Abstract
Background
Ultra-processed foods (UPFs), often high in sodium, sugar, and unhealthy fats, compose more than half of total dietary energy consumption in the United States. A diet composed of a high amount of UPFs can contribute to glucose dysregulation and insulin resistance, which may lead to prediabetes and type 2 diabetes (T2D). However, few studies have assessed the associations between UPFs and T2D or obesity in young people. The goal of this study is to examine associations between UPF consumption and prediabetes and related biomarkers in youth.
Methods
Young adults (age 17–22, n = 85) with a history of overweight or obesity from the Metabolic and Asthma Incidence Research (Meta-AIR) study, a subset of the Children’s Health Study, were enrolled between 2014 and 2018 and returned for a second visit between 2020 and 2022. Participants completed two 24-hour dietary recalls and an oral glucose tolerance test at each visit. Food items were categorized as either an UPF or non-UPF according to NOVA classification guidelines. The proportion of the diet composed of UPFs was calculated for each participant. Regression models were used to assess relationships of UPF consumption at baseline and change between visits with markers of glucose homeostasis at follow-up, adjusting for demographics, physical activity, and total energy intake.
Results
A 10%-point increase in UPF consumption between visits was associated with a 51% (OR: 1.51, 95% Cl: 1.04, 2.31) higher odds of having prediabetes and 158% (OR: 2.58, 95% CI: 1.43, 5.85) higher odds of impaired glucose tolerance at follow-up. Higher baseline UPF consumption was significantly positively associated with 2-hour insulin ( = 45.11, 95% CI: 22.42, 67.80) and insulin area under the curve (
= 45.11, 95% CI: 22.42, 67.80) and insulin area under the curve (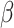 = 63.56, 95% CI: 34.95, 92.17) at follow-up.
= 63.56, 95% CI: 34.95, 92.17) at follow-up.
Conclusion
UPF consumption may increase the risk for T2D among young adults. Our findings suggest that limiting UPF consumption could be an important strategy for T2D prevention in this population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12986-025-01036-6.
Keywords: Ultra-processed food, Type 2 diabetes, Prediabetes, Young adults, Body composition
Introduction
Prediabetes has become more common among young adults in recent years, which increases the risk for early onset type 2 diabetes (T2D) [1, 2]. In the United States (U.S.), the prevalence of T2D is estimated to be about 17.9 per 100,000 in youth under 20 years of age [3]. T2D is also a significant global public health concern because it can affect individuals’ quality of life, lead to many comorbidities, and increase mortality risk [2, 4]. The early onset of T2D among young adults can lead to more long-term health issues compared to onset of T2D in later adulthood [1, 5–9]. Obesity greatly increases the risk for prediabetes and T2D, and poor diet and other lifestyle factors can be risk factors for all three conditions [2, 4, 5]. Since prediabetes, T2D, and obesity are closely related to each other and share similar risk factors, the assessment of modifiable risk factors like diet is crucial for prevention and treatment of these conditions.
In the US, more than half of total dietary energy consumption is composed of ultra-processed foods (UPFs) [10, 11]. UPFs are food items that go through multiple industrial processes before people purchase or eat them [12]. Examples of common UPFs include soft drinks, packaged snacks, margarine, and sausages [12]. Most UPFs are calorie-dense and high in sugar, salt, and unhealthy fats, while low in protein, vitamins, and minerals [10, 13–16]. Studies have demonstrated that higher consumption of UPFs results in poor nutritional diet quality and increased risk for the development of chronic diseases, including T2D and hypertension [13–18]. It is important to limit UPF consumption in childhood and adolescence due to the high content of added sugar and saturated fats in UPFs and their possible contribution to weight gain, T2D, cardiovascular disease, and hypertension [16–18].
Though many risk factors for metabolic disease first appear in early life, most research studies have focused on the effects of UPFs on metabolic disease in middle-aged and older adults [19–22]. Many of these studies show that diets with a higher proportion of UPFs or increasing consumption of UPFs is associated with a higher risk of T2D and obesity among adults [19–22]. In addition, most previous studies examining UPF consumption and metabolic disease have been conducted in Brazil and primarily used cross-sectional analyses, with only a few using a longitudinal study design [23–27]. However, few studies have assessed the associations between UPFs and T2D or obesity in young people, and those that have are cross-sectional and report mixed results [18, 20–22]. Some studies have shown that limiting the consumption of UPFs can reduce T2D and obesity risk among children and young adults, while others found no association between UPF consumption and obesity or overweight [18, 20–22]. Because of the limited studies on UPF consumption among young adults, and the importance of early lifestyle changes in preventing T2D among high risk populations, more research is needed to understand the relationship between UPF consumption and risk for T2D and obesity in young adults [28].
The purpose of this study is to assess the longitudinal associations between UPF consumption and prediabetes and obesity in young adults, using glucose and insulin measurements, body composition, and diet assessment over four years of follow-up. We hypothesized that increases in UPF consumption would be associated with a higher risk of altered glucose homeostasis, insulin resistance, obesity, and prediabetes.
Methods
Cohort
Between 2014 and 2018, 155 young adults aged 17–22 who had previously participated in the Children’s Health Study in Southern California were invited to enroll in the Metabolic and Asthma Incidence Research (Meta-AIR) study to assess the impact of air pollution on obesity and cardiometabolic health status [9, 29, 30]. To be eligible, participants had a history of overweight or obesity in early adolescence, were not diagnosed with either type 1 or type 2 diabetes, were not taking medications that influence glucose metabolism, and had no other significant medical diagnosis [9]. Of these, 85 returned for a follow-up visit between 2020 and 2022 (the MetaCHEM study, Fig. 1) [9]. This study was approved by the Institutional Review Board at the University of Southern California and written informed consents or assents were obtained from participants and their guardians.
Fig. 1.

Flowchart for participants recruitment
Dietary assessment and UPF classification
Participants completed two non-consecutive 24-hour dietary recalls on one weekday and one weekend day at each visit [9]. Trained interviewers used the Nutritional Data System for Research (NDSR) software version 2014 to complete the baseline recalls, while participants used the Automated Self-Administered 24-hr Dietary Assessment Tool (ASA24) version 2018 to conduct the recalls at the follow-up visit [9, 31, 32]. At baseline, 10.3% (n = 16) of participants completed only one recall, while at follow-up 10.2% (n = 9) of participant completed only one recall.
In this study, a total of 1,167 unique food items were reported at the baseline visit and a total of 807 unique food items were reported the at the follow-up visit. Some food items reported at the follow-up visit contained mixed dishes with multiple ingredients. When possible, these foods were disaggregated into individual ingredients using the 2017–2018 Food and Nutrient Database for Dietary Studies (FNDDS) Ingredients database and matched to the food codes from the FNDDS Foods and Beverages database that were provided by ASA24. [9, 33] Each ingredient or food was classified as ultra-processed or not by two independent reviewers (YL, EC). Foods were classified according to the NOVA group definitions of (1) unprocessed and minimally processed foods, (2) processed culinary ingredients, (3) processed foods, and (4) ultra-processed foods (UPFs) [10, 12, 34]. Any disagreements on UPF classification were discussed, and the final classification decision was made by consensus.
Briefly, unprocessed and minimally processed foods in NOVA group 1 are edible parts of plants or animals and foods that have been through basic processing such as drying, griding and freezing (e.g., fruits and vegetables, eggs, meat and seafood, flour, spices) [12]. Processed culinary ingredients in NOVA group 2 mainly composed of vegetable oils, butter, salt, sugar, and honey for the main purpose of seasoning and cooking [12]. Processed foods in NOVA group 3 contain food products that are made by adding items in group 2 to group 1 for the purpose of preservation (e.g., canned vegetables, cured or smoked meat and fish, freshly made bread) [12]. UPFs in NOVA group 4 are made with series of industrial techniques that cannot be replicated at home and may involve molding or pre-frying, the addition of colors and flavors, and often includes added sugars, salt, oils, and fats [12]. This group also includes most branded and packaged foods, pre-prepared ready-to-eat products, and instant foods [12]. However, enriched food items, like milk or flour with added vitamins or minerals, are not included in this group. Examples of UPFs are soft drinks, candies, cereal, ice-cream, mass-produced cookies or pastries, margarines, packaged and shelf-stable spreads, milk drinks, flavored yogurts, pizza, and sausages.
UPF classification was made with the following considerations: how the food is typically prepared in the U.S. and how most people obtain the food (if purchased from a store, restaurant, or homemade). Items from fast food restaurants were categorized as UPFs. Bread and rolls were presumed to be shelf-stable and purchased from grocery stores or wholesalers were classified as ultra-processed unless reported to be homemade. A list of all foods reported by participants at each visit, and their classification as UPF or non-UPF, is included in the Excel Supplement.
UPF percentage calculation
The proportion of the diet composed of UPFs was calculated by weight rather than calories, to account for some foods that provide no contribution to energy intake, such as diet soda. The percent of diet that was ultra-processed (UPF%) was calculated as the total amount of UPFs in grams divided by the total amount of foods and beverages consumed in grams, multiplied by 100% and averaged across both recall days. If a participant only completed one dietary recall, the dietary information from the single day was used. The change in UPF consumption between visits (UPF% ) was calculated as UPF%baseline – UPF%follow−up.
) was calculated as UPF%baseline – UPF%follow−up.
Study outcomes
Glucose homeostasis was assessed using hemoglobin A1c (HbA1c) and a 2-hour oral glucose tolerance test (OGTT) glucose and insulin related measures [9]. During the OGTT, glucose and insulin were measured in plasma while fasting and at 30-, 60-, 90- and 120 min after glucose administration [9, 29]. HbA1c was measured in fasting whole blood samples [9]. The glucose and insulin area under the curves (AUCs) were calculated using the trapezoidal method with the 5 time points from the OGTT [9, 29, 35].
Prediabetes or T2D was categorized according to the American Diabetes Association criteria based on OGTT or HbA1c values measured during the study. Participants were considered to have prediabetes if their HbA1c was between 5.7% and 6.4%, their fasting glucose was between 100 mg/dL and 125 mg/dL, or their 2-hour glucose was between 140 mg/dL and 199 mg/dL. Participants were considered to have T2D if their HbA1c was 6.5% or higher, their fasting glucose was 126 mg/dL or higher, or their 2-hour glucose was 200 mg/dL or higher [29, 36]. Impaired fasting glucose (IFG) was defined as having a fasting glucose value greater than 100 mg/dL and impaired glucose tolerance (IGT) was defined as having a 2-hour glucose value greater than 140 mg/dL [37].
To assess insulin resistance and beta-cell function, the homeostatic model assessment of insulin resistance (HOMA-IR) and homeostatic model assessment of β-cell function (HOMA-β) were calculated from fasting glucose and fasting insulin values [38]. The Matsuda Index was used to estimate the insulin sensitivity of the entire body using the 5 time points from the OGTT [29, 39].
Body composition was assessed using body mass index (BMI, kg/m2) and dual-energy X-ray absorptiometry (DEXA) [9, 40]. DEXA measures included body fat percentage, android to gynoid ratio, fat mass to height ratio (kg/m2), and visceral adipose tissue (VAT) mass (g) [9, 40]. BMI was categorized as normal weight (< 25 kg/m2), overweight (25–29.9 kg/m2), and obesity  30 kg/m2) using body weight and height measured at each visit.
30 kg/m2) using body weight and height measured at each visit.
Covariates
Demographic information including age, sex, and ethnicity were self-reported though questionnaires at baseline and follow-up [9]. Ethnicity was categorized as White, Hispanic/Latino, and Other. Physical activity level at the follow-up visit was assessed using the International Physical Activity Questionnaire and categorized into High, Medium, and Low [9, 41]. Smoking status and alcohol consumption were also assessed at the follow-up visit. Smoking was categorized as ever or never, and alcohol consumption was included as number of drinking consumed per month. Covariates were selected using a Directed Acyclic Graph (DAG) (Figure S1). All proposed covariates were selected as part of the minimally sufficient set.
Statistical analysis
Descriptive statistics for %UPF consumption, outcomes, and covariates at both visits were calculated. Differences between categorical variables at each visit were assessed using McNamar’s test and differences between continuous variables at each visit were assessed using paired t-tests. Few participants were found to have T2D, so prediabetes and T2D were combined into one outcome group (Prediabetes/T2D) for analysis.
Linear and logistic regressions were used to evaluate the effects of UPF consumption on each outcome, measured at the follow-up visit. Each model contained UPF% consumption at baseline, UPF% , and adjusted for covariates as follows:
, and adjusted for covariates as follows:
 |
All models adjusted for age, sex, ethnicity, energy intake at the follow-up visit, and exercise at the follow-up visit. Beta estimates and odds ratios were scaled by 10 units. An additional logistic regression was performed for the prediabetes/T2D outcome restricting the study population to only those who did not have prediabetes/T2D at baseline.
We also performed a sensitivity analysis to examine the impact of smoking (ever or never) and alcohol intake (number of drinks per month), by including them as covariates. All analyses were performed using R (version 2022.02.3 + 492; R Core Development Team).
Results
Descriptive statistics
Descriptive statistics for participants’ characteristics are presented in Table 1. The proportion of the diet composed of UPFs increased, on average, from about 20% at baseline to almost 24% at follow-up (p = 0.02) (Table 1). At the baseline visit, 28% (n = 24) of participants were free of either prediabetes or T2D, while 38% (n-32) of participants had either prediabetes or T2D at follow-up. Of those who did not have prediabetes/T2D at baseline (n = 61), 16 of these participants had prediabetes/T2D at follow-up, while some participants with prediabetes/T2D at baseline reverted to no diabetes (Table 2). IGT were not significantly different between visits, while more participants had IFG at the follow-up than at baseline (Table 2). Fasting glucose increased by 4.91 mg/dL from the baseline to the follow-up visit (p < 0.05), while HbA1c, two-hour glucose, and glucose AUC also increased between visits, though the increase was not statistically significant (Table 2). Similar patterns between visits were observed where fasting insulin, two-hour insulin, HOMA-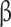 , and HOMA-IR increased between visits, though Matsuda index significantly decreased (p < 0.05) (Table 2). All body composition measurements significantly increased from the baseline to the follow-up visit, except android/gynoid ratio (Table 2).
, and HOMA-IR increased between visits, though Matsuda index significantly decreased (p < 0.05) (Table 2). All body composition measurements significantly increased from the baseline to the follow-up visit, except android/gynoid ratio (Table 2).
Table 1.
Descriptive statistics for participant characteristics at baseline and follow-up visits
| Baseline | Follow-up | p-value1 | |
|---|---|---|---|
| UPF%, mean (SD) | 20.40 (12.68) | 23.60 (17.73) | 0.02 |
| Total Energy Intake (kcal), mean (SD) | 2094 (669) | 2252 (770) | - |
| Age (years), mean (SD) | 19.97 (1.20) | 24.07 (0.75) | - |
|
Sex, n (%) Female Male |
43 (50.59) 42 (49.41) |
- | - |
|
Ethnicity White /Latino Other |
30 (35.29) 49 (57.65) 6 (7.06) |
- | - |
|
BMI category Normal weight Overweight Obesity |
13 (15.29) 35 (41.18) 37 (43.53) |
11 (12.94) 32 (37.65) 42 (49.41) |
0.47 |
|
Physical activity Low Medium High N/A |
- |
16 (18.82) 21 (24.71) 47 (55.29) 1 (1.18) |
- |
|
Smoking status Ever Never |
- |
30 (35%) 55 (65%) |
- |
|
Alcohol consumption (drinks per month), mean (SD) |
- | 4.3 (4.3) | - |
1p-values were calculated using a paired t-test for UPF% and McNemar’s test for BMI category, Type 2 Diabetes, IFG and IGT
*Abbreviations: UPF%: percent of the diet from ultra-processed foods; BMI: body mass index; T2D: type 2 diabetes; IFG: impaired fasting glucose; IGT: impaired glucose tolerance; SD: standard deviation
Table 2.
Descriptive statistics for outcome measurements at baseline and follow-up visits
| Mean (SD) | Change between v(SD) | p-value1 | ||
|---|---|---|---|---|
| Baseline | Follow-up | |||
| Glucose measurements | ||||
| HbA1c | 5.22 (0.28) | 5.26 (0.52) | 0.04 (0.46) | 0.35 |
|
Fasting Glucose (mg/dL) Missing, n(%) |
90.41 (7.55) 0 |
95.32 (16.61) 1 (1.2%) |
5.04 (15.0) | 0.003 |
|
Two-Hour Glucose (mg/dL) Missing, n (%) |
119.0 (26.4) 0 |
121.3 (35.0) 4 (4.7%) |
3.1 (31.8) | 0.39 |
|
Glucose AUC Missing, n (%) |
263.1 (45.0) 0 |
270.5 (45.1) 4 (4.7%) |
10.7 (39.8) | 0.02 |
| Insulin measurements | ||||
|
Fasting Insulin (µIU/mL) Missing, n (%) |
9.6 (8.6) 12 (14.1%) |
13.3 (11.2) 0 |
4.56 (13.1) | 0.02 |
|
Two-Hour Insulin (µIU/mL) Missing, n (%) |
57.2 (49.6) 28 (32.9%) |
88.3 (128.5) 0 |
24.6 (81.0) | 0.03 |
|
Insulin AUC Missing, n (%) |
129.6 (82.3) 34 (40.0%) |
186.5 (176.9) 3 (3.5%) |
60.7 (157.0) | 0.01 |
|
HOMA- Missing, n (%) |
100.0 (63.2) 34 (40.0%) |
151.4 (120.3) 1 (1.2%) |
59.2 (139.0) | 0.004 |
|
HOMA-IR Missing, n (%) |
1.7 (1.2) 34 (40.0%) |
3.4 (3.7) 1 (1.2%) |
2.1 (4.2) | 0.001 |
|
Matsuda Index Missing, n (%) |
5.9 (3.6) 34 (40.0%) |
4.3 (2.8) 6 (7.1%) |
−1.9 (2.9) | 0.009 |
| Body composition | ||||
|
BMI (kg/m2) Missing, n (%) |
30.1 (5.0) 0 (0%) |
31.8 (7.0) 0 (0%) |
1.75 (4.3) | < 0.001 |
|
Body Fat Percent Missing, n (%) |
35.2 (8.1) 0 (0%) |
38.3 (8.4) 2 (2.4%) |
3.1 (4.7) | < 0.001 |
|
Android/Gynoid Ratio Missing, n (%) |
0.99 (0.14) 46 (54.1%) |
1.01 (0.15) 2 (2.4%) |
0.015 (0.085) | 0.47 |
|
Fat Mass/Height2 Missing, n (%) |
10.6 (3.7) 46 (54.1%) |
12.2 (4.8) 2 (2.4%) |
1.6 (2.1) | < 0.001 |
|
VAT mass (g) Missing, n (%) |
506.4 (197.8) 46 (54.1%) |
594.3 (301.4) 2 (2.4%) |
80.5 (137.0) | 0.06 |
| Dichotomous outcomes, n (%) | ||||
|
Type 2 Diabetes No Diabetes Prediabetes/T2D |
61 (71.76) 24 (28.24) |
53 (62.35) 32 (37.65) |
0.17 | |
|
IFG Normal Abnormal Missing |
80 (94.12) 5 (5.88) 0 |
68 (80.00) 16 (18.82) 1 (1.18) |
0.003 | |
|
IGT Normal Abnormal Missing |
69 (81.18) 16 (18.82) 0 |
66 (77.64) 15 (17.65) 4 (4.71) |
0.82 | |
1p-values were calculated using paired t-tests and McNemar’s test for Type 2 Diabetes, IFG and IGT
*Abbreviations: SD: standard deviation; BMI: body mass index; VAT: visceral adipose tissue; HbA1c: Hemoglobin A1c; AUC: Area Under the Curve; HOMA-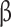 : homeostatic model assessment of
: homeostatic model assessment of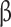 -cell function; HOMA-IR: homeostatic model assessment for insulin resistance
-cell function; HOMA-IR: homeostatic model assessment for insulin resistance
Prediabetes/T2D and insulin resistance
Table 3 shows the associations between UPF%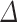 and UPF% at baseline and prediabetes/T2D, IFG, and IGT after adjusting for covariates. A 10-unit increase in UPF%
and UPF% at baseline and prediabetes/T2D, IFG, and IGT after adjusting for covariates. A 10-unit increase in UPF%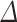 was significantly associated with a 51% higher odds of having prediabetes/T2D (OR: 1.51, 95% CI: 1.04, 2.31), and with a 158% higher odds of having IGT (OR: 2.58, 95% CI: 1.43, 5.85). UPF%
was significantly associated with a 51% higher odds of having prediabetes/T2D (OR: 1.51, 95% CI: 1.04, 2.31), and with a 158% higher odds of having IGT (OR: 2.58, 95% CI: 1.43, 5.85). UPF% was also significantly positively associated with 2-hour glucose (β = 6.21, 95% CI: 0.71–11.71) (Table 4). These findings were similar when participants with prediabetes/T2D at baseline were excluded (Table 3). Significant positive associations were also observed between baseline UPF% and 2-hour insulin and insulin AUC, and a significant negative association was observed between baseline UPF% and Matsuda index (Table 4). Further adjustment for smoking and alcohol consumption did not appreciably change the magnitude of these associations (Table S1, Table S2).
was also significantly positively associated with 2-hour glucose (β = 6.21, 95% CI: 0.71–11.71) (Table 4). These findings were similar when participants with prediabetes/T2D at baseline were excluded (Table 3). Significant positive associations were also observed between baseline UPF% and 2-hour insulin and insulin AUC, and a significant negative association was observed between baseline UPF% and Matsuda index (Table 4). Further adjustment for smoking and alcohol consumption did not appreciably change the magnitude of these associations (Table S1, Table S2).
Table 3.
Odds ratios for the effect of change in UPF consumption and baseline UPF consumption on prediabetes/type 2 diabetes, impaired fasting glucose, and impaired glucose tolerance
| OR (95% CI) | ||
|---|---|---|
UPF%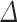 1 1
|
UPF% at baseline 2 | |
| All participants (n = 85) | ||
| Prediabetes/T2D | 1.51 (1.04, 2.31) | 1.41 (0.90, 2.26) |
| IFG | 1.21 (0.83, 1.79) | 1.22 (0.71, 2.05) |
| IGT | 2.58 (1.43, 5.85) | 1.31 (0.65, 2.69) |
| Excluding participants with Prediabetes/T2D at baseline (n= 61) | ||
| Prediabetes/T2D | 1.56 (1.00, 2.59) | 1.54 (0.93, 2.64) |
| IFG | 1.24 (0.74, 2.08) | 1.67 (0.86, 3.40) |
| IGT | 2.30 (1.28, 5.01) | 1.42 (0.67, 3.12) |
*All outcomes were measured at the follow-up visit. Effects are scaled by 10 units
1Models were adjusted for age, sex, ethnicity, exercise at follow-up, total energy intake at follow-up, and UPF% at baseline
2Models were adjusted for age, sex, ethnicity, exercise at follow-up, total energy intake at follow-up, and UPF%Δ
**Abbreviations: UPF%: percent of diet from ultra-processed foods; UPF%Δ: change in percent of diet from ultra-processed foods between visits; T2D: type 2 diabetes; IFG: impaired fasting glucose; IGT: impaired glucose tolerance
Table 4.
Effect estimates for UPF percentage change and baseline UPF consumption on body composition and glucose, insulin, and body composition measurements
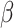 (95% CI) (95% CI) |
||
|---|---|---|
UPF% 1 1
|
UPF% at Baseline 2 | |
| Glucose measurements | ||
| HbA1c | 0.02 (−0.06, 0.11) | −0.01 (−0.12, 0.09) |
| Fasting glucose | 1.42 (−1.23, 4.06) | 0.37 (−2.89, 3.63) |
| Two-hour glucose | 6.21 (0.71, 11.71) | 0.98 (−5.77, 7.72) |
| Glucose AUC | 4.75 (−2.33, 11.84) | 3.31 (−5.37, 12.00) |
| Insulin measurements | ||
| Fasting insulin | 0.25 (−1.39, 1.88) | 1.97 (−0.04, 3.98) |
| Insulin after 120 min | 6.41 (−12.15, 24.97) | 45.11 (22.42, 67.80) |
| Insulin AUC | 4.05 (−19.36, 27.45) | 63.56 (34.95, 92.17) |
HOMA-
|
−5.45 (−22.05, 11.15) | 17.54 (−2.91, 37.99) |
| HOMA-IR | 0.16 (−0.40, 0.73) | 0.40 (−0.29, 1.09) |
| Matsuda index | −0.42 (−0.85, 0.01) | −0.63 (−1.13, −0.14) |
| Body composition | ||
| BMI | 0.10 (−0.99, 1.18) | 0.73 (−0.61, 2.06) |
| Body fat percentage | 0.60 (−0.35, 1.55) | 1.08 (−0.07, 2.23) |
| Android/gynoid ratio | 0.005 (−0.02, 0.03) | 0.006 (−0.02, 0.03) |
| Fat mass/height2 | 0.002 (−0.70, 0.71) | 0.41 (−0.44, 1.26) |
| VAT mass | −13.01 (−61.79, 35.77) | 20.26 (−38.71, 79.23) |
*All outcomes were measured at the follow-up visit. Effects are scaled by 10 units
1Models were adjusted for age, sex, ethnicity, exercise at follow-up, total energy intake at follow-up, and UPF% at baseline
2Models were adjusted for age, sex, ethnicity, exercise at follow-up, total energy intake at follow-up, and UPF%Δ
**Abbreviations: UPF%: percent of diet from ultra-processed foods; UPF%Δ: change in percent of diet from ultra-processed foods between visits; BMI: body mass index; VAT: visceral adipose tissue; HbA1c: Hemoglobin A1c; AUC: Area Under the Curve; HOMA-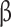 : homeostatic model assessment of
: homeostatic model assessment of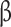 -cell function; HOMA-IR: homeostatic model assessment for insulin resistance
-cell function; HOMA-IR: homeostatic model assessment for insulin resistance
Body composition
The associations between UPF% and UPF% at baseline and body composition measurements are shown in Table 4. There were no statistically significant associations between UPF%
and UPF% at baseline and body composition measurements are shown in Table 4. There were no statistically significant associations between UPF% or baseline UPF% and body composition. However, we observed positive but non-statistically significant associations between UPF%
or baseline UPF% and body composition. However, we observed positive but non-statistically significant associations between UPF%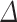 and baseline UPF consumption and BMI, body fat percent, android/gynoid ratio, fat mass/height [2] and VAT mass. Results were similar after adjustment for smoking and alcohol consumption (Table S2).
and baseline UPF consumption and BMI, body fat percent, android/gynoid ratio, fat mass/height [2] and VAT mass. Results were similar after adjustment for smoking and alcohol consumption (Table S2).
Discussion
In this novel longitudinal analysis, we found that increasing UPF consumption over a four-year period increased the odds of having prediabetes and IGT in young adults. Higher UPF consumption was associated with significant increases in some markers of insulin sensitivity including fasting insulin, 2-hour insulin, and insulin AUC. Positive but non-significant associations with most measures of adiposity were also observed. Increases in UPF consumption between study visits was also associated with decreasing Matsuda index, a measure of insulin sensitivity that describes insulin secretion relative to blood glucose [39]. These findings suggest that UPF consumption is associated with increased odds of having insulin resistance. Importantly, since early prevention and T2D treatment among young adults can be highly effective, our results highlight the adverse impact of UPF consumption on T2D development and emphasize the importance on dietary habits for young adults [28].
Previous studies have investigated associations between UPF consumption and T2D, though none have included detailed glucose and insulin measurements to explore possible mechanisms of T2D development or the changes in glucose homeostasis that could lead to T2D [27, 42, 43]. In addition to positive associations between UPF consumption and IGT and prediabetes, we also found associations between UPF consumption and insulin resistance by multiple measures: lower Matsuda indices, higher insulin concentrations across the OGTT, and positive, but non-significant, association with HOMA-IR. Insulin resistance and beta-cell dysfunction are important physiological characteristics of T2D and can be influenced by diet. Some nutritional components of many UPFs, including saturated fat, free fatty acids, and added sugars, are known to affect beta-cell function. If consumed in excess, these nutrients could exhaust beta-cells, inhibiting their function and further contributing to insulin resistance and eventually T2D [44]. Though we did not observe any inverse associations between UPF consumption and HOMA-β, this does not exclude beta-cell exhaustion as a possible mechanism underlying the relationship between UPF consumption and T2D; relationships between HOMA-β and risk for T2D are inconsistent across different populations and among populations at different stages of T2D progression [45, 46]. It is important to continue to explore the impact of UPF consumption and its underlying role on developing T2D.
Growing proportions of the diet are composed of UPFs, which are usually high in added sugars, saturated fats, or other nutrients, leading to lower diet quality and increasing prevalence of diet-associated chronic diseases [15–18]. Existing studies on UPF consumption have primarily focused on middle-aged or older adults, yet the early onset of T2D among young adults is rising, suggesting the need for evaluation and interventions targeted at youth [1]. Studies in older adults have showed that higher UPF consumption is associated with an increased risk for T2D, which is consistent with our findings [47–51]. However, evidence for associations between overweight or obesity, both risk factors for T2D, and UPF consumption is inconsistent across different age groups. While many studies have reported a positive association between UPF consumption and overweight and/or obesity among adults, others did not observe the same pattern in children [21, 22, 42, 51, 52]. We observed mostly positive, but non-significant, relationships between UPF consumption and obesity-related body compositive measurements, which is consistent with previous studies in adults [23–25]. These studies suggested that reducing UPF consumption may also benefit adults by preventing excess weight gain [23–25]. However, the lack of statistical significance in our findings may be due to the limited sample size, suggesting larger sample sizes are needed for future studies of the impact of UPF consumption on young adults.
Many UPFs are high in salt, fat, and sugar, which could independently contribute to metabolic diseases, and thus are especially relevant targets of public health interventions [53–55]. High salt intake may be a contributor to obesity and diabetes [56–58], and dietary fat, especially trans fatty acids and saturated fats, is positively associated with T2D and obesity risks due to its effects on insulin sensitivity [58–61]. Consumption of soft drinks and foods that contain high amounts of added sugar were also found to increase risk for T2D and obesity, and limiting added sugar intake and UPFs may prevent chronic disease in children and adolescents [16, 61, 62]. Previous work and the findings from our present study suggest that the nutrients common to UPFs increase the risk for obesity, which may be a mechanism by which UPFs increase the risk for T2D.
This study has many strengths. Firstly, gold-standard outcome measurements were obtained using OGTT and DEXA at both time points [9]. Secondly, our study focuses on young adults: an age group not often included in previous work. Young adults in their late teens and twenties have only recently reached physically mature and are undergoing significant lifestyle changes that may affect their risk for obesity and T2D. Thirdly, this study is one of the few longitudinal studies to examine the relationship between UPFs and risk for prediabetes, insulin resistance, and obesity, where diet and each outcome was assessed at each time point. This study design allowed us to evaluate the changes in UPF consumption over several years to follow up and assess the resulting impacts on glucose homeostasis and insulin sensitivity.
However, this study also has some limitations. Our relatively small sample size may have limited our statistical power to detect associations between UPF consumption and some outcomes. Only 85 participants returned for a follow-up visit and completed all study activities, though we have previously reported that participants’ characteristics were similar in those who completed follow-up compared to the overall baseline population [9]. Despite this, we did have enough power to consistently detect associations between UPF% consumption and prediabetes, IGT, and markers of insulin resistance. The sample size also limited our ability to use more robust models, such as log-binomial regression and Poisson, to estimate the true risk ratios. Our logistic regression models generated odds ratios, which may overestimate the true relative risk when the prevalence of the outcome is high. Additionally, dietary recalls may be subject to recall bias and may not represent long-term eating habits, though administration of multiple recalls improves estimates of nutrient intake and the variety of foods consumed [9]. We also used two different dietary recall systems, NDSR and ASA24, for the baseline and follow-up visits, which use different databases for reported foods. However, both systems provided a similar level of detail about food processing and sources, and we do not expect that one or the other would systematically encourage classification into higher NOVA processing categories [63, 64]. While it is possible that some misclassification of UPFs occurred, though we minimized this by preforming the classification using two independent researchers, and we would expect any misclassification to be non-differential. Additionally, recall days were not random. Specifically, the first dietary recall was conducted at the study visit, and the second was scheduled ahead of time to accommodate participants’ schedules. This may introduce bias if participants adjusted their eating patterns in anticipation of their recall. However, we expect that any dietary changes would be made to make the diet appear healthful and would result in lower reported intake of foods perceived to be unhealthy (which may include UPFs such as sugar sweetened beverages, fast food, etc.). Because all participants’ recalls were scheduled in the same way, we would not expect any differential misclassification. Finally, this study was conducted in a population with a history of overweight and obesity in adolescence, which may limit generalization to other populations.
Findings from this study suggest that reducing UPF consumption may reduce the risk for prediabetes and T2D in youth. Young adults may also benefit from limiting foods that contain high amounts of salt, fat, and sugar as they all potentially contribute to obesity, which also increases the risk for T2D [4, 5]. Metabolic diseases such as T2D and obesity are significant public health problems and are becoming more prevalent among young adults [1,65]. Our study also shows that UPF consumption is associated with insulin resistance, a risk factor for T2D and a condition not commonly assessed in previous studies of UPFs and T2D risk. Because more than half of the total daily energy in consumption in the US is from UPFs, this modifiable risk factor is a possible target for both individual and public health interventions in preventing metabolic diseases. Future studies may incorporate additional methods of diet assessment and larger sample sizes to improve our understanding of the eating habits of young adults and the mechanisms underlying associations between UPF consumption and metabolic diseases.
Conclusions
This prospective study found that UPF consumption is positively associated with increased odds of having prediabetes among young adults. Increasing UPF consumption was also associated with impaired glucose tolerance and insulin resistance, known risk factors for the future development of T2D. This study evaluated a unique population of the youth with detailed longitudinal measurements of diet and glucose homeostasis. These findings indicate that limiting the consumption of UPFs may be an important strategy for T2D prevention among young adults.
Supplementary Information
Author contributions
Y.L., E.C. and L.C. designed research; E.C., W.B.P. and S.R. conducted research; Y.L. and E.C. performed statistical analysis; Y.L. and E.C. wrote the paper; Y.L. had primary responsibility for final content; W.B.P., Z.C., F.G., M.I.G., T.L.A., J.A.G., D.V.C, N.S. and L.C. reviewed and edited the paper. All authors have read and approved the final manuscript.
Funding
This publication was supported by the National Institute of Environmental Health Science (NIEHS) under Award Number P42ES036506 and P30ES007048. Funding for the MetaAir study came from the Southern California Children’s Environmental Health Center grants funded by NIEHS (5P01ES022845-03, P30ES007048, 5P01ES011627), the United States Environmental Protection Agency (RD83544101), and the Hastings Foundation. Additional funding from NIEHS supported Dr. Chatzi (R01ES029944, R01ES030364, and U01HG013288), and Dr. Costello (T32ES013678 and U01HG013288). Other support from the European Union supported Dr. Chatzi (The Advancing Tools for Human Early Lifecourse Exposome Research and Translation (ATHLETE) project: 874583), Dr. Alderete (NIEHS: R01ES035035 and R01ES035056 and National Institute on Minority Health and Health Disparities: P50MD017344), and Dr. Stratakis (Horizon Europe Research and Innovation Program under the Marie Skłodowska-Curie Actions Postdoctoral Fellowships: 101059245).
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request. The data are not publicly available to protect participants’ identifiable information.
Declarations
Ethics approval and consent to participate
Informed consent and/or assent were obtained from all participants and their guardians, if participants were under the age of 18. This study was conducted in accordance with the Declaration of Helsinki and approved by the University of California Institutional Review Board (MetaAir IRB#: HS-13-00283; Meta-CHEM IRB#: HS-19-00338).
Competing interests
The authors declare no competing interests.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors did not use any AI tool during the preparation of this work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in incidence of type 1 and type 2 diabetes among youths — selected counties and Indian Reservations, United States, 2002–2015. MMWR Morb Mortal Wkly Rep. 2020;69(6):161–5. 10.15585/mmwr.mm6906a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuster-Parra P, Yañez AM, López-González A, Aguiló A, Bennasar-Veny M. Identifying risk factors of developing type 2 diabetes from an adult population with initial prediabetes using a bayesian network. Front Public Health. 2023;10:1035025. 10.3389/fpubh.2022.1035025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagenknecht LE, Lawrence JM, Isom S, et al. Trends in incidence of youth-onset type 1 and type 2 diabetes in the USA, 2002–18: results from the population-based SEARCH for diabetes in youth study. Lancet Diabetes Endocrinol. 2023;11(4):242–50. 10.1016/S2213-8587(23)00025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes – Global burden of disease and forecasted trends. J Epidemiol Glob Health. 2019;10(1):107. 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes AS. The epidemic of obesity and diabetes: trends and treatments. Tex Heart Inst J. 2011;38(2):142–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Symptoms & Causes of Diabetes | NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases. Accessed February 4. 2023. https://www.niddk.nih.gov/health-information/diabetes/overview/symptoms-causes
- 7.Shah AS, Nadeau KJ. The changing face of paediatric diabetes. Diabetologia. 2020;63(4):683–91. 10.1007/s00125-019-05075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magliano DJ, Sacre JW, Harding JL, Gregg EW, Zimmet PZ, Shaw JE. Young-onset type 2 diabetes mellitus — implications for morbidity and mortality. Nat Rev Endocrinol. 2020;16(6):321–31. 10.1038/s41574-020-0334-z. [DOI] [PubMed] [Google Scholar]
- 9.Costello E, Goodrich J, Patterson WB, et al. Diet quality is associated with glucose regulation in a cohort of young adults. Nutrients. 2022;14(18):3734. 10.3390/nu14183734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monteiro CA, Cannon G, Levy RB, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22(5):936–41. 10.1017/S1368980018003762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baraldi LG, Martinez Steele E, Canella DS, Monteiro CA. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study. BMJ Open. 2018;8(3):e020574. 10.1136/bmjopen-2017-020574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monteiro CA, Cannon G, Lawrence M. Maria Laura da Costa Louzada, Priscila Pereira Machado. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System.
- 13.Moubarac JC, Batal M, Louzada ML, Martinez Steele E, Monteiro CA. Consumption of ultra-processed foods predicts diet quality in Canada. Appetite. 2017;108:512–20. 10.1016/j.appet.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Rauber F, da Costa Louzada ML, Steele E, Millett C, Monteiro CA, Levy RB. Ultra-Processed food consumption and chronic Non-Communicable Diseases-Related dietary nutrient profile in the UK (2008–2014). Nutrients. 2018;10(5):587. 10.3390/nu10050587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louzada ML, da Ricardo C, Steele CZ, Levy EM, Cannon RB, Monteiro G. The share of ultra-processed foods determines the overall nutritional quality of diets in Brazil. Public Health Nutr. 2018;21(1):94–102. 10.1017/S1368980017001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cediel G, Reyes M, da Costa Louzada ML, et al. Ultra-processed foods and added sugars in the Chilean diet (2010). Public Health Nutr. 2018;21(1):125–33. 10.1017/S1368980017001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rauber F, Campagnolo PDB, Hoffman DJ, Vitolo MR. Consumption of ultra-processed food products and its effects on children’s lipid profiles: a longitudinal study. Nutr Metab Cardiovasc Dis. 2015;25(1):116–22. 10.1016/j.numecd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Louzada ML, da Baraldi C, Steele LG. Consumption of ultra-processed foods and obesity in Brazilian adolescents and adults. Prev Med. 2015;81:9–15. 10.1016/j.ypmed.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Askari M, Heshmati J, Shahinfar H, Tripathi N, Daneshzad E. Ultra-processed food and the risk of overweight and obesity: a systematic review and meta-analysis of observational studies. Int J Obes. 2020;44(10):2080–91. 10.1038/s41366-020-00650-z. [DOI] [PubMed] [Google Scholar]
- 20.Handakas E, Chang K, Khandpur N, et al. Metabolic profiles of ultra-processed food consumption and their role in obesity risk in British children. Clin Nutr. 2022;41(11):2537–48. 10.1016/j.clnu.2022.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Asgari E, Askari M, Bellissimo N, Azadbakht L. Association between ultraprocessed food intake and overweight, obesity, and malnutrition among children in Tehran, Iran. Int J Clin Pract. 2022;2022:8310260. 10.1155/2022/8310260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carla Cristina ENES. Carolina Moura de CAMARGO, Maraisa Isabela Coelho JUSTINO. Ultra-processed food consumption and obesity in adolescents. Rev Nutr. 2019;32:e18170. 10.1590/1678-9865201932e180170. [Google Scholar]
- 23.Juul F, Martinez-Steele E, Parekh N, Monteiro CA, Chang VW. Ultra-processed food consumption and excess weight among US adults. Br J Nutr. 2018;120(1):90–100. 10.1017/S0007114518001046. [DOI] [PubMed] [Google Scholar]
- 24.Nardocci M, Leclerc BS, Louzada ML, Monteiro CA, Batal M, Moubarac JC. Consumption of ultra-processed foods and obesity in Canada. Can J Public Health. 2019;110(1):4–14. 10.17269/s41997-018-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavigne-Robichaud M, Moubarac JC, Lantagne-Lopez S, et al. Diet quality indices in relation to metabolic syndrome in an Indigenous Cree (Eeyouch) population in Northern Québec, Canada. Public Health Nutr. 2018;21(1):172–80. 10.1017/S136898001700115X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canhada SL, Vigo Á, Luft VC, et al. Ultra-processed food consumption and increased risk of metabolic syndrome in adults: the ELSA-Brasil. Diabetes Care. 2023;46(2):369–76. 10.2337/dc22-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llavero-Valero M, Escalada-San Martín J, Martínez-González MA, Basterra-Gortari FJ, de la Fuente-Arrillaga C, Bes-Rastrollo M. Ultra-processed foods and type-2 diabetes risk in the SUN project: a prospective cohort study. Clin Nutr. 2021;40(5):2817–24. 10.1016/j.clnu.2021.03.039. [DOI] [PubMed] [Google Scholar]
- 28.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–50. 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 29.Kim JS, Chen Z, Alderete TL, et al. Associations of air pollution, obesity and cardiometabolic health in young adults: the meta-AIR study. Environ Int. 2019;133:105180. 10.1016/j.envint.2019.105180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McConnell R, Shen E, Gilliland FD, et al. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California children’s health study. Environ Health Perspect. 2015;123(4):360–6. 10.1289/ehp.1307031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park Y, Dodd KW, Kipnis V, et al. Comparison of self-reported dietary intakes from the automated self-administered 24-h recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers. Am J Clin Nutr. 2018;107(1):80–93. 10.1093/ajcn/nqx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann K, Boeing H, Dufour A, et al. Estimating the distribution of usual dietary intake by short-term measurements. Eur J Clin Nutr. 2002;56(S2):S53–62. 10.1038/sj.ejcn.1601429. [DOI] [PubMed] [Google Scholar]
- 33.FNDDS download databases: USDA ARS. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds-download-databases/
- 34.Overweight & Obesity Statistics | NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases. Accessed February 23. 2023. https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity
- 35.Floch JPL, Escuyer P, Baudin E, Baudon D, Perlemuter L. Blood glucose area under the curve: methodological aspects. Diabetes Care. 1990;13(2):172–5. 10.2337/diacare.13.2.172. [DOI] [PubMed] [Google Scholar]
- 36.Diagnosis | ADA. Accessed February 23, 2023. https://diabetes.org/diabetes/a1c/diagnosis
- 37.Rao SS, Disraeli P, McGregor T. Impaired glucose tolerance and impaired fasting glucose. Am Fam Physician. 2004;69(8):1961–8. [PubMed] [Google Scholar]
- 38.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and ?-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 40.Lorente Ramos RM, Azpeitia Armán J, Arévalo Galeano N, Muñoz Hernández A, García Gómez JM, Gredilla Molinero J. Dual energy X-ray absorptimetry: fundamentals, methodology, and clinical applications. Radiologia. 2012;54(5):410–23. 10.1016/j.rx.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 41.Craig CL, Marshall AL, SJÖSTRÖM M, et al. International physical activity questionnaire: 12-Country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed]
- 42.Dicken SJ, Batterham RL. The role of diet quality in mediating the association between ultra-processed food intake, obesity and health-related outcomes: a review of prospective cohort studies. Nutrients. 2021;14(1):23. 10.3390/nu14010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costa CS, Del-Ponte B, Assunção MCF, Santos IS. Consumption of ultra-processed foods and body fat during childhood and adolescence: a systematic review. Public Health Nutr. 2018;21(1):148–59. 10.1017/S1368980017001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol. 2013. 10.3389/fendo.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khalili D, Khayamzadeh M, Kohansal K, et al. Are HOMA-IR and HOMA-B good predictors for diabetes and pre-diabetes subtypes? BMC Endocr Disord. 2023;23(1):39. 10.1186/s12902-023-01291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373(9682):2215–21. 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almarshad MI, Algonaiman R, Alharbi HF, Almujaydil MS, Barakat H. Relationship between ultra-processed food consumption and risk of diabetes mellitus: a mini-review. Nutrients. 2022;14(12):2366. 10.3390/nu14122366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moradi S, Hojjati Kermani Mali, Bagheri R, et al. Ultra-processed food consumption and adult diabetes risk: a systematic review and dose-response meta-analysis. Nutrients. 2021;13(12):4410. 10.3390/nu13124410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delpino FM, Figueiredo LM, Bielemann RM, et al. Ultra-processed food and risk of type 2 diabetes: a systematic review and meta-analysis of longitudinal studies. Int J Epidemiol. 2022;51(4):1120–41. 10.1093/ije/dyab247. [DOI] [PubMed] [Google Scholar]
- 50.Popkin BM, Ng SW. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes Rev. 2022. 10.1111/obr.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elizabeth L, Machado P, Zinöcker M, Baker P, Lawrence M. Ultra-processed foods and health outcomes: a narrative review. Nutrients. 2020;12(7):1955. 10.3390/nu12071955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan F, Wang Z, Wang H, et al. Association between ultra-processed food consumption and metabolic syndrome among adults in China—results from the China health and nutrition survey. Nutrients. 2023;15(3):752. 10.3390/nu15030752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poti JM, Mendez MA, Ng SW, Popkin BM. Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? Am J Clin Nutr. 2015;101(6):1251–62. 10.3945/ajcn.114.100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: epidemiology and treatment. Curr Diab Rep. 2014;14(8):508. 10.1007/s11892-014-0508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maggio CA, Pi-Sunyer FX. Obesity and type 2 diabetes. Endocrinol Metab Clin North Am. 2003;32(4):805–22. 10.1016/S0889-8529(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 56.Lanaspa MA, Kuwabara M, Andres-Hernando A, et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc Natl Acad Sci U S A. 2018;115(12):3138–43. 10.1073/pnas.1713837115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma Y, He FJ, MacGregor GA. High salt intake: independent risk factor for obesity? Hypertension Dallas Tex 1979. 2015;66(4):843–9. 10.1161/HYPERTENSIONAHA.115.05948. [DOI] [PubMed] [Google Scholar]
- 58.Rouhani P, Mirzaei S, Asadi A, Akhlaghi M, Saneei P. Nutrient patterns in relation to metabolic health status in overweight and obese adolescents. Sci Rep. 2023;13(1):119. 10.1038/s41598-023-27510-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48(1):44–51. 10.1016/j.plipres.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rice Bradley BH. Dietary fat and risk for type 2 diabetes: a review of recent research. Curr Nutr Rep. 2018;7(4):214–26. 10.1007/s13668-018-0244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sami W, Ansari T, Butt NS, Hamid MRA. Effect of diet on type 2 diabetes mellitus: a review. Int J Health Sci. 2017;11(2):65–71. [PMC free article] [PubMed] [Google Scholar]
- 62.Rippe JM, Angelopoulos TJ. Relationship between added sugars consumption and chronic disease risk factors: current understanding. Nutrients. 2016;8(11):697. 10.3390/nu8110697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maitre L, de Bont J, Casas M, et al. Human early life exposome (HELIX) study: a European population-based exposome cohort. BMJ Open. 2018;8(9):e021311. 10.1136/bmjopen-2017-021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith-Warner SA, Spiegelman D, Ritz J, et al. Methods for pooling results of epidemiologic studies. Am J Epidemiol. 2006;163(11):1053–64. 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 65. Poobalan A, Aucott L. Obesity Among Young Adults in Developing Countries: A Systematic Overview. Curr Obes Rep. 2016;5(1):2–13. 10.1007/s13679-016-0187-x. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request. The data are not publicly available to protect participants’ identifiable information.



