Abstract
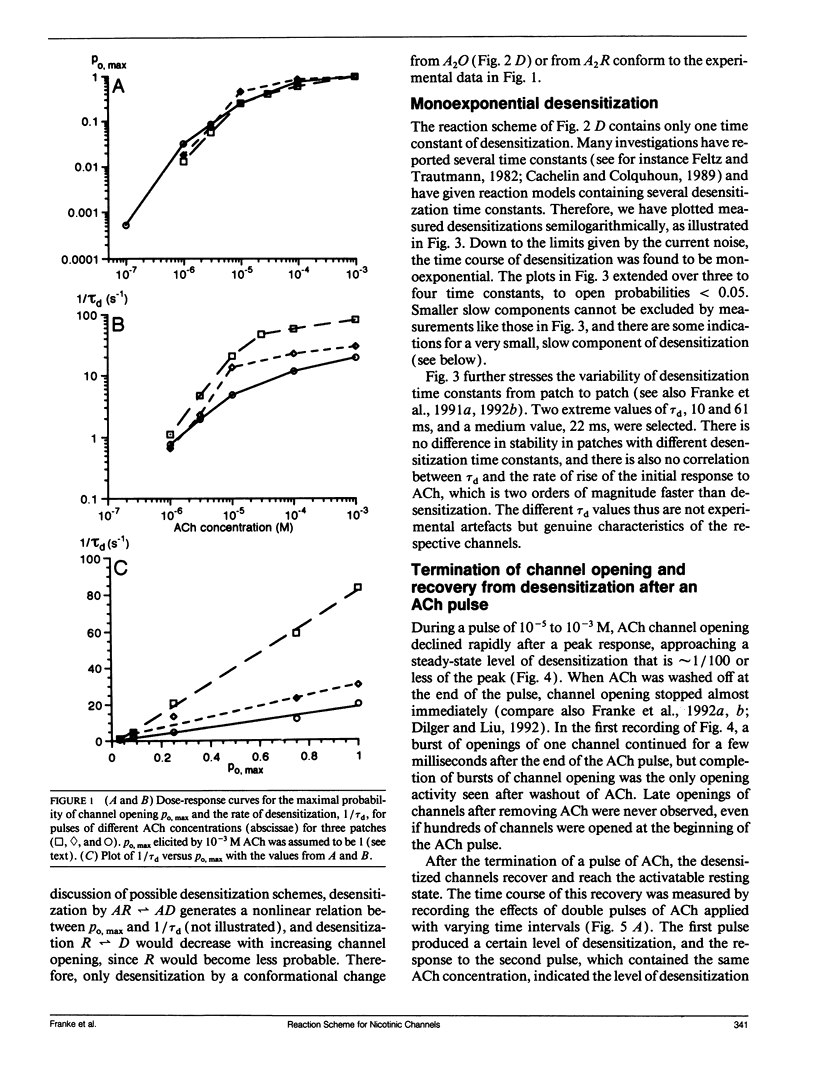
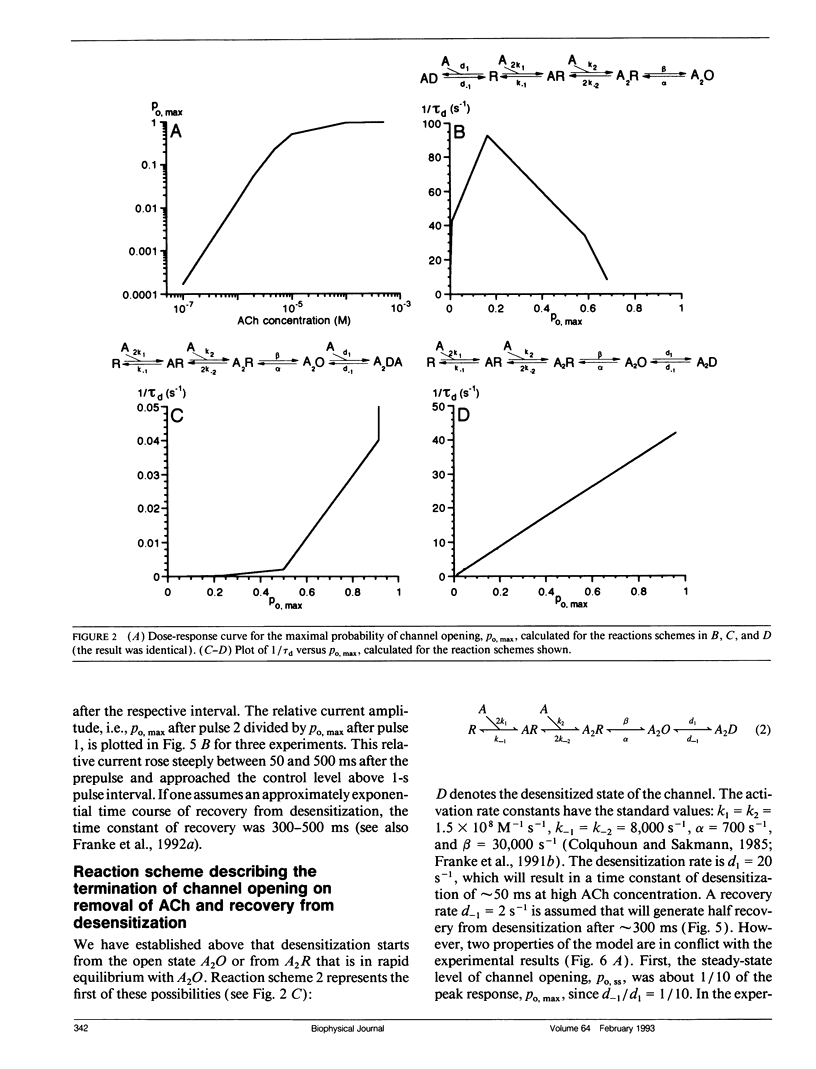
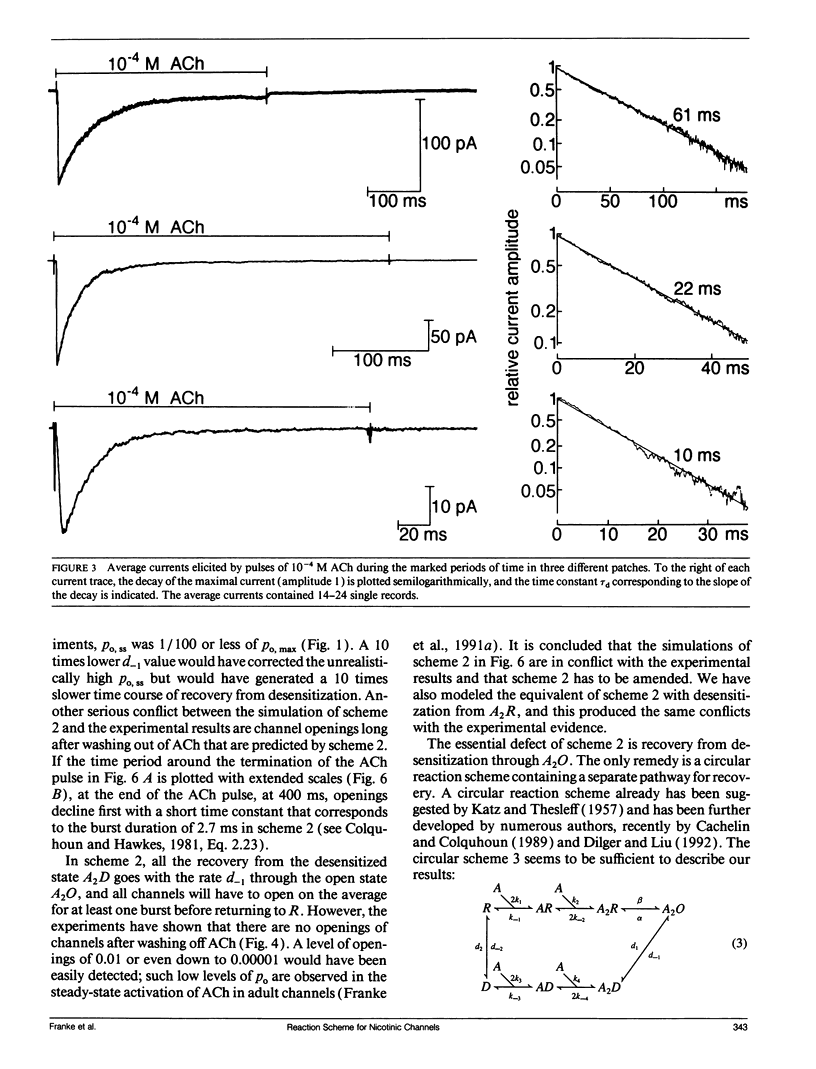
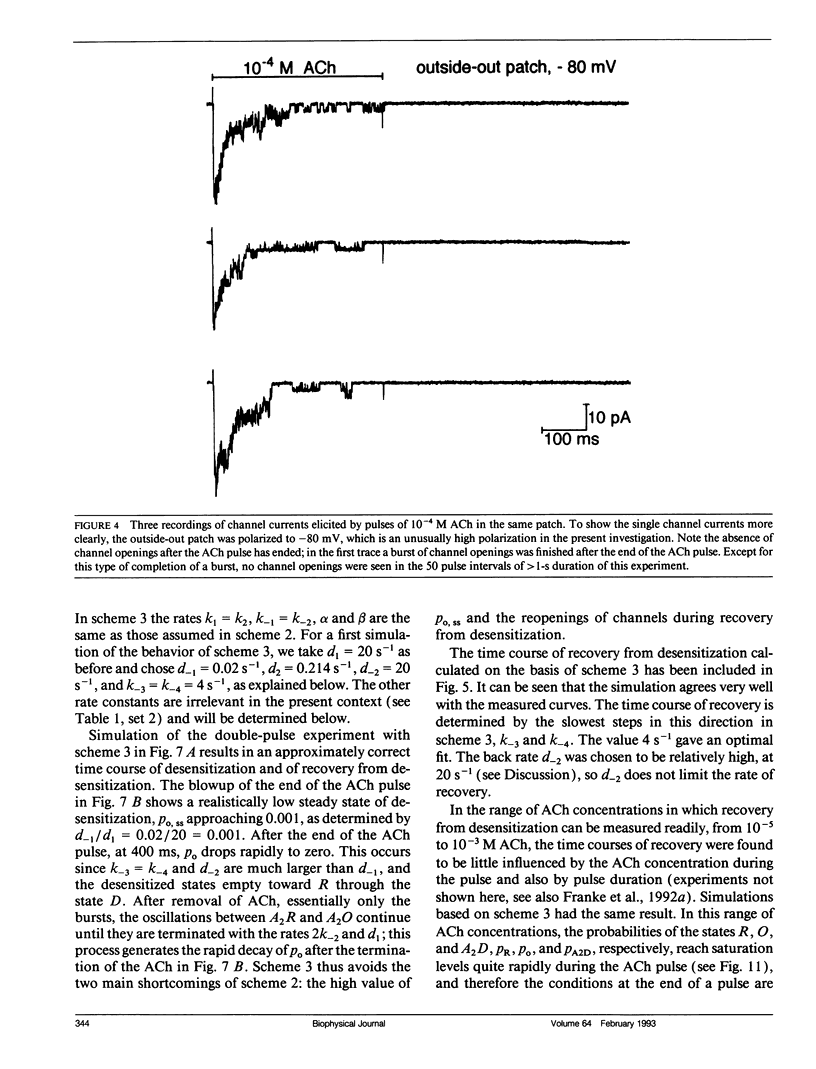
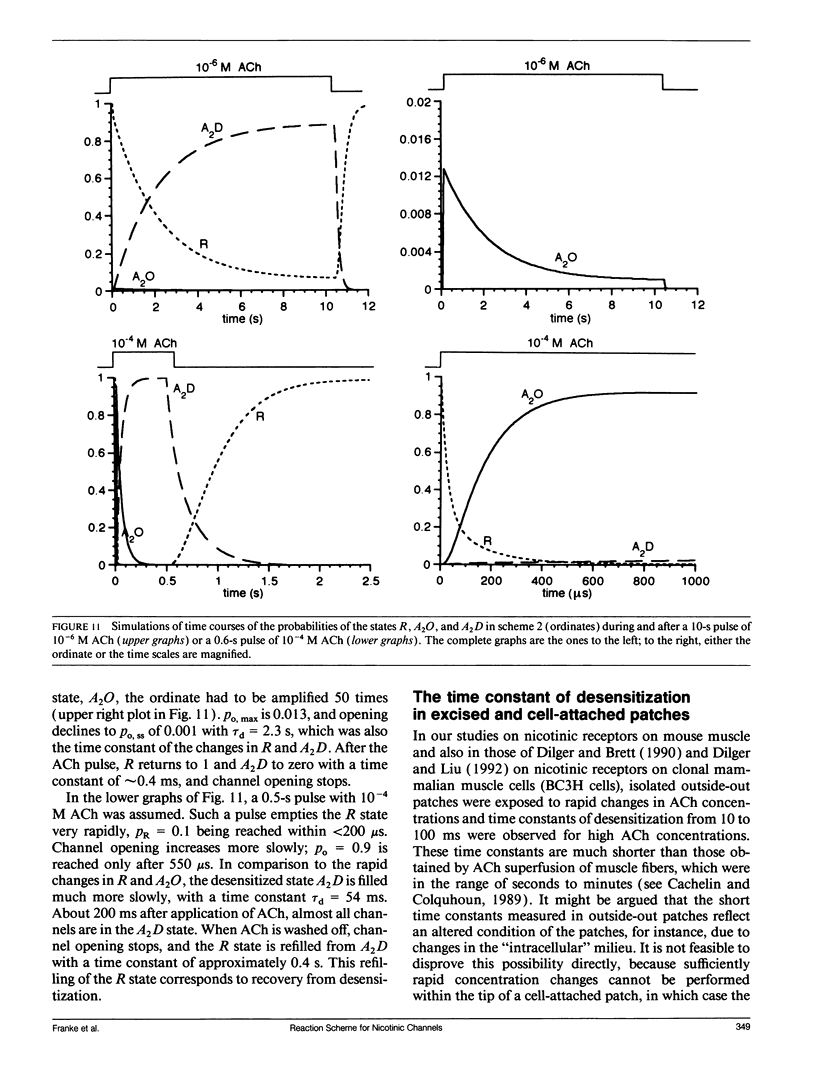
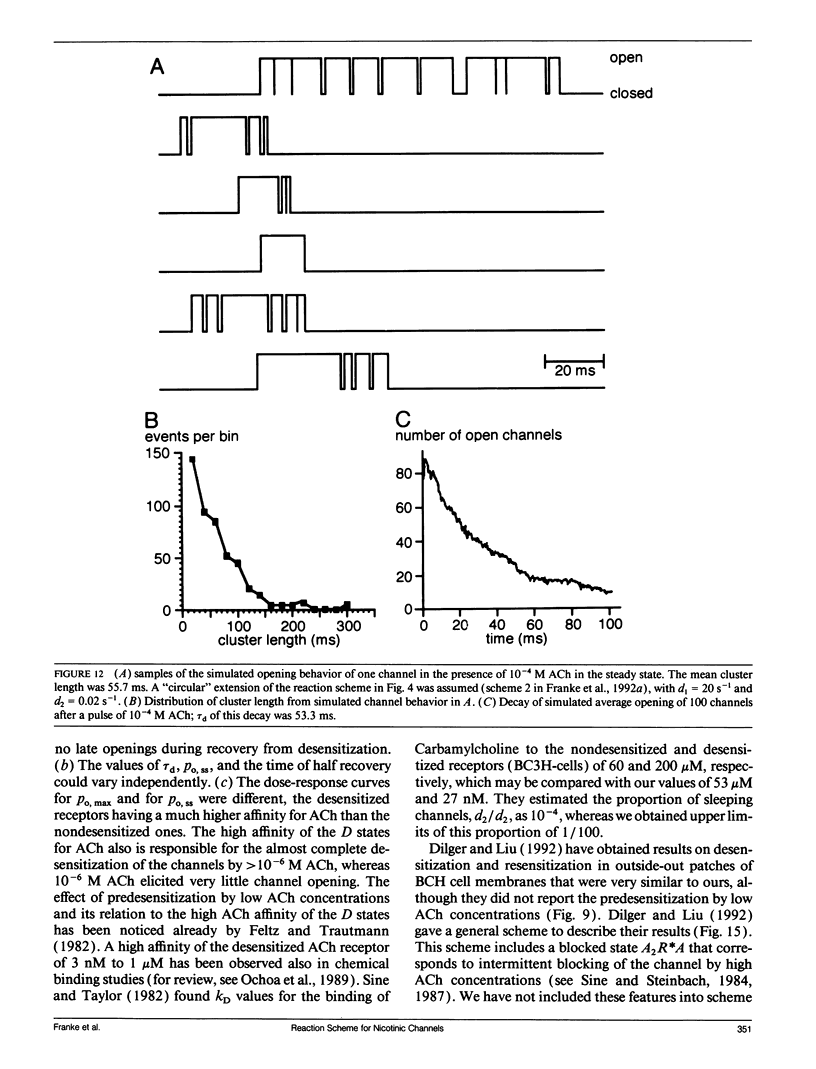
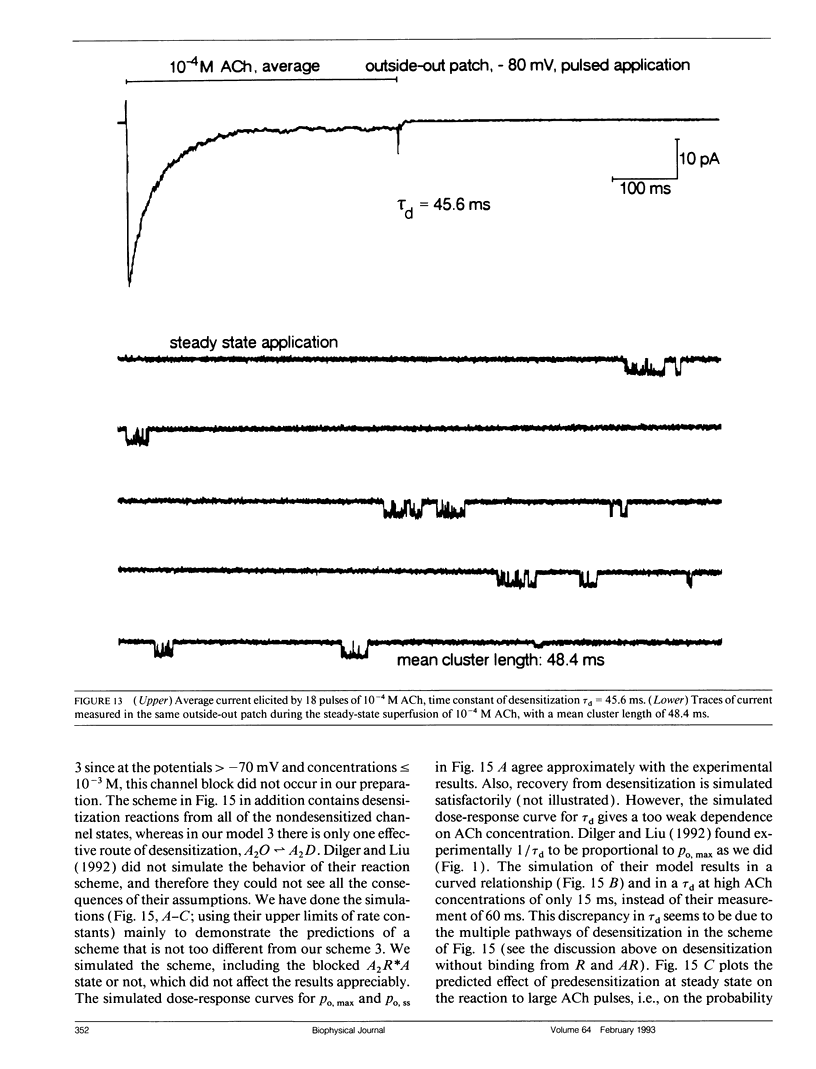
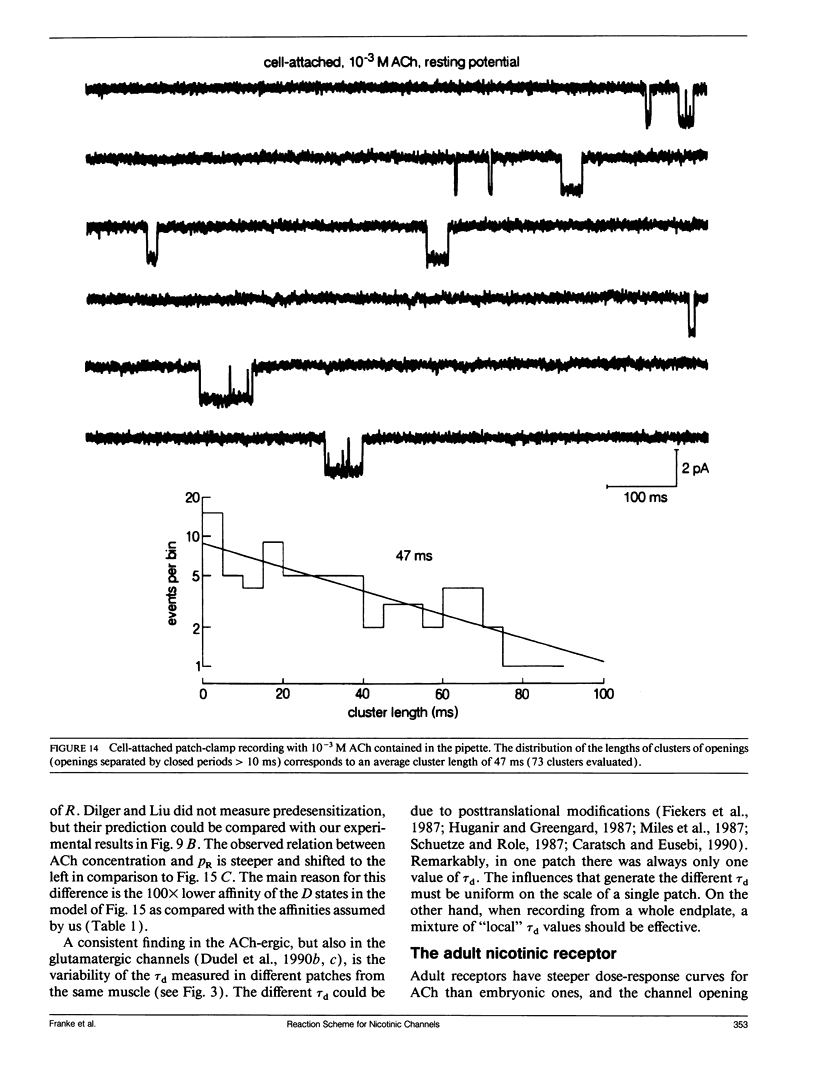
In outside-out patches of mouse-muscle membrane, embryonic-like channels were activated by pulses of acetylcholine (ACh). On increasing the ACh concentration, the rate of desensitization, 1/tau d, increased linearly with the peak open probability, indicating desensitization from the open state. Desensitization had only one time constant tau d at each ACh concentration. Recovery from desensitization was only approximately 10 times slower than desensitization, whereas the probability of steady-state channel opening, declined to < 0.01 with > 10(-6) M ACh. The peak probability of opening in > 10(-4) M ACh pulse was close to 1. A linear reaction scheme was not compatible with these results. The scheme had to be expanded resulting in a circular scheme with two additional ACh binding steps to desensitized channel states. The approximate rate constants of all reaction steps in the circular scheme could be determined using computer simulations. The model predicted that clusters of channel opening had the average duration tau d at the respective ACh concentration. In cell-attached patches on intact muscle fibers, similar average cluster durations were observed at the respective ACh concentration. This indicates that tau d in the intact muscle fibers has similar values as in outside-out patches.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blau H. M., Webster C. Isolation and characterization of human muscle cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachelin A. B., Colquhoun D. Desensitization of the acetylcholine receptor of frog end-plates measured in a Vaseline-gap voltage clamp. J Physiol. 1989 Aug;415:159–188. doi: 10.1113/jphysiol.1989.sp017717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caratsch C. G., Eusebi F. Effect of calcitonin gene-related peptide on synaptic transmission at the neuromuscular junction of the frog. Neurosci Lett. 1990 Apr 6;111(3):344–350. doi: 10.1016/0304-3940(90)90286-i. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Ogden D. C. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J Physiol. 1988 Jan;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger J. P., Brett R. S. Direct measurement of the concentration- and time-dependent open probability of the nicotinic acetylcholine receptor channel. Biophys J. 1990 Apr;57(4):723–731. doi: 10.1016/S0006-3495(90)82593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
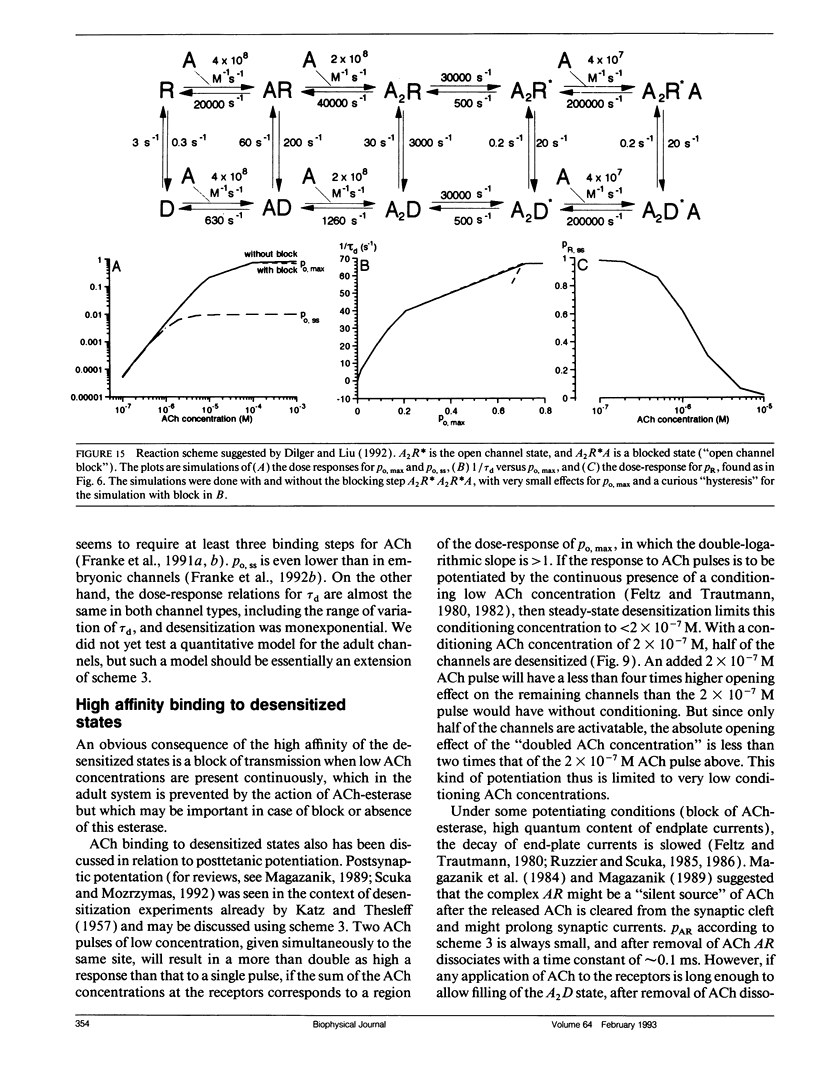
- Dilger J. P., Liu Y. Desensitization of acetylcholine receptors in BC3H-1 cells. Pflugers Arch. 1992 Apr;420(5-6):479–485. doi: 10.1007/BF00374622. [DOI] [PubMed] [Google Scholar]
- Dudel J., Franke C., Hatt H., Ramsey R. L., Usherwood P. N. Glutamatergic channels in locust muscle show a wide time range of desensitization and resensitization characteristics. Neurosci Lett. 1990 Jul 3;114(2):207–212. doi: 10.1016/0304-3940(90)90073-i. [DOI] [PubMed] [Google Scholar]
- Dudel J., Franke C., Hatt H. Rapid activation, desensitization, and resensitization of synaptic channels of crayfish muscle after glutamate pulses. Biophys J. 1990 Mar;57(3):533–545. doi: 10.1016/S0006-3495(90)82569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P. The electromotive action of acetylcholine at the motor end-plate. J Physiol. 1950 Oct 16;111(3-4):408–422. doi: 10.1113/jphysiol.1950.sp004492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Interaction between nerve-related acetylcholine and bath applied agonists at the frog end-plate. J Physiol. 1980 Feb;299:533–552. doi: 10.1113/jphysiol.1980.sp013141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiekers J. F., Neel D. S., Parsons R. L. Acceleration of desensitization by agonist pre-treatment in the snake. J Physiol. 1987 Oct;391:109–124. doi: 10.1113/jphysiol.1987.sp016729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C., Hatt H., Dudel J. Liquid filament switch for ultra-fast exchanges of solutions at excised patches of synaptic membrane of crayfish muscle. Neurosci Lett. 1987 Jun 15;77(2):199–204. doi: 10.1016/0304-3940(87)90586-6. [DOI] [PubMed] [Google Scholar]
- Franke C., Hatt H., Dudel J. Steep concentration dependence and fast desensitization of nicotinic channel currents elicited by acetylcholine pulses, studied in adult vertebrate muscle. Pflugers Arch. 1991 Jan;417(5):509–516. doi: 10.1007/BF00370947. [DOI] [PubMed] [Google Scholar]
- Franke C., Hatt H., Parnas H., Dudel J. Kinetic constants of the acetylcholine (ACh) receptor reaction deduced from the rise in open probability after steps in ACh concentration. Biophys J. 1991 Nov;60(5):1008–1016. doi: 10.1016/S0006-3495(91)82138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C., Hatt H., Parnas H., Dudel J. Recovery from the rapid desensitization of nicotinic acetylcholine receptor channels on mouse muscle. Neurosci Lett. 1992 Jun 22;140(2):169–172. doi: 10.1016/0304-3940(92)90094-n. [DOI] [PubMed] [Google Scholar]
- Franke C., Költgen D., Hatt H., Dudel J. Activation and desensitization of embryonic-like receptor channels in mouse muscle by acetylcholine concentration steps. J Physiol. 1992;451:145–158. doi: 10.1113/jphysiol.1992.sp019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jackson M. B. Dependence of acetylcholine receptor channel kinetics on agonist concentration in cultured mouse muscle fibres. J Physiol. 1988 Mar;397:555–583. doi: 10.1113/jphysiol.1988.sp017019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Nikolsky E. E., Giniatullin R. A. End-plate currents evoked by paired stimuli in frog muscle fibres. Pflugers Arch. 1984 Jun;401(2):185–192. doi: 10.1007/BF00583880. [DOI] [PubMed] [Google Scholar]
- Miles K., Anthony D. T., Rubin L. L., Greengard P., Huganir R. L. Regulation of nicotinic acetylcholine receptor phosphorylation in rat myotubes by forskolin and cAMP. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6591–6595. doi: 10.1073/pnas.84.18.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa E. L., Chattopadhyay A., McNamee M. G. Desensitization of the nicotinic acetylcholine receptor: molecular mechanisms and effect of modulators. Cell Mol Neurobiol. 1989 Jun;9(2):141–178. doi: 10.1007/BF00713026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas H., Flashner M., Spira M. E. Sequential model to describe the nicotinic synaptic current. Biophys J. 1989 May;55(5):875–884. doi: 10.1016/S0006-3495(89)82886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzzier F., Scuka M. The effect of repetitive neuromuscular activity on the sensitivity of acetylcholine receptors. Pflugers Arch. 1986 Feb;406(2):99–103. doi: 10.1007/BF00586669. [DOI] [PubMed] [Google Scholar]
- Schuetze S. M., Role L. W. Developmental regulation of nicotinic acetylcholine receptors. Annu Rev Neurosci. 1987;10:403–457. doi: 10.1146/annurev.ne.10.030187.002155. [DOI] [PubMed] [Google Scholar]
- Scuka M., Mozrzymas J. W. Postsynaptic potentiation and desensitization at the vertebrate end-plate receptors. Prog Neurobiol. 1992;38(1):19–33. doi: 10.1016/0301-0082(92)90033-b. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by high concentrations of agonist. J Physiol. 1987 Apr;385:325–359. doi: 10.1113/jphysiol.1987.sp016496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by low concentrations of agonist. J Physiol. 1986 Apr;373:129–162. doi: 10.1113/jphysiol.1986.sp016039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Agonists block currents through acetylcholine receptor channels. Biophys J. 1984 Aug;46(2):277–283. doi: 10.1016/S0006-3495(84)84022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Taylor P. Local anesthetics and histrionicotoxin are allosteric inhibitors of the acetylcholine receptor. Studies of clonal muscle cells. J Biol Chem. 1982 Jul 25;257(14):8106–8104. [PubMed] [Google Scholar]
- THESLEFT S. The mode of neuromuscular block caused by acetylcholine, nicotine, decamethonium and succinylcholine. Acta Physiol Scand. 1955 Oct 27;34(2-3):218–231. doi: 10.1111/j.1748-1716.1955.tb01242.x. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991 Jun 21;252(5013):1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]


