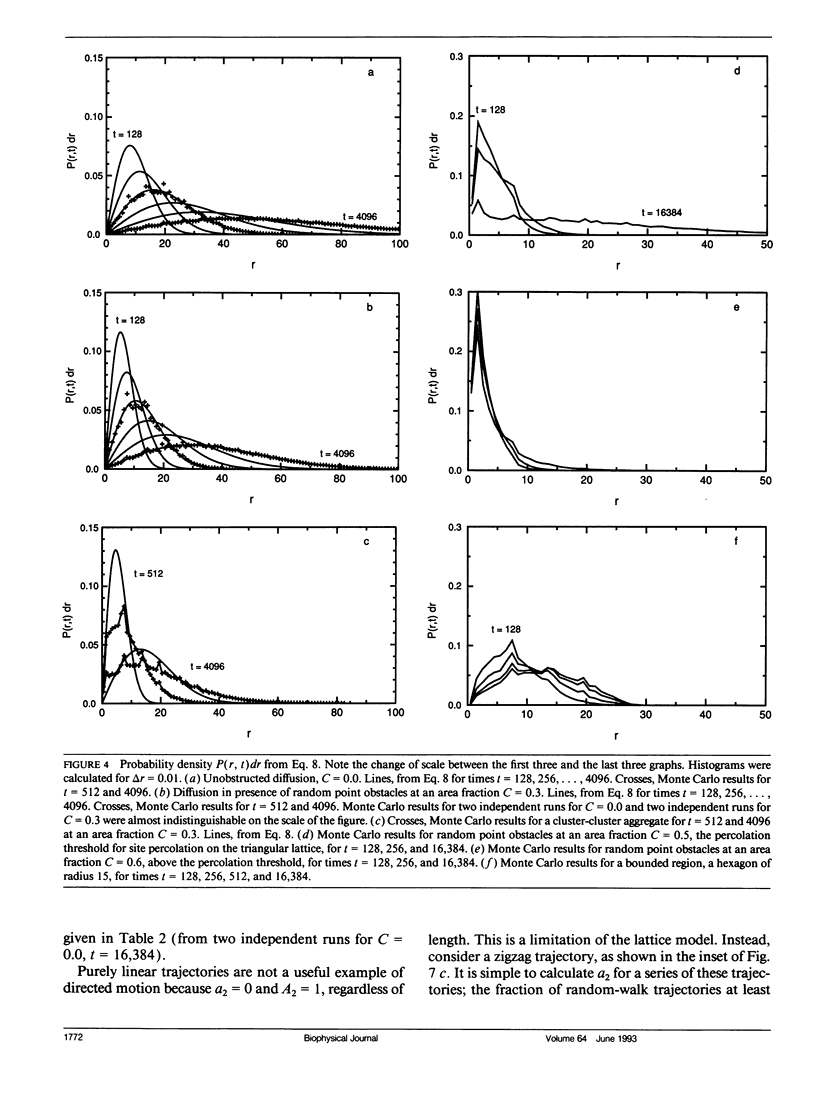
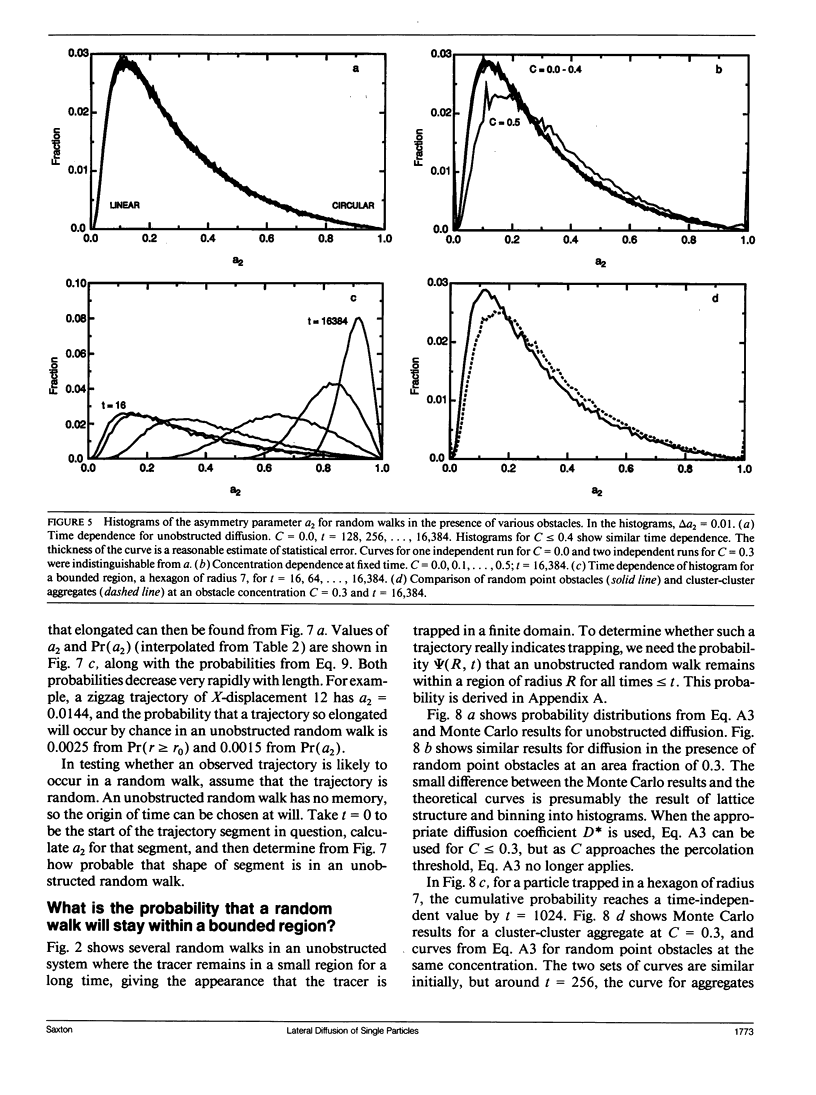
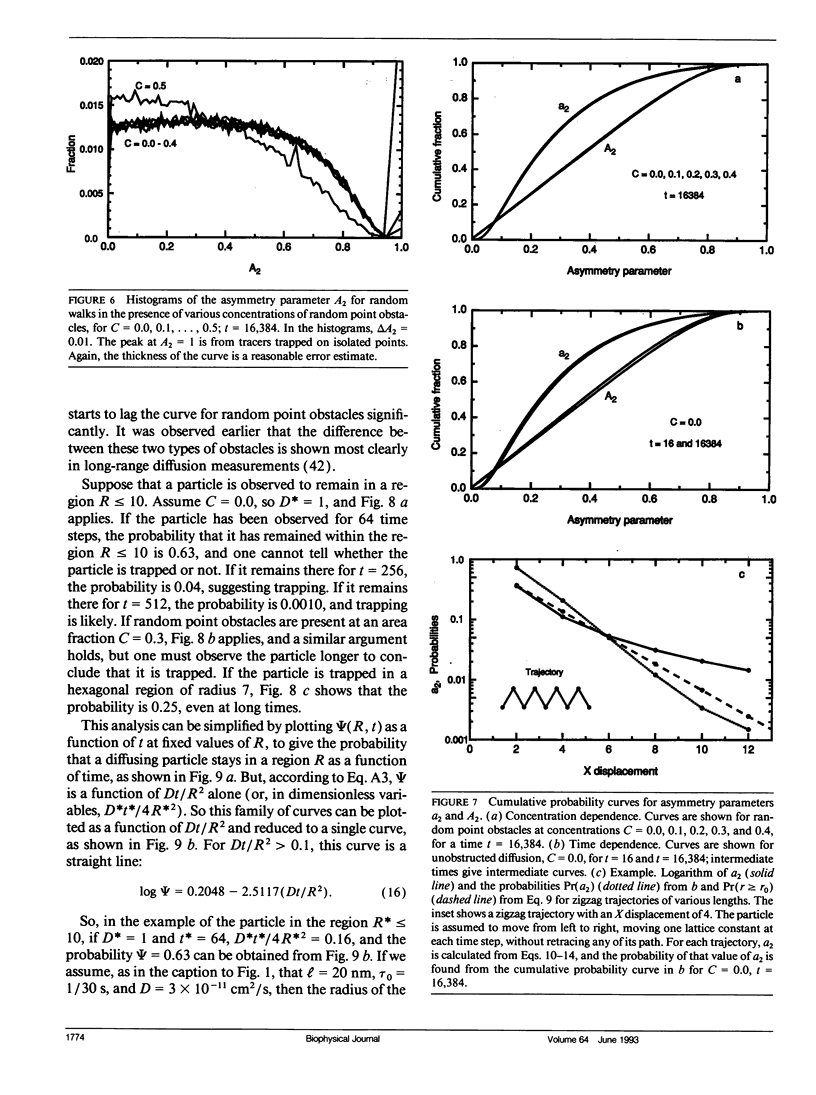
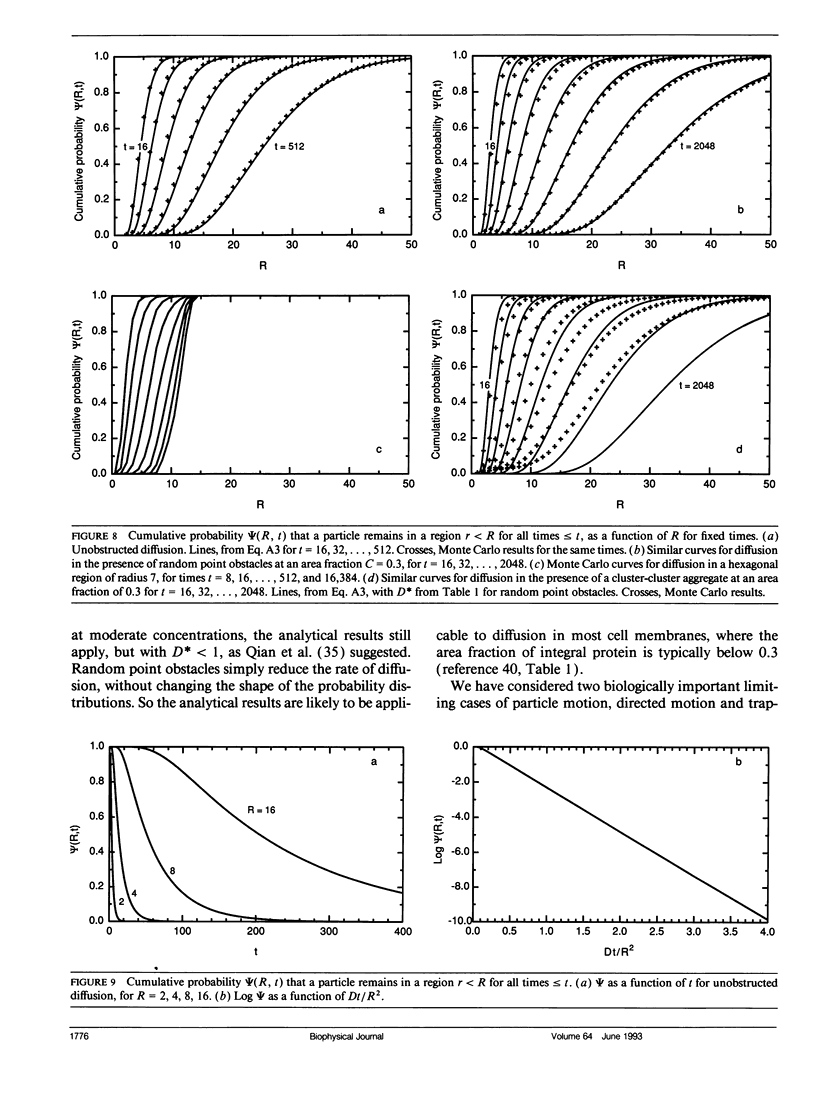
Abstract
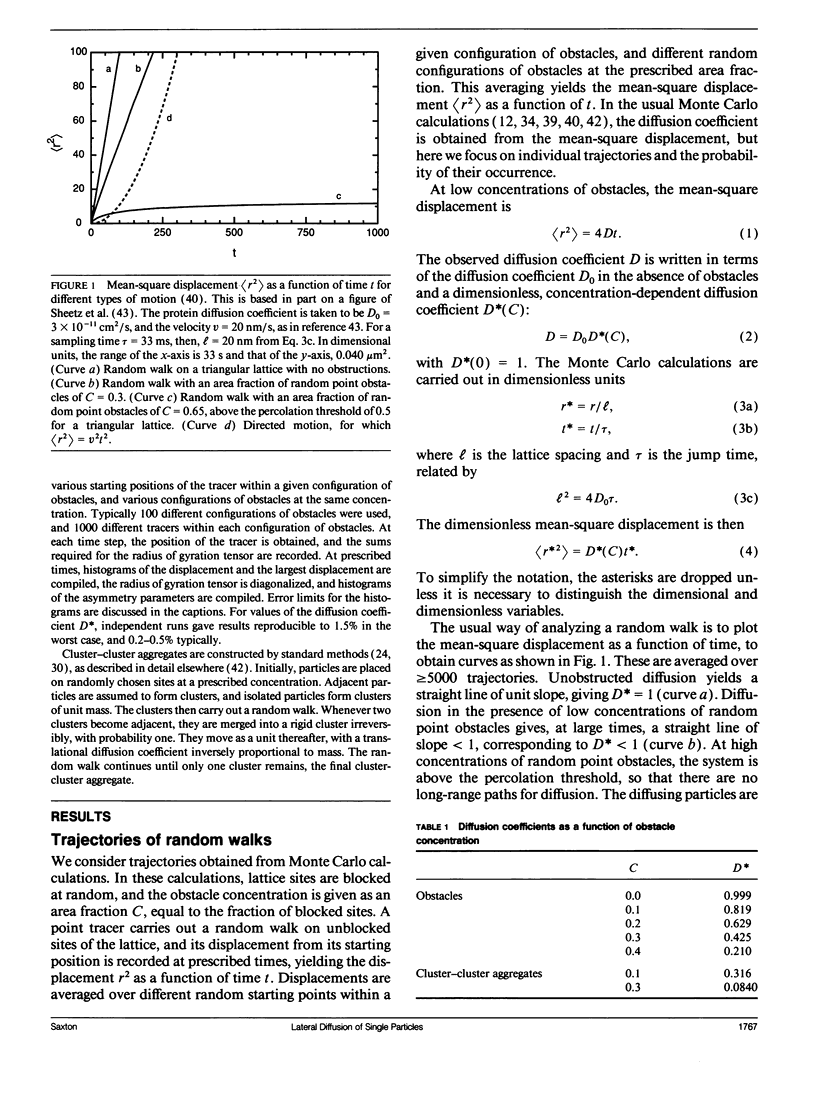
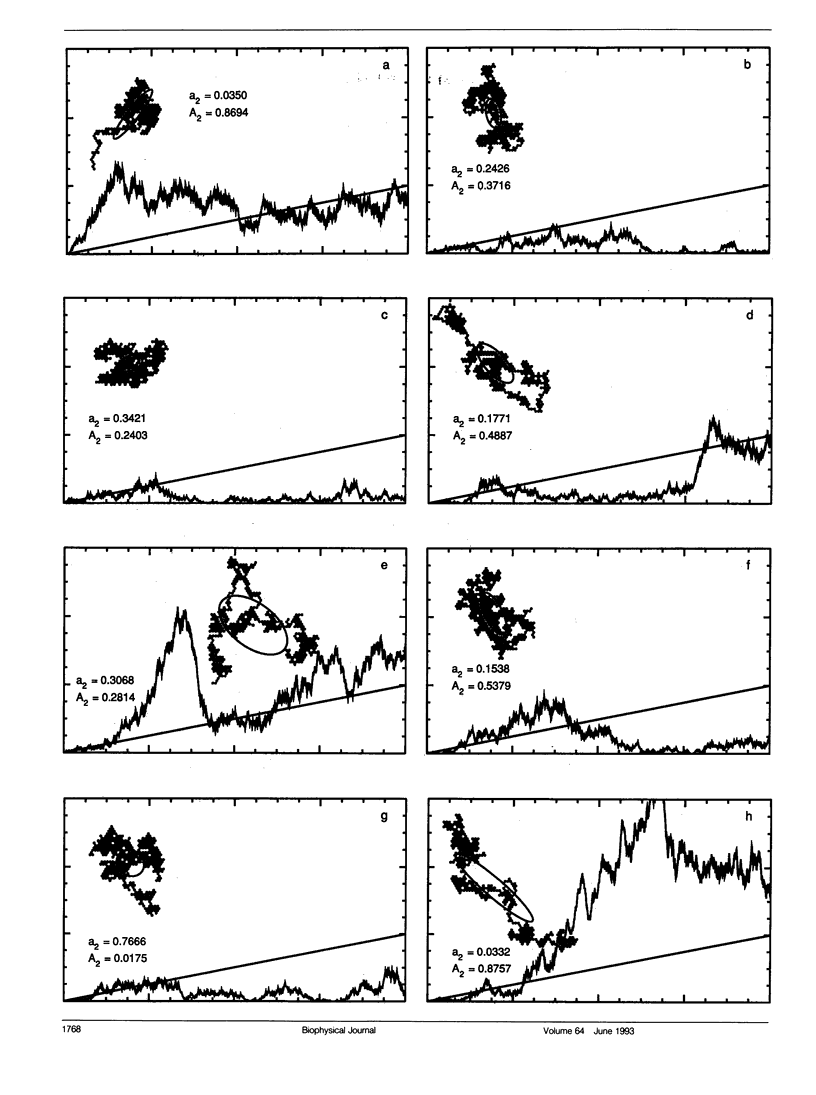
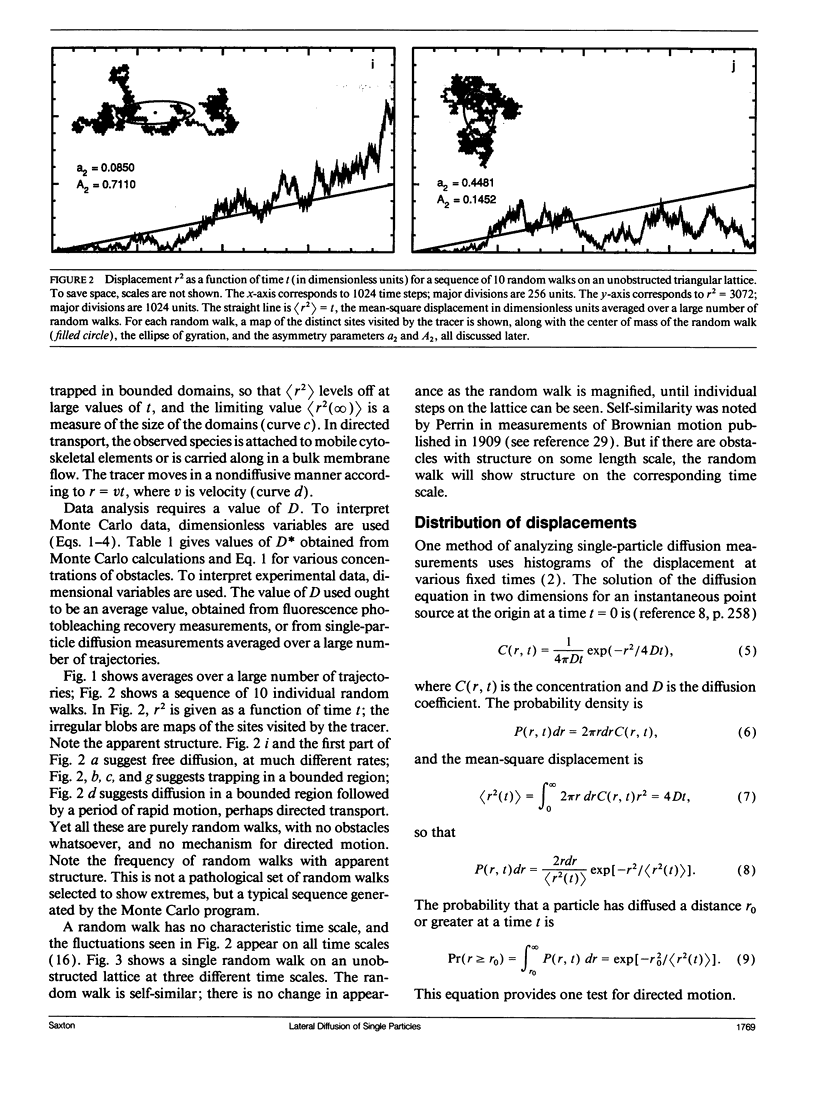
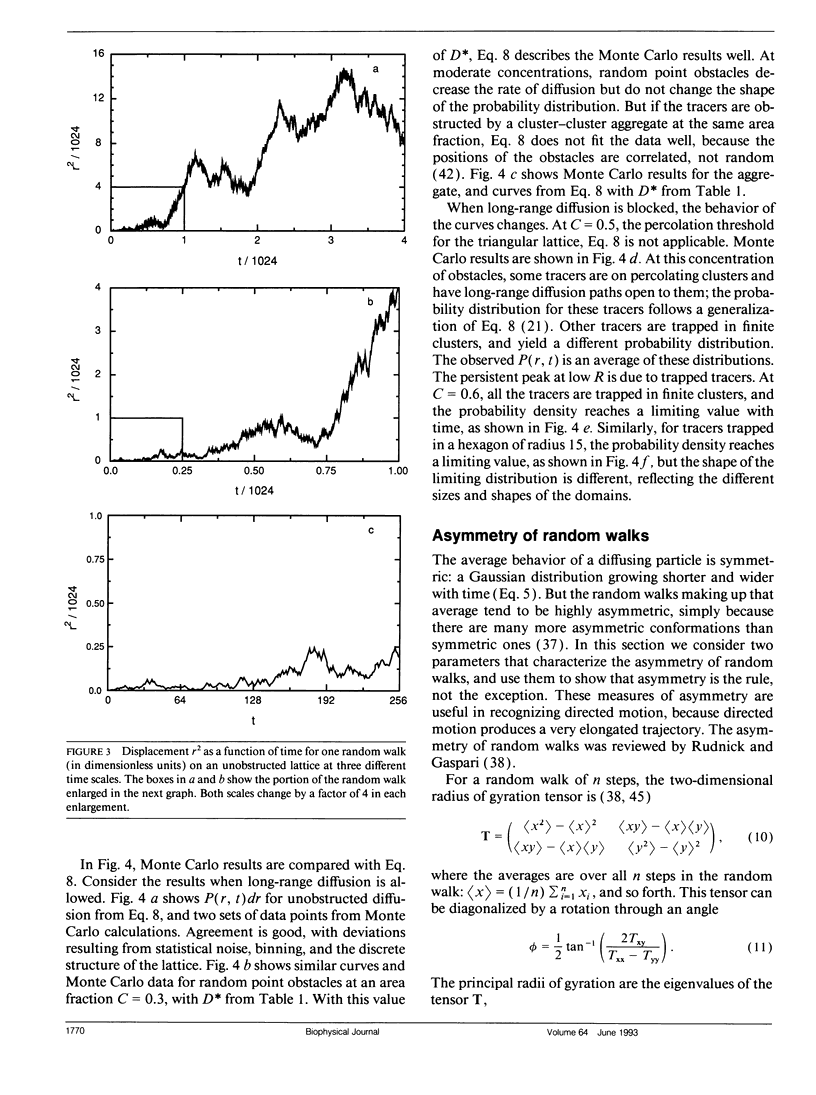
Several laboratories have measured lateral diffusion of single particles on the cell surface, and these measurements may reveal an otherwise inaccessible level of submicroscopic organization of cell membranes. Pitfalls in the interpretation of these experiments are analyzed. Random walks in unobstructed systems show structure that could be interpreted as free diffusion, obstructed diffusion, directed motion, or trapping in finite domains. To interpret observed trajectories correctly, one must consider not only the trajectories themselves but also the probabilities of occurrence of various trajectories. Measures of the asymmetry of obstructed and unobstructed random walks are calculated, and probabilities are evaluated for random trajectories that resemble either directed motion or diffusion in a bounded region.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. M., Georgiou G. N., Morrison I. E., Stevenson G. V., Cherry R. J. Tracking of cell surface receptors by fluorescence digital imaging microscopy using a charge-coupled device camera. Low-density lipoprotein and influenza virus receptor mobility at 4 degrees C. J Cell Sci. 1992 Feb;101(Pt 2):415–425. doi: 10.1242/jcs.101.2.415. [DOI] [PubMed] [Google Scholar]
- Edidin M., Kuo S. C., Sheetz M. P. Lateral movements of membrane glycoproteins restricted by dynamic cytoplasmic barriers. Science. 1991 Nov 29;254(5036):1379–1382. doi: 10.1126/science.1835798. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Flores J., Petersen W. P. A milling crowd model for local and long-range obstructed lateral diffusion. Mobility of excimeric probes in the membrane of intact erythrocytes. Biophys J. 1986 May;49(5):987–1001. doi: 10.1016/S0006-3495(86)83727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Family F, Vicsek T, Meakin P. Are random fractal clusters isotropic? Phys Rev Lett. 1985 Aug 12;55(7):641–644. doi: 10.1103/PhysRevLett.55.641. [DOI] [PubMed] [Google Scholar]
- Kucik D. F., Elson E. L., Sheetz M. P. Cell migration does not produce membrane flow. J Cell Biol. 1990 Oct;111(4):1617–1622. doi: 10.1083/jcb.111.4.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavergne J., Joliot P. Restricted diffusion in photosynthetic membranes. Trends Biochem Sci. 1991 Apr;16(4):129–134. doi: 10.1016/0968-0004(91)90054-y. [DOI] [PubMed] [Google Scholar]
- Lee G. M., Ishihara A., Jacobson K. A. Direct observation of brownian motion of lipids in a membrane. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6274–6278. doi: 10.1073/pnas.88.14.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecham R. P., Whitehouse L., Hay M., Hinek A., Sheetz M. P. Ligand affinity of the 67-kD elastin/laminin binding protein is modulated by the protein's lectin domain: visualization of elastin/laminin-receptor complexes with gold-tagged ligands. J Cell Biol. 1991 Apr;113(1):187–194. doi: 10.1083/jcb.113.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F. Long tail kinetics in biophysics? Biophys J. 1992 Aug;63(2):366–370. doi: 10.1016/S0006-3495(92)81602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Sheetz M. P., Elson E. L. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophys J. 1991 Oct;60(4):910–921. doi: 10.1016/S0006-3495(91)82125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick J., Gaspari G. The shapes of random walks. Science. 1987 Jul 24;237(4813):384–389. doi: 10.1126/science.237.4813.384. [DOI] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion and aggregation. A Monte Carlo study. Biophys J. 1992 Jan;61(1):119–128. doi: 10.1016/S0006-3495(92)81821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Distance dependence of the diffusion coefficient. Biophys J. 1989 Sep;56(3):615–622. doi: 10.1016/S0006-3495(89)82708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. The effect of mobile obstacles. Biophys J. 1987 Dec;52(6):989–997. doi: 10.1016/S0006-3495(87)83291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. The membrane skeleton of erythrocytes. A percolation model. Biophys J. 1990 Jun;57(6):1167–1177. doi: 10.1016/S0006-3495(90)82636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Turney S., Qian H., Elson E. L. Nanometre-level analysis demonstrates that lipid flow does not drive membrane glycoprotein movements. Nature. 1989 Jul 27;340(6231):284–288. doi: 10.1038/340284a0. [DOI] [PubMed] [Google Scholar]
- Thomas J. L., Feder T. J., Webb W. W. Effects of protein concentration on IgE receptor mobility in rat basophilic leukemia cell plasma membranes. Biophys J. 1992 May;61(5):1402–1412. doi: 10.1016/S0006-3495(92)81946-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Crise B., Su B., Hou Y., Rose J. K., Bothwell A., Jacobson K. Lateral diffusion of membrane-spanning and glycosylphosphatidylinositol-linked proteins: toward establishing rules governing the lateral mobility of membrane proteins. J Cell Biol. 1991 Oct;115(1):75–84. doi: 10.1083/jcb.115.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brabander M., Nuydens R., Ishihara A., Holifield B., Jacobson K., Geerts H. Lateral diffusion and retrograde movements of individual cell surface components on single motile cells observed with Nanovid microscopy. J Cell Biol. 1991 Jan;112(1):111–124. doi: 10.1083/jcb.112.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]


