Abstract
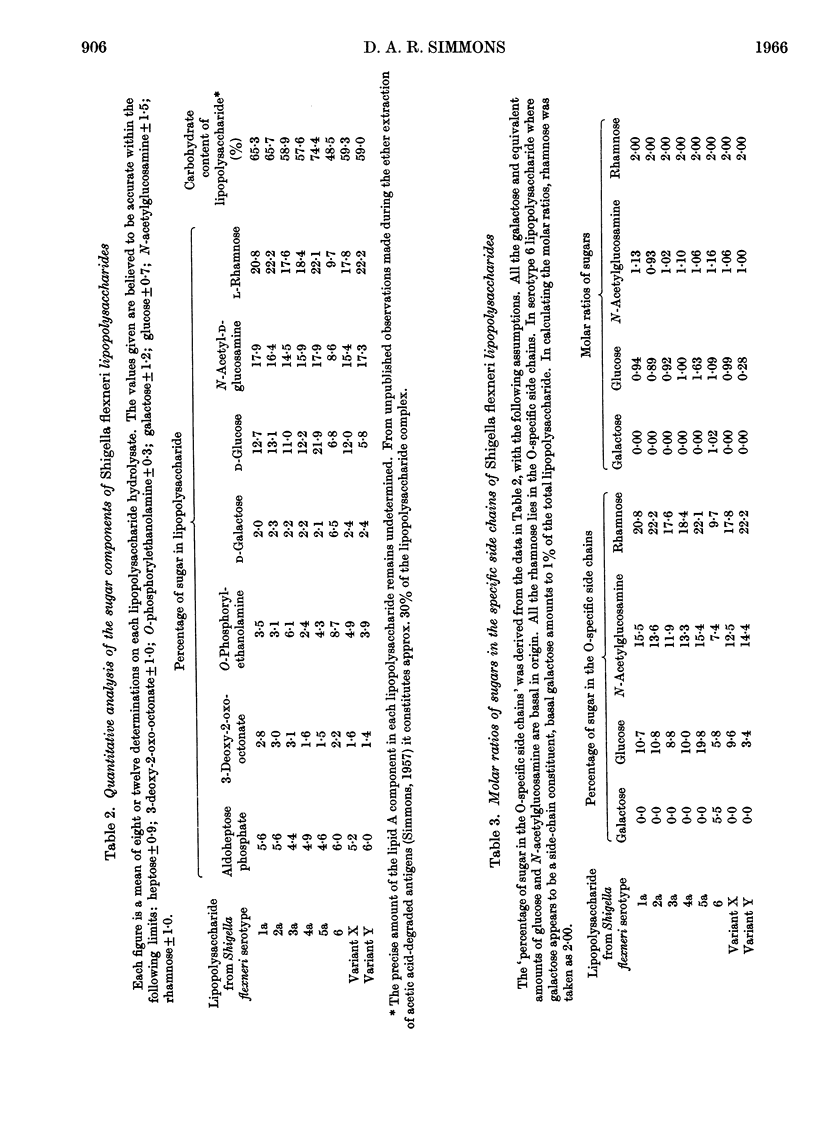
1. The lipopolysaccharides of a representative selection of Shigella flexneri serotypes all contain the same constituents as Salmonella chemotype VII, namely, aldoheptose phosphate, 3-deoxy-2-oxo-octonate, O-phosphorylethanolamine, d-galactose, d-glucose, N-acetyl-d-glucosamine and l-rhamnose. 2. The presence of all the Salmonella basal sugars in Sh. flexneri lipopolysaccharides is consistent with the view that the latter contain a basal structure or core which is similar to the common basal structure of Salmonella lipopolysaccharides. 3. Although the Sh. flexneri lipopolysaccharides belong to one chemotype, there appear to be quantitative differences in the composition of their O-specific side chains. The repeating units of Sh. flexneri serotypes 1a, 2a, 3a, 4a, and variant X contain d-glucose, N-acetyl-d-glucosamine and l-rhamnose in the proportions 1:1:2 respectively. The analogous repeating units of serotypes 5a and 6 contain an additional mole of d-glucose and d-galactose respectively and that of variant Y 1 mole of d-glucose less.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Consden R., Gordon A. H., Martin A. J. Qualitative analysis of proteins: a partition chromatographic method using paper. Biochem J. 1944;38(3):224–232. doi: 10.1042/bj0380224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFIMOVA A. A. [Carbohydrate composition of specific polysaccharides of certain types of pathogenic bacteria of the enteric group]. Zh Mikrobiol Epidemiol Immunobiol. 1959 Nov;30:100–107. [PubMed] [Google Scholar]
- Edstrom R. D., Heath E. C. Sugar nucleotide transferases in Escherichia coli lipopolysaccharide biosynthesis. Biochem Biophys Res Commun. 1964 Aug 11;16(6):576–581. doi: 10.1016/0006-291x(64)90195-0. [DOI] [PubMed] [Google Scholar]
- FISCHER W., ZAPF J. QUANTITATIVE BESTIMMUNG DER GALAKTOSE MITTELS GALATOSEOXYDASE AUS DACTYLIUM DENDROIDES. I. Hoppe Seylers Z Physiol Chem. 1964;337:186–195. doi: 10.1515/bchm2.1964.337.1.186. [DOI] [PubMed] [Google Scholar]
- GHUYSEN J. M., STROMINGER J. L. STRUCTURE OF THE CELL WALL OF STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. I. PREPARATION OF FRAGMENTS BY ENZYMATIC HYDROLYSIS. Biochemistry. 1963 Sep-Oct;2:1110–1119. doi: 10.1021/bi00905a035. [DOI] [PubMed] [Google Scholar]
- GROLLMAN A. P., OSBORN M. J. O-PHOSPHORYLETHANOLAMINE: A COMPONENT OF LIPOPOLYSACCHARIDE IN CERTAIN GRAM-NEGATIVE BACTERIA. Biochemistry. 1964 Oct;3:1571–1574. doi: 10.1021/bi00898a031. [DOI] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Jermyn M. A., Isherwood F. A. Improved separation of sugars on the paper partition chromatogram. Biochem J. 1949;44(4):402–407. doi: 10.1042/bj0440402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFFMANN F., LUEDERITZ O., STIERLIN H., WESTPHAL O. [On the immunochemistry of O antigens of Enterobacteriaceae. I. Analysis of the sugar component of Salmonella O antigens]. Zentralbl Bakteriol. 1960 May;178:442–458. [PubMed] [Google Scholar]
- KRUEGER L., LUEDERITZ O., STROMINGER J. L., WESTPHAL O. [On the immunochemistry of O antigens of Enterobacteriaceae. VII. The relation of hexoses and 6-desoxyhexoses in Salmonella lipopolysaccharides to the D and L group]. Biochem Z. 1962;335:548–558. [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LUEDERITZ O., SIMMONS D. A., WESTPHAL O., STROMINGER J. L. A SPECIFIC MICRODETERMINATION OF GLUCOSAMINE AND THE ANALYSIS OF OTHER HEXOSAMINES IN THE PRESENCE OF GLUCOSAMINE. Anal Biochem. 1964 Nov;9:263–271. doi: 10.1016/0003-2697(64)90184-8. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Simmons D. A., Westphal G. The immunochemistry of Salmonella chemotype VI O-antigens. The structure of oligosaccharides from Salmonella group U (o 43) lipopolysaccharides. Biochem J. 1965 Dec;97(3):820–826. doi: 10.1042/bj0970820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge S. M. Filter-paper partition chromatography of sugars: 1. General description and application to the qualitative analysis of sugars in apple juice, egg white and foetal blood of sheep. with a note by R. G. Westall. Biochem J. 1948;42(2):238–250. doi: 10.1042/bj0420238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- ROBBINS P. W., UCHIDA T. Studies on the chemical basis of the phage conversion of O-antigens in the E-group Salmonellae. Biochemistry. 1962 Mar;1:323–335. doi: 10.1021/bi00908a020. [DOI] [PubMed] [Google Scholar]
- SIMMONS D. A. The glucosidic linkages of the Shigella flexneri polysaccharides. Biochem J. 1962 Aug;84:353–360. doi: 10.1042/bj0840353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMMONS D. A. The hexose constituents of some Shigella polysaccharide hydrolysates. J Gen Microbiol. 1957 Dec;17(3):650–657. doi: 10.1099/00221287-17-3-650. [DOI] [PubMed] [Google Scholar]
- SLEIN M. W., SCHNELL G. W. An aldoheptose phosphate in a polysaccharide isolated from Shigella flexneri. Proc Soc Exp Biol Med. 1953 Apr;82(4):734–738. doi: 10.3181/00379727-82-20231. [DOI] [PubMed] [Google Scholar]
- STOFFYN P. J., JEANLOZ R. W. Identification of amino sugars by paper chromatography. Arch Biochem Biophys. 1954 Oct;52(2):373–379. doi: 10.1016/0003-9861(54)90137-x. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L., PARK J. T., THOMPSON R. E. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959 Dec;234:3263–3268. [PubMed] [Google Scholar]
- Simmons D. A., Lüderitz O., Westphal O. The immunochemistry of Salmonella chemotype VI O-antigens. The structure of oligosaccharides from Salmonella group G (o 13,22) lipopolysaccharides. Biochem J. 1965 Dec;97(3):807–814. doi: 10.1042/bj0970807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. A., Lüderitz O., Westphal O. The immunochemistry of Salmonella chemotype VI O-antigens. The structure of oligosaccharides from Salmonella group N (o 30) lipopolysaccharides. Biochem J. 1965 Dec;97(3):815–819. doi: 10.1042/bj0970815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W., Lüderitz O., Westphal O. Studies on the structure of lipopolysaccharides of Salmonella minnesota and Salmonella typhimurium R strains. Biochem J. 1965 Aug;96(2):439–448. doi: 10.1042/bj0960439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- WALLENFELS K., KURZ G. [On the specificity of galactose dehydrogenase from Pseudomonas saccharophila and its use as an analytical aid]. Biochem Z. 1962;335:559–573. [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- WESTPHAL O., KAUFFMANN F., LUEDERITZ O., STIERLIN H. [On the immunochemistry of the O-antigen of Enterobacteriaceae. III. Analysis of the sugar components of cross-reacting O-antigens of Salmonella, Arizona and Escherichia]. Zentralbl Bakteriol. 1960 Jul;179:336–342. [PubMed] [Google Scholar]


