Abstract
The blood vessels supplying the central-peripheral transitional zone of rat fifth lumbar ventral spinal nerve rootlets were examined during development and at maturity. At all stages all vessels were either capillaries or postcapillary venules. They lay in the spaces between the rootlets, being entirely absent from the endoneurial spaces. A proportion of these vessels communicated with those supplying the adjacent spinal cord. In this respect they differed from those supplying the dorsal rootlet transitional zone, at least in the cat, where no such communication occurs. During the first week after birth, at least one capillary was directly related to each rootlet, generally over about half the length of the transitional zone. Subsequently vascularity increased considerably. At three weeks postnatum, and subsequently, capillaries outnumbered rootlets by up to 50% and almost the entire length of the transitional zone was related to one capillary or more. This change was related to the maturation of the transitional nodes of gamma axons, which is likely to be related to increased alpha and gamma motoneuron activity. These changes were somewhat offset due to the fact that rootlet diameter increased with age. As a result, the distance between the capillary wall and the centre of the rootlet almost doubled between 20 and 300 days postnatum. The diameter of the capillaries did not change with age but that of the postcapillary venules increased.
Full text
PDF


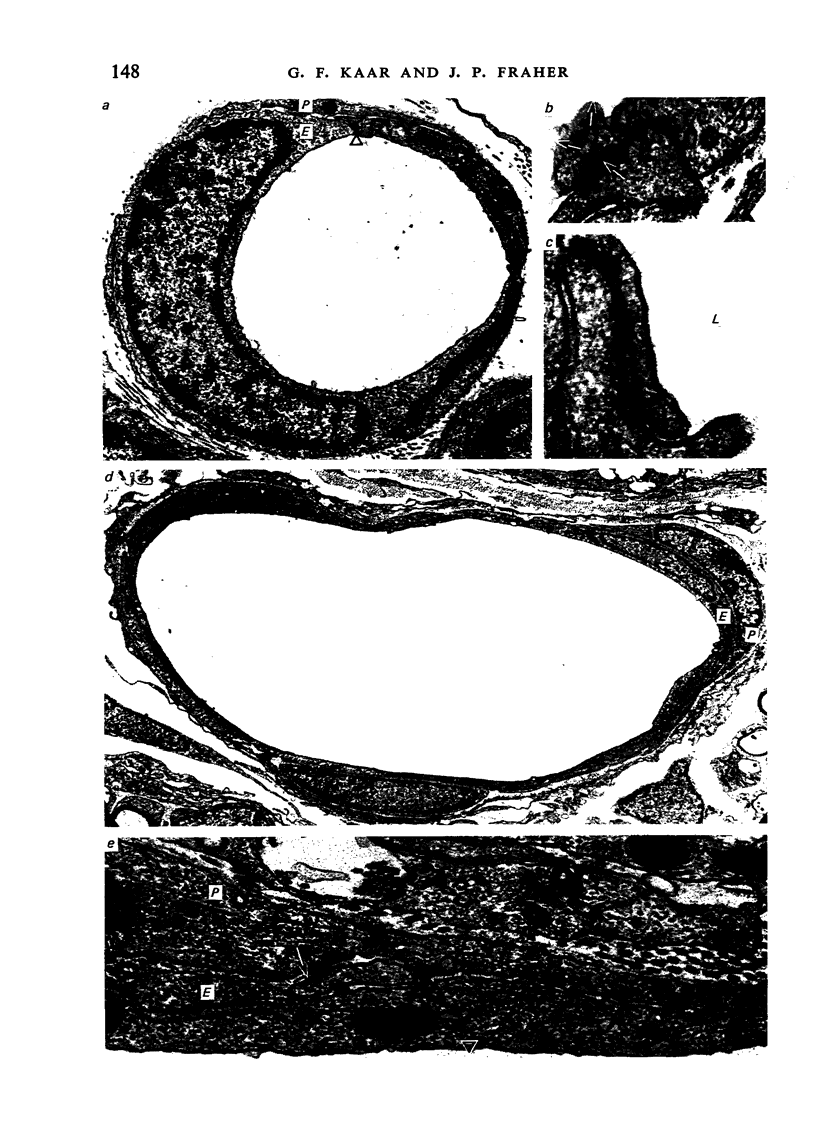
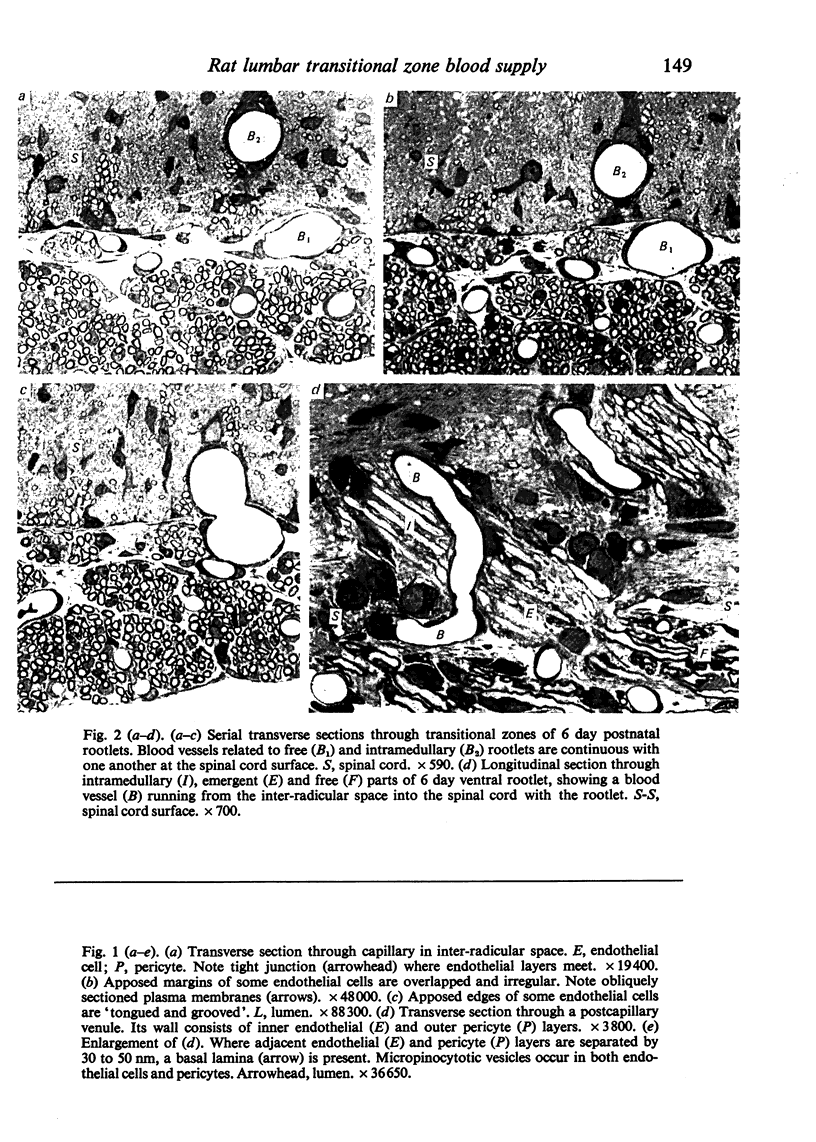




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthold C. H., Carlstedt T. Observations on the morphology at the transition between the peripheral and the central nervous system in the cat. II. General organization of the transitional region in S1 dorsal rootlets. Acta Physiol Scand Suppl. 1977;446:23–42. [PubMed] [Google Scholar]
- Burkel W. E. The histological fine structure of perineurium. Anat Rec. 1967 Jun;158(2):177–189. doi: 10.1002/ar.1091580207. [DOI] [PubMed] [Google Scholar]
- Carlstedt T. An electron-microscopical study of the developing transitional region in feline S1 dorsal rootlets. J Neurol Sci. 1981 Jun;50(3):357–372. doi: 10.1016/0022-510x(81)90148-9. [DOI] [PubMed] [Google Scholar]
- Fraher J. P., Kaar G. F. The development of alpha and gamma motoneuron fibres in the rat. II. A comparative ultrastructural study of their central and peripheral myelination. J Anat. 1985 Aug;141:89–103. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P., Kaar G. F. The lumbar ventral root-spinal cord transitional zone in the rat. A morphological study during development and at maturity. J Anat. 1986 Apr;145:109–122. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P., Kaar G. F. The transitional node of Ranvier at the junction of the central and peripheral nervous systems: an ultrastructural study of its development and mature form. J Anat. 1984 Sep;139(Pt 2):215–238. [PMC free article] [PubMed] [Google Scholar]
- Hannah R. S., Nathaniel E. J. The postnatal development of blood vessels in the substantia gelatinosa of rat cervical cord--an ultrastructural study. Anat Rec. 1974 Apr;178(4):691–709. doi: 10.1002/ar.1091780404. [DOI] [PubMed] [Google Scholar]
- Kaar G. F., Fraher J. P. The development of alpha and gamma motoneuron fibres in the rat. I. A comparative ultrastructural study of their central and peripheral axon growth. J Anat. 1985 Aug;141:77–88. [PMC free article] [PubMed] [Google Scholar]
- Kaar G. F., Fraher J. P. The sheaths surrounding the attachments of rat lumbar ventral roots to the spinal cord: a light and electron microscopical study. J Anat. 1986 Oct;148:137–146. [PMC free article] [PubMed] [Google Scholar]
- Olsson Y., Reese T. S. Permeability of vasa nervorum and perineurium in mouse sciatic nerve studied by fluorescence and electron microscopy. J Neuropathol Exp Neurol. 1971 Jan;30(1):105–119. doi: 10.1097/00005072-197101000-00011. [DOI] [PubMed] [Google Scholar]
- Phelps C. H. The development of glio-vascular relationships in the rat spinal cord. An electron microscopic study. Z Zellforsch Mikrosk Anat. 1972;128(4):555–563. doi: 10.1007/BF00306988. [DOI] [PubMed] [Google Scholar]
- Rhodin J. A. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967 Apr;18(1):181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- Rhodin J. A. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J Ultrastruct Res. 1968 Dec;25(5):452–500. doi: 10.1016/s0022-5320(68)80098-x. [DOI] [PubMed] [Google Scholar]
- Steer J. M. Some observations on the fine structure of rat dorsal spinal nerve roots. J Anat. 1971 Sep;109(Pt 3):467–485. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. A quantitative and morphological study of vascularisation of the developing mouse spinal cord. J Anat. 1981 Mar;132(Pt 2):203–221. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. A quantitative study of vascularisation of the prenatal rabbit spinal cord. J Anat. 1982 Aug;135(Pt 1):89–96. [PMC free article] [PubMed] [Google Scholar]
- THOMAS P. K. The connective tissue of peripheral nerve: an electron microscope study. J Anat. 1963 Jan;97:35–44. [PMC free article] [PubMed] [Google Scholar]