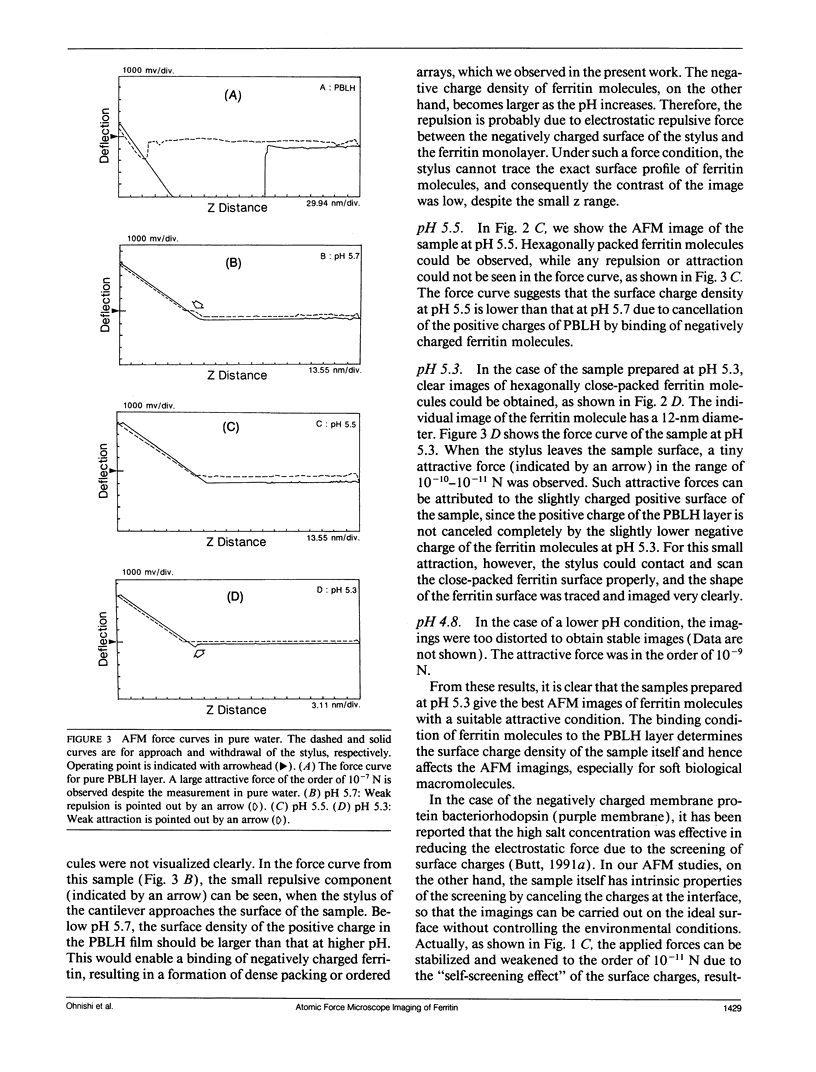
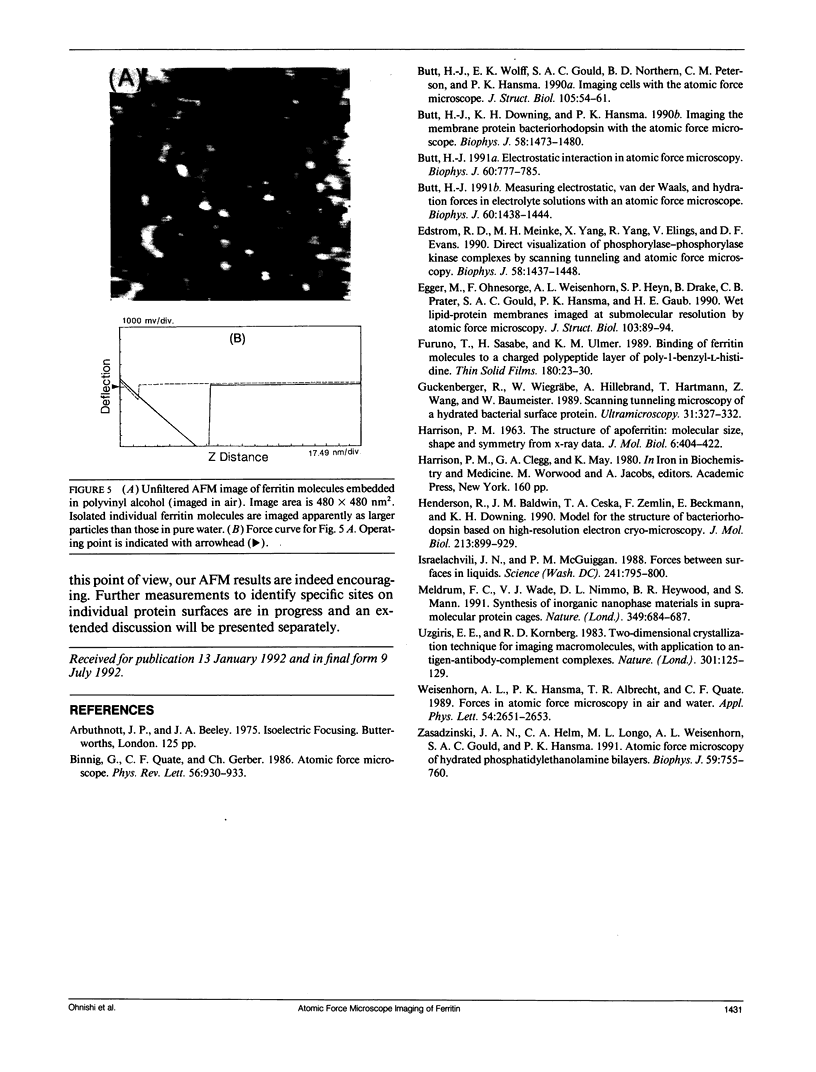
Abstract
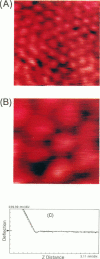
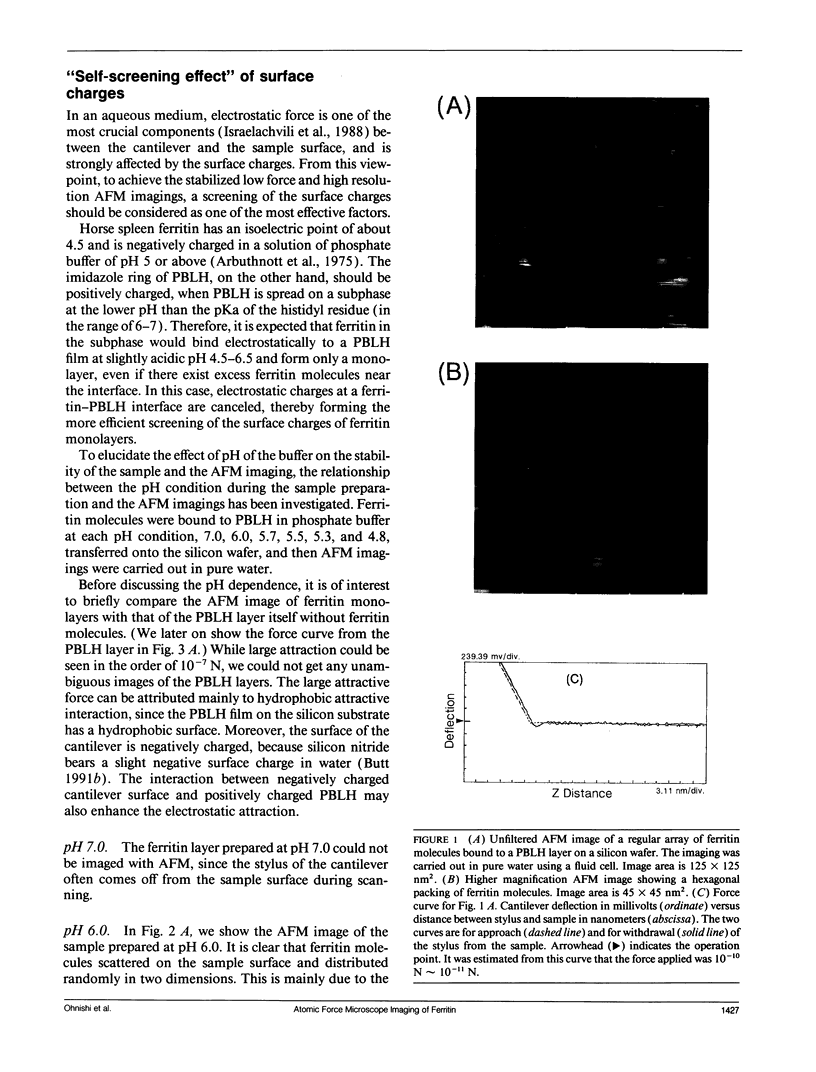
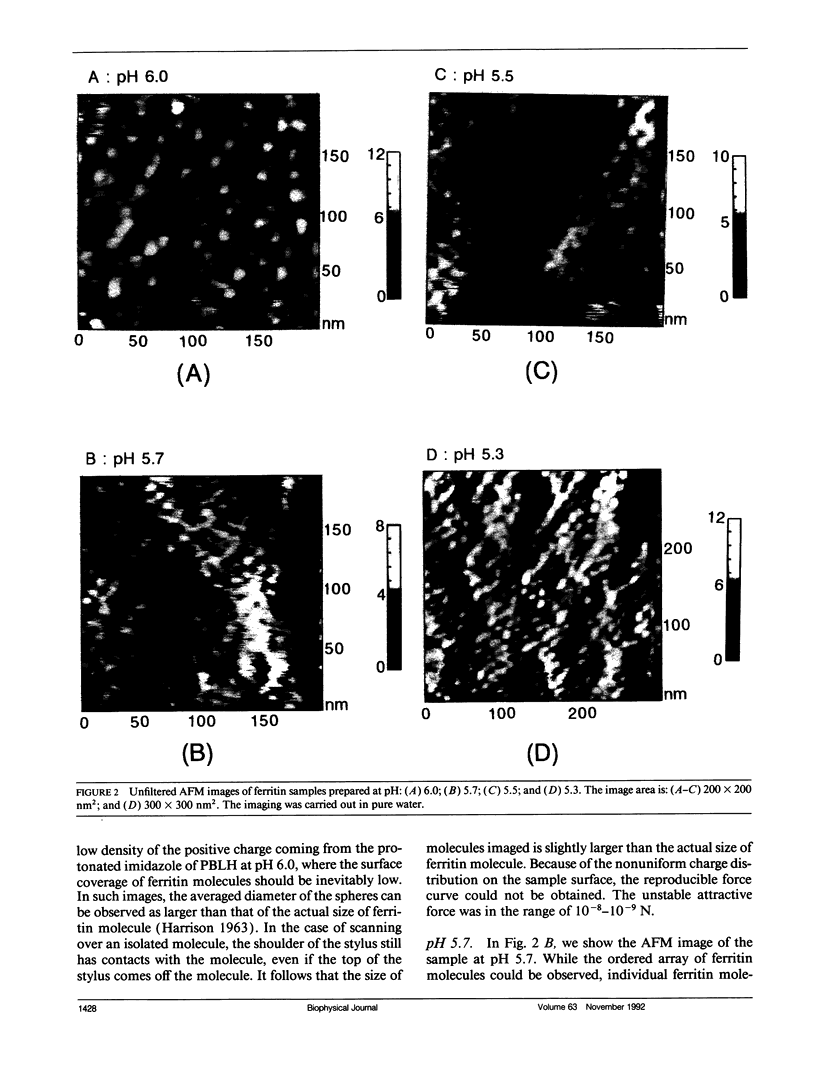
Individual water-soluble molecules of the protein ferritin have been imaged on a silicon surface in pure water at room temperature with the atomic force microscope (AFM). The ferritin molecules formed an ordered monolayer by binding to a charged polypeptide monolayer of poly-1-benzyl-L-histidine (PBLH) spread at the air-water interface. The film, fully wetted with water, was horizontally transferred onto an alkylated silicon wafer for AFM imagings. The hexagonal arrangement of ferritin molecules was imaged with high reproducibility on the whole surface of the film, since the forces between cantilever and the sample could be kept sufficiently smaller than 10-10 N, mainly due to a “self-screening effect” of the surface charges of the ferritin-PBLH layer. This is the first observation of two-dimensional ordered arrays of water-soluble protein molecules directly confirmed by AFM with molecular resolution.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Butt H. J., Downing K. H., Hansma P. K. Imaging the membrane protein bacteriorhodopsin with the atomic force microscope. Biophys J. 1990 Dec;58(6):1473–1480. doi: 10.1016/S0006-3495(90)82492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt H. J. Electrostatic interaction in atomic force microscopy. Biophys J. 1991 Oct;60(4):777–785. doi: 10.1016/S0006-3495(91)82112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt H. J., Wolff E. K., Gould S. A., Dixon Northern B., Peterson C. M., Hansma P. K. Imaging cells with the atomic force microscope. J Struct Biol. 1990 Oct-Dec;105(1-3):54–61. doi: 10.1016/1047-8477(90)90098-w. [DOI] [PubMed] [Google Scholar]
- Edstrom R. D., Meinke M. H., Yang X. R., Yang R., Elings V., Evans D. F. Direct visualization of phosphorylase-phosphorylase kinase complexes by scanning tunneling and atomic force microscopy. Biophys J. 1990 Dec;58(6):1437–1448. doi: 10.1016/S0006-3495(90)82489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON P. M. The structure of apoferritin: molecular size, shape and symmetry from x-ray data. J Mol Biol. 1963 May;6:404–422. doi: 10.1016/s0022-2836(63)80052-2. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., McGuiggan P. M. Forces between surfaces in liquids. Science. 1988 Aug 12;241(4867):795–800. doi: 10.1126/science.241.4867.795. [DOI] [PubMed] [Google Scholar]
- Uzgiris E. E., Kornberg R. D. Two-dimensional crystallization technique for imaging macromolecules, with application to antigen--antibody--complement complexes. Nature. 1983 Jan 13;301(5896):125–129. doi: 10.1038/301125a0. [DOI] [PubMed] [Google Scholar]
- Zasadzinski J. A., Helm C. A., Longo M. L., Weisenhorn A. L., Gould S. A., Hansma P. K. Atomic force microscopy of hydrated phosphatidylethanolamine bilayers. Biophys J. 1991 Mar;59(3):755–760. doi: 10.1016/S0006-3495(91)82288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]