicine into modern healthcare systems, ensuring safety, efficacy, dosage standardization, and quality control. Additionally, understanding administration routes informs conservation strategies by clarifying which plant parts are used and the sustainability implications of harvesting.
Use of fresh versus dried plant materials
In Menz Keya Gebreal District, most traditional remedies are prepared from fresh plant materials (90 cases, 57.7%), while 63 remedies (40.4%) use dried plants, and a small fraction (1.9%) combine both forms. The preference for fresh materials reflects local beliefs that they retain higher potency, aroma, and bioactive compounds. Similar patterns have been reported across Ethiopia, including Dawuro, Addi Arkay, Asagirt, Wadla, and Sekela districts, where fresh plant use predominates for immediate treatment of acute ailments, facilitated by the ready availability of plant parts in natural habitats [7, 12, 41, 75, 82].
Internationally, studies from East Africa, South Asia, and Latin America corroborate this trend. In Kenya, Tanzania, India, and China, fresh plants are preferred for their perceived efficacy, particularly for infections and inflammatory conditions, while drying is employed to ensure off-season availability. Indigenous communities in Latin America often use fresh or dried materials according to seasonal availability and treatment needs [5, 45, 49, 50, 69, 79].
The use of dried materials in Menz Keya Gebreal highlights community recognition of preservation benefits, ensuring medicinal access during dry periods or when fresh plants are scarce. Combined fresh and dried preparations are rare, likely reflecting specific cultural practices or disease-specific protocols. Heavy reliance on fresh plants may exert seasonal pressure on wild populations, raising the risk of overharvesting. Promoting effective drying and storage techniques could alleviate this pressure, ensuring year-round availability and sustainable use of medicinal species.
Ingredients and solvents used in medicinal preparations
Nearly half of remedies in Menz Keya Gebreal (47.9%) are prepared solely from raw plant material. Among formulations with additives, water was the primary solvent (25.6%), followed by butter (8.3%), honey (4.2%), and milk, coffee, or citrus juice (each 2.5%). Less common solvents included traditional foods (Enjera, Besso) and local beverages (Tella, < 2%), with salt and oil rarely used (0.8%) (Table 3).
Table 3.
Ingredients or solvents used for medicinal Preparation in Menz Keya district
| Types of ingredient | Frequency of preparations | Percentage (%) |
|---|---|---|
| Besso | 2 | 1.7 |
| Butter | 10 | 8.3 |
| Citrus juice | 3 | 2.5 |
| Coffee | 3 | 2.5 |
| Enjera | 2 | 1.7 |
| Honey | 5 | 4.2 |
| Milk | 3 | 2.5 |
| Oil | 1 | 0.8 |
| Salt | 1 | 0.8 |
| Tella | 2 | 1.7 |
| Water | 31 | 25.6 |
| Without ingredient | 58 | 47.9 |
| Total | 121 | 100 |
This pattern reflects a cultural preference for simple prepa rations and reliance on the inherent medicinal potency of plants. Water’s predominance aligns with studies across Ethiopia and internationally, where its accessibility, safety, and extraction efficiency make it the primary solvent for both topical and oral remedies [61, 67, 83, 84]. Animal-derived products such as butter and milk, and sweeteners like honey, enhance therapeutic efficacy, bioavailability, or palatability. Honey, for example, synergizes with antimicrobial plant compounds, while butter and milk facilitate absorption of fat-soluble constituents [47, 52, 72, 85]. Acidic solvents like citrus juice may aid extraction or preservation, as reported in Ethiopia and India [6, 54]. The inclusion of culturally significant foods and beverages highlights the integration of dietary and medicinal traditions, a feature shared with other African and Latin American indigenous medical systems [67, 85].
The substantial proportion of remedies prepared without solvents suggests many applications are topical or involve direct use as powders, pastes, or chewing, consistent with documented administration practices [38, 59, 64, 86]. Understanding ingredient and solvent choices is essential for standardizing and validating traditional medicine. Knowledge of traditional solvents informs pharmacological investigations, ensuring authentic assessment of bioactivity. For healthcare providers and policymakers, these insights support culturally sensitive interventions and the integration of community-based traditional medicine into broader health systems. Educational programs emphasizing both the cultural significance and practical aspects of medicinal preparation can enhance safe, sustainable use while preserving vital ethnomedical knowledge.
Dosage and antidotes
In Menz Keya Gebreal District, traditional healers determine medicinal dosages using indigenous units and flexible timeframes, as no standardized system exists. Common measures include finger length, spoons, teacups, calabashes, and counts of plant parts such as leaves, seeds, fruits, or bulbs. Dosages are adjusted based on illness severity and patient characteristics, particularly age and physical condition. For topical applications, specific dosages are rarely defined, and treatment duration depends on symptom resolution or the healer’s judgment. Such variability mirrors findings from other Ethiopian districts, including Guraferda, Ensaro, and Quara, where experiential adjustment predominates [8, 11, 13, 36, 42]. For example, recommendations for Lepidium sativum against malaria ranged from two tea glasses to four or five cups, reflecting healer discretion.
Although side effects are generally considered minimal, some adverse reactions such as diarrhea or vomiting have been reported. Traditional antidotes include cooked teff flour, honey, boiled coffee, milk, butter, and “Tella,” a fermented beverage. These are believed to neutralize or reduce side effects, a practice documented in southern Ethiopia and parts of East Africa [21, 36, 39, 47, 50, 60, 72, 76, 87]. While Ethiopian remedies are largely considered safe [88], the lack of standardized dosing and limited pharmacological validation highlights safety concerns. International literature similarly emphasizes the need for toxicological assessments and dosage standardization in traditional medicine [38, 58, 67].
This variability in dosage underscores challenges for integration into formal healthcare systems. Systematic documentation of local dosing practices, coupled with biochemical and pharmacological validation, is essential to ensure safer and more effective use. Training traditional healers on dosage standardization and potential toxicities, alongside community awareness programs, can enhance therapeutic outcomes and patient safety.
Marketability of medicinal plants
A market survey conducted in the Monday market of Zemero Town and the Sunday market of Qolaqo Town revealed that while some medicinal plants are actively traded, many are valued primarily for non-medicinal uses. Species such as Ruta chalepensis, Citrus aurantifolia, Foeniculum vulgare, Hordeum vulgare, Ocimum basilicum, Combretum collinum, Allium sativum, and Allium cepa were mainly regarded as aromatic or culinary plants rather than strictly medicinal. Only a few species, including Hagenia abyssinica, Withania somnifera, Echinops kebericho, and Verbena officinalis, were specifically collected for medicinal purposes.
These findings align with Ethiopian studies in Bita and Yeki districts, where many medicinal plants serve multifunctional roles as spices, flavorings, or household materials [2, 20, 21, 36]. Similar multifunctionality has been documented internationally, reflecting integration of medicinal plants into food, ritual, and economic domains [49, 60, 72]. In Menz Keya District, of 33 documented species, many were primarily sold as foodstuffs (Capsicum annuum, Lycopersicon esculentum, Mangifera indica, Persea americana), spices (Schinus molle, Allium sativum, Allium cepa), or aromatics (Citrus aurantifolia, Myrtus communis, Foeniculum vulgare) (Table 4). Others served household uses, including timber and firewood (Eucalyptus globulus, Cordia africana, Pennisetum sphacelatum), stimulants (Catha edulis, Coffea arabica), soap production (Phytolacca dodecandra), clothing (Gossypium herbaceum), and agricultural tools (Olea europaea) [20, 36, 49].
Table 4.
Medicinal plants that traded in the open market for different uses
| Local name | Scintific name | Uses |
|---|---|---|
| Key shinkurt | Allium cepa | food, spice |
| Nech shinkurt | Allium sativum | food, spice |
| Habesha gomen | Brassica carinata | Food |
| Koba | Canna indica | Bread making |
| Berbere | Capsicum annum | Food |
| Papaya | Carica papaya | Food |
| Khat | Catha edulis | Stimulant |
| Lomi | Citrus aurantifolia | Aromatics |
| Tirngo | Citrus medica | Food |
| Birtukan | Citrus sinensis | Food |
| Buna | Coffea arabica | Stimulant |
| Abalo | Combretum collinum | Aromatics |
| Wanza | Cordia africana | house hold material |
| Duba | Cucurbita pepo | Food |
| Nech bahir zaf | Eucalyptus globulus | house hold materials |
| Ensilal | Foeniculum vulgare | Aromatics |
| Titi | Gossypium herbaceum | Clothing |
| Gebs | Hordium vulgare | Food |
| Timatim | Lycopersicon esculentum | Food |
| Mango | Mangifera indica | Food |
| Adese | Myrtus communis | Aromatics |
| Besobila | Ocimum bacilicum | Aromatics |
| Woira | Olea europia | farmer tools |
| Bahir qulqual | Opuntiaficus indica | Food |
| Tinjut | Oteostegia integrifolia | Aromatics |
| Sindedo | pennisetum sphacelatum | house hold material |
| Avocado | Persea americana | Food |
| Endod | Phytolaca dodecandra | soap berry |
| Gesho | Rhamnus prinoides | beverage production |
| Enjori | Rubus steudneri | Food |
| Tiena adam | Ruta chalepensis | Aromatics |
| Kundo berbere | Schinus mulle | Spices |
| Bakela | Vicia faba | Food |
The multifunctional nature of these plants underscores their central role in rural livelihoods and community economies, while also highlighting potential overharvesting risks that could threaten their sustainability as medicinal resources [20, 63, 87]. Recognizing that species traded primarily for food or aromatic purposes often have medicinal value provides an opportunity to integrate conservation with sustainable economic development. Promoting cultivation of high-demand multifunctional species, raising awareness among harvesters and traders, and supporting value addition, market access, and livelihood diversification can strengthen both biodiversity conservation and socio-economic resilience [17, 74, 80, 89].
Informant consensus factor (ICF)
The Informant Consensus Factor (ICF) analysis revealed varying levels of agreement among informants regarding medicinal plant use for different disease categories in Menz Keya Gebreal District. The highest consensus was recorded for skin diseases (ICF = 0.87), based on 33 species and 250 use reports, followed by digestive disorders (ICF = 0.82; 44 species, 246 use reports) and respiratory diseases (ICF = 0.78; 12 species, 52 use reports) (Table 5). In contrast, musculoskeletal disorders showed the lowest consensus (ICF = 0.33; 3 species, 4 use reports), indicating limited shared knowledge or inconsistent remedy use for this category. Intermediate ICF values were found for nervous system diseases (0.76), cardiovascular diseases (0.73), miscellaneous ailments (0.61), and infective diseases (0.54).
Table 5.
ICF values of medicinal plants used for treating human ailments in Menz Keya
| Diseases category | Nt | Nur | Nur-Nt | Nur-1 | ICF | % | Rank |
|---|---|---|---|---|---|---|---|
| Cardiovascular diseases | 6 | 20 | 14 | 19 | 0.73 | 73 | 5th |
| Digestive diseases | 44 | 246 | 202 | 245 | 0.82 | 82 | 2nd |
| Infective diseases | 12 | 25 | 13 | 24 | 0.54 | 54 | 7th |
| Miscellaneous | 8 | 19 | 11 | 18 | 0.61 | 61 | 6th |
| Musculoskeletal diseases | 3 | 4 | 1 | 3 | 0.33 | 33 | 8th |
| Nervous system diseases | 6 | 22 | 16 | 21 | 0.76 | 76 | 4th |
| Respiratory systems | 12 | 52 | 40 | 51 | 0.78 | 78 | 3rd |
| Skin diseases | 33 | 250 | 217 | 249 | 0.87 | 87 | 1st |
ICF Informant consensus Factor, Nur number of use reports by informants, Nt number of plant taxa or species used
These results suggest that skin and digestive conditions are both the most commonly treated ailments and those for which medicinal plant knowledge is most widely shared. Similar patterns have been observed in other Ethiopian districts. In Gurafrrda, high ICF values were recorded for gastrointestinal and dermatological disorders [8]. In Gera District, Oromia, skin infections and digestive ailments also exhibited strong consensus [85], while Quarit District reported comparable trends, highlighting the reliability of specific species for stomach and skin conditions [90]. Internationally, studies in Nepal and rural Uganda similarly demonstrate high consensus for digestive and dermatological ailments, reflecting the frequent occurrence of these conditions and the established efficacy of local remedies [91, 92].
The high ICF values in Menz Keya Gebreal likely reflect both the prevalence of these ailments and the perceived effectiveness of a limited number of well-known remedies, making associated species promising candidates for pharmacological and phytochemical research. Conversely, low ICF scores for musculoskeletal and infective diseases may result from a more diverse set of species being used or from less frequent occurrence of these conditions, leading to fragmented knowledge. These findings underscore the importance of systematic documentation and standardization, particularly for categories with low consensus, to preserve indigenous knowledge and support its integration into broader healthcare frameworks.
Index of RFC, FL, RPL, ROP, and CVI analysis
Quantitative ethnobotanical analysis in Menz Keya Gebreal District revealed notable variation in the cultural significance and therapeutic consensus of medicinal plants (Table 6). Hagenia abyssinica exhibited the highest Fidelity Level (FL = 1.00), reflecting unanimous agreement on its use against tapeworm infestations. Ocimum lamiifolium (FL = 0.97) and Echinops kebericho (FL = 0.97) followed closely, primarily used for febrile illnesses and spiritual ailments, respectively. Justicia schimperiana also showed high fidelity (FL = 0.94) for malaria treatment.
Table 6.
Index of RFC, FL, RPL, ROP, CVI analysis
| Medicinal plant species | Disease categories | IP | IU | FL | RPL | ROP | RFC | CVI |
|---|---|---|---|---|---|---|---|---|
| Allium sativum L. | Eczema | 36 | 39 | 0.92 | 0.84 | 77.2 | 0.48 | 0.29 |
| Artemisia abyssinica Sch.Bip. ex Oliv. & Hiern | Evil sprit | 34 | 38 | 0.89 | 0.82 | 72.9 | 0.47 | 0.19 |
| Capsicum annuum L. | Hepatitis | 19 | 24 | 0.79 | 0.52 | 41.1 | 0.3 | 0.18 |
| Carica papaya L. | Gastric | 17 | 21 | 0.80 | 0.45 | 36 | 0.26 | 0.21 |
| Carissa spinarum L. | Snake bite | 24 | 28 | 0.85 | 0.60 | 51 | 0.35 | 0.14 |
| Citrus × aurantium L. | Eye disease | 16 | 31 | 0.51 | 0.67 | 34.1 | 0.38 | 0.38 |
| Citrus medica L. | Hepatitis | 18 | 28 | 0.64 | 0.60 | 38.4 | 0.35 | 0.35 |
| Clutia abyssinica Jaub. & Spach | Jaundice | 19 | 23 | 0.82 | 0.50 | 41.0 | 0.28 | 0.11 |
| Coffea arabica L. | Headache | 29 | 34 | 0.85 | 0.73 | 62.0 | 0.42 | 0.42 |
| Cordia africana Lam. | Wound | 17 | 22 | 0.77 | 0.47 | 36.2 | 0.27 | 0.27 |
| Croton macrostachyus Hochst. ex Delile | Eczema | 26 | 32 | 0.81 | 0.69 | 55.8 | 0.40 | 0.40 |
| Dodonaea angustifolia L.f. | Skin rash | 20 | 26 | 0.76 | 0.56 | 42.5 | 0.32 | 0.13 |
| Echinops kebericho Mesfin | Evil Eye | 43 | 44 | 0.97 | 0.95 | 92.1 | 0.55 | 0.33 |
| Eucalyptus globulus Labill. | Fibril illness | 33 | 36 | 0.91 | 0.78 | 70.9 | 0.45 | 0.45 |
| Euclea divinorum Hiern | Eye disease | 13 | 24 | 0.54 | 0.52 | 28.1 | 0.3 | 0.12 |
| Hagenia abyssinica (Bruce) J.F.Gmel. | Tape warm | 26 | 26 | 1.00 | 0.56 | 56.0 | 0.32 | 0.32 |
| Justicia schimperiana (Hochst. ex Nees) | Malaria | 33 | 35 | 0.94 | 0.76 | 71.4 | 0.43 | 0.17 |
| Kalanchoe petitiana A.Rich. | Hemorrhoid | 14 | 24 | 0.58 | 0.52 | 30.1 | 0.3 | 0.06 |
| Lepidium sativum L. | Malaria | 15 | 22 | 0.68 | 0.47 | 31.9 | 0.27 | 0.16 |
| Ocimum lamiifolium Hochst. ex Benth. | Febrile illness | 45 | 46 | 0.97 | 1 | 97.0 | 0.57 | 0.23 |
| Olea europaea subsp. cuspidata (Wall. ex G.Don) Cif. | Gastric | 11 | 26 | 0.42 | 0.56 | 23.5 | 0.32 | 0.32 |
| Otostegia integrifolia Benth. | Snake bite | 24 | 32 | 0.75 | 0.69 | 51.7 | 0.4 | 0.16 |
| Plantago lanceolata L. | Wound | 26 | 28 | 0.92 | 0.60 | 55.2 | 0.35 | 0.07 |
| Rumex nepalensis Spreng. | Rabbies | 13 | 29 | 0.44 | 0.63 | 27.7 | 0.36 | 0.072 |
| Rumex nervosus Vahl | Wound | 15 | 21 | 0.71 | 0.45 | 31.9 | 0.26 | 0.10 |
| Ruta chalepensis L. | Evil eye | 36 | 41 | 0.87 | 0.89 | 77.4 | 0.51 | 0.20 |
| Stephania abyssinica (Quart.-Dill. & A.Rich.) Walp. | Swelling | 18 | 29 | 0.62 | 0.63 | 39.0 | 0.36 | 0.072 |
| Verbascum sinaiticum Benth. | Jaundice | 29 | 36 | 0.80 | 0.78 | 62.4 | 0.45 | 0.18 |
| Vernonia amygidalia Del. | Dandruff | 37 | 43 | 0.86 | 0.93 | 79.9 | 0.53 | 0.32 |
| Withania somnifera (L.) Dunal | Rabies | 16 | 29 | 0.55 | 0.63 | 34.6 | 0.36 | 0.14 |
| Zehneria scabra (L.f.) Sond. | Evil eye | 15 | 33 | 0.45 | 0.71 | 31.9 | 0.41 | 0.08 |
Key: FL Fidelity Level, Ip number of informants who independently cited the importance of a species for treating a particular disease, Iu total number of informants who reported the plant for any given disease, RPL Relative popularity level, ROP Rank-order priority, CVI Cultural Value Index, RFC Relative frequency of citation
Relative Popularity Level (RPL) was highest for Ocimum lamiifolium (1.00), with Echinops kebericho (0.95) and Vernonia amygdalina (0.93) also ranking prominently. Corresponding Rank Order Priority (ROP) values highlighted their dominant cultural and therapeutic roles. Relative Frequency of Citation (RFC) supported these findings, with Ocimum lamiifolium (0.57) and Echinops kebericho (0.55) cited most frequently. The Cultural Value Index (CVI) emphasized the broader cultural relevance of Eucalyptus globulus (0.45), Coffea arabica (0.42), Echinops kebericho (0.33), and Vernonia amygdalina (0.32), reflecting their dual medicinal and socio-cultural significance.
The perfect FL of Hagenia abyssinica aligns with reports from Amhara and Oromia regions, affirming its national reputation as a trusted anthelmintic [7, 93]. High FL values for Ocimum lamiifolium and Echinops kebericho are consistent with studies from southern Ethiopia, where these species are associated with febrile and spiritual treatments, respectively [3]. Elevated RPL and ROP indices indicate widespread recognition and prioritization within the community, mirroring findings from Bench-Sheko Zone and northern Ethiopia [2, 7, 8]. Internationally, high RPL values similarly reflect cultural prominence, as observed in West African ethnobotanical surveys [94], while RFC trends correspond with reports from Kenya and Uganda, where Ocimum species are frequently cited for febrile and respiratory conditions [95–97].
CVI scores further highlight the integration of certain plants into cultural identity, with species like Echinops kebericho serving both therapeutic and ritualistic roles, consistent with observations from Gojam and Sheka zones [7, 36, 47, 90]. Multidimensional indices collectively illuminate the nuanced relationships between the community and medicinal plants: high FL and RPL values confirm the centrality and perceived reliability of species such as Ocimum lamiifolium, Hagenia abyssinica, and Echinops kebericho, whereas plants with moderate FL but high RFC, like Coffea arabica, likely fulfill broader medicinal or cultural functions.
These findings underscore the need to prioritize conservation and pharmacological validation of high FL, RPL, and RFC species, given their consistent and prominent use. The observed variability in citation frequencies also highlights uneven knowledge transmission, emphasizing the importance of ethnobotanical education, youth engagement, and community-based knowledge revitalization programs to sustain indigenous medicinal knowledge. Such measures are essential for preserving ethnopharmacological heritage and strengthening resilience in rural healthcare systems in Ethiopia and comparable settings worldwide [5, 10, 12, 23].
Paired comparison analysis of medicinal plant preferences
Paired comparison analysis with ten key informants (R1–R10) revealed a clear hierarchy in the perceived importance of five commonly used medicinal plants in Menz Keya Gebreal District (Table 7). Dracaena fischeri Baker emerged as the most preferred species, scoring 45 points, reflecting its perceived efficacy, broad therapeutic applications, or cultural significance. It was followed by Croton macrostachyus (41 points), Ficus vasta (26 points), Allium sativum (21 points), and Festuca glauca (17 points), the least preferred.
Table 7.
Paired comparison analysis of medicinal plant preferences
| The respondents that are labeled from R1 to R10 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Types of medicinal plant | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | Total | Rank |
| Alium sativum | 3 | 4 | 1 | 1 | 2 | 1 | 1 | 5 | 2 | 1 | 21 | 4th |
| Croton macrostachyus | 4 | 3 | 5 | 5 | 4 | 5 | 4 | 3 | 4 | 4 | 41 | 2nd |
| Festuca glauca | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 17 | 5th |
| Ficus vasta | 2 | 1 | 4 | 3 | 3 | 3 | 3 | 1 | 3 | 3 | 26 | 3rd |
| Sanseveria fischeri | 5 | 5 | 3 | 4 | 5 | 4 | 5 | 4 | 5 | 5 | 45 | 1st |
The high ranking of Dracaena fischeri suggests it is highly valued for treating specific ailments, while the consistent preference for Croton macrostachyus aligns with its historical medicinal use across Ethiopia [2, 11, 36, 47]. Lower scores for Allium sativum and Festuca glauca may indicate limited use, narrower therapeutic scope, or lower familiarity among community members.
These findings are consistent with paired comparison studies elsewhere in Ethiopia. In the Sheka Zone, Croton macrostachyus was among the top five preferred medicinal plants due to its effectiveness in treating skin and gastrointestinal disorders [47]. Similarly, in Oromia, traditional healers favored species such as Vernonia amygdalina and Croton macrostachyus for their perceived reliability and accessibility [57, 84, 93]. Internationally, paired comparisons have also highlighted community preferences; in Kenya, Dracaena species were prioritized for wound healing and infection management [98], while in Zimbabwe, preference rankings reflected rapid healing efficacy and minimal side effects [99].
Paired comparison analysis is particularly valuable in ethnobotanical research, offering insights into community-level prioritization of medicinal resources. The prominence of Dracaena fischeri and Croton macrostachyus identifies them as prime candidates for pharmacological and phytochemical validation. Targeted in-situ conservation and integration into community-based healthcare strategies are recommended for these culturally and therapeutically significant species. However, lower-ranked plants like Festuca glauca and Allium sativum should not be overlooked, as they may possess underutilized or context-specific medicinal potential.
These results provide a strategic framework for aligning traditional knowledge with scientific validation and conservation initiatives. Incorporating community preferences into healthcare planning can enhance sustainable use of medicinal plants while supporting rural health systems where traditional remedies remain primary sources of treatment.
Preference ranking of medicinal plants for treating hepatitis
A preference ranking analysis involving eight key informants (R1–R8) identified the most favored medicinal plants for treating hepatitis in Menz Keya Gebreal District (Table 8). Clutia abyssinica emerged as the top-ranked species with 54 points, followed by Euphorbia abyssinica (48 points), and Allium sativum (garlic) in third place with 40 points. Citrus aurantifolia scored 33 points, ranking fourth, while Arundo donax, Capsicum annuum, and Morella salicifolia ranked fifth to seventh with 23, 14, and 12 points, respectively.
Table 8.
Preference ranking of human medicinal plants reported for treating hepatitis
| List of Medicinal plants | Respondants from R1-R8 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | Total | Rank | |
| Allium sativum | 6 | 5 | 5 | 6 | 4 | 5 | 5 | 4 | 40 | 3rd |
| Arundo donax | 3 | 3 | 4 | 3 | 2 | 3 | 3 | 2 | 23 | 5th |
| Capsicum annum | 2 | 1 | 1 | 2 | 3 | 1 | 1 | 3 | 14 | 6th |
| Citrus aurantfollia | 4 | 4 | 3 | 4 | 5 | 4 | 4 | 5 | 33 | 4th |
| Clutia abyssinica | 7 | 7 | 6 | 7 | 7 | 7 | 6 | 7 | 54 | 1st |
| Euphorbia abyssinica | 5 | 6 | 7 | 5 | 6 | 6 | 7 | 6 | 48 | 2nd |
| Morella salicifolia | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 12 | 7th |
The strong preference for Clutia abyssinica reflects its perceived efficacy, cultural familiarity, and local availability. Similarly, the high ranking of Euphorbia abyssinica underscores its recognized therapeutic role within the community’s ethnomedicinal system. Although Allium sativum is globally acknowledged for its hepatoprotective, antioxidant, and antimicrobial properties, its third-place ranking highlights the influence of cultural perception and localized therapeutic experience on plant preference [2, 100]. Lower scores for Arundo donax, Capsicum annuum, and Morella salicifolia likely indicate limited traditional use, lower perceived efficacy, or reduced accessibility for hepatitis treatment in the district.
Comparable studies in Ethiopia and beyond support these observations. In central Ethiopia, Croton macrostachyus ranked highest for wound treatment, demonstrating the utility of preference ranking in identifying culturally and therapeutically significant species [2, 7, 90]. In the Sheka Zone of southwestern Ethiopia, Euphorbia abyssinica and Clutia abyssinica were also cited among the most effective plants for liver-related ailments [36, 47]. Similarly, in Uganda, Vernonia amygdalina and Securidaca longipedunculata were highly preferred for liver and digestive disorders, illustrating the role of local knowledge and belief systems in shaping medicinal plant use [66].
This analysis underscores preference ranking as a powerful ethnobotanical tool for identifying locally prioritized species based on efficacy, availability, and cultural trust. The prominent positions of Clutia abyssinica and Euphorbia abyssinica indicate they are strong candidates for phytochemical and pharmacological validation in the context of hepatitis and liver disorders. Their high demand and cultural significance also highlight the need for sustainable harvesting and community-based conservation strategies to ensure long-term availability.
Although Allium sativum received a moderate preference score, its established pharmacological profile, including antioxidant and hepatoprotective properties, suggests further potential that could be enhanced through educational and integrative healthcare initiatives [100]. Lower-ranked species should not be overlooked, as they may harbor untapped bioactive compounds or hold greater cultural relevance in neighboring communities.
Direct matrix ranking of multipurpose medicinal plants
A direct matrix ranking of nine multipurpose medicinal plant species in Menz Keya Gebreal District revealed that Cordia africana achieved the highest total score (47), reflecting its extensive utility across various community needs. This species scored highest in construction (6), furniture (6), fencing (4), charcoal/firewood (4), and food (4) (Table 9). Olea europaea subsp. cuspidata followed with 26 points, and Juniperus procera ranked third with 25 points. Mid-ranking species included Dovyalis abyssinica (24), Osyris quadripartita (22), and Arundo donax (21), while Canna indica (20), Morella salicifolia (19), and Dodonaea angustifolia (18) scored the lowest. Among use categories, fencing (47), construction (43), and firewood/charcoal (42) received the highest cumulative scores, whereas food ranked lowest (12).
Table 9.
Direct matrix ranking for nine plant species and main use in the study area
| Main Use | Plant species | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cordia africana | Canna indica | Dodonea angustifolia | Arundo donax | Osyris quadripartita | Dovyalis abyssica | Olea europaea | Morella salicifolia | Juniperus procera | Total | Rank | |
| Charcoal and firewood | 4 | 5 | 5 | 2 | 6 | 5 | 4 | 5 | 6 | 42 | 3rd |
| Construction | 6 | 4 | 4 | 5 | 4 | 5 | 6 | 4 | 5 | 43 | 2nd |
| Fence | 4 | 6 | 6 | 6 | 5 | 6 | 5 | 5 | 4 | 47 | 1st |
| Food | 4 | 0 | 0 | 1 | 0 | 5 | 2 | 0 | 0 | 12 | 6th |
| Furniture | 6 | 1 | 3 | 4 | 5 | 0 | 4 | 4 | 4 | 31 | 4th |
| Ornamental | 3 | 4 | 2 | 3 | 2 | 3 | 5 | 1 | 6 | 29 | 5th |
| Sum | 27 | 20 | 18 | 21 | 22 | 24 | 26 | 19 | 25 | ||
| Rank | 1st | 7th | 9th | 6th | 5th | 4th | 2nd | 8th | 3rd | ||
The prioritization of Cordia africana reflects its multifunctional importance in rural livelihoods, particularly for construction, fencing, and energy. This aligns with findings from other Ethiopian regions, such as Asagirt District, where Cordia africana is valued for timber, shade, and construction [75]. Similarly, in the Bonga Forest of southwest Ethiopia, Olea europaea and Juniperus procera are frequently cited for durable wood and energy uses [20]. The high ranking of these species underscores their socio-economic significance beyond medicinal applications.
However, extensive exploitation for timber, firewood, and construction exerts considerable pressure on these populations, raising conservation concerns. Previous studies have identified Cordia africana and Olea europaea among Ethiopia’s most vulnerable medicinal plants due to combined medicinal and non-medicinal uses [2, 7, 8, 36, 90]. Comparable trends are reported in Kenya and Uganda, where heavy use of Cordia africana and Juniperus procera for construction and fuel leads to resource depletion [101, 102].
The low scores for food-related uses indicate that, although some species provide edible parts, their primary importance lies in non-food functions. These findings highlight the need for integrated management strategies that consider both medicinal and utilitarian demands. Direct matrix ranking proves to be an effective participatory tool for identifying species central to healthcare and community infrastructure.
Policies should prioritize high-demand species such as Cordia africana and Olea europaea, ensuring their long-term availability while maintaining ecosystem services. These insights are crucial for guiding rural development planning, balancing socio-economic needs with biodiversity conservation.
Jaccard’s similarity index
Jaccard’s Similarity Index (JSI) was used to assess the overlap in medicinal plant species composition between Menz Keya Gebreal District and other previously studied districts in Ethiopia. The analysis revealed considerable variation, with similarity values ranging from a high of 58.1% with Ensaro, sharing 47 species [11], to a low of 5.7% with Metema [2]. Other notable similarities included Asagirt (38.5%; 40 species) [75], Habru (35.8%; 44 species) [49], and Wadla (35.1%; 39 species) [12], whereas minimal overlap was observed with Quarit (7.3%) [90] and Fadis (8.6%) [84] (Table 10).
Table 10.
Jaccard similarity index comparing the present study with earlier research
| Study Area | a | b | Common species(c) | Jaccard index similarity (%) | References |
|---|---|---|---|---|---|
| Addi arkay | 95 | 104 | 17 | 9.3 | [41] |
| Ameya | 55 | 98 | 23 | 17.7 | [13] |
| Armachiho | 60 | 103 | 18 | 12.4 | [48] |
| Asagirt | 63 | 81 | 40 | 38.5 | [75] |
| Bita | 101 | 100 | 21 | 11.6 | [20] |
| Borecha | 61 | 101 | 20 | 14.1 | [93] |
| Dawuro | 234 | 81 | 40 | 14.5 | [82] |
| Dibatie | 132 | 83 | 38 | 21.4 | [76] |
| Ensaro | 54 | 74 | 47 | 58.1 | [11] |
| Fadis | 29 | 110 | 11 | 8.6 | [84] |
| Fofa | 139 | 96 | 25 | 11.9 | [22] |
| Gamo | 154 | 89 | 32 | 15.2 | [72] |
| Ganta | 147 | 95 | 26 | 12.0 | [103] |
| Gechi | 52 | 103 | 18 | 13.1 | [57] |
| Gera | 48 | 106 | 15 | 10.8 | [85] |
| Goro | 59 | 96 | 25 | 19.2 | [104] |
| Guraferda | 65 | 105 | 16 | 10.3 | [8] |
| Gurage | 210 | 87 | 34 | 12.9 | [105] |
| Habru | 90 | 77 | 44 | 35.8 | [49] |
| Hamar | 120 | 96 | 25 | 13.1 | [83] |
| Kelala | 56 | 95 | 26 | 20.8 | [61] |
| Metema | 75 | 111 | 10 | 5.7 | [2] |
| Mojana | 30 | 95 | 26 | 26.2 | [17] |
| Nensebo | 91 | 85 | 36 | 25.7 | [24] |
| Quara | 104 | 97 | 24 | 13.5 | [62] |
| Quarit | 98 | 107 | 14 | 7.3 | [90] |
| Sekela | 87 | 87 | 34 | 24.3 | [7] |
| Sheka | 232 | 87 | 34 | 11.9 | [47] |
| Sodo | 86 | 101 | 20 | 11.9 | [21] |
| Ticho | 59 | 102 | 19 | 13.4 | [106] |
| Tulo | 94 | 101 | 20 | 11.4 | [107] |
| Wadla | 68 | 82 | 39 | 35.1 | [12] |
| Yeki | 79 | 102 | 19 | 11.7 | [36] |
| Zuway | 53 | 101 | 20 | 14.9 | [51] |
Key: a number of medicinal plant species only in previous study, b number of medicinal plant species only in current study, c number of common medicinal plant species in current study and previous studies
These differences largely reflect the number of unique species in each area, influenced by local biodiversity, ecological conditions, and cultural practices. The high similarity with Ensaro and other ecologically adjacent districts suggests shared floristic composition, environmental conditions, and overlapping ethnocultural heritage. Previous studies indicate that geographical proximity often facilitates the exchange of ethnobotanical knowledge, promoting convergence in medicinal plant use [11, 20, 36]. Conversely, districts with low similarity likely differ in vegetation types, altitude, climate, or cultural dynamics, resulting in distinct medicinal floras and ethnobotanical practices [2].
Comparable patterns have been documented in other Ethiopian regions. For instance, Yeki and Bita districts exhibited high species similarity due to similar ecological conditions, whereas highland and lowland zones of Oromia showed markedly different plant communities [20, 36]. Internationally, similar trends in Nigeria and Nepal indicate that high JSI values correlate with cultural and ecological resemblance, while low values reflect unique regional knowledge systems [108, 109].
These findings highlight the dual influence of environmental and cultural factors on medicinal plant composition and use. Widely shared species across multiple sites underscore the reliance on versatile plants that may be prioritized for pharmacological validation. In contrast, locally unique species emphasize the importance of comprehensive documentation and site-specific conservation to preserve ethnobotanical diversity.
From a conservation and policy perspective, the JSI results advocate for regionally tailored strategies. Areas with high similarity may benefit from collaborative conservation and sustainable harvesting initiatives, whereas districts with low overlap require targeted preservation of culturally significant species. Furthermore, high JSI values provide opportunities for cross-regional knowledge-sharing platforms, supporting cooperative efforts in medicinal plant conservation, research, and sustainable use.
Rahman’s similarity index
Rahman’s Similarity Index (RSI) was used to evaluate the overlap in medicinal plant knowledge between Menz Keya Gebreal District and other Ethiopian regions. RSI values ranged from a maximum of 16.66% with Ensaro [11] to a minimum of 1.67% with Borecha [93]. Other notable similarities were observed with Asagirt (14.72%) [75] and Mojana (13.53%) [17], whereas Quara (1.81%) [62] and Ganta (1.90%) [103] exhibited the lowest similarity values (Table 11).
Table 11.
RSI comparing the current study (Menz Keya gebreal) with prior research reports
| Study Area | PS | Na | Nb | Nc | Nd | RSI (%) | References |
|---|---|---|---|---|---|---|---|
| Addi arkay | 112 | 95 | 104 | 17 | 6 | 2.85 | [41] |
| Ameya | 78 | 55 | 98 | 23 | 7 | 4.14 | [13] |
| Armachiho | 78 | 60 | 103 | 18 | 8 | 4.62 | [48] |
| Asagirt | 103 | 63 | 81 | 40 | 24 | 14.72 | [75] |
| Bita | 122 | 101 | 100 | 21 | 7 | 3.25 | [20] |
| Borecha | 81 | 61 | 101 | 20 | 3 | 1.67 | [93] |
| Dawuro | 274 | 234 | 81 | 40 | 13 | 3.80 | [82] |
| Dibatie | 170 | 132 | 83 | 38 | 14 | 5.85 | [76] |
| Ensaro | 101 | 54 | 74 | 47 | 25 | 16.66 | [11] |
| Fadis | 40 | 29 | 110 | 11 | 4 | 2.73 | [84] |
| Fofa | 164 | 139 | 96 | 25 | 6 | 2.36 | [22] |
| Gamo | 188 | 154 | 89 | 32 | 10 | 3.77 | [72] |
| Ganta | 173 | 147 | 95 | 26 | 5 | 1.90 | [103] |
| Gechi | 70 | 52 | 103 | 18 | 5 | 2.97 | [57] |
| Gera | 63 | 48 | 106 | 15 | 5 | 3.04 | [85] |
| Goro | 84 | 59 | 96 | 25 | 8 | 4.65 | [104] |
| Guraferda | 81 | 65 | 105 | 16 | 4 | 2.19 | [8] |
| Gurage | 244 | 210 | 87 | 34 | 13 | 4.08 | [105] |
| Habru | 134 | 90 | 77 | 44 | 23 | 12.23 | [49] |
| Hamar | 145 | 120 | 96 | 25 | 8 | 3.43 | [83] |
| Kelala | 82 | 56 | 95 | 26 | 15 | 9.23 | [61] |
| Metema | 85 | 75 | 111 | 10 | 8 | 4.25 | [2] |
| Mojana | 56 | 30 | 95 | 26 | 18 | 13.53 | [17] |
| Nensebo | 127 | 91 | 85 | 36 | 16 | 8.16 | [24] |
| Quara | 128 | 104 | 97 | 24 | 4 | 1.81 | [62] |
| Quarit | 112 | 98 | 107 | 14 | 12 | 5.79 | [90] |
| Sekela | 121 | 87 | 87 | 34 | 15 | 7.77 | [7] |
| Sheka | 266 | 232 | 87 | 34 | 11 | 3.21 | [47] |
| Sodo | 106 | 86 | 101 | 20 | 6 | 2.98 | [21] |
| Ticho | 78 | 59 | 102 | 19 | 6 | 3.44 | [106] |
| Tulo | 104 | 94 | 101 | 20 | 6 | 2.87 | [107] |
| Wadla | 107 | 68 | 82 | 39 | 21 | 12.5 | [12] |
| Yeki | 98 | 79 | 102 | 19 | 8 | 4.16 | [36] |
| Zuway | 73 | 53 | 101 | 20 | 9 | 5.45 | [51] |
Key: PS Number of Medicinal Plants Reported in Previous Studies, Na Medicinal Plants Only in Previous Studies, Nb Medicinal Plants Only in current Study, Nc Medicinal Plants Common in Both Areas, Nd Common Medicinal Plants with Similar Uses, RSI Rahman’s Similarity Index in %
These variations reflect differences in shared species (Nc) and their reported uses (Nd) among districts, highlighting the influence of geographical proximity, ecological conditions, and cultural exchange on traditional medicinal knowledge. Higher RSI values with Ensaro, Asagirt, and Mojana suggest floristic and cultural affinities, likely shaped by similar agroecological zones and inter-community interactions. Comparable patterns have been reported in Amhara and Oromia, where neighboring districts exhibited higher RSI due to shared climate, vegetation, and social connectivity [2, 11, 17].
Conversely, low RSI values in districts such as Borecha and Quara indicate ecological divergence, cultural specificity, or linguistic barriers, resulting in distinct medicinal practices. This aligns with other Ethiopian ethnobotanical studies showing that regions separated by elevation gradients or ethnic boundaries display significant variation in plant use [8, 20, 22]. Internationally, similar trends are observed. In Nepal, RSI comparisons across mountain, hill, and Terai regions revealed that ecological diversity and cultural heterogeneity strongly influence medicinal plant knowledge, with similarity declining as environmental and cultural distances increase [11]. Likewise, studies in Cameroon showed that distinct ethnic groups possess unique species usage patterns, emphasizing the role of cultural heritage in shaping ethnobotanical knowledge [110].
High-RSI districts indicate communities with shared species and therapeutic practices, suggesting potential for collaborative conservation and ethnopharmacological validation. In contrast, low-RSI districts highlight the need for localized documentation to safeguard rare or culturally significant species. Given the threats posed by environmental degradation and modernization, understanding these inter-regional knowledge patterns is critical for targeted biocultural conservation.
From a policy perspective, the RSI analysis underscores the importance of regionally tailored conservation and sustainable use strategies. Cross-regional collaboration can facilitate knowledge sharing, resource exchange, and harmonized conservation initiatives, supporting both biodiversity preservation and the maintenance of traditional medicinal systems.
Novel ethnobotanical findings
The ethnobotanical survey in Menz Keya Gebreal District, North Shewa Zone, Ethiopia, documented previously unreported medicinal uses of several plant species, enriching the region’s traditional pharmacopoeia and highlighting either localized innovation or underreported traditional knowledge. Unique applications were recorded for both well-known and lesser-studied plants.
Notable findings include the use of Pennisetum pedicellatum for leishmaniasis (locally “Kunchir”), a neglected tropical disease, and Hordeum vulgare L. for dandruff management, reflecting adaptation to contemporary health challenges. Other documented uses include Becium grandiflorum for abdominal pain; Laggera crispa and Premna schimperi for seasonal allergies (“Choq”); Cardiospermum corindum for infertility; oral administration of Tacazzea conferta for snakebite; Glinus lotoides for tapeworm infections; Vernonia leopoldi for hemorrhoids; and Verbascum sinaiticum for jaundice.
These findings correspond with reports from other Ethiopian districts such as Metema, Bita, Quara, and Addi Arkay, where novel uses of Glinus lotoides, Tacazzea conferta, and Cardiospermum corindum have been observed [2, 20, 41, 62]. Similar discoveries in countries like Nigeria and Nepal suggest that traditional knowledge evolves in response to emerging health needs.
The use of Pennisetum pedicellatum against leishmaniasis is particularly noteworthy given the global disease burden and limited therapeutic options. Likewise, Cardiospermum corindum and Premna schimperi show potential in addressing infertility and allergic conditions, highlighting the need for scientific validation and potential pharmaceutical development. The anthelmintic application of Glinus lotoides aligns with previous ethnopharmacological studies, while the reported uses of Tacazzea conferta and Verbascum sinaiticum for snakebite and jaundice warrant further phytochemical and toxicological investigation.
These findings demonstrate a dynamic and sophisticated ethnomedical system in Menz Keya Gebreal, capable of addressing diverse ailments, including infectious and parasitic diseases, reproductive, gastrointestinal, and dermatological disorders. Frequent citation of certain species reflects strong community consensus regarding their perceived efficacy.
The novel insights presented here have significant implications for ethnopharmacology, conservation, and public health. Priority should be given to medicinal plants such as Glinus lotoides, Tacazzea conferta, and Cardiospermum corindum for scientific validation and exploration of drug development potential. Simultaneously, conservation strategies must target ecologically and culturally important species at risk of overharvesting and habitat degradation due to increasing human pressures.
Comparison of informant groups based on the botanical ethnoknowledge index (BEI)
The application of the Botanical Ethnoknowledge Index (BEI) and t-test in Menz Keya Gebreal District revealed significant variation in medicinal plant knowledge across gender, informant status, education, and age.
Male informants (N = 53) demonstrated significantly greater knowledge than females (N = 27), with mean scores of 4.8 ± 1.6 and 2.4 ± 1.5, respectively (t = 6.7, p < 0.05) (Table 12). Males also reported more species (ms = 6.5) and citations per species (mc = 9.5), citing 114 species in total (BEI = 0.196). Females cited 90 species (ms = 2.5; mc = 5.3; BEI = 0.147) (Table 13). This disparity reflects sociocultural roles, as men traditionally engage more in farming and foraging, which increases exposure to medicinal plants. Similar gendered patterns have been reported in Ethiopia (Bita, Yeki) [20, 36] and internationally in Uganda and India [71, 111, 112]. However, other studies document equal or greater female knowledge [64, 86, 103, 113], underscoring the importance of inclusive ethnobotanical research that fully incorporates women’s contributions [22, 72, 76].
Table 12.
Comparison of informant groups using the botanical ethnoknowledge index (BEI) in the study area
| Informant groups | N | Ms | Sg | mc | St | F | BEI | |
|---|---|---|---|---|---|---|---|---|
| Gender | Male | 53 | 6.5 | 114 | 9.5 | 121 | 0.885 | 0.196 |
| Female | 27 | 2.5 | 90 | 5.3 | 0.147 | |||
| Informant type | Key | 20 | 7.2 | 121 | 12.3 | 121 | 0.899 | 0.606 |
| General | 60 | 2.4 | 63 | 2.5 | 0.212 | |||
| Education level | Illiterate | 59 | 5.3 | 120 | 12.1 | 121 | 0.897 | 0.220 |
| Literate | 21 | 2.1 | 62 | 2.7 | 0.073 | |||
| Age | 20–30 | 15 | 1.3 | 21 | 1.1 | 121 | 0.873 | 0.0203 |
| 31–50 | 25 | 3.5 | 84 | 4.7 | 0.137 | |||
| 52–85 | 40 | 7.9 | 112 | 12.5 | 0.308 | |||
Key: N Number of participants in a particular group, ms Mean number of species reported per participant in a particular group, sg Total number of species reported by all participants in a particular group, mc Mean number of citations per species in a particular group, st Total number of species reported by all compared groups in the study, BEI Botanical Ethnoknowledge Index, F correcting factor
Table 13.
Medicinal plants knowledge among informant groups (t-test)
| Characters | Informant groups | N | Mean ± SD | t –value | p – value |
|---|---|---|---|---|---|
| Gender | Male | 53 | 4.8 ± 1.6 | 6.7 | P < 0.05 |
| Female | 27 | 2.4 ± 1.5 | |||
| Literacy level | Illiterate | 59 | 4.1 ± 1.9 | 5.7 | P < 0.05 |
| Literate | 21 | 2.1 ± 1.3 | |||
| Experience of Informant | Key informant | 20 | 5.5 ± 1.4 | 9.4 | P < 0.05 |
| General informant | 60 | 2.4 ± 1.3 |
Key informants (N = 20), primarily traditional healers and elders, displayed substantially higher knowledge (mean score 5.5 ± 1.4) compared to general informants (N = 60; 2.4 ± 1.3; t = 9.4, p < 0.05). They reported an average of 7.2 species per person (121 species total, mc = 12.3, BEI = 0.606), whereas general informants cited only 2.4 species per person (BEI = 0.212). This concentration of knowledge among specialists is consistent with findings from other Ethiopian districts (Ganta Afeshum, Asagirt) [75, 103] and from Kenya and Nepal [114, 115]. The vulnerability of this knowledge pool highlights the urgency of documentation and mentorship initiatives to safeguard intergenerational transfer [47, 75, 111].
Illiterate informants (N = 59) reported significantly higher knowledge (4.1 ± 1.9) than literate informants (N = 21; 2.1 ± 1.3; t = 5.7, p < 0.05). Illiterate respondents cited more species (ms = 5.3; BEI = 0.220), whereas literate individuals averaged only 2.1 species (BEI = 0.073). Similar patterns, where formal education correlates with reduced ethnobotanical knowledge, have been observed in Ethiopia [2, 8, 20, 69, 76], Pakistan, and Brazil [116, 117]. This inverse relationship reflects the reliance of formal education on written systems, which often marginalizes orally transmitted traditions. Integrating ethnobotanical content into curricula and promoting interdisciplinary approaches could help preserve knowledge while supporting cultural resilience [2, 36, 41].
Age was a strong predictor of ethnobotanical knowledge. Older informants (52–85 years, N = 40) reported the highest knowledge (MS = 7.9; BEI = 0.308), followed by middle-aged participants (31–50 years, N = 25; MS = 3.5; BEI = 0.137), and younger informants (20–30 years, N = 15; MS = 1.3; BEI = 0.0203). A one-way ANOVA (Table 14) confirmed significant differences between age groups (p < 0.05), while Pearson correlation (r = 0.653, p < 0.001) and linear regression (B₁ = 0.148, R² = 0.575) indicated that age explained 57.5% of the variation in knowledge (Fig. 5). These findings suggest knowledge accumulation through prolonged exposure and cultural immersion. Comparable trends of generational decline have been reported across Ethiopia [2, 51, 103] and globally in Nepal, Mexico, China, and Kenya [77, 118, 119]. Modernization, migration, and reduced interaction with natural environments contribute to this decline. Urgent documentation, youth engagement, and intergenerational programs such as apprenticeships, workshops, and community-based learning are therefore essential to ensure continuity of ethnobotanical practices [11, 12, 47].
Table 14.
Age categories with medicinal plants knowledge (One way ANOVA)
| Source of Variation | Df | SS | MS = SS/Df | F Ratio | P-value |
|---|---|---|---|---|---|
| Between Groups | 2 | 381.5 | 190.75 | 48.7 | P < 0.05 |
| Residual (within) | 77 | 301.2 | 3.91 | ||
| Total | 79 | 682.7 | 194.66 |
Df degree of freedom, SS Sum of Squares, MS Mean of Square, Significant codes: 0.05
Fig. 5.
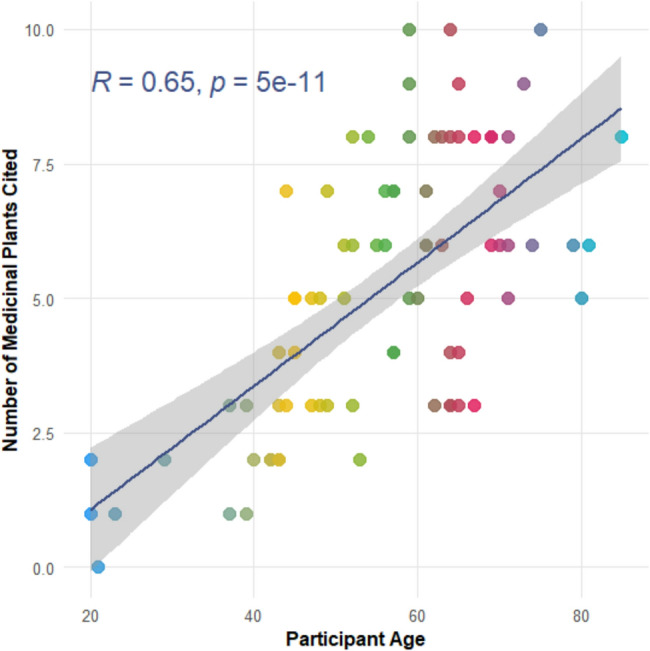
Correlation models of informants age categories with medicinal plant knowledge
Transmission of traditional medicinal knowledge
In Menz Keya Gebreal District, traditional medicinal knowledge is predominantly transmitted within families. Parents were the most frequently cited source (42.5%, n = 34), followed by grandparents (23.7%, n = 19), siblings (17.5%, n = 14), and uncles or aunts (15%, n = 12). Only one respondent (1.3%) mentioned neighbors, highlighting the limited role of community transmission beyond the family. This indicates a primarily intergenerational, family-centered mode of knowledge transfer, occurring through oral instruction, observation, and hands-on experience, with elders serving as key custodians.
Similar patterns have been reported in Ethiopian districts such as Quarit, Metema, and Ensaro, where medicinal knowledge is closely guarded and selectively passed within trusted family circles, sometimes under ritual secrecy [2, 11, 90]. In Bita and Yeki, traditional healers intentionally restrict information flow to preserve the integrity and exclusivity of remedies [20, 36]. Although religious institutions like the Ethiopian Orthodox Church contribute to preserving knowledge through ancient manuscripts, these formal channels remain secondary to oral traditions [11, 41]. Globally, many indigenous societies rely on oral transmission, though some have incorporated written records and formal education to enhance preservation and accessibility [71, 114, 120–122].
While the familial model supports cultural continuity and mentorship, it also poses risks. Knowledge that is undocumented and closely held is vulnerable to loss, particularly if younger generations disengage or if elders die before transmitting it [11, 64]. The minimal involvement of non-family members further limits dissemination and institutional support, a challenge also observed in South Asia and Latin America [44, 45].
These findings underscore the urgent need for documentation and community-driven initiatives to safeguard ethnomedicinal heritage. Engaging elders, youth, and researchers in respectful, collaborative efforts can facilitate knowledge transfer without compromising cultural values [20, 51, 61, 69]. Incorporating ethnomedicinal knowledge into education, conservation, and public health planning can enhance its relevance and ensure its survival. Approaches such as storytelling, apprenticeships, and participatory learning have proven effective in promoting intergenerational dialogue and sustaining traditional practices [18, 19, 62, 64]. Supporting these systems preserves cultural identity while strengthening the resilience and accessibility of healthcare in rural Ethiopia and similar global contexts.
Threats to medicinal plants in Menz Keya Gebreal District
The analysis of threats to medicinal plants in Menz Keya Gebreal District revealed that agricultural expansion is the most significant factor, receiving the highest total score of 68 from respondents. This was followed by firewood collection (58), charcoal production (52), and construction activities (50), highlighting that direct human activities are the primary drivers of medicinal plant depletion in the area. Other factors, including overgrazing (47), fodder collection (43), urbanization (41), and drought (34), were perceived as less immediate threats, indicating that while environmental pressures affect medicinal plants, anthropogenic impacts dominate (Table 15).
Table 15.
Threating factors of medicinal plants in Menz Keya district
| Threats to medicinal plant | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | Total | Rank |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agricultural expansion | 7 | 6 | 8 | 6 | 8 | 7 | 7 | 6 | 6 | 7 | 68 | 1st |
| Charcoal production | 6 | 5 | 6 | 7 | 5 | 4 | 4 | 3 | 5 | 7 | 52 | 3rd |
| Construction | 6 | 5 | 5 | 6 | 5 | 4 | 5 | 4 | 4 | 6 | 50 | 4th |
| Drought | 3 | 4 | 4 | 5 | 2 | 3 | 4 | 3 | 4 | 2 | 34 | 8th |
| Firewood | 7 | 5 | 6 | 8 | 4 | 5 | 5 | 5 | 6 | 7 | 58 | 2nd |
| Fodder | 4 | 5 | 4 | 5 | 5 | 4 | 5 | 4 | 4 | 3 | 43 | 6th |
| Overgrazing | 5 | 5 | 4 | 4 | 6 | 4 | 5 | 4 | 5 | 5 | 47 | 5th |
| Urbanization | 4 | 4 | 5 | 5 | 3 | 4 | 5 | 3 | 4 | 4 | 41 | 7th |
R Respondent’s
These findings are consistent with broader ethnobotanical research in Ethiopia. Studies from Bita District and Addi Arkay similarly identify land-use change, particularly agricultural expansion, and unsustainable biomass harvesting as major threats to medicinal plant biodiversity [20, 21, 41]. Research from Bita District also highlights the detrimental effects of agrochemicals such as herbicides and pesticides, which harm medicinal plants directly and disrupt pollinator populations essential for plant reproduction [20]. Such ecological disturbances can reduce seed set, genetic diversity, and population viability, reflecting patterns observed in Menz Keya Gebreal.
Comparable threat patterns have been reported internationally. In Uganda, Kenya, and Tanzania, agricultural encroachment, fuelwood collection, and charcoal production are major causes of medicinal plant habitat loss and population decline [39, 66, 123–125]. In Menz Keya, the prominence of firewood and charcoal production aligns with widespread reliance on biomass fuels in rural settings, contributing to forest degradation and loss of medicinal plant species [24, 26, 79]. Construction activities, often involving selective logging, further degrade habitats and limit regeneration potential.
These findings underscore the urgent need for coordinated, multifaceted conservation interventions. Sustainable land management approaches, such as agroforestry integrating medicinal plants with crops and trees, have proven effective in mitigating habitat loss and promoting biodiversity conservation in Ethiopia and neighboring countries [27, 28]. Regulated and community-managed access to forest resources, alongside promotion of alternative energy sources such as biogas or solar, can reduce dependence on firewood and charcoal, alleviating harvesting pressure [36, 41]. Raising public awareness about the ecological and cultural importance of medicinal plants is essential to foster community stewardship. Supporting eco-friendly livelihoods that reduce resource extraction can complement conservation goals, as demonstrated by integrated community-based projects in Ethiopia and other African countries [50, 79, 83].
Threatened medicinal plants in Menz Keya Gebreal District
The analysis of threatened medicinal plants in Menz Keya Gebreal District identified Echinops kebercho as the most critically endangered species, receiving the highest total score of 38 and consistently ranked by respondents as the most at risk. Other highly threatened species included Cordia africana (33), Withania somnifera (20), Gossypium herbaceum (16), and Dodonaea angustifolia (13) (Table 16). These rankings reflect local perceptions of species facing the greatest threats from overharvesting, habitat loss, and environmental pressures.
Table 16.
Threatened medicinal plant in the district
| Threatened medicinal plant | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | Total | Rank |
|---|---|---|---|---|---|---|---|---|---|---|
| Cordia africana | 4 | 5 | 4 | 5 | 3 | 4 | 4 | 4 | 33 | 2nd |
| Dodonea angustifolia | 1 | 3 | 1 | 3 | 2 | 1 | 1 | 1 | 13 | 5th |
| Echinops kebercho | 5 | 4 | 5 | 4 | 5 | 5 | 5 | 5 | 38 | 1st |
| Gossypium herbaceum | 2 | 1 | 2 | 1 | 4 | 2 | 2 | 2 | 16 | 4th |
| Withania somnifera | 3 | 2 | 3 | 2 | 1 | 3 | 3 | 3 | 20 | 3rd |
R Respondants
The pronounced vulnerability of Echinops kebercho and Cordia africana likely stems from high medicinal demand, extensive traditional use, and overexploitation, compounded by limited regeneration and habitat degradation. Conversely, species such as Dodonaea angustifolia were perceived as less threatened, possibly due to broader distribution or lower harvesting pressure. These findings are consistent with other ethnobotanical studies in Ethiopia, which report that culturally and pharmacologically valuable medicinal plants are often the most at risk [20, 22, 49]. For example, research in Adi Arkay District documented Echinops kebercho as Near Threatened (NT) under the IUCN Red List, alongside other endemic species like Millettia ferruginea and Thymus schimperi [41].
Internationally, similar patterns are observed. In India, Withania somnifera faces increasing threat from unsustainable harvesting and habitat loss [126], while Cordia africana populations in Sidama have declined due to logging and fuelwood collection [127]. Comparable challenges exist across South Asia, highlighting the global need to conserve culturally important medicinal plants [128].
The recognition of these species’ threatened status by local communities presents an opportunity to integrate community-based conservation with scientific approaches. Recommended strategies include in situ conservation through community-managed forest reserves, coupled with ex situ propagation in home gardens, nurseries, and botanical gardens to support population recovery [26, 27, 36]. Promoting sustainable harvesting techniques, seasonal collection restrictions, and awareness campaigns can further reduce pressure on wild populations. Integrating indigenous knowledge with ethnopharmacological research enhances understanding of species ecology and sustainable use, enabling culturally appropriate conservation programs [7, 15, 28].
Capacity-building initiatives for local stakeholders, alongside strengthened policy and legal frameworks, are essential to ensure effective conservation and sustainable resource management [75, 76].
Management and conservation of medicinal plants in Menz Keya Gebreal District
Local knowledge in Menz Keya Gebreal District demonstrates active community involvement, particularly by traditional healers, in managing and conserving medicinal plants. These practices support a range of needs, including healthcare, food, construction, fuel, fodder, trade, and spiritual rituals. Informants exhibited extensive ecological knowledge, encompassing plant habitat preferences, seasonal harvesting patterns, sustainable collection methods, and awareness of species’ conservation status. Traditional healers often lead conservation efforts by cultivating medicinal plants in home gardens and protecting them in sacred spaces, particularly around Orthodox Tewahedo churches.
Church forests function as culturally protected in situ conservation zones, preserving medicinal and multipurpose species. Spiritual and utilitarian values attached to these areas reinforce conservation through cultural norms and social cohesion. Field observations confirm that sacred groves harbor better-preserved plant populations than unmanaged wildlands, a pattern also documented in southern Ethiopia and parts of East Africa [20, 66]. Ethiopia’s Green Legacy campaign and comparable community-based tree planting initiatives in Nepal, Kenya, and Uganda further underscore the role of grassroots environmental stewardship [39, 41, 66, 129]. Cultural gatherings such as Mahiber, conducted in sacred sites, strengthen conservation by linking traditional authority with ecological protection. Species thriving in these environments include Podocarpus falcatus, Juniperus procera, Olea europaea, Phoenix reclinata, and Hagenia abyssinica.
Despite these efforts, some species, including Echinops kebericho, Withania somnifera, and Cordia africana, remain threatened due to overharvesting and habitat degradation, reflecting challenges observed elsewhere in Ethiopia and tropical Africa [20, 22, 45, 49]. Home gardens, live fences, and roadside plantings (e.g., Rosmarinus halepensis, Vernonia amygdalina, Eucalyptus globulus) expand the cultivation of medicinal plants, yet these practices remain largely informal and lack systematic support. Similar techniques are reported in Oromia and Southern Nations regions, though consistent community-led conservation structures are limited [12, 22, 93].
The gradual erosion of indigenous knowledge, particularly among youth, accelerated by modernization and shifting cultural values, poses additional challenges. Addressing this requires integrating ethnobotanical knowledge into education, raising awareness, and promoting the ecological, cultural, and health significance of medicinal plants. Studies from West Africa and South Asia highlight the effectiveness of combining traditional wisdom with formal education and policy frameworks to ensure sustainability [71, 79, 129, 130]. Empowering communities especially youth through apprenticeships, workshops, and participatory conservation projects can revitalize traditional practices.
Implications of medicinal plant utilization in Menz Keya Gebreal District
Medicinal plants remain a cornerstone of primary healthcare in the district, particularly in rural areas with limited access to formal medical services. They provide affordable, culturally accepted treatments for common ailments such as digestive disorders, respiratory illnesses, and skin infections. Frequently used species include Otostegia integrifolia, Withania somnifera, Verbascum sinaiticum, Rumex nervosus, and Cordia africana, consistent with reports from Ethiopia [8, 36, 41, 90] and other Sub-Saharan and South Asian regions [44, 70, 71, 79, 121].
Beyond therapeutic use, medicinal plants support preventive health by enhancing immunity and general well-being, reflecting the community’s intricate understanding of local ecology and health management. Many species also provide nutritional benefits, offering vitamins, minerals, and antioxidants that support food security and mitigate malnutrition. Examples include Cordia africana, Persea americana, Urtica simensis, Rosa abyssinica, Dovyalis abyssinica, Carica papaya, Citrus medica, Mangifera indica, Carissa spinarum, and Cucurbita pepo. This dual role in health and nutrition mirrors findings from other Ethiopian districts and similar ecological contexts in West Africa and South Asia [41, 103, 131, 132]. Traditional knowledge promoting sustainable harvesting and cultivation further enhances agricultural biodiversity and ecosystem resilience.
Environmental benefits of medicinal plants include biodiversity conservation and ecosystem balance. Practices such as selective harvesting and in situ cultivation prevent depletion. For instance, cultivating Festuca glauca (“Guassa”) aids land restoration by reducing soil erosion and promoting regeneration of native flora, sustaining local food chains. Similar restoration initiatives using native medicinal plants have been reported in Kenya and Nepal [39, 115, 120].
Culturally, medicinal plants are embedded in the social fabric, supporting spiritual rituals, ceremonies, and identity. Intergenerational transmission by elders and traditional healers preserves intangible cultural heritage, strengthening community cohesion and linking current generations to ancestral wisdom [38, 87, 106, 132]. However, modernization, urban migration, and changing cultural values threaten this knowledge transfer, risking loss of traditional health practices and cultural identity, a trend observed globally [94, 133–135].
Preserving and revitalizing medicinal plant knowledge is therefore critical for sustaining cultural continuity, promoting community health, and fostering resilience against socio-environmental changes.
Study limitations
Despite providing comprehensive insights into the ethnobotanical knowledge, use, and conservation of medicinal plants in Menz Keya Gebreal District, this study has some limitations. Data were primarily based on self-reported information from key informants, which may introduce recall bias or subjective interpretation. Quantitative indices such as ICF, FL, RFC, and ROP, while informative, do not assess pharmacological efficacy or chemical composition. Seasonal variations and accessibility constraints may have limited plant collection, potentially overlooking rare or less-utilized species. Additionally, the study focused on specific localities, limiting broader generalization. Future research should incorporate phytochemical analyses and longitudinal monitoring to validate and expand these findings.
Conclusion and recommendations
This study provides the first comprehensive ethnobotanical documentation of Menz Keya Gebreal District, revealing a rich diversity of medicinal plants and the sociocultural factors shaping their use. Quantitative analyses indicated that age, gender, education, and healer status significantly influence traditional knowledge, with elders and traditional healers serving as primary custodians. High informant consensus and indices (ICF, FL, RFC, ROP, CVI) highlighted culturally and therapeutically important species, including Hagenia abyssinica, Ocimum lamiifolium, Echinops kebericho, and Clutia abyssinica, which are promising candidates for pharmacological and phytochemical validation. Remedies predominantly employ fresh plant materials, typically administered orally, reflecting local beliefs in their potency.
The study identified major threats to medicinal plants, including agricultural expansion, overharvesting, habitat degradation, and limited intergenerational knowledge transfer. Multipurpose species such as Echinops kebericho, Cordia africana, and Withania somnifera were perceived as highly vulnerable. Knowledge transmission remains largely family-based, increasing the risk of erosion under modernization and reduced youth engagement.
To ensure the sustainability of medicinal plants and associated knowledge, community-based conservation strategies are recommended. These include in situ protection in sacred groves and home gardens, ex situ propagation in nurseries, sustainable harvesting practices, seasonal collection restrictions, and promotion of alternative energy sources to reduce pressure on wild populations. Integrating ethnobotanical knowledge into educational programs, apprenticeships, and workshops can strengthen intergenerational transfer and encourage youth participation.
Policy support and legal frameworks should promote sustainable utilization, market regulation, and biodiversity conservation. Coupling traditional knowledge with scientific validation and formal healthcare strategies will safeguard medicinal plant diversity, enhance rural healthcare, preserve cultural heritage, and ensure long-term sustainability in Menz Keya Gebreal District and comparable contexts.
Supplementary Information
Acknowledgements
We sincerely thank the residents of Menz keya Gebreal district, North shewa zone, Ethiopia, especially the local community, for their generosity in sharing their knowledge about medicinal plants and for their warm hospitality throughout our research. We are also grateful to the cultural elders for openly sharing their valuable insights about these plants. Our appreciation extends to the local administrative and agricultural offices of the district, along with the health center and kebele administrators, for their support, cooperation, and for providing essential information.
Clinical trial number
Not applicable.
Abbreviations
- BEI
Botanical Ethnoknowledge Index
- RFC
Relative frequency of citation
- RPL
Relative Popularity Level
- ROP
Rank order priority
- FGD
Focus group discussion
- JSI
Jaccard similarity index
- PR
Preference ranking
- DMR
Direct matrix ranking
- RSI
Rahman’s Similarity Index
- FL
Fidelity level
- ICF
Informant consensus factor
- NMSA
National meteorology service agency
Authors’ contributions
All authors contributed signifcantly to this original research. MY was responsible for drafting the manuscript and methodology, as well as managing data collection. AT concentrated on language editing, verifying the botanical names of plants, and conducting a comprehensive review. ZK also focused on language editing and the verifcation of botanical names. AA verifed the data analysis, created the climatogram for the study area, and prepared the map of the study area. Each author has reviewed and approved the fnal manuscript.
Funding
No funding.
Data availability
Data is provided within the manuscript.
Declarations
Ethics approval and consent to participate
Prior to starting data collection, we secured permission from the Menz Keya Gebreal district administration offices. Informants were verbally asked for their consent before interviews and group discussions, and their data was recorded only upon their approval. We also obtained explicit consent from them for the publication of any personal information collected. Our study strictly followed the ethical guidelines outlined by the Debre Birhan University declaration, which governs research involving human and animal subjects, and was approved by the university’s Institutional Review Board. We guaranteed that all participants provided informed consent and that their rights and welfare were protected throughout the research process, in accordance with the principles of the Declaration of Helsinki. Participants were informed about the study’s objectives, expected outcomes, potential benefits, and any risks involved. Written consent was obtained from each individual prior to data collection. The plant collection was conducted on both private and public land, with the necessary permissions from landowners and the relevant authorities in Menz Keya Gebreal district.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thakur N, Kumar N, Kumar S, Sharma A, Ahlawat YK, Malik A, et al. Ethnobiological survey on medicinal plants used by Gaddi and Gujjar tribes of riparian region of Beas river of Himachal Pradesh in North Western Himalayas, India. Clin Phytoscience. 2025;11(1):4. [Google Scholar]
- 2.Tadesse D, Lulekal E, Masresha G. Ethnopharmacological study of traditional medicinal plants used by the people in Metema district, Northwestern Ethiopia. Front Pharmacol. 2025;16:1535822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie J, Wang P, Jiang Q, Chen Q, Xiao M, He W, et al. Medicinal plant use by the Tujia people in Northeastern Guizhou, China: an ethnobotanical study. Front Pharmacol. 2025;16:1522456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO global report on traditional and complementary medicine. World Health Organization. Accessed 16 May 2019.
- 5.Lu Z, Chen H, Lin C, Ou G, Li J, Xu W. Ethnobotany of medicinal plants used by the Yao people in Gongcheng County, Guangxi, China. J Ethnobiol Ethnomed. 2022;18(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laldingliani TB, Thangjam NM, Zomuanawma R, Bawitlung L, Pal A, Kumar A. Ethnomedicinal study of medicinal plants used by Mizo tribes in Champhai district of Mizoram, India. J Ethnobiol Ethnomed. 2022;18(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dessie Y, Amsalu N. Ethnobotanical study of medicinal plants in Sekela District, Northwestern Ethiopia. Phytomedicine Plus. 2024;4(3):100602. [Google Scholar]
- 8.Awoke A, Gudesho G, Akmel F, Shanmugasundaram P. Traditionally used medicinal plants for human ailments and their threats in Guraferda District, Benchi-Sheko zone, Southwest Ethiopia. J Ethnobiol Ethnomed. 2024;20(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayalew H, Tewelde E, Abebe B, Alebachew Y, Tadesse S. Endemic medicinal plants of Ethiopia: ethnomedicinal uses, biological activities and chemical constituents. J Ethnopharmacol. 2022;293:115307. [DOI] [PubMed] [Google Scholar]
- 10.Abebe BA, Chane Teferi S. Ethnobotanical study of medicinal plants used to treat human and livestock ailments in Hulet Eju Enese Woreda, East Gojjam zone of Amhara region, Ethiopia. Evidence-Based Complement Altern Med. 2021;2021(1):6668541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teshome M, Kebede F, Yohannes T. An ethnobotanical survey of Indigenous knowledge on medicinal plants used by communities to treat various diseases around Ensaro District, North Shewa zone of Amhara regional State, Ethiopia. Scientifica. 2023;2023(1):5575405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yosef S, Lulekal E, Debela A, Tahir M. Medicinal plants used to treat human ailments in Wadla District, Northern Ethiopia: an ethnobotanical approach. Phytomedicine Plus. 2025;5(1):100683. [Google Scholar]
- 13.Tadesse T, Teka A. Ethnobotanical study on medicinal plants used by the local communities of Ameya District, Oromia Regional State, Ethiopia. Evidence-Based Complementary and Alternative Medicine. 2023;2023(1):5961067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamene S, Negash M, Makonda FB, Chiwona-Karltun L, Kibret KS. Ethnobotanical study on medicinal plant knowledge among three ethnic groups in peri-urban areas of south-central Ethiopia. J Ethnobiol Ethnomed. 2023;19(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aragaw HS, Nohr D, Callo-Concha D. Nutritional potential of underutilized edible plant species in coffee agroforestry systems of Yayu, Southwestern Ethiopia. Agroforest Syst. 2021;95(6):1047–59. [Google Scholar]
- 16.Asfaw A, Lulekal E, Bekele T, Debella A, Debebe E, Sisay B. Medicinal plants used to treat livestock ailments in Ensaro district, North Shewa Zone, Amhara regional state, Ethiopia. BMC Vet Res. 2022;18(1):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.HAILE AA. Ethnobotanical study of medicinal plants used by local people of mojana wadera woreda, north shewa zone, amhara region, Ethiopia. Asian J Ethnobiol. 2022;5(1).
- 18.Agize M, Asfaw Z, Nemomissa S, Gebre T. Ethnobotany of vascular plants use, conservation and management practice in the homegardens by the people of Dawuro in Southwestern Ethiopia. J Ethnobiol Ethnomed. 2025;21(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaynemsa KG. Plant Biodiversity Conservation in Ethiopia. Cham: Springer International Publishing. 2022. 10.1007/978-3-031-20225-4.
- 20.Awoke A, Gudesho G, Chane K, Siyum Y, Tilahun W, Gebremedhin H, et al. Traditionally used phytomedicines and their associated threats in Bita district, Southwestern Ethiopia. J Ethnobiol Ethnomed. 2025;21(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tessema ZK, Nibret E, Awoke A. Ethnobotanical study of medicinal plants used for the treatment of human ailments in the Sodo district of East Gurage zone central Ethiopia. Ethnobot Res Appl. 2025;30:1–45. [Google Scholar]
- 22.Lulesa F, Alemu S, Kassa Z, Awoke A. Ethnobotanical investigation of medicinal plants utilized by Indigenous communities in the Fofa and Toaba sub-districts of the yem Zone, central Ethiopian region. J Ethnobiol Ethnomed. 2025;21(1):1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geta O, Hansha H, Asafa O, Amde A. Ethnobotanical study of medicinal plants in ale Woreda, South West Ethiopia. J Med Plants. 2020;8(5):121–9. [Google Scholar]
- 24.Girma Z, Abdela G, Awas T. Ethnobotanical study of medicinal plant species in Nensebo District, south-eastern Ethiopia. Ethnobot Res Appl. 2022;24:1–25. [Google Scholar]
- 25.Yirga G, Teferi M, Gebreslassea Y. Ethnozoological study of traditional medicinal animals used by the people of Kafta-Humera District, Northern Ethiopia. Int J Med Med Sci. 2011;3(10):316–20. [Google Scholar]
- 26.Tefera Y, Lulekal E, Warkineh B. Human–forest interaction of useful plants in the Wof Ayzurish Forest, North Showa Zone, ethiopia: cultural significance index, conservation, and threats. J Ethnobiol Ethnomed. 2025;21(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alemneh D. Ethnobotanical study of medicinal plants used for the treatment of domestic animal diseases in Yilmana densa and Quarit districts, West Gojjam zone, Amhara region, Ethiopia. Ethnobot Res Appl. 2021;22:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayele AH, Seid A, Mekonnen AB, Adnew WW, Yemata G. Ethnobotanical study of the traditional use of medicinal plants used for treating human diseases in selected districts of West Gojjam Zone, Amhara Region, Ethiopia. Phytomedicine Plus. 2024;4(3):100620. [Google Scholar]
- 29.Hailemariam M, Mekonen S. Extent and awareness to use animals for traditional medicine and attitudes towards ethnozoological knowledge among communities of Menz Keya Gabriel District, North Ethiopia. Egyptian Academic Journal of Biological Sciences, B Zoology. 2021;13(2):77–88. [Google Scholar]
- 30.Awoke A, Gudesho G, Akmel F, Tessema ZK, Tilahun W, Abdu AA, et al. Medicinal plants used for treatment of domestic animal diseases and their threats in Guraferda District, Bench-Sheko Zone, South West Ethiopia. Ethnobot Res Appl. 2024;29:1–37. [Google Scholar]
- 31.Hedberg I. Flora of Ethiopia and eritrea. InThe Biodiversity of African Plants: Proceedings XIVth AETFAT Congress 22–27 August 1994, Wageningen, The Netherlands: Springer Netherlands; 1996. pp. 802–804.
- 32.Alexiades MN. Collecting ethnobotanical data: an introduction to basic concepts and techniques. Adv Economic Bot. 1996;10:53–94. [Google Scholar]
- 33.Cotton CM. Ethnobotany: principles and applications. 1996 Oct 4.
- 34.Martin GJ. Ethnobotany: a methods manual. Routledge. Accessed 23 Sept 2010.
- 35.Kelbessa E, Demissew S. Diversity of vascular plant taxa of the flora of Ethiopia and Eritrea. Ethiop J Biol Sci. 2014;13(1S):37–45. [Google Scholar]
- 36.Awoke A, Siyum Y, Awoke D, Gebremedhin H, Tadesse A. Ethnobotanical study of medicinal plants and their threats in Yeki district, Southwestern Ethiopia. J Ethnobiol Ethnomed. 2024;20(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sulaiman N. Botanical ethnoknowledge index: a new quantitative assessment method for cross-cultural analysis. J Ethnobiol Ethnomed. 2025;21(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tardío J, Pardo-de-Santayana M. Cultural importance indices: a comparative analysis based on the useful wild plants of Southern Cantabria (Northern Spain). Econ Bot. 2008;62:24–39. [Google Scholar]
- 39.Shaheen H, Qureshi R, Qaseem MF, Amjad MS, Bruschi P. The cultural importance of indices: a comparative analysis based on the useful wild plants of Noorpur Thal Punjab, Pakistan. Eur J Integr Med. 2017;12:27–34. [Google Scholar]
- 40.Osman A, Sbhatu DB, Giday M. Medicinal plants used to manage human and livestock ailments in Raya Kobo district of Amhara regional State, Ethiopia. Evidence-Based Complementary and Alternative Medicine. 2020;2020(1):1329170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Misganaw W, Masresha G, Alemu A, Lulekal E. Ethnobotanical study of medicinal plants used to treat human and livestock ailments in Addi Arkay district, Northwest Ethiopia. J Ethnobiol Ethnomed. 2025;21(1):1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tadesse D, Masresha G, Lulekal E, Alemu A. Ethnobotanical study of wild edible plants in Metema and Quara districts, Northwestern Ethiopia. J Ethnobiol Ethnomed. 2025;21(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kisangau DP, Kauti M, Mwobobia R, Kanui T, Musimba N, License CA. Traditional knowledge on use of medicinal plants in Kitui County, Kenya. Int J Ethnobiol Ethnomed. 2017;4(1):1–0. [Google Scholar]
- 44.Adebo GM, Alfred SD. Gender dimension of herbal medicine’s knowledge and practice in Ekiti and Ondo States, Nigeria. J Med Plants Res. 2011;5(8):1283–90. [Google Scholar]
- 45.de Boer HJ, Cotingting C. Medicinal plants for women’s healthcare in Southeast Asia: a meta-analysis of their traditional use, chemical constituents, and pharmacology. J Ethnopharmacol. 2014;151(2):747–67. [DOI] [PubMed] [Google Scholar]
- 46.Megersa M, Tamrat N. Medicinal plants used to treat human and livestock ailments in Basona Werana district, North Shewa zone, Amhara region, Ethiopia. Evidence-Based Complement Altern Med. 2022;2022(1):5242033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kassa Z, Asfaw Z, Demissew S. An ethnobotanical study of medicinal plants in Sheka zone of Southern nations nationalities and peoples regional state. Ethiop J Ethnobiol Ethnomed. 2020;16:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chekole G, Masresha G, Tamiru W. Ethnobotanical study on medicinal plant species uses against human ailments in Lay Armachiho District, Northwest Ethiopia. Ethiop J Nat Comput Sci. 2023;3(1):375–98. [Google Scholar]
- 49.Alemu M, Asfaw Z, Lulekal E, Warkineh B, Debella A, Sisay B, et al. Ethnobotanical study of traditional medicinal plants used by the local people in Habru District, North Wollo Zone, Ethiopia. J Ethnobiol Ethnomed. 2024;20(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mwingira FW, Matiya DJ, Mogha NG. Ethnobotanical survey on the knowledge and use of medicinal plants for malaria management among university students. Tanzan J Sci. 2023;49(3):576–86. [Google Scholar]
- 51.Megersa M, Nedi T, Belachew S. Ethnobotanical study of medicinal plants used against human diseases in Zuway Dugda District, Ethiopia. Evidence-Based Complementary and Alternative Medicine. 2023;2023(1):5545294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma Y, Liu D, Cheng H, Bussmann RW, He H, Guo Z, et al. Ethnobotanical study of medicinal plants used by Miao people in Jijiezi, Yunnan, China. Ethnobot Res Appl. 2019;18:1–4. [Google Scholar]
- 53.Abebe M. The study of ethnoveterinary medicinal plants at mojana Wodera district, central Ethiopia. PLoS ONE. 2022;17(5):e0267447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sikarwar RL, Tiwari AP. A review of plants used in ethnoveterinary medicine in Central India. Indian J Tradit Knowl (IJTK). 2020;19(3):617–34.
- 55.Amsalu N, Bezie Y, Fentahun M, Alemayehu A, Amsalu G. Use and conservation of medicinal plants by indigenous people of Gozamin Wereda, East Gojjam zone of Amhara region, Ethiopia: an ethnobotanical approach. Evidence-Based Complementary and Alternative Medicine. 2018;2018(1):2973513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tefera BN, Kim YD. Ethnobotanical study of medicinal plants in the Hawassa zuria district, Sidama zone, Southern Ethiopia. J Ethnobiol Ethnomed. 2019;15:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Desalegn A, Egigu MC, Sasikumar JM. Ethnobotanical study on medicinal plants used by ethnic people of Gechi District, South West Oromia, Ethiopia. Nusantara Bioscience. 2022;14(1).
- 58.Zhou H, Zhang J, Kirbis BS, Mula Z, Zhang W, Kuang Y, Huang Q, Yin L. Ethnobotanical study on medicinal plants used by Bulang people in Yunnan, China. Journal of Ethnobiology and Ethnomedicine. 2023;19(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prabhu S, Vijayakumar S, Yabesh JM, Prakashbabu R, Murugan R. An ethnobotanical study of medicinal plants used in Pachamalai hills of Tamil Nadu, India. J Herb Med. 2021;25:100400. [Google Scholar]
- 60.Chekole G. Ethnobotanical study of medicinal plants used against human ailments in Gubalafto District, Northern Ethiopia. J Ethnobiol Ethnomed. 2017;13(1):55. [DOI] [PMC free article] [PubMed]
- 61.Assen Y, Woldearegay M, Haile A. An ethnobotanical study of medicinal plants in Kelala District, South Wollo zone of Amhara region, Northeastern Ethiopia. Evidence-based Complement Altern Med. 2021;2021(1):6651922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tadesse D, Masresha G, Lulekal E. Ethnobotanical study of medicinal plants used to treat human ailments in Quara district, Northwestern Ethiopia. J Ethnobiol Ethnomed. 2024;20(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eshete MA, Molla EL. Traditional medicine practices of Guji semi-pastoralist people to treat livestock ailments in Suro Barguda District, West Guji zone, Ethiopia. Ethnobiol Conserv. 2022;11.
- 64.Tahir M, Gebremichael L, Beyene T, Van Damme P. Ethnobotanical study of medicinal plants in Adwa district, central zone of Tigray regional state, Northern Ethiopia. J Ethnobiol Ethnomed. 2021;17:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cámara-Leret R, Paniagua-Zambrana N, Balslev H, Macía MJ. Ethnobotanical knowledge is vastly under-documented in northwestern South America. PLoS ONE. 2014;9(1):e85794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Njoroge GN, Kaibui IM, Njenga PK, Odhiambo PO. Utilisation of priority traditional medicinal plants and local people’s knowledge on their conservation status in arid lands of Kenya (Mwingi District). J Ethnobiol Ethnomed. 2010;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bai Y, Zhang Q, He X, Wang H, Li W, Zhu J, et al. An ethnobotanical study on medicinal plants of Shexian dryland stone terraced system in Northern China. J Ethnobiol Ethnomed. 2022;18(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hussain M, Alam J, Shah GM, Gul A, Majid A, Shafqat N, et al. Assessment of traditional knowledge of medicinal plants practiced by rural communities residing around Musk Deer National Park, Kashmir Himalaya, Pakistan. Ethnobot Res Appl. 2024;28:1–23. [Google Scholar]
- 69.Ralte L, Sailo H, Singh YT. Ethnobotanical study of medicinal plants used by the Indigenous community of the Western region of Mizoram, India. J Ethnobiol Ethnomed. 2024;20(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghimire SK, Bista G, Lama NS, Craig SR, Lama W, Gurung TN. Without the plants, we have no medicine: Sowa Rigpa, ethnobotany, and conservation of threatened species in Nepal. Kathmandu Nepal: WWF Nepal and Himalayan Amchi Association; 2021. [Google Scholar]
- 71.Ssenku JE, Okurut SA, Namuli A, Kudamba A, Tugume P, Matovu P, et al. Medicinal plant use, conservation, and the associated traditional knowledge in rural communities in Eastern Uganda. Trop Med Health. 2022;50(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zemede J, Mekuria T, Ochieng CO, Onjalalaina GE, Hu GW. Ethnobotanical study of traditional medicinal plants used by the local Gamo people in Boreda Abaya District, Gamo Zone, Southern Ethiopia. J Ethnobiol Ethnomed. 2024;20(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sultan S, Telila H, Kumsa L. Ethnobotany of traditional cosmetics among the Oromo women in Madda Walabu District, Bale Zone, southeastern Ethiopia. J Ethnobiol Ethnomed. 2024;20(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hankiso M, Asfaw Z, Warkineh B, Abebe A, Sisay B, Debella A. Ethnoveterinary medicinal plants and their utilization by the people of Soro District, Hadiya Zone, Southern Ethiopia. J Ethnobiol Ethnomed. 2024;20(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tahir M, Asnake H, Beyene T, Van Damme P, Mohammed A. Ethnobotanical study of medicinal plants in Asagirt district, Northeastern Ethiopia. Trop Med Health. 2023;51(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anbessa B, Lulekal E, Debella A, Hymete A. Ethnobotanical study of medicinal plants in Dibatie district, Metekel zone, Benishangul Gumuz regional State, Western Ethiopia. J Ethnobiol Ethnomed. 2024;20(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu S, Zhang B, Lei Q, Zhou J, Ali M, Long C. Diversity and traditional knowledge of medicinal plants used by shui people in Southwest China. J Ethnobiol Ethnomed. 2023;19(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li H, Huang C, Li Y, Wang P, Sun J, Bi Z, et al. Ethnobotanical study of medicinal plants used by the Yi people in Mile, Yunnan, China. J Ethnobiol Ethnomed. 2024;20(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pedrollo CT, Kinupp VF, Shepard G Jr, Heinrich M. Medicinal plants at Rio Jauaperi, Brazilian amazon: ethnobotanical survey and environmental conservation. J Ethnopharmacol. 2016;186:111–24. [DOI] [PubMed] [Google Scholar]
- 80.Jima KB, Asfaw Z, Demissew S, Dalle G. Medicinal plant Composition, Distribution, usage and conservation status in Nole Kaba District, West Wollega, West Ethiopia. 2021.
- 81.Tahir M, Egzabher YG, Yonas M, Giday K, Lemma H, Kidane L, et al. Ethnobotanical study of medicinal plants of the Abohoy Gara Mountains, Northern Ethiopia. Pharmacol Res. 2024;4:100069. [Google Scholar]
- 82.Agize M, Asfaw Z, Nemomissa S, Gebre T. Ethnobotany of traditional medicinal plants and associated indigenous knowledge in Dawuro zone of Southwestern Ethiopia. J Ethnobiol Ethnomed. 2022;18(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bekele M, Woldeyes F, Lulekal E, Bekele T, Demissew S. Ethnobotanical investigation of medicinal plants in Buska mountain range, Hamar district, Southwestern Ethiopia. J Ethnobiol Ethnomed. 2022;18(1):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kindie B, Tamiru C, Abdala T. Ethnobotanical study of medicinal plants and conservation status used to treat human and livestock ailments in Fadis District, Eastern Ethiopia. International Journal of Homeopathy & Natural Medicines. 2021;7(1):7–17. [Google Scholar]
- 85.Gonfa N, Tulu D, Hundera K, Raga D. Ethnobotanical study of medicinal plants, its utilization, and conservation by Indigenous people of Gera district. Ethiopia. Cogent Food Agric. 2020;6(1):1852716. [Google Scholar]
- 86.Ralte L, Singh YT. Ethnobotanical survey of medicinal plants used by various ethnic tribes of Mizoram, India. PLoS ONE. 2024;19(5):e0302792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giday M, Asfaw Z, Woldu Z. Medicinal plants of the Meinit ethnic group of Ethiopia: an ethnobotanical study. J ethnopharmacol. 2009;124(3):513–21. [DOI] [PubMed]
- 88.Alves RR, Rosa IL, Albuquerque UP, Cunningham AB. Medicine from the wild: an overview of the use and trade of animal products in traditional medicines. Animals in traditional folk medicine: implications for conservation. Sep. 2012;19:25–42. [Google Scholar]
- 89.Kassaye KD, Amberbir A, Getachew B, Mussema Y. A historical overview of traditional medicine practices and policy in Ethiopia. Ethiop J Health Dev. 2006;20(2):127–34. [Google Scholar]
- 90.Alemneh D. Ethnobotanical study of plants used for human ailments in Yilmana densa and Quarit districts of West Gojjam Zone, Amhara region, Ethiopia. BioMed Res Int. 2021;2021(1):6615666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hishe M, Asfaw Z, Giday M. Review on value chain analysis of medicinal plants and the associated challenges. J Med Plants Stud. 2016;4(3):45–55. [Google Scholar]
- 92.Kunwar RM, Shrestha KP, Bussmann RW. Traditional herbal medicine in Far-west Nepal: a pharmacological appraisal. J Ethnobiol Ethnomed. 2010;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tassew G. Ethnobotanical study of medicinal plants in Borecha woreda, Buno Bedele zone Southwestern Ethiopia. Int J Sci Res. 2019;8(9):1484–98. [Google Scholar]
- 94.Tugume P, Kakudidi EK, Buyinza M, Namaalwa J, Kamatenesi M, Mucunguzi P, et al. Ethnobotanical survey of medicinal plant species used by communities around Mabira central forest reserve, Uganda. J Ethnobiol Ethnomed. 2016;12:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Diallo MS, Traore MS, Balde MA, Camara AK, Baldé ES, Traore S, et al. Prevalence, management and ethnobotanical investigation of hypertension in two Guinean urban districts. J Ethnopharmacol. 2019;231:73–9. [DOI] [PubMed] [Google Scholar]
- 96.Kipkore W, Wanjohi B, Rono H, Kigen G. A study of the medicinal plants used by the Marakwet community in Kenya. J Ethnobiol Ethnomed. 2014;10:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Omambia L, Amadi J, Wabuyele E, Onyambu M, Orwa J, Mwafaida J, et al. Abundance and threats to medicinal plants used in managing respiratory illnesses in Migori County, Kenya: a need for increased local sensitization. Biol Environ Sci J Tropics. 2024;21(3):180–91. [Google Scholar]
- 98.Tabuti JR, Kukunda CB, Waako PJ. Medicinal plants used by traditional medicine practitioners in the treatment of tuberculosis and related ailments in Uganda. J Ethnopharmacol. 2010;127(1):130–6. [DOI] [PubMed] [Google Scholar]
- 99.Omara T, Odero MP, Obakiro SB. Medicinal plants used for treating cancer in Kenya: an ethnopharmacological overview. Bull Natl Res Cent. 2022;46(1):148. [Google Scholar]
- 100.Nyagumbo E, Nyirenda T, Mawere C, Mutaramutswa A, Kapanga D, Ngorima G, et al. Medicinal plants used for the treatment and management of malaria in Zimbabwe–review and perspectives. Ethnobot Res Appl. 2025;30:1–41. [Google Scholar]
- 101.Rauf A, Abu-Izneid T, Thiruvengadam M, Imran M, Olatunde A, Shariati MA, et al. Garlic (Allium sativum L.): its chemistry, nutritional composition, toxicity, and anticancer properties. Curr Top Med Chem. 2022;22(11):957–72. [DOI] [PubMed] [Google Scholar]
- 102.Ojelel S, Mucunguzi P, Katuura E, Kakudidi EK, Namaganda M, Kalema J. Wild edible plants used by communities in and around selected forest reserves of Teso-Karamoja region, Uganda. J Ethnobiol Ethnomed. 2019;15:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kidane L, Gebremedhin G, Beyene T. Ethnobotanical study of medicinal plants in Ganta Afeshum district, Eastern zone of Tigray, Northern Ethiopia. J Ethnobiol Ethnomed. 2018;14:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Usman KA, Egigu MC, Mahalingam JS. Ethnobotanical study on traditional medicinal plants used by Oromo ethnic people of Goro district, Bale zone of Oromia region, Ethiopia. Ethnobot Res Appl. 2022;24:1–21. [Google Scholar]
- 105.Teka A, Asfaw Z, Demissew S, Van Damme P. Medicinal plant use practice in four ethnic communities (Gurage, Mareqo, Qebena, and Silti), south central Ethiopia. J Ethnobiol Ethnomed. 2020;16:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bushra MK, Beressa TB, Yimer SM. Ethnobotanical study of medicinal plants used by Indigenous people in Ticho District, Arsi Zone, Oromia regional State, Ethiopia. J Med Plants By-Products. 2025;14(1):44–53. [Google Scholar]
- 107.Bogale M, Sasikumar JM, Egigu MC. An ethnomedicinal study in tulo district, west hararghe zone, oromia region, Ethiopia. Heliyon. 2023;9(4). [DOI] [PMC free article] [PubMed]
- 108.Maiyo ZC, Njeru SN, Toroitich FJ, Indieka SA, Obonyo MA. Ethnobotanical study of medicinal plants used by the people of Mosop, Nandi County in Kenya. Front Pharmacol. 2024;14:1328903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sonibare MA, Moody JO, Adesanya EO. Use of medicinal plants for the treatment of measles in Nigeria. J Ethnopharmacol. 2009;122(2):268–72. [DOI] [PubMed]
- 110.Kunwar RM, Subedi BP, Baral SR, Maraseni T, LeBoa C, Adhikari YP, et al. Ethnobotany of the himalayas: the Nepal, Bhutanese, and Tibetan himalayas. In: Ethnobotany of the Himalayas. Cham: Springer International Publishing; 2020. p. 1–39. [Google Scholar]
- 111.Kefalew A, Asfaw Z, Kelbessa E. Ethnobotany of medicinal plants in Ada’a District, East Shewa Zone of Oromia regional state, Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):25. [DOI] [PMC free article] [PubMed]
- 112.Avana-Tientcheu ML, Sime CH, Tsobou R, Tchoundjeu Z. Diversity, ethnobotanical potential and sustainability assessment of plants used by traditional healers to treat cancer in Boyo division, North-West region, Cameroon. Eur J Med Plants. 2019;27:1–22. [Google Scholar]
- 113.GNAHORE E, KOUADIO KR, AMBA AJ, KONE M, BAKAYOKO A. Ethnobotanical survey of plants used by the riparian population of Banco National Park (Abidjan, Ivory Coast). Asian J Ethnobiol. 2022;5(2).
- 114.Singh V, Rawat H, Sharma S, Mohan J, Singh A. Ethnobotanical survey of medicinal plants in the Garhwal region of Uttarakhand, India. Int J Environ Stud. 2025;21:1–9. [Google Scholar]
- 115.Wanjohi BK, Sudoi V, Njenga EW, Kipkore WK. An ethnobotanical study of traditional knowledge and uses of medicinal wild plants among the Marakwet community in Kenya. Evidence-Based Complementary and Alternative Medicine. 2020;2020(1):3208634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Adhikari M, Thapa R, Kunwar RM, Devkota HP, Poudel P. Ethnomedicinal uses of plant resources in the Machhapuchchhre rural municipality of Kaski district, Nepal. Medicines (Basel). 2019;6(2):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ahmad K, Ahmad M, Huber FK, Weckerle CS. Traditional medicinal knowledge and practices among the tribal communities of Thakht-e-Sulaiman Hills, Pakistan. BMC Complement Med Ther. 2021;21:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dhakal RH. Modernization in medical practices in rural Nepal: an ethnographic study of Hyolmos. Molung Educational Frontier. 2021;11:169–87. [Google Scholar]
- 119.Diantaris MT. Conservation Strategies for Endangered Ethnobotanical Species in Tropical Regions. Int J Adv Ethnobotany Res Appl. 2024;1(1).
- 120.Awuchi CG. Medicinal plants: the medical, food, and nutritional biochemistry and uses. Int J Adv Acad Res. 2019;5(11):220–41. [Google Scholar]
- 121.Blaikie C. Mainstreaming marginality: traditional medicine and primary healthcare in Himalayan India. Asian Med. 2019;14(1):145–72. [Google Scholar]
- 122.Mattalia G, Stryamets N, Pieroni A, Sõukand R. Knowledge transmission patterns at the border: ethnobotany of Hutsuls living in the Carpathian mountains of Bukovina (SW Ukraine and NE Romania). J Ethnobiol Ethnomed. 2020;16:1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mbuni YM, Wang S, Mwangi BN, Mbari NJ, Musili PM, Walter NO, et al. Medicinal plants and their traditional uses in local communities around Cherangani Hills, Western Kenya. Plants. 2020;9(3):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Francis JW, Wale CS. Conservation and sustainable use of medicinal plants in Mbale district: a case of Bukiende sub-county, Mbale District, Eastern Uganda. J Pharm Sci Drug Discov. 2025;4(1):1–4. [Google Scholar]
- 125.Said MK, Peter KH. The use of medicinal plants and its implication on plants conservation: the case of West Usambara, Tanzania. J Geogr Assoc Tanzania. 2022;42(1).
- 126.Kumar R, Sahu H, Singh SB, Chandra N, Kumar S, Arunachalam K. Harmony in habitats: unveiling the conservation insights through maxent modeling of Withania somnifera (L.) Dunal in Uttarakhand. In: Threatened medicinal plants in the Indian Himalayan region: sustainability challenges and conservation strategies. Cham: Springer Nature Switzerland; 2024. p. 237–49. [Google Scholar]
- 127.Lelamo LL, Kemal AW. Distribution and regeneration status of Cordia africana (Lam.) tree in agroforestry practices along agroecology and farmers’ wealth status in Sidama zone Southern Ethiopia. Univer J Agric Res. 2021;9(3):70–8. [Google Scholar]
- 128.Kumar A, Venugopal S, Jnanesha AC, Lal RK. Agricultural-based challenges, genetic enhancement, and obstacles to an industrially important medicinal plant, Ashwagandha (Withania somnifera (L.) Dunal): a review. Ecol Genet Genomics. 2023;28:100183. [Google Scholar]
- 129.Poudel B, Bhandari J, Poudel A, Gautam D. Ethnomedicinal use of common garden species in Arghakhanchi district, Western Nepal. Asian J Pharmacognocy. 2021;4:31–65. [Google Scholar]
- 130.Saggar S, Mir PA, Kumar N, Chawla A, Uppal J, Kaur A. Traditional and herbal medicines: opportunities and challenges. Pharmacognosy Res. 2022;14(2).
- 131.Obakiro SB, Kiprop A, Kowino I, Kigondu E, Odero MP, Omara T, et al. Ethnobotany, ethnopharmacology, and phytochemistry of traditional medicinal plants used in the management of symptoms of tuberculosis in East Africa: a systematic review. Trop Med Health. 2020;48:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ulian T, Diazgranados M, Pironon S, Padulosi S, Liu U, Davies L, et al. Unlocking plant resources to support food security and promote sustainable agriculture. Plants People Planet. 2020;2(5):421–45. [Google Scholar]
- 133.Agnes KN, Boeff DD, de Oliveira Carvalho L, Konrath EL. Ethnobotanical knowledge on native Brazilian medicinal plants traditionally used as anthelmintic agents–A review. Exp Parasitol. 2023;249:108531. [DOI] [PubMed] [Google Scholar]
- 134.Manzoor M, Ahmad M, Zafar M, Gillani SW, Shaheen H, Pieroni A, et al. The local medicinal plant knowledge in Kashmir Western Himalaya: a way to foster ecological transition via community-centred health seeking strategies. J Ethnobiol Ethnomed. 2023;19(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Benyei P, Arreola G, Reyes-García V. Storing and sharing: a review of Indigenous and local knowledge conservation initiatives. Ambio. 2020;49(1):218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript.