Abstract
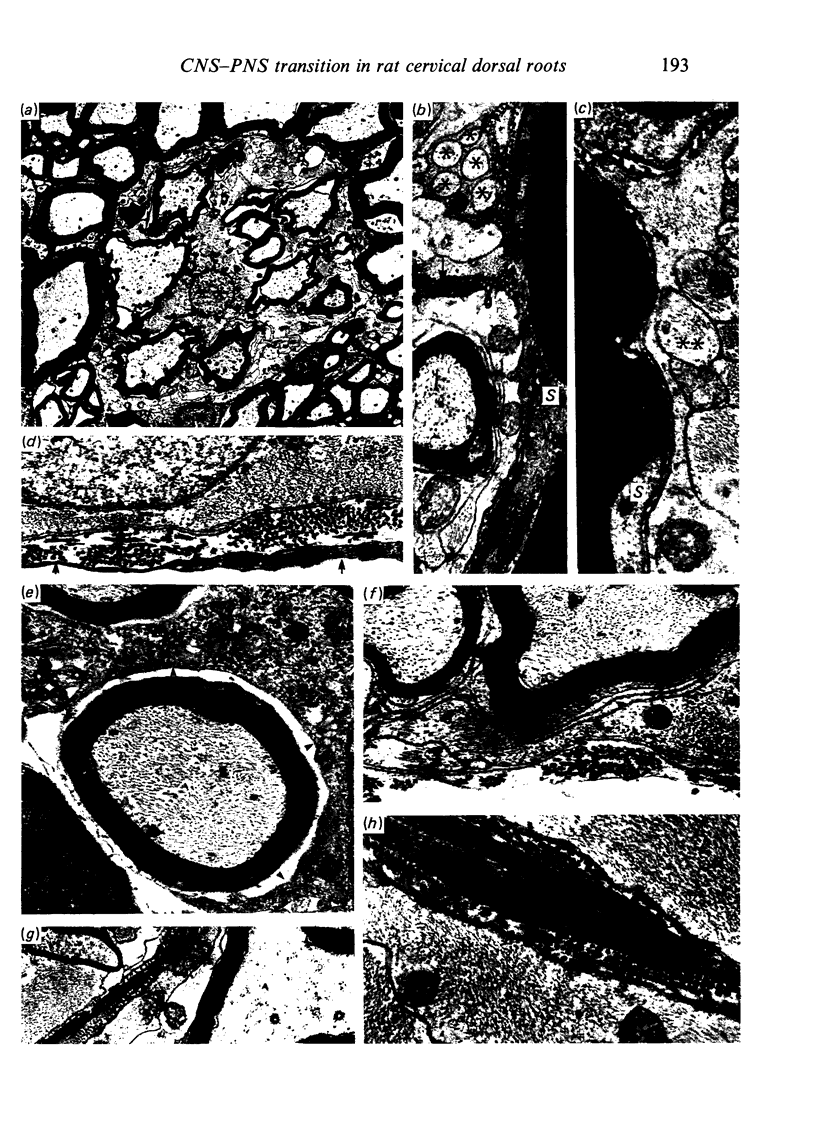
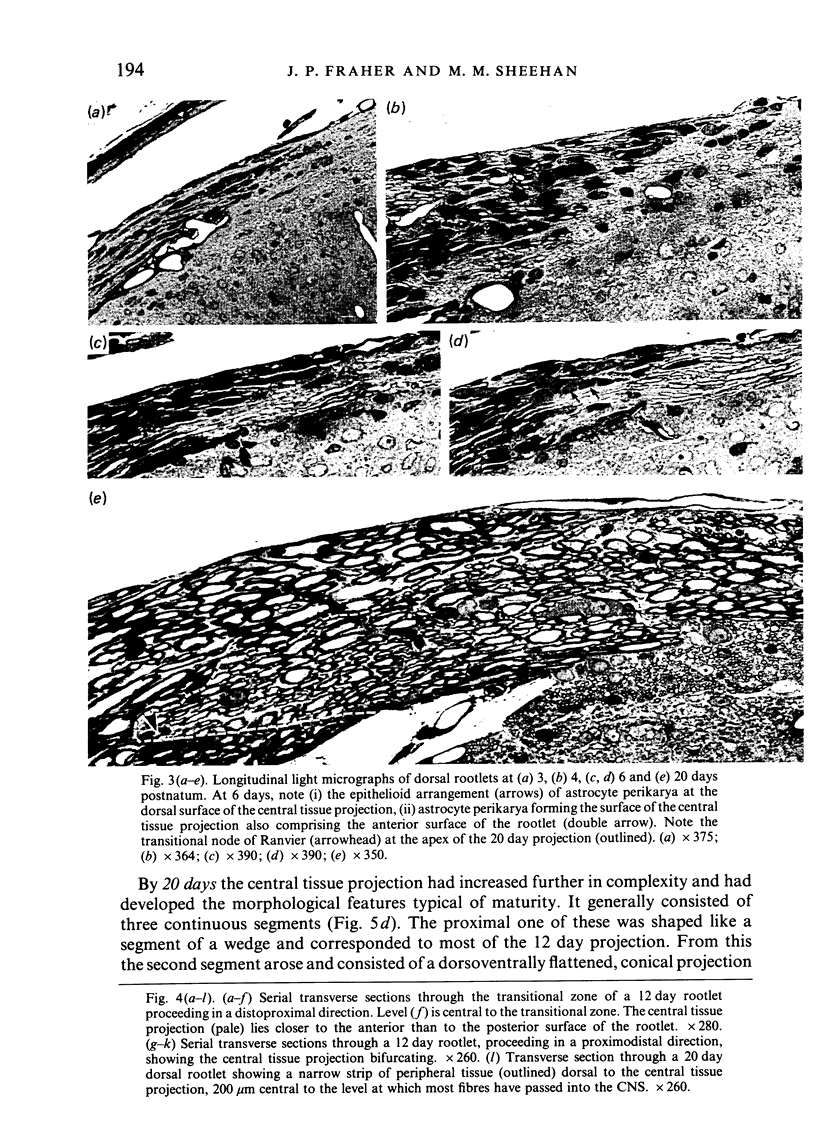
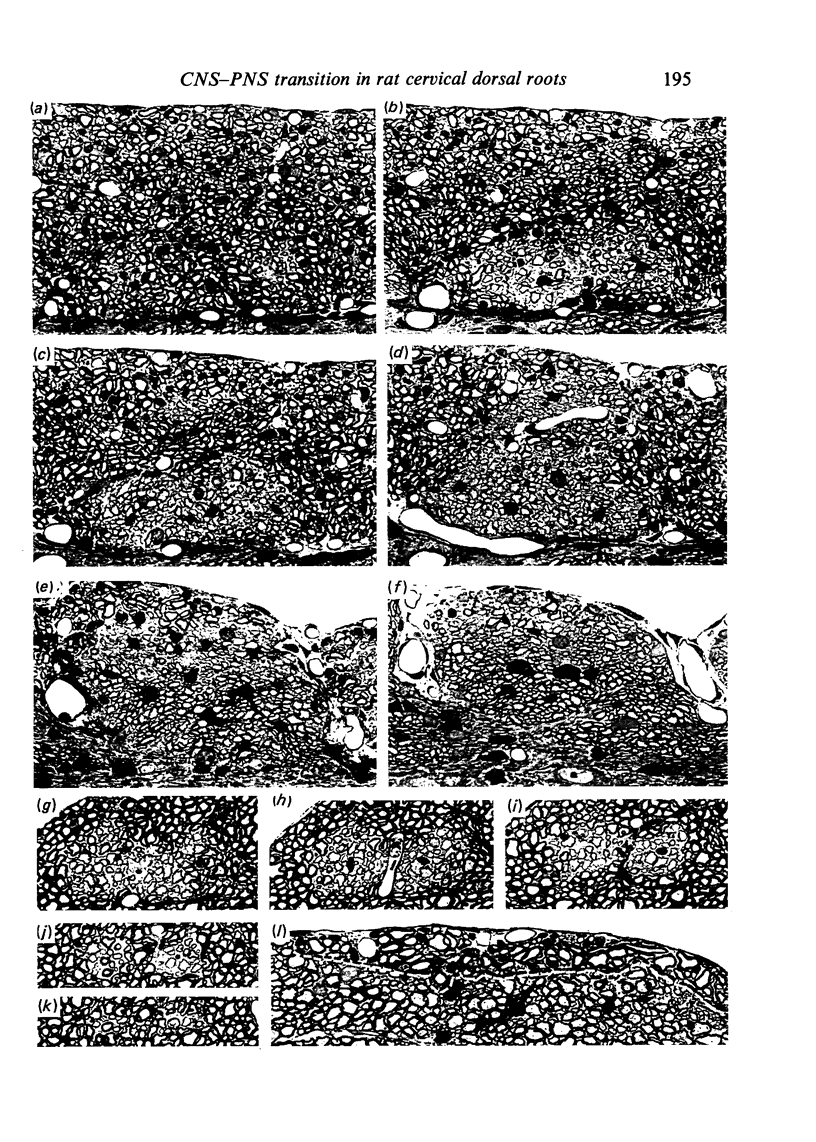
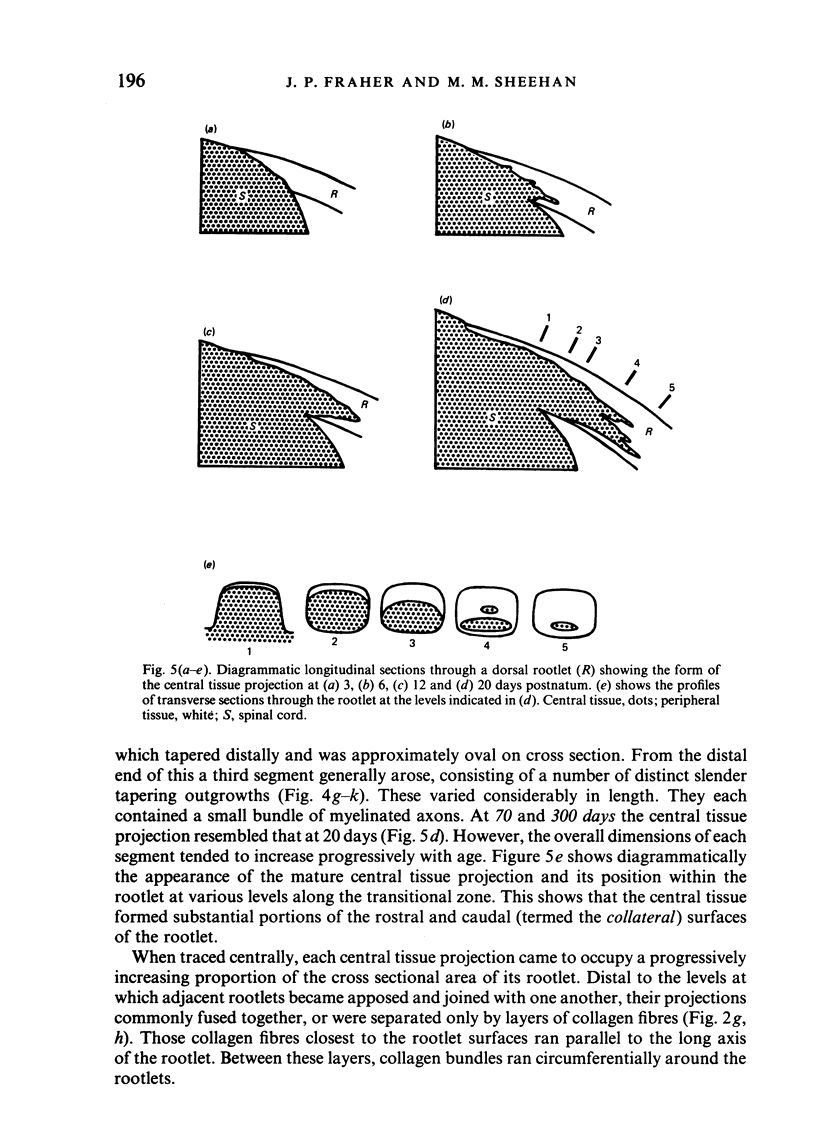
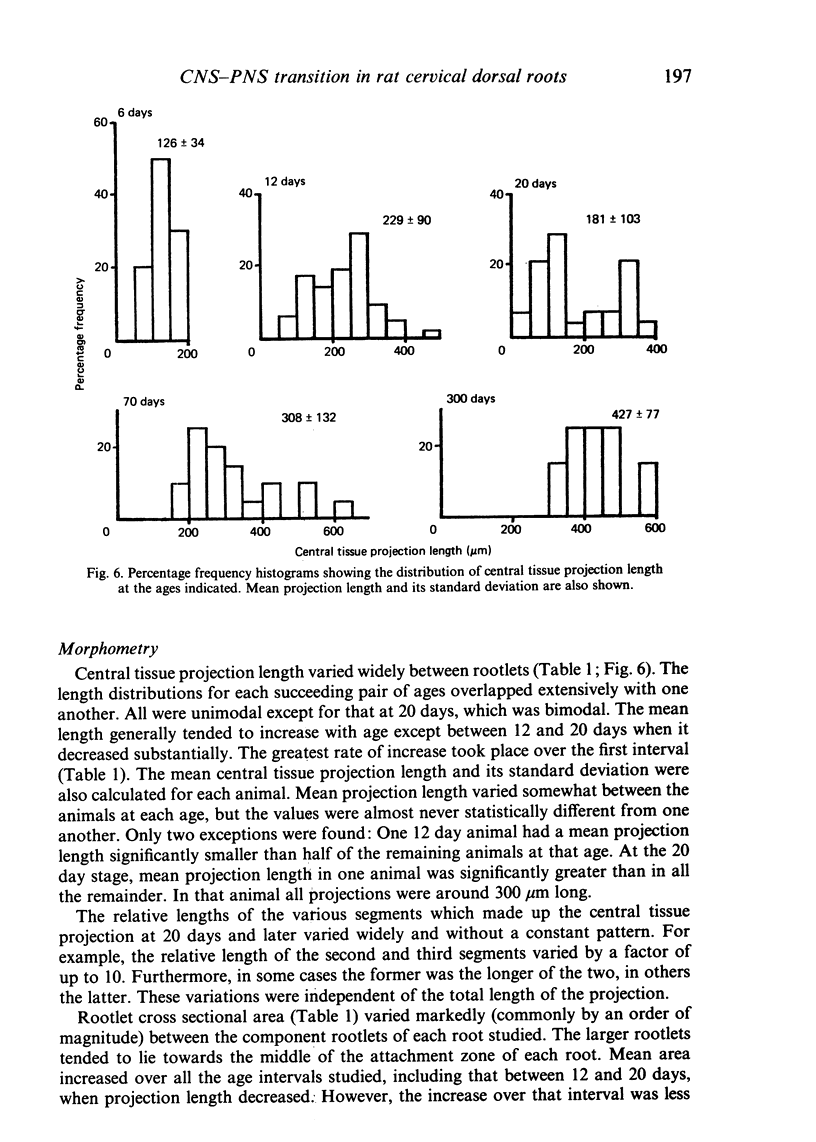
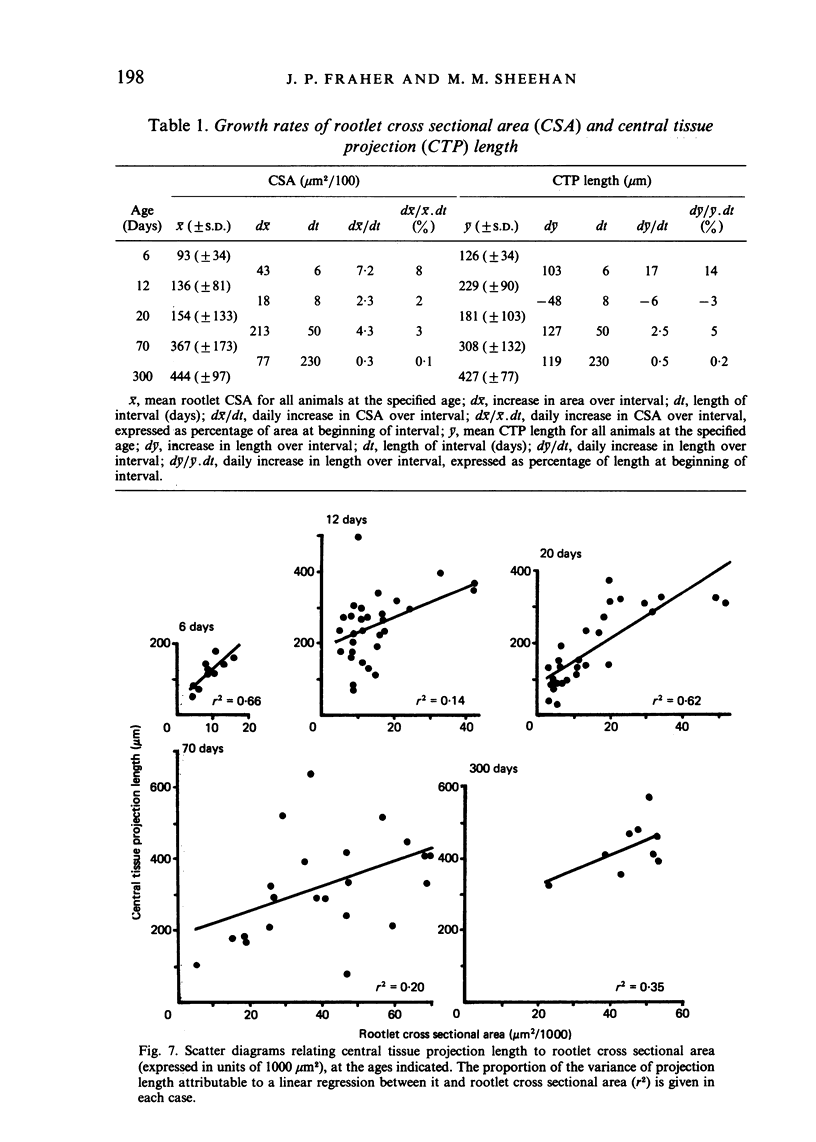
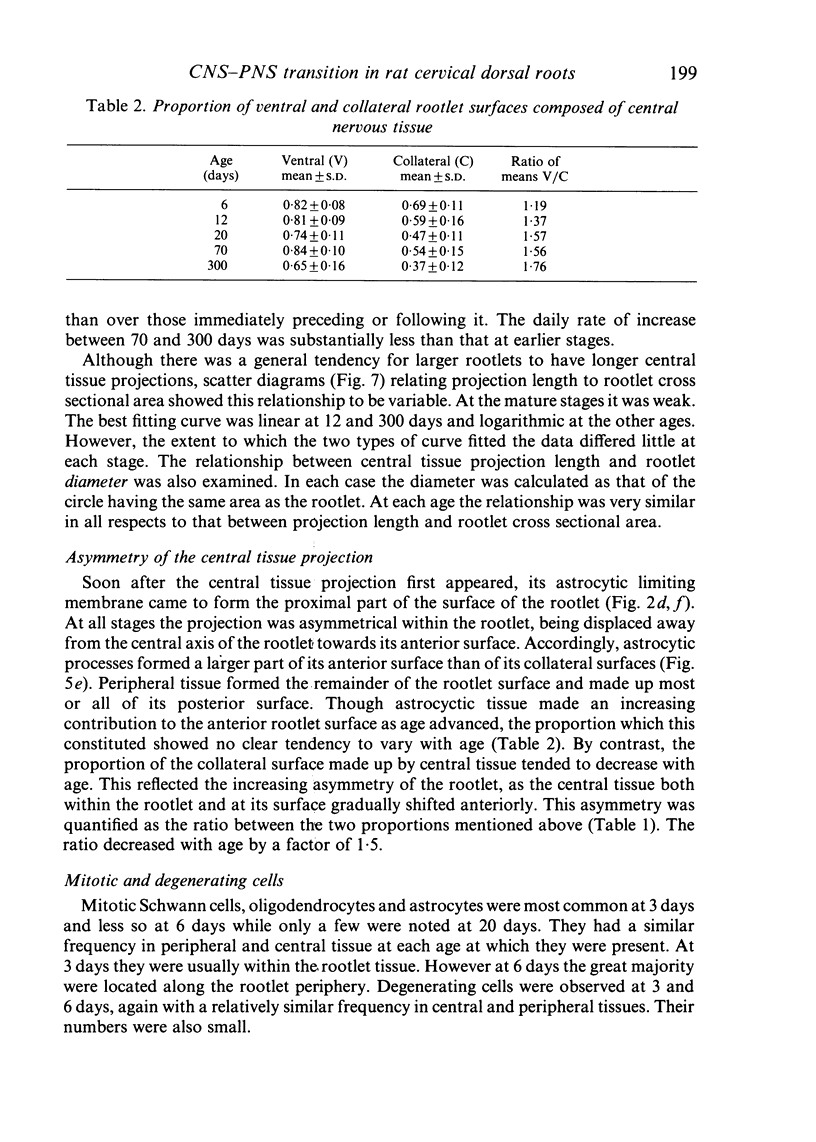
Each seventh cervical dorsal nerve root is attached to the spinal cord surface by four to eight rootlets. A tapering outgrowth of central nervous tissue, the central tissue projection, extends distally into the proximal part of each rootlet in the immediate postnatal period. The central ends of the most proximal peripheral internodes surround this projection. Thus a length of rootlet contains both CNS and PNS tissue. This is termed the transitional zone. Material was processed by standard preparative techniques for electron microscopy. Serial semithin and ultrathin sections were made over the entire extent of several transitional zones at ages ranging from 2 to 300 days postnatum. Central tissue projections were reconstructed in three dimensions and analysed morphometrically. The morphology of the central tissue projection varies during development. At first, it forms an irregular projection into the anterior portion of the rootlet. It than elongates and takes the form of a dorsoventrally flattened, distally tapering wedge. By 20 days postnatum it has attained its definitive form. This consists of three segments: a proximal wedge-shaped portion, similar to that described above; continuous with this is a distally tapering, dorsoventrally flattened, cone-shaped segment which generally branches into two or more slender projections of central tissue. The latter comprise the third segment. The projection comes to form a substantial proportion of the anterior, proximal and distal surfaces of the dorsal rootlet from an early stage. The mean length of the central tissue projection increases progressively over all intervals studied, except that between 12 and 30 days postnatum, when a reduction in length is associated with reorganisation of the morphology of the projection. Projection length varies considerably between rootlets and is relatively weakly correlated with rootlet cross sectional area. There is a great deal of overlap between the distributions of projection lengths at all stages between 20 and 300 days.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthold C. H., Carlstedt T. Observations on the morphology at the transition between the peripheral and the central nervous system in the cat. II. General organization of the transitional region in S1 dorsal rootlets. Acta Physiol Scand Suppl. 1977;446:23–42. [PubMed] [Google Scholar]
- Carlstedt T. An electron-microscopical study of the developing transitional region in feline S1 dorsal rootlets. J Neurol Sci. 1981 Jun;50(3):357–372. doi: 10.1016/0022-510x(81)90148-9. [DOI] [PubMed] [Google Scholar]
- Fraher J. P., Kaar G. F. The development of alpha and gamma motoneuron fibres in the rat. II. A comparative ultrastructural study of their central and peripheral myelination. J Anat. 1985 Aug;141:89–103. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P., Kaar G. F. The lumbar ventral root-spinal cord transitional zone in the rat. A morphological study during development and at maturity. J Anat. 1986 Apr;145:109–122. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P., Kaar G. F. The maturation of the ventral root-spinal cord transitional zone. Part 2. A quantitative ultrastructural study of the dynamics of its early development. J Neurol Sci. 1982 Jan;53(1):63–75. doi: 10.1016/0022-510x(82)90080-6. [DOI] [PubMed] [Google Scholar]
- Fraher J. P., Kaar G. F. The transitional node of Ranvier at the junction of the central and peripheral nervous systems: an ultrastructural study of its development and mature form. J Anat. 1984 Sep;139(Pt 2):215–238. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P. The maturation of the ventral root-spinal cord transitional zone. An ultrastructural study. J Neurol Sci. 1978 May;36(3):427–449. doi: 10.1016/0022-510x(78)90049-7. [DOI] [PubMed] [Google Scholar]
- Kaar G. F., Fraher J. P. The development of alpha and gamma motoneuron fibres in the rat. I. A comparative ultrastructural study of their central and peripheral axon growth. J Anat. 1985 Aug;141:77–88. [PMC free article] [PubMed] [Google Scholar]
- Moll C., Meier C. The central-peripheral transition zone of cervical spinal nerve roots in Jimpy mutant and normal mice. Light- and electron-microscopic study. Acta Neuropathol. 1983;60(3-4):241–251. doi: 10.1007/BF00691872. [DOI] [PubMed] [Google Scholar]
- PEACHEY L. D. Thin sections. I. A study of section thickness and physical distortion produced during microtomy. J Biophys Biochem Cytol. 1958 May 25;4(3):233–242. doi: 10.1083/jcb.4.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]