Abstract
Defining signals that can support the self-renewal of multipotential hemopoietic progenitor cells (MHPCs) is pertinent to understanding leukemogenesis and may be relevant to developing stem cell-based therapies. Here we define a set of signals, JAK2 plus either c-kit or flt-3, which together can support extensive MHPC self-renewal. Phenotypically and functionally distinct populations of MHPCs were obtained, depending on which receptor tyrosine kinase, c-kit or flt-3, was activated. Self-renewal was abrogated in the absence of STAT5a/b, and in the presence of inhibitors targeting either the mitogen-activated protein kinase or phosphatidylinositol 3′ kinase pathways. These findings suggest that a simple two-component signal can drive MHPC self-renewal.
Keywords: JAK2/leukemia/self-renewal/stem cell
Introduction
Hemopoietic stem cells (HSCs) possess the ability to self-renew or to differentiate into multiple distinct cell lineages. Substantial HSC self-renewal has been demonstrated in transplantation models (Pawliuk et al., 1996; Iscove and Nawa, 1997), in stroma-containing long-term marrow cultures supplemented with thrombopoietin (Yagi et al., 1999) and upon the ectopic expression of HoxB4 (Sauvageau et al., 1995) or a constitutively active derivative of Notch1 (Varnum-Finney et al., 2000). Sub stantially lesser HSC self-renewal (<10-fold) has been achieved using defined culture conditions (Holyoake et al., 1996; Miller and Eaves, 1997; Ema et al., 2000; Audet et al., 2001), and thus the processes that govern HSC self-renewal remain poorly understood.
The boundary separating HSCs from their relatively more mature counterparts, the multipotential hemopoietic progenitor cells (MHPCs), is blurred by the phenotypic and functional heterogeneity that exists within the HSC compartment itself (Metcalf, 1999). Phenotypically distinct classes of murine and human HSCs have been described (Zijlmans et al., 1995; Osawa et al., 1996; Bhatia et al., 1998). Cells with either short- or long-term repopulating ability have been identified within the HSC compartment of mice (Lemischka et al., 1986; Snodgrass and Keller, 1987; Jordan and Lemischka, 1990). Similarly, retroviral marking of human cord blood cells transplanted into severe combined immunodeficient (SCID) mice has uncovered substantial functional heterogeneity within the HSC compartment of man (Guenechea et al., 2001). In comparison to HSCs, MHPCs are more restricted in their potential for differentiation, and are generally considered to possess a lesser capacity for self-renewal. Nevertheless, we have reported extensive MHPC self-renewal upon activating an ectopically expressed derivative of the thrombopoietin receptor (mpl) in primary murine bone marrow cells (Jin et al., 1998).
Several lines of evidence suggest that the tyrosine kinase JAK2 (Harpur et al., 1992) might play a significant role in HSC/MHPC self-renewal. The aforementioned ability of mpl to support MHPC self-renewal is JAK2 dependent (Otto et al., 2001). Furthermore, two receptors that are not dependent upon JAK2 for their activity, flt-3 and the granulocyte colony-stimulating factor (GCSF) (Parganas et al., 1998), were unable to support MHPC self-renewal (Zeng et al., 2001). Finally, considering leukemia as the self-renewal of a stem cell gone awry (Bonnet and Dick, 1997; Reya et al., 2001), JAK2 derivatives rendered constitutively active through fusion with the oligomerization domain of TEL cause either myeloid or lymphoid leukemias in mice (Schwaller et al., 1998, 2000) and in humans (Lacronique et al., 1997; Peeters et al., 1997). We therefore tested whether JAK2 itself is capable of supporting MHPC self-renewal.
Results
A conditional JAK2 allele stimulates growth in a factor-dependent cell line
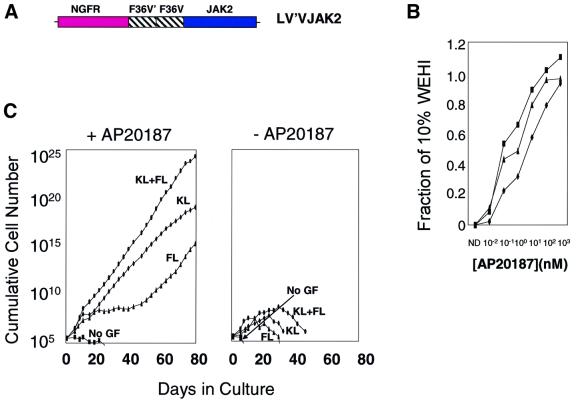
To construct a conditional allele of JAK2, we employed the principle of chemically induced dimerization (Spencer et al., 1993), exploiting the fact that the protein is naturally activated by induced proximity. The catalytic JH-1 domain was incorporated into a fusion protein, designated LV′VJAK2 (Figure 1A), which also includes a tandem binding site for the membrane-permeable synthetic ligand AP20187 (Jin et al., 2000), and the trans-membrane and extracellular portions of the low-affinity nerve growth factor receptor (LNGFR), which serves as a marker of transduced cells (Mavilio et al., 1994). In the presence of AP20187, a chemical inducer of dimerization (CID) (Spencer et al., 1993; Neff and Blau, 2001), two or more copies of the LV′VJAK2 fusion are brought into close juxtaposition. To determine whether CID-dependent aggregation of the LV′VJAK2 fusion could trigger cell growth, the LV′VJAK2 construct was inserted into a murine stem cell virus (MSCV)-based vector (Hawley et al., 1994), which was in turn used to infect the interleukin (IL)-3-dependent cell line Ba/F3 (Palacios and Steinmetz, 1985). Treatment of Ba/F3 cells expressing the LV′VJAK2 fusion with AP20187 resulted in AP20187 concentration-dependent cell growth (Figure 1B). These results support a previous report in demonstrating that chemically induced dimerization of the JH-1 domain is capable of stimulating growth of Ba/F3 cells, replacing IL-3 (Mohi et al., 1998).
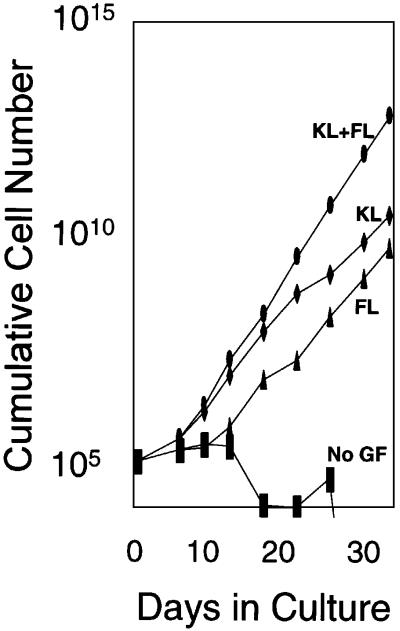
Fig. 1. (A) Schematic diagram of the LV′VJAK2 construct. LNGFR, the extracellular and transmembrane domains (residues 1–274) of the human low-affinity nerve growth factor receptor; F36V, Phe36→Val36 point mutant of human FKBP12; F36V′, codon-wobbled F36V; JAK2, the catalytic JH-1 domain of JAK2 (317 amino acids). The LV′VJAK2 construct was inserted into an MSCV-based retroviral vector and a high-titer ecotropic producer cell line was generated. (B) Cell proliferation (MTT) assays of three clones of retrovirally transduced Ba/F3 cells expressing the LV′VJAK2 vector. Cells were tested in the absence of IL-3 and in the presence of AP20187 at the concentrations indicated. Results are plotted as a fraction of OD 570–630 nm values obtained using the same clone cultured in 10% IL-3 containing WEHI conditioned medium. (C) JAK2 signaling, in combination with either c-kit or flt-3, stimulates expansion of genetically modified bone marrow cells. Results of a representative experiment (from a total of five) are shown. After retroviral transduction, marrow cells were cultured in IMDM containing 10% FCS, in the presence (left panel) or absence (right panel) of AP20187 (100 nM) and either no growth factor (No GF), or with the addition of flt-3 ligand (FL; 100 ng/ml), murine c-kit ligand (KL; 50 ng/ml) or both flt-3 ligand and c-kit ligand (KL + FL) at the same concentrations. Cells were counted at various times during culture. Note that the y-axis is on a logarithmic scale.
JAK2 alone is insufficient to stimulate growth in primary murine bone marrow cells: requirement for c-kit and/or flt-3
In contrast to its effect in Ba/F3 cells, the LV′VJAK2 fusion failed to induce an AP20187-dependent proliferative response in transduced primary murine bone marrow cells (Figure 1C, left panel, No GF). This result is in keeping with a previous report by our laboratory (Zeng et al., 2001) indicating that not all signals capable of inducing growth of Ba/F3 cells are able to induce growth in primary murine bone marrow cells. To test whether marrow cell growth could be induced by providing signals in addition to JAK2, we tested AP20187 in combination with ligands for the receptor tyrosine kinases c-kit and flt-3. A significant sustained outgrowth of transduced marrow cells was accomplished using AP20187 (AP) plus flt-3 ligand (FL), AP plus c-kit ligand (KL) or AP plus FL plus KL (Figure 1C, left panel). In each of five experiments, cells cultured for 80 days expanded up to 1020-fold, averaging one cell division every 30 h. Cells cultured in AP/KL/FL displayed the fastest rate of growth, while cell growth was least rapid with the AP/FL combination. On the contrary, sustained growth failed to occur using FL alone, KL alone or FL plus KL in combination (Figure 1C, right panel).
In contrast to the sustained growth of transduced marrow cells consistently observed when AP was combined with either KL or FL, sustained growth failed to occur when AP was combined with GCSF, IL-3 or the IL-6–soluble IL-6 receptor fusion protein, an artificial gp130 ligand (Fischer et al., 1997) (data not shown). These findings demonstrate that JAK2 can induce sustained growth of primary marrow cells when complemented by signals common to receptor tyrosine kinases (c-kit and flt-3), and that these complementary signals are not provided by three members of the cytokine receptor superfamily (GCSF receptor, IL-3 receptor and gp130).
JAK2 plus either c-kit or flt-3 induces the self-renewal of distinct classes of MHPCs
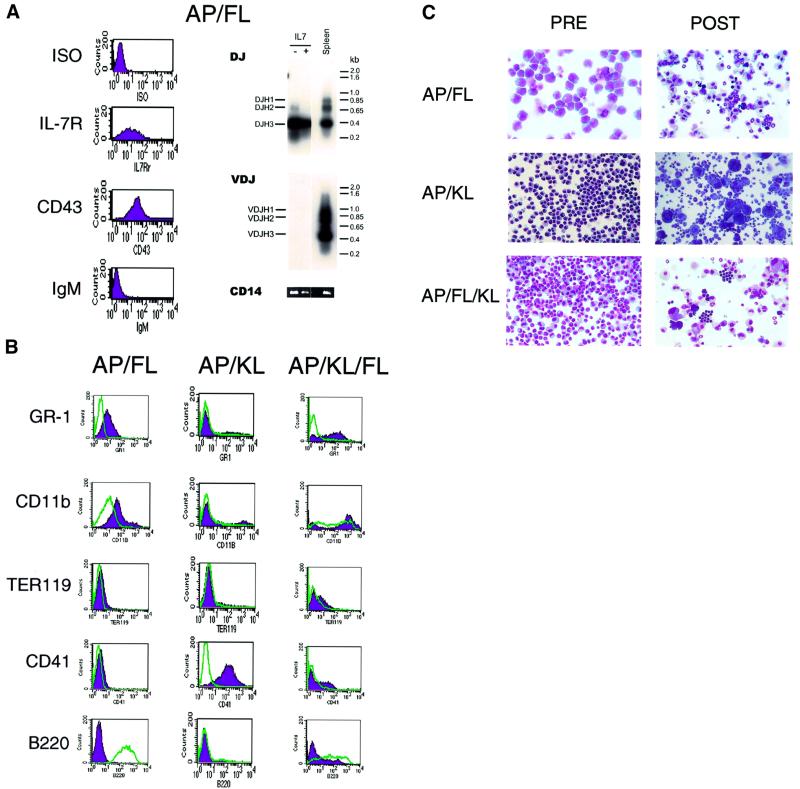
In each of five separate experiments, cells emerging in response to AP plus KL and/or FL displayed distinctive phenotypic and functional characteristics (Figure 2; Table I). Cells expanded in AP plus FL displayed a pro-B-cell phenotype (Figure 2A). c-kit, CD34, Sca-1 and CD11b were expressed at intermediate to low levels, while markers for CD3, Gr-1, Ter119 and CD41 were all negative (Figure 2B; data not shown). CD43 and the IL-7 receptor were expressed, while surface IgM expression was absent (Figure 2A). Up to 100% of cells expressed B220 (Figure 2B, left panel). DJ gene rearrangement was detected by PCR, while VDJ rearrangement was absent (Figure 2A, right panel). Cells expanded in AP plus FL formed colonies in semisolid media containing Epo/IL3/IL6/KL (CFU-C) and in the spleens of irradiated mice (day 12 CFU-S) (Table I). AP/FL cells demonstrated a substantial potential for myeloid differentiation, as demonstrated by the acquisition of Gr-1 and CD11b expression upon addition of myeloid growth factors (Figure 2B, left panel and C, upper panel). Unlike cells cultured under other conditions, AP/FL cells efficiently formed B-lymphoid colonies in semisolid cultures containing IL-7 (Table I). These findings indicate that marrow cells expanded in AP plus FL include hemopoietic progenitor cells with both myeloid and B-lymphoid potential.
Fig. 2. Phenotype of primary murine bone marrow cells transduced with the LV′VJAK2 vector and expanded in the presence of AP20187 (AP) plus either FL or KL. (A) Cells expanded in the presence of AP plus FL are pro-B cells. Left: flow cytometric analysis using antibodies directed against IL-7 receptor, CD43 and IgM. Right: PCR for rearrangement of the IgH locus demonstrates DJ rearrangement but no complete VDJ rearrangements. Spleen DNA provides a positive control for VDJ rearrangement (right panel), and amplification of the CD14 gene (bottom) provides a positive control for PCR. (B) Flow cytometric analysis of cells expanded in AP plus FL (left panel), AP plus KL (middle panel) and AP plus both FL and KL (right panel), prior to (green) and following (purple) differentiation using conditions described in Materials and methods. (C) Photomicrographs of cells before and after differentiation (20× magnification).
Table I. Functional potential of cells expanded for more than 100 days in AP/KL, AP/FL and AP/KL/FL.
| Experiment | CFU-C | BFUe | IL-7-responsive colonies | Day 12 CFU-S | |
|---|---|---|---|---|---|
| AP/KL | |||||
| 1 | 3 (5) | 0 | 0 | ND | |
| 2 | 1689 (96) | 0 | 0 | 8.23 | |
| 3 | 20 (20) | 0 | 0 | ND | |
| Clone 1 | 0 | 0 | 0 | ND | |
| Clone 2 | 60 (25) | 0 | 0 | 0.1 | |
| AP/FL | |||||
| 1 | 676 (60) | 0 | 176 (12) | 1.15 | |
| 2 | 596 (144) | 0 | 292 (24) | 1.06 | |
| Clone 1 | 872 | 0 | 424 (28) | 3.5 | |
| Clone 2 | 573 (120) | 0 | 535 (24) | 1.3 | |
| Clone 3 | 399 (71) | 0 | 457 (20) | ND | |
| Clone 4 | 381 (28) | 0 | 401 (12) | 4.6 | |
| AP/KL/FL | |||||
| 1 | 900 (12) | 0 | 0 | 1.3 | |
| 2 | 1072 (368) | 88 (16) | 32 (8) | 2.2 | |
| 3 | 468 (76) | 68 (8) | 0 | 4 | |
| Clone 1 | 0 | 0 | 0 | ND | |
| Clone 2 | 373 (49) | 0 | 0 | 3 |
Clones were derived by limiting dilution of LV′VJAK2-transduced marrow cells that were initially expanded in AP/KL, AP/FL or AP/KL/FL. For colony-forming unit cells (CFU-C), erythroid burst-forming units (BFUe) and IL-7-responsive colonies, results indicate the number colonies per 105 cells plated. For day 12 spleen colonies (CFU-S), results indicate the number of spleen colonies per 107 cells injected. Numbers in parentheses indicate standard deviations.
AP plus KL generated a phenotypically homogeneous population of blast-like cells that were positive for c-kit and CD34, and strongly positive for Sca1 (data not shown). Cells expressing erythroid (Ter119) and megakaryocytic (CD41) antigens were present on relatively few cells (3–22%), while granulocytic (Gr-1), macrophage (CD11b), B-cell (B220) and T-cell (CD3) markers were uniformly absent (Figure 2B, middle panel; data not shown). Functional assays demonstrated that these cells could form CFU-C and day 12 CFU-S (Table I). Prominent megakaryocytic differentiation was observed upon culturing AP/KL-expanded marrow cells in the presence of thrombopoietin (Figure 2B and C, middle panels). Marrow cells expanded in the presence of AP plus KL exhibited no demonstrable B-cell potential (Table I; data not shown). These findings are consistent with their identity as myeloid-restricted progenitor cells.
Cells expanded in AP/KL/FL exhibited substantial heterogeneity (Figure 2B, right panel and C, lower panel). Sca-1 was expressed at low levels in 34–96% of cells, while 30–60% of cells expressed c-kit, 30–85% expressed CD34, 12–60% expressed B220, 9–20% expressed Gr-1, 0–22% expressed Ter119, 5–40% expressed CD41, 45–72% expressed CD11b and none expressed CD3 (Figure 2B, right panel; data not shown). AP/KL/FL cells formed CFU-C, including well-hemoglobinized erythroid colonies, B-cell colonies in IL-7 (albeit inefficiently) and day 12 CFU-S (Table I), compatible with their identity as multipotential hemopoietic progenitor cells with both B-lymphoid and myeloid potential.
Examination of subclones derived from each of these culture conditions confirmed that AP/KL induced the outgrowth of MHPCs with myeloid, erythroid and megakaryocytic potential, while AP/FL and AP/KL/FL induced growth of MHPCs with B-lymphoid as well as myeloid potential (Table I). None of the culture conditions examined yielded cells with the potential to form T cells following injection into the thymuses of sublethally irradiated mice, and none demonstrated long-term repopulating ability in transplantation assays (data not shown).
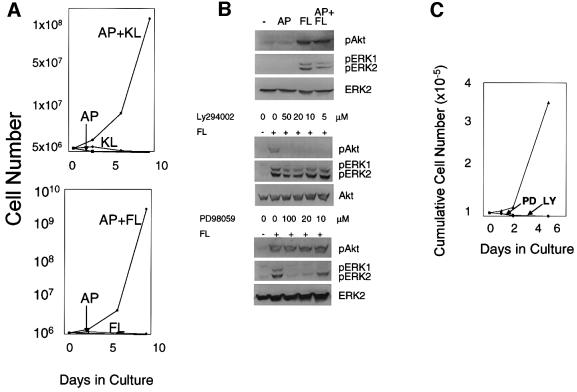
Withdrawal experiments demonstrated that the combination of JAK2 plus either KL or FL was essential for maintaining cell growth (Figure 3A). Cells expanded in the presence of AP/KL could not be converted to AP/FL responsiveness and, similarly, AP/FL cells were not responsive to the AP/KL combination (data not shown). These findings demonstrate an absolute requirement for the combination of JAK2 plus either c-kit or flt-3 to support self-renewal.
Fig. 3. (A) Two signals are required to maintain growth of AP/KL and AP/FL expanded murine bone marrow cells. Murine bone marrow cells transduced with the LV′VJAK2 vector and expanded in the presence of either AP + KL (upper panel) or AP + FL (lower panel) were washed, and then cultured either in AP alone, KL (or FL) alone and AP + KL or AP + FL. (B and C) Inhibitors of either MAP kinase or PI3 kinase inhibit growth of marrow cells expanded in AP + FL. (B) Upper panel: mouse bone marrow cells transduced with the LV′VJAK2 vector were washed, starved for 14 h and then tested for tyrosine phosphorylation of Akt or ERK1/2 10 min following the addition of either AP20187 (AP; 100 nM), flt-3 ligand (FL; 100 ng/ml) or both AP + FL. (–), neither AP nor FL was added. Antibodies specific for phospho-Akt (pAkt) and ERK1/2 (pERK1, pERK2) are indicated. Middle panel: identification of the minimum concentration of Ly294002 sufficient to inhibit tyrosine phosphorylation of Akt. Five micromolar Ly294002 was sufficient to prevent tyrosine phosphorylation of Akt. Lower panel: identification of the minimum concentration of PD98059 sufficient to prevent tyrosine phosphorylation of ERK1/2. Twenty micromolar PD98059 was sufficient to prevent tyrosine phosphorylation of ERK1/2. (C) Growth curves of AP/FL-expanded marrow cells cultured either in the presence or absence (triangles) of the lowest concentrations of PD98059 (PD) or Ly294002 (LY) sufficient to inhibit tyrosine phosphorylation of ERK1/2 and Akt, respectively.
Inhibition of either MAPK or PI3K abrogates MHPC self-renewal
To examine the complementation between FL and JAK2 in supporting MHPC self-renewal, marrow cells expanded in AP/FL were used to test the effects of AP and FL on activation of phosphatidylinositol 3′ kinase (PI3K) and mitogen-activated protein kinase (MAPK) (Figure 3B, upper panel). Neither Akt nor ERK1/2 was appreciably tyrosine phosphorylated in response to AP, whereas FL induced prompt and significant tyrosine phosphorylation of both Akt and ERK1/2. To identify signaling pathways that are essential for MHPC self-renewal, we examined the effect of Ly294002, an inhibitor of PI3K (Geddis et al., 2001), and PD98059, an inhibitor of MAPK (Miyakawa et al., 2001), on the growth of AP/FL cells (Figure 3B, middle and lower panels). After identifying the minimal concentrations of Ly294002 and PD98059 necessary to prevent tyrosine phosphorylation of Akt and ERK1/2, respectively, the effects of these inhibitors on AP/FL cell growth were examined. Minimal concentrations of either inhibitor completely abrogated growth of the AP/FL-expanded cells (Figure 3C).
Requirement for STAT5a/b
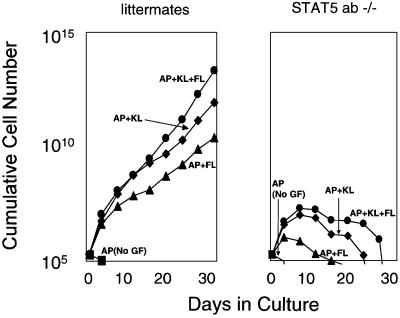
Members of the family of signal transducers and activators of transcription (STATs) are well-characterized targets of JAK signaling (Leonard and O’Shea, 1998). Two STATs that are 95% identical to each other, STAT5a and STAT5b, are essential mediators of JAK2-regulated cell growth (Schwaller et al., 2000). To test whether JAK2-mediated MHPC self-renewal requires STAT5a/b, we tested marrow cells from mice nullizygous for both STAT5a and STAT5b (Teglund et al., 1998). AP-mediated JAK2 signaling, in combination with KL, FL or KL plus FL, induced sustained growth of marrow cells from littermate controls (Figure 4, left panel). In contrast, none of these combinations supported sustained growth of marrow cells from the STAT5a/b knockout mice (Figure 4, right panel), demonstrating a direct role for STAT5a/b in supporting JAK2-mediated MHPC self-renewal.
Fig. 4. The ability of JAK2 to mediate MHPC self-renewal is STAT5a/b dependent. Marrow cells from two STAT5a/b nullizygous mice and two wild-type littermate controls were transduced with the LV′VJAK2 vector as described in Materials and methods. The transduction efficiency into progenitors was 39% for marrow cells from the STAT5a/b knockout mice and 42% for the littermate controls. Following transduction, marrow cells were cultured in suspension in serum containing medium in 100 nM AP in the absence (squares) or presence of FL (100 ng/ml), KL (50 ng/ml) or both growth factors. AP alone fails to induce cell growth either in the STAT5a/b nullizygous mice or the littermate controls, consistent with our previous results. Marrow cells from the littermate controls exhibited sustained growth responses in AP/FL, AP/KL and AP/KL/FL, similar to results depicted in Figure 2. In contrast, each of these conditions produced only transient growth in the transduced marrow cells of STAT5a/b knockout mice.
TEL–JAK2 reproduces the effects of the conditional JAK2
Based on our findings using a conditional JAK2 allele, we hypothesized that mouse marrow cells expressing a constitutively active JAK2 tyrosine kinase would exhibit sustained growth only if the constitutive JAK2 signal were supplemented by signals provided either by KL, FL or both growth factors in combination. Mouse marrow cells were transduced with an MSCV-based vector encoding a TEL–JAK2 variant that contains JAK2 sequences identical to those present in our LV′VJAK2 fusion ([TEL– JAK2 5–19]; Schwaller et al., 1998). As shown in Figure 5, sustained growth was achieved only when the TEL–JAK2-transduced cells were cultured in the presence of KL, FL or both growth factors. The presence of the green fluorescent protein gene within the vector allowed for the demonstration that sustained growth was restricted to the transduced cell population (data not shown). Flow cytometry revealed that the types of cells emerging from each of the cultures were the same as those obtained using the conditionally activated LV′VJAK2.
Fig. 5. Sustained growth of marrow cells expressing a constitutively active TEL–JAK2 requires FL, KL or both growth factors. Marrow cells from C57BL/6 mice were transfected with an MSCV–TEL–JAK2 vector (Schwaller et al., 1998) using a transient packaging system. Following transduction, marrow cells were cultured in suspension in IMDM with 10% FCS either in the absence (rectangles) or presence of FL (triangles), KL (diamonds) or KL + FL (ellipses). The y-axis is on a logarithmic scale.
Discussion
The capacity for extensive self-renewal is commonly regarded as a property that is reserved for stem cells. While the MHPCs described in the present studies did not meet the full functional criteria for stem cells, these cells nevertheless retained a tremendous capacity for self-renewal. These findings indicate that MHPCs may, under certain circumstances, exhibit extensive self-renewal. Alternatively, it has been proposed that traditional assays for stem cells may miss classes of multipotential cells capable of contributing to hematopoiesis for prolonged periods of time (Metcalf, 1999).
Our system for assessing self-renewal provides information unobtainable by other approaches. Traditional cell culture systems using cytokines cannot examine the consequences of activating specific intracellular signaling molecules. Conversely, mouse models that evaluate the effect of deleting or overexpressing specific genes are subject to the compensatory or confounding influences of the wide assortment of other signaling pathways that are available in vivo. By contrast, direct chemical activation allows a single molecular event to be activated specifically and in real time. Since induced proximity is a common mechanism in intracellular signal transduction, similar approaches could possibly be used to evaluate the roles of many other signaling molecules in self-renewal.
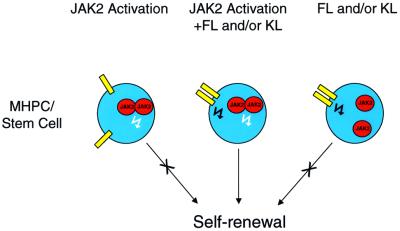
Very recent studies in Drosophila have identified an essential role for the JAK–STAT pathway in specifying stem cell self-renewal in spermatogenesis (Kiger et al., 2001; Tulina and Matunis, 2001). Our findings show that JAK2 plays a pivotal role in the self-renewal of MHPCs in mammals. In the absence of the JAK2 signal, KL and FL, alone or in combination, can support a transient expansion of bone marrow cells but are incapable of supporting sustained marrow cell growth (Figure 1C). These findings, depicted schematically in Figure 6, are consistent with a previous report by our group (Zeng et al., 2001) indicating that the signaling requirements for achieving self-renewal of MHPCs are distinct from those sufficient to support transient cell growth.
Fig. 6. Schematic diagram depicting the requirement for two signals to direct self-renewal of stem and multipotential progenitor cells.
The implications of our findings for JAK2 function in normal hemopoiesis should be viewed in the context of several caveats. First, the JAK2 fusions evaluated here contain only the JH-1 kinase domain and lack domains implicated in auto-regulation (JH-2), receptor binding and other poorly defined functions (JH-3–JH-7) (Leonard and O’Shea, 1998). We confined our analysis to the JH-1 domain because a similar construct incorporating full-length JAK2 failed to confer CID-dependent growth in Ba/F3 cells (data not shown), a finding that has also been reported by others (Mohi et al., 1998). Secondly, CID-induced activation of the JAK2 kinase domain may generate signals that are qualitatively or quantitatively different from those arising from endogenous JAK2 activation. Thirdly, our retrovirally transferred JAK2 fusions are not subject to the transcriptional control mechanisms that regulate endogenous JAK2 expression. Fourthly, the subcellular location of our LV′VJAK2 fusion is at the membrane, whereas JAK2 (and the TEL–JAK2 fusion used here) are in the cytoplasm.
Our results provide a potential inroad to understanding leukemogenesis. Our findings suggest that leukemias associated with expression of an oncogenic fusion protein may require two types of signals: those arising from the oncogenic protein itself, and those provided by the environment. In support of this hypothesis is the longstanding observation that HSCs/MHPCs expressing the BCR–ABL fusion protein enjoy a tremendous selective advantage in vivo that is promptly lost when the same cells are cultured in vitro (Udomsakdi et al., 1992; Petzer et al., 1997). An implication of this hypothesis is that one approach to treating leukemia may be to target supportive signals that originate from the environment. Alternatively, the receptor tyrosine kinase signal may result from mutations that cause constitutive activation of the receptor. In this regard, it is noteworthy that activating mutations involving flt-3 have been identified in up to 20% of adults with acute myeloid leukemia (Nakao et al., 1996; Yokota et al., 1997) and in cases of acute lymphocytic leukemia (ALL) (Xu et al., 1999).
Leukemias associated with TEL–JAK2 are variable in phenotype and include atypical chronic myeloid leukemic cells, pre-B-cell ALL and T-cell ALL (Lacronique et al., 1997; Peeters et al., 1997). Our findings suggest a potential mechanism whereby different leukemic phenotypes may arise from a common oncogenic event. We find that signaling by JAK2 in combination with FL generates cells with a pro-B cell phenotype, while JAK2 plus KL produces cells with a myeloid-restricted phenotype. These findings are consistent with the lineages most responsive to these two ligands (Lyman and Jacobsen, 1998). We hypothesize that the phenotype of an oncogene-associated leukemia is determined by the environmental cues to which a cell with tremendous proliferative capacity is poised to respond.
Two approaches were used to identify the events downstream of JAK2 that are necessary to support MHPC self-renewal. AP20187 failed to induce detectable tyrosine phosphorylation of STAT5 (data not shown), PI3K or MAPK (Figure 3B). Nevertheless, results using marrow cells from STAT5ab nullizygous mice demonstrated that STAT5 was absolutely required for sustained cell growth arising from the JAK2-mediated self-renewal of MHPCs (Figure 4). In contrast, despite the absence of STAT5, marrow cells could nevertheless expand transiently in response to KL and/or FL (Figure 4, right panel). These findings suggest that while STAT5 is not necessary for transient cell growth, STAT5 nevertheless plays a pivotal role in the JAK2-mediated self-renewal of primitive hemopoietic cells. This observation is consistent with a previous report showing that STAT5 is essential for the development of TEL–JAK2-mediated leukemia in mice (Schwaller et al., 2000). Oncostatin M has been proposed as a potential target for STAT5-induced HSC expansion (Schwaller et al., 2000). We are currently evaluating whether oncostatin M is an essential intermediary of the JAK2-initiated self-renewal of MHPCs. We also attempted to identify which aspect of receptor tyrosine kinase signaling was necessary to complement the JAK2 signal in support of MHPC self-renewal. Our results suggest that both PI3K and MAPK are necessary to support JAK2-mediated self-renewal.
In summary, our studies describe an experimental system for examining the signaling requirements that induce self-renewal among primitive multipotential hemopoietic cells. In contrast to measurements of HSC activity, which require in vivo assays, the system described here allows individual signaling pathways to be examined in isolation. Our findings demonstrating a cooperative effect between JAK2 and a second signal, provided either by KL or FL, may be relevant to understanding leukemogenesis (Reya et al., 2001) and to developing stem cell-based therapies (Neff and Blau, 2001).
Materials and methods
Vector construction
The LV′VJAK2 construct encodes, from N- to C-terminus: (i) the extracellular and transmembrane domains of the human LNGFR (p75) (Amara et al., 1999); (ii) two copies of the human FK506 binding protein FKBP12, each modified with the point mutation F36V and with modified codon sequences in one sequence to prevent recombination in a retroviral context (Thomis et al., 2001); and (iii) the JH-1 kinase domain of JAK2. The nucleotide sequences for JAK2 were of murine origin, nucleotides 2530–3483, however the murine and human genes in this region of JAK2 encode identical amino acid sequences. The vector was constructed by inserting an XbaI–SpeI linked JAK2 PCR fragment into the previously described vector LV′V (Thomis et al., 2001). The construct was then inserted as an EcoRI–BglII fragment into the retroviral vector MSCVneo (Hawley et al., 1994).
Cell proliferation assay
Cell proliferation assays were evaluated using an MTT colorimetric assay as described previously (Blau et al., 1997).
Retroviral producer lines
For the LV′VJAK2 vector, a high-titer, genetically stable GpE+86 retroviral producer cell line was generated exactly as described previously (Jin et al., 1998). For the TEL–JAK2 vector, ecotropic retroviral supernatant was generated by transient transfection of 293T cells (Phoenix; Orbigen, San Diego, CA). Eighteen to 24 h prior to transfection, ecotropic Phoenix cells were plated at a concentration of 3–5 × 106 cells per 10 cm2 plate in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum (FCS) and supplemented with glutamine and penicillin/streptomycin. An MSCV expression plasmid containing TEL–JAK2 (ex5–ex19) (Schwaller et al., 1998) was purified using the Qiagen system and calcium phosphate transfection was performed using 25 µg of DNA and a kit purchased from Gibco-BRL, in accordance with the manufacturer’s instructions. Twelve to 16 h following transfection, the Phoenix cells were irradiated (1500 rads) and co-cultivated with pre-stimulated marrow cells from C57BL/6J mice marrow cells prepared as described in the following section, except for the addition of polybrene (8 µg/ml).
Retroviral transduction
5-fluorouracil (150 mg/kg) was injected intraperitoneally into female C57BL/6J mice (Jackson Laboratory). Forty-eight hours later, marrow cells were harvested and cultured for 48 h in DMEM containing 16% FCS (Intergen), 5% IL-3-conditioned medium (Stem Cell Technologies), 100 ng/ml recombinant human IL-6 and 50 ng/ml recombinant murine stem cell factor in a 37°C, 5% CO2 incubator. After 48 h of pre-stimulation, cells were transferred onto irradiated (1500 cGy) producer cells and co-cultivated using identical growth conditions except for the addition of polybrene (8 µg/ml). Marrow cells were harvested after 48 h of co-cultivation.
Suspension cultures
Following retroviral transduction, marrow cells were cultured in Iscove’s modified Dulbecco’s medium (IMDM) containing 10% FCS, 50 U/ml penicillin, 50 µg/ml streptomycin either in the presence or absence of AP (100 nM), murine KL (50 ng/ml) or human FL (100 ng/ml) in a 37°C, 5% CO2 incubator. Both KL and FL were purchased from PeproTech. Cell numbers were determined on the days indicated.
Flow cytometry
Antibodies were purchased from PharMingen (San Diego, CA). Prior to antibody staining, cells were pre-incubated with FcγRII block. Monoclonal antibodies directed against Sca1, TER119, CD11b, Thy1.2, Gr-1, CD3, B220 and isotype controls were directly conjugated to phycoerythrin (PE), while monoclonals directed against CD19, CD41, CD34 and c-kit and isotype controls were directly conjugated to fluorescein isothiocyanate (FITC).
Differentiation assays
For myeloid differentiation, cells were cultured in the presence of KL (50 ng/ml), GM-CSF (100 ng/ml) and IL-3 (10 ng/ml) and, after 5 days, myeloid differentiation was assessed using antibodies directed against Gr1 and CD11b. For erythroid differentiation, cells were cultured in KL plus human erythropoietin (3 U/ml) and, after 5 days, erythroid differentiation was assessed using an antibody directed against TER119. For megakaryocytic differentiation, cells were cultured in human KL, human thrombopoietin (PeproTech) (50 ng/ml) and IL-11 (50 ng/ml) and, after 5 days, megakaryocytic differentiation was assessed using an antibody directed against CD41. For B-lymphoid differentiation, cells were cultured in the presence of KL (50 ng/ml), IL-7 (10 ng/ml) and IL-3 (10 ng/ml), and B-lymphoid differentiation was assessed after 5 days using an antibody directed against B220. For AP/FL cells, differentiation assays were performed in methylcellulose-containing semisolid medium supplemented with human erythropoietin (3 U/ml), murine IL-3 (10 ng/ml), murine IL-6 (10 ng/ml) and KL (50 ng/ml) (Stem Cell Technologies) according to the manufacturer’s protocol. After 8 days of culture, colonies were picked and flow cytometry was performed.
Clonogenic assays
Cells were plated at a concentration of 2.5 × 103 cells per ml in cultures containing 30% FCS, 1% bovine serum albumin, 5 × 10–4 M 2-mercaptoethanol (Sigma), human erythropoietin (3 U/ml; Amgen), 10 ng/ml murine IL-3 (PeproTech), 10 ng/ml human IL-6 (PeproTech), 50 ng/ml murine KL (PeproTech) and 1% methylcellulose (Fisher Scientific, Pittsburgh, PA). To evaluate IL-7-responsive colony formation, 2.5 × 104 cells per ml were cultured as above except that the only growth factor used was 10 ng/ml IL-7 (PeproTech). Cultures were plated in triplicate and incubated in a highly humidified 37°C incubator, flushed continuously with 5% CO2. Colonies were evaluated on day 8.
Day 12 CFU-S assays
Day 12 CFU-S assays were performed as described previously (Blau et al., 1996). In brief, C57BL/6 mice were irradiated with 850 cGy, a dose that was determined to consistently prevent the formation of endogenous day 12 CFU-S in control animals. Following irradiation, 1 × 107 cells were injected via the tail vein into each recipient. Spleen colonies were identified following fixation in Bouin’s solution (15 parts picric acid, 5 parts formaldehyde and 1 part glacial acetic acid; all from Sigma) for 10 min, rinsed in 10% formaldehyde and CFU-S were counted.
Signaling studies
Anti-phosphoAkt (Ser473) antibody and Anti-phospho ERK were obtained from New England Biolabs (Beverly, MA). All chemical inhibitors were purchased from Calbiochem (La Jolla, CA). Cells were deprived of serum and stimuli for14 h, stimulated with the indicated agents for 10 min, washed once with ice-cold phosphate-buffered saline (PBS) and lysed in a buffer containing 0.5% NP-40 as reported previously (Miyakawa et al., 2001). The protein concentration of lysates was measured by Protein/DC assay (Bio-Rad, Hercules, CA). Equal amounts of protein lysate were subjected to SDS–PAGE and western blot analysis as described previously (Miyakawa et al., 2001).
Transplantation assays
LV′VJAK2-transduced marrow cells from C57BL/6(Ly5.2) mice were cultured in the presence of AP/KL, AP/FL or AP/KL/FL for between 6 and 14 weeks, then injected into lethally (1050cGy) irradiated C57BL/6 (Ly5.1) recipients. For transplantation, 1 × 107 cultured Ly5.2 cells were mixed with 1 × 105 fresh Ly5.1 bone marrow cells and injected intravenously via the tail vein. The percentages of cells from either donor were determined by dual labeling using a PE-conjugated anti-mouse CD45.1 antibody (clone A20) and a FITC-conjugated anti-mouse CD45.2 antibody (clone 104) (both purchased from PharMingen).
Thymic injections
Four- to 6-week-old Ly5.1 recipient mice received a single dose of 600 cGy using a linear accelerator. They were anesthetized with halothane and a mid-line incision was made in the upper thoracic region to expose the sternum. A small longitudinal incision of the sternum was made to expose the top of the thymic lobes and a 10–20 µl solution containing between 1 × 106 and 4.5 × 107 AP/FL-expanded (Ly5.2) cells was injected using a Hamilton syringe and a 30-gauge needle. Three weeks following intra-thymic injection, mice were killed and thymuses were resected and used to generate single-cell suspensions. Cells were washed once in PBS/10% FCS, pre-incubated for 10 min at 4°C with FcγRII to block non-specific binding, and stained with a FITC-conjugated anti-NGFR antibody. Positive controls were provided for using HSCN1cl10 cells, which have been demonstrated to differentiate into T cells using this assay (Varnum-Finney et al., 2000).
Acknowledgments
Acknowledgements
We thank Cynthia Nourigat, Irv Bernstein and James Yan for advice and technical support, Juerg Schwaller for the TEL–JAK2 cDNA, Stefan Rose-John for the IL-6–soluble IL-6 protein, and Horst von Recum, Bob Richard and Tobias Neff for their advice and comments regard ing the manuscript. AP20187 and plasmids for regulated dimerization are available upon request from ARIAD Pharmaceuticals (www. ariad.com/regulationkits). This work was supported by grant numbers 5R01DK52997, 1R01DK57525, R01DK61844, 2P01HL53750, 1P01DK55820 and 2P01DK47754 from the National Institutes of Health, an American Society of Hematology Junior Faculty Scholar Award, and an award from the Fanconi Anemia Research Fund.
References
- Amara J.F., Courage,N.L. and Gilman,M. (1999) Cell surface tagging and a suicide mechanism in a single chimeric human protein. Hum. Gene Ther., 10, 2651–2655. [DOI] [PubMed] [Google Scholar]
- Audet J., Miller,C.L., Rose-John,S., Piret,J.M. and Eaves,C.J. (2001) Distinct role of gp130 activation in promoting self-renewal divisions in mitogenically stimulated murine hematopoietic stem cells. Proc. Natl Acad. Sci. USA, 98, 1757–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M., Bonnet,D., Murdoch,B., Gan,O.I. and Dick,J.E. (1998) A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nature Med., 4, 1038–1045. [DOI] [PubMed] [Google Scholar]
- Blau C.A., Neff,T. and Papayannopoulou,Th. (1996) The hematological effects of folate analogs: implications for using the dihydrofolate reductase gene for in vivo selection. Hum. Gene Ther., 7, 2069–2078. [DOI] [PubMed] [Google Scholar]
- Blau C.A., Peterson,K.R., Drachman,J.G. and Spencer,D.M. (1997) A proliferation switch for genetically modified cells. Proc. Natl Acad. Sci. USA, 94, 3076–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D. and Dick,J.E. (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Med., 3, 730–737. [DOI] [PubMed] [Google Scholar]
- Ema H., Takano,H., Sudo,K. and Nakauchi,H. (2000) In vitro self-renewal division of hematopoietic stem cells. J. Exp. Med., 192, 1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M., Goldschmitt,J., Peschel,C., Brakenhoff,J.P., Kallen,K.J., Wollmer,A., Grotzinger,J. and Rose-John,S. (1997) A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nature Biotechnol., 15, 142–145. [DOI] [PubMed] [Google Scholar]
- Geddis A.E., Fox,N.E. and Kaushansky,K. (2001) Phosphatidylinositol 3-kinase (PI3K) is necessary but not sufficient for thrombopoietin-induced proliferation in engineered Mpl bearing cell lines as well as in primary megakaryocytic progenitors. J. Biol. Chem., 276, 34473–34479. [DOI] [PubMed] [Google Scholar]
- Guenechea G., Gan,O.I., Dorrell,C. and Dick,J.E. (2001) Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nature Immunol., 2, 75–82. [DOI] [PubMed] [Google Scholar]
- Harpur A.G., Andres,A.C., Ziemiecki,A., Aston,R.R. and Wilks,A.F. (1992) JAK2, a third member of the JAK family of protein tyrosine kinases. Oncogene, 7, 1347–1353. [PubMed] [Google Scholar]
- Hawley R.G., Lieu,F.H., Fong,A.Z. and Hawley,T.S. (1994) Versatile retroviral vectors for potential use in gene therapy. Gene Ther., 1, 136–138. [PubMed] [Google Scholar]
- Holyoake T.L., Freshney,M.G., McNair,L., Parker,A.N., McKay,P.J., Steward,W.P., Fitzsimons,E., Graham,G.J. and Pragnell,I.B. (1996) Ex vivo expansion with stem cell factor and interleukin-11 augments both short-term recovery post-transplant and the ability to serially transplant marrow. Blood, 87, 4589–4595. [PubMed] [Google Scholar]
- Iscove N.N. and Nawa,K. (1997) Hematopoietic stem cells expand during serial transplantation in vivo without apparent exhaustion. Curr. Biol., 7, 805–808. [DOI] [PubMed] [Google Scholar]
- Jin L., Siritanaraktul,N., Emery,D.W., Richard,R.E., Kaushansky,K., Papayannopoulou,Th. and Blau,C.A. (1998) Targeted expansion of genetically modified bone marrow cells. Proc. Natl Acad. Sci. USA, 95, 8093–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., Zeng,H., Chien,S., Otto,K.G., Richard,R.E., Emery,D.W. and Blau,C.A. (2000) In vivo selection using a cell-growth switch. Nature Genet., 26, 64–66. [DOI] [PubMed] [Google Scholar]
- Jordan C. and Lemischka,I.R. (1990) Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev., 4, 220–232. [DOI] [PubMed] [Google Scholar]
- Kiger A.A., Jones,D.L., Schulz,C., Rogers,M.B. and Fuller,M.T. (2001) Stem cell self-renewal specified by JAK–STAT activation in response to a support cell cue. Science, 294, 2542–2545. [DOI] [PubMed] [Google Scholar]
- Lacronique V. et al. (1997) A TEL–JAK2 fusion protein with constitutive kinase activity in human leukemia. Science, 278, 1309–1312. [DOI] [PubMed] [Google Scholar]
- Lemischka I.R., Raulet,D.H. and Mulligan,R.C. (1986) Developmental potential and dynamic behavior of hematopoietic stem cells. Cell, 45, 917–927. [DOI] [PubMed] [Google Scholar]
- Leonard W.J. and O’Shea,J.J. (1998) JAKS and STATs: biological implications. Annu. Rev. Immunol., 16, 293–322. [DOI] [PubMed] [Google Scholar]
- Lyman S.D. and Jacobsen,S.E.W. (1998) C-kit ligand and flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood, 91, 1101–1134. [PubMed] [Google Scholar]
- Mavilio F., Ferrari,G., Rossini,S., Nobili,N., Bonini,C., Casorati,G., Traversari,C. and Bordignon,C. (1994) Peripheral blood lympho cytes as target cells of retroviral vector-mediated gene transfer. Blood, 83, 1988–1997. [PubMed] [Google Scholar]
- Metcalf D. (1999) Stem cells, pre-progenitor cells and lineage-committed cells: are our dogmas correct? Ann. N. Y. Acad. Sci., 872, 289–304. [DOI] [PubMed] [Google Scholar]
- Miller C.L. and Eaves,C.J. (1997) Expansion in vitro of adult murine hematopoietic stem cells with transplantable lympho-myeloid reconstituting ability. Proc. Natl Acad. Sci. USA, 94, 13648–13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa Y., Rojnuckarin,P., Habib,T. and Kaushansky,K. (2001) Thrombopoietin induces PI3K and SHP2 activation through Gab and IRS proteins in BaF3 cells and primary murine megakaryocytes. J. Biol. Chem., 276, 2494–2502. [DOI] [PubMed] [Google Scholar]
- Mohi M.G., Arai,K. and Watanabe,S. (1998) Activation and functional analysis of Janus kinase 2 in BA/F3 cells using the coumermycin/gyrase B system. Mol. Biol. Cell, 9, 3299–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M., Yokota,S., Iwai,T., Kaneko,H., Horiike,S., Kashima,K., Sonoda,Y., Fujimoto,T. and Misawa,S. (1996) Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia, 10, 1911–1918. [PubMed] [Google Scholar]
- Neff T. and Blau,C.A. (2001) Pharmacologically regulated cell therapy. Blood, 97, 2535–2540. [DOI] [PubMed] [Google Scholar]
- Osawa M., Hanada,K., Hamada,H. and Nakauchi,H. (1996) Long-term lymphohematopoietic reconstitution by a single CD34– low/negative hematopoietic stem cell. Science, 273, 242–245. [DOI] [PubMed] [Google Scholar]
- Otto K.G., Broudy,V.C., Lin,N., Parganas,E., Drachman,J.G., Luthi,J.N., Ihle,J.N. and Blau,C.A. (2001) Membrane localization is not required for mpl function in normal hematopoietic cells. Blood, 98, 2077–2083. [DOI] [PubMed] [Google Scholar]
- Palacios R. and Steinmetz,M. (1985) IL3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration and generate B lymphocytes in vivo. Cell, 41, 727–734. [DOI] [PubMed] [Google Scholar]
- Parganas E. et al. (1998) Jak2 is essential for signaling through a variety of cytokine receptors. Cell, 93, 385–395. [DOI] [PubMed] [Google Scholar]
- Pawliuk R., Eaves,C. and Humphries,R.K. (1996) Evidence of both ontogeny and transplant dose-regulated expansion of hematopoietic stem cells in vivo. Blood, 88, 2852–2858. [PubMed] [Google Scholar]
- Peeters P. et al. (1997) Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood, 90, 2535–2540. [PubMed] [Google Scholar]
- Petzer A.L., Eaves,C.J., Barnett,M.J. and Eaves,A.C. (1997) Selective expansion of primitive normal hematopoietic cells in cytokine-supplemented cultures of purified cells from patients with chronic myeloid leukemia. Blood, 90, 64–69. [PubMed] [Google Scholar]
- Reya T., Morrison,S.J., Clarke,M.F. and Weissman,I.L. (2001) Stem cells, cancer and cancer stem cells. Nature, 414, 105–111. [DOI] [PubMed] [Google Scholar]
- Sauvageau G., Thorsteinsdottir,U., Eaves,C.J., Lawrence,H.J., Largman,C., Lansdorp,P.M. and Humphries,R.K. (1995) Over expression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev., 15, 1753–1765. [DOI] [PubMed] [Google Scholar]
- Schwaller J. et al. (1998) Transformation of hematopoietic cell lines to growth-factor independence and induction of a fatal myelo- and lymphoproliferative disease in mice by retrovirally transduced TEL/JAK2 fusion genes. EMBO J., 17, 5321–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaller J. et al. (2000) Stat5 is essential for the myelo- and lymphoproliferative disease induced by TEL/JAK2. Mol. Cell, 6, 693–704. [DOI] [PubMed] [Google Scholar]
- Snodgrass R. and Keller,G. (1987) Clonal fluctuation within the haematopoietic system of mice reconstituted with retrovirus-infected stem cells. EMBO J., 6, 3955–3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D.M., Wandless,T.J., Schreiber,S.L. and Crabtree,G.R. (1993) Controlling signal transduction with synthetic ligands. Science, 262, 1019–1024. [DOI] [PubMed] [Google Scholar]
- Teglund S. et al. (1998) Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell, 93, 841–850. [DOI] [PubMed] [Google Scholar]
- Thomis D.C., Marktel,S., Bonini,C., Traversari,C., Gilman,M., Bordignon,C. and Clackson,T. (2001) A Fas-based suicide switch in human T cells for the treatment of graft-versus-host disease. Blood, 97, 1249–1257. [DOI] [PubMed] [Google Scholar]
- Tulina N. and Matunis,E. (2001) Control of stem cell self-renewal in Drosophila spermatogenesis by JAK–STAT signaling. Science, 294, 2546–2549. [DOI] [PubMed] [Google Scholar]
- Udomsakdi C., Eaves,C.J., Swolin,B., Reid,D.S., Barnett,M.J. and Eaves,A.C. (1992) Rapid decline of chronic myeloid leukemic cells in long-term culture due to a defect at the leukemic stem cell level. Proc. Natl Acad. Sci. USA, 89, 6192–6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum-Finney B., Xu,L., Brasham-Stein,C., Nourigat,C., Flowers,D., Bakkour,S., Pear,W.S. and Bernstein,I.D. (2000) Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nature Med., 6, 1278–1281. [DOI] [PubMed] [Google Scholar]
- Xu F., Yang,H.W., Hanada,R., Hongo,T., Ohnishi,H., Kobayashi,M., Bessho,F., Yanagisawa,M. and Hayashi,Y. (1999) Tandem duplication of the FLT3 gene is found in acute lymphoblastic leukaemia as well as acute myeloid leukaemia but not in myelodysplastic syndrome or juvenile chronic myelogenous leukaemia in children. Br. J. Haematol., 105, 155–162. [PubMed] [Google Scholar]
- Yagi M., Ritchie,K.A., Sitnicka,E., Storey,C., Roth,G.J. and Bartelmez,S. (1999) Sustained ex vivo expansion of hematopoietic stem cells mediated by thrombopoietin. Proc. Natl Acad. Sci. USA, 96, 8126–8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S. et al. (1997) Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia, 11, 1605–1609. [DOI] [PubMed] [Google Scholar]
- Zeng H., Masuko,M., Jin,L., Neff,T., Otto,K.G. and Blau,C.A. (2001) Receptor specificity in the self-renewal and differentiation of primary multipotential hemopoietic cells. Blood, 98, 328–334. [DOI] [PubMed] [Google Scholar]
- Zijlmans J.M., Visser,J.W., Kleiverda,K., Kluin,P.M., Willemze,R. and Fibbe,W.E. (1995) Modification of rhodamine staining allows identification of hematopoietic stem cells with preferential short-term or long-term bone marrow-repopulating ability. Proc. Natl Acad. Sci. USA, 92, 8901–8905. [DOI] [PMC free article] [PubMed] [Google Scholar]