Abstract
Extended-spectrum β-lactamases (ESBLs) are a rapidly evolving group of β-lactamases which share the ability to hydrolyze third-generation cephalosporins and aztreonam yet are inhibited by clavulanic acid. Typically, they derive from genes for TEM-1, TEM-2, or SHV-1 by mutations that alter the amino acid configuration around the active site of these β-lactamases. This extends the spectrum of β-lactam antibiotics susceptible to hydrolysis by these enzymes. An increasing number of ESBLs not of TEM or SHV lineage have recently been described. The presence of ESBLs carries tremendous clinical significance. The ESBLs are frequently plasmid encoded. Plasmids responsible for ESBL production frequently carry genes encoding resistance to other drug classes (for example, aminoglycosides). Therefore, antibiotic options in the treatment of ESBL-producing organisms are extremely limited. Carbapenems are the treatment of choice for serious infections due to ESBL-producing organisms, yet carbapenem-resistant isolates have recently been reported. ESBL-producing organisms may appear susceptible to some extended-spectrum cephalosporins. However, treatment with such antibiotics has been associated with high failure rates. There is substantial debate as to the optimal method to prevent this occurrence. It has been proposed that cephalosporin breakpoints for the Enterobacteriaceae should be altered so that the need for ESBL detection would be obviated. At present, however, organizations such as the Clinical and Laboratory Standards Institute (formerly the National Committee for Clinical Laboratory Standards) provide guidelines for the detection of ESBLs in klebsiellae and Escherichia coli. In common to all ESBL detection methods is the general principle that the activity of extended-spectrum cephalosporins against ESBL-producing organisms will be enhanced by the presence of clavulanic acid. ESBLs represent an impressive example of the ability of gram-negative bacteria to develop new antibiotic resistance mechanisms in the face of the introduction of new antimicrobial agents.
INTRODUCTION
The introduction of the third-generation cephalosporins into clinical practice in the early 1980s was heralded as a major breakthrough in the fight against β-lactamase-mediated bacterial resistance to antibiotics. These cephalosporins had been developed in response to the increased prevalence of β-lactamases in certain organisms (for example, ampicillin hydrolyzing TEM-1 and SHV-1 β-lactamases in Escherichia coli and Klebsiella pneumoniae) and the spread of these β-lactamases into new hosts (for example, Haemophilus influenzae and Neisseria gonorrhoeae). Not only were the third-generation cephalosporins effective against most β-lactamase-producing organisms but they had the major advantage of lessened nephrotoxic effects compared to aminoglycosides and polymyxins.
The first report of plasmid-encoded β-lactamases capable of hydrolyzing the extended-spectrum cephalosporins was published in 1983 (197). The gene encoding the β-lactamase showed a mutation of a single nucelotide compared to the gene encoding SHV-1. Other β-lactamases were soon discovered which were closely related to TEM-1 and TEM-2, but which had the ability to confer resistance to the extended-spectrum cephalosporins (62, 373). Hence these new β-lactamases were coined extended-spectrum β-lactamases (ESBLs). In the first substantial review of ESBLs in 1989, it was noted by Philippon, Labia, and Jacoby that the ESBLs represented the first example in which β-lactamase-mediated resistance to β-lactam antibiotics resulted from fundamental changes in the substrate spectra of the enzymes (308).
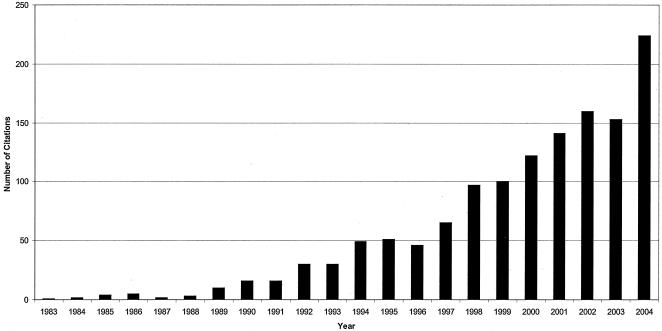
In 2001, the ESBLs were reviewed in this journal by Patricia Bradford (51). The body of knowledge pertaining to ESBLs has grown rapidly since that time (Fig. 1). A PubMed search using the key-words extended-spectrum β-lactamase reveals more than 1,300 relevant articles, with more than 600 published since the time Bradford's review was written.
FIG. 1.
Explosion of knowledge on extended-spectrum β-lactamases.
The total number of ESBLs now characterized exceeds 200. These are detailed on the authorative website on the nomeclature of ESBLs hosted by George Jacoby and Karen Bush (http://www.lahey.org/studies/webt.htm). Published research on ESBLs has now originated from more than 30 different countries, reflecting the truly worldwide distribution of ESBL-producing organisms.
The purpose of this review is to focus on the clinical aspects of ESBL production, with an emphasis on the implications for clinical microbiology laboratories, hospital molecular epidemiology laboratories, infection control practitioners, and infectious disease physicians.
DEFINITION OF EXTENDED-SPECTRUM β-LACTAMASES
β-Lactamases are most commonly classified according to two general schemes: the Ambler molecular classification scheme and the Bush-Jacoby-Medieros functional classification system (10, 66, 338). The Ambler scheme divides β-lactamases into four major classes (A to D). The basis of this classification scheme rests upon protein homology (amino acid similarity), and not phenotypic characteristics. In the Ambler classification scheme, β-lactamases of classes A, C, and D are serine β-lactamases. In contrast, the class B enzymes are metallo-β-lactamases. The Bush-Jacoby-Medeiros classification scheme groups β-lactamases according to functional similarities (substrate and inhibitor profile). There are four main groups and multiple subgroups in this system. This classification scheme is of much more immediate relevance to the physician or microbiologist in a diagnostic laboratory because it considers β-lactamase inhibitors and β-lactam substrates that are clinically relevant.
There is no consensus of the precise definition of ESBLs. A commonly used working definition is that the ESBLs are β-lactamases capable of conferring bacterial resistance to the penicillins, first-, second-, and third-generation cephalosporins, and aztreonam (but not the cephamycins or carbapenems) by hydrolysis of these antibiotics, and which are inhibited by β-lactamase inhibitors such as clavulanic acid. For the purpose of this review, the term ESBL will be taken to mean those β-lactamases of Bush-Jacoby-Medeiros group 2be and those of group 2d which share most of the fundamental properties of group 2be enzymes (66).
The 2be designation shows that these enzymes are derived from group 2b β-lactamases (for example, TEM-1, TEM-2, and SHV-1); the e of 2be denotes that the β-lactamases have an extended spectrum. Group 2b enzymes hydrolyze penicillin and ampicillin, and to a lesser degree carbenicillin or cephalothin (66). They are not able to hydrolyze extended-spectrum cephalosporins or aztreonam to any significant degree. TEM-1 is the most common plasmid-mediated β-lactamase of ampicillin resistant enteric gram-negative bacilli (for example, Escherichia coli), while SHV-1 is produced by the vast majority of Klebsiella pneumoniae (219). TEM-2 is a less common member of the same group with identical biochemical properties to TEM-1. The ESBLs derived from TEM-1, TEM-2, or SHV-1 differ from their progenitors by as few as one amino acid. This results in a profound change in the enzymatic activity of the ESBLs, so that they can now hydrolyze the third-generation cephalosporins or aztreonam (hence the extension of spectrum compared to the parent enzymes).
With the exception of OXA-type enzymes (which are class D enzymes), the ESBLs are of molecular class A, in the classification scheme of Ambler. They are able to hydrolyze the penicillins, narrow-spectrum and third-generation cephalosporins, and monobactams. The ESBLs have hydrolysis rates for ceftazidime, cefotaxime, or aztreonam (aminothiazoleoxime β-lactam antibiotics) at least 10% that for benzylpenicillin. They are inhibited by clavulanic acid (66).
This property differentiates the ESBLs from the AmpC-type β-lactamases (group 1) produced by organisms such as Enterobacter cloacae which have third-generation cephalosporins as their substrates but which are not inhibited by clavulanic acid. Selection of stably derepressed mutants which hyperproduce the AmpC-type β-lactamases has been associated with clinical failure when third-generation cephalosporins are used to treat serious infections with Enterobacter spp. (86, 92, 192). In general, the fourth-generation cephalosporin, cefepime, is clinically useful against organisms producing Amp C-type β-lactamases (354), but may be less useful in treating ESBL-producing organisms (440). This is discussed in more detail later in this article. Additionally, the metalloenzymes (group 3) produced by organisms such as Stenotrophomonas maltophilia can hydrolyze third-generation cephalosporins (and carbapenems), but are inhibited by EDTA (a heavy metal chelator) but not clavulanic acid (413a).
Some enzymes generally regarded as ESBLs (for example, TEM-7 and TEM-12) do not rigorously meet the hydrolysis criteria above. However, large increases in hydrolysis rates for ceftazidime are seen compared to the parent TEM-1 and TEM-2 enzymes, resulting in increased MICs of ceftazidime for organisms bearing such β-lactamases. Hence, these TEM β-lactamases are included in group 2be and are widely regarded as ESBLs (66).
In common with the ESBLs are other groups of β-lactamases (2d, 2e, and 2f) that hydrolyze cephalosporins and are inhibited by clavulanic acid. However, group 2e β-lactamases (for example, the inducible cephalosporinases of Proteus vulgaris) hydrolyze cefotaxime well but lack good penicillin-hydrolyzing activity, and do not have a high affinity for aztreonam, in contrast to the cephalosporinases in group 1. Group 2f β-lactamases (for example, Sme-1 from Serratia marcescens) are carbapenem-hydrolyzing enzymes that are weakly inhibited by clavulanic acid. Extension of the spectrum of OXA-type β-lactamases (group 2d) towards the extended-spectrum cephalosporins has been observed, and many authorities regard some of these enzymes as ESBLs (243).
DIVERSITY OF ESBL TYPES
SHV
The SHV-type ESBLs may be more frequently found in clinical isolates than any other type of ESBLs (177). SHV refers to sulfhydryl variable. This designation was made because it was thought that the inhibition of SHV activity by p-chloromercuribenzoate was substrate-related, and was variable according to the substrate used for the assay (385). (This activity was never confirmed in later studies with purified enzyme.) Reviews focusing on the SHV-type β-lactamases summarizing kinetic properties of β-lactamases of this family have been published (162, 399).
In 1983, a Klebsiella ozaenae isolate from Germany was discovered which possessed a β-lactamase which efficiently hydrolyzed cefotaxime, and to a lesser extent ceftazidime (197). Sequencing showed that the β-lactamase differed from SHV-1, by replacement of glycine by serine at the 238 position. This mutation alone accounts for the extended-spectrum properties of this β-lactamase, designated SHV-2. Within 15 years of the discovery of this enzyme, organisms harboring SHV-2 were found in every inhabited continent (288), implying that selection pressure from third-generation cephalosporins in the first decade of their use was responsible. SHV-type ESBLs have been detected in a wide range of Enterobacteriaceae and outbreaks of SHV-producing Pseudomonas aeruginosa and Acinetobacter spp. have now been reported (170, 321)
TEM
The TEM-type ESBLs are derivatives of TEM-1 and TEM-2. TEM-1 was first reported in 1965 from an Escherichia coli isolate from a patient in Athens, Greece, named Temoneira (hence the designation TEM) (101). TEM-1 is able to hydrolyze ampicillin at a greater rate than carbenicillin, oxacillin, or cephalothin, and has negligible activity against extended-spectrum cephalosporins. It is inhibited by clavulanic acid. TEM-2 has the same hydrolytic profile as TEM-1, but differs from TEM-1 by having a more active native promoter and by a difference in isoelectric point (5.6 compared to 5.4). TEM-13 also has a similar hydrolytic profile to TEM-1 and TEM-2 (180).
TEM-1, TEM-2, and TEM-13 are not ESBLs. However, in 1987 Klebsiella pneumoniae isolates detected in France as early as 1984 were found to harbor a novel plasmid-mediated β-lactamase coined CTX-1 (62, 373). The enzyme was originally named CTX-1 because of its enhanced activity against cefotaxime. The enzyme, now termed TEM-3, differed from TEM-2 by two amino acid substitutions (377).
In retrospect, TEM-3 may not have been the first TEM-type ESBL. Klebsiella oxytoca, harboring a plasmid carrying a gene encoding ceftazidime resistance, was first isolated in Liverpool, England, in 1982 (117). The responsible β-lactamase was what is now called TEM-12. Interestingly, the strain came from a neonatal unit which had been stricken by an outbreak of Klebsiella oxytoca producing TEM-1. Ceftazidime was used to treat infected patients, but subsequent isolates of Klebsiella oxytoca from the same unit harbored the TEM-type ESBL (117). This is a good example of the emergence of ESBLs as a response to the selective pressure induced by extended-spectrum cephalosporins.
Well over 100 TEM-type β-lactamases have been described, of which the majority are ESBLs. Their isoelectric points range from 5.2 to 6.5. The amino acid changes in comparison with TEM-1 or TEM-2 are documented at http://www.lahey.org/studies/temtable.htm.
A number of TEM derivatives have been found which have reduced affinity for β-lactamase inhibitors. These enzymes have been reviewed elsewhere (78). With very few exceptions (see below), TEM-type enzymes which are less susceptible to the effects of β-lactamase inhibitors have negligible hydrolytic activity against the extended-spectrum cephalosporins and are not considered ESBLs.
However, interesting mutants of TEM β-lactamases are being recovered that maintain the ability to hydrolyze third-generation cephalosporins but which also demonstrate inhibitor resistance. These are referred to as complex mutants of TEM (CMT-1 to -4) (128, 273, 322, 372). A unique TEM-derived enzyme, TEM-AQ, has been found in Italy (301). This enzyme has an amino acid deletion not seen in other TEM enzymes plus several amino acid substitutions.
CTX-M and Toho β-Lactamases
The CTX-M enzymes have been previously reviewed in detail (43). The name CTX reflects the potent hydrolytic activity of these β-lactamases against cefotaxime. Organisms producing CTX-M-type β-lactamases typically have cefotaxime MICs in the resistant range (>64 μg/ml), while ceftazidime MICs are usually in the apparently susceptible range (2 to 8 μg/ml). However, some CTX-M-type ESBLs may actually hydrolyze ceftazidime and confer resistance to this cephalosporin (MICs as high as 256 μg/ml) (22, 318, 382). Aztreonam MICs are variable. CTX-M-type β-lactamases hydrolyze cefepime with high efficiency (400), and cefepime MICs are higher than observed in bacteria producing other ESBL types (436). Tazobactam exhibits an almost 10-fold greater inhibitory activity than clavulanic acid against CTX-M-type β-lactamases (67). It should be noted that the same organism may harbor both CTX-M-type and SHV-type ESBLs or CTX-M-type ESBLs and AmpC-type β-lactamases, which may alter the antibiotic resistance phenotype (429).
Toho-1 and Toho-2 are β-lactamases related structurally to CTX-M-type β-lactamases. (Toho refers to the Toho University School of Medicine Omori Hospital in Tokyo, where a child was hospitalized who was infected with Toho-1 β-lactamase-producing Escherichia coli.) Like most CTX-M-type β-lactamases, the hydrolytic activity of the Toho-1 and Toho-2 enzymes is more potent against cefotaxime than ceftazidime (204, 227).
It appears that the CTX-M-type β-lactamases are closely related to β-lactamases of Kluyvera spp. (109, 112, 171, 278, 319, 353). For example, a chromosomally encoded β-lactamase gene of Kluyvera georgiana encoded an extended-spectrum β-lactamase, KLUG-1, which shares 99% amino acid identity with CTX-M-8 (319). CTX-M-type β-lactamases have 40% or less identity with TEM and SHV-type ESBLs.
The number of CTX-M-type ESBLs is rapidly expanding. They have now been detected in every populated continent (9, 22, 23, 49, 58, 70, 71, 81, 90, 120, 140, 190, 226, 253, 284, 288, 305, 333, 353, 414, 428, 437). For some years, CTX-M ESBLs were predominantly found in three geographic areas: South America, the Far East, and Eastern Europe. In Western Europe and North America, CTX-M-type β-lactamases have previously appeared to be infrequent (105). However, in recent years, a number of authors have reported the advent of CTX-M-type ESBLs in these regions (9, 43, 253, 254, 263, 313, 324). Given the widespread findings of CTX-M-type ESBLs in China and India, it could be speculated that CTX-M-type ESBLs are now actually the most frequent ESBL type worldwide.
The relationship between antibiotic consumption and occurrence of CTX-M-type β-lactamases has not been studied, although the prevalence of the enzymes in agents of community-acquired diarrhea raises speculation that oxyimino cephalosporins available outside the hospital (such as ceftriaxone) may be important. Interestingly, identical β-lactamases have been discovered in widely separated parts of the world (for example, CTX-M-3 has been discovered in Poland and Taiwan), suggesting independent evolution of these enzymes (144, 429). Clonal spread of CTX-M-type β-lactamase producing bacteria has been well-documented (144).
OXA
The OXA-type β-lactamases are so named because of their oxacillin-hydrolyzing abilities. These β-lactamases (group 2d) are characterized by hydrolysis rates for cloxacillin and oxacillin greater than 50% that for benzylpenicillin (66). They predominantly occur in Pseudomonas aeruginosa (417) but have been detected in many other gram-negative bacteria. In fact, the most common OXA-type β-lactamase, OXA-1 has been found in 1 to 10% of Escherichia coli isolates (219).
Most OXA-type β-lactamases do not hydrolyze the extended-spectrum cephalosporins to a significant degree and are not regarded as ESBLs. However, OXA-10 hydrolyzes (weakly) cefotaxime, ceftriaxone, and aztreonam, giving most organisms reduced susceptibility to these antibiotics. Other OXA ESBLS include: OXA-11, -14, -16, -17, -19, -15, -18, -28, -31, -32, -35, and -45 (396). These confer frank resistance to cefotaxime and sometimes ceftazidime and aztreonam (97-100, 156, 396). The simultaneous production of a carbapenem-hydrolyzing metalloenzyme and an aztreonam hydrolyzing OXA enzyme can readily lead to resistance to all β-lactam antibiotics (396).
The OXA-type ESBLs were originally discovered in Pseudomonas aeruginosa isolates from a single hospital in Ankara, Turkey. In France, a novel derivative of OXA-10 (numbered OXA-28) was found in a Pseudomonas aeruginosa isolate (317). A novel ESBL (OXA-18) and an extended-spectrum derivative of the narrow-spectrum OXA-13 β-lactamase (numbered OXA-19) have also been discovered in France in Pseudomonas aeruginosa isolates (309). The evolution of ESBL OXA-type β-lactamases from parent enzymes with narrower spectra has many parallels with the evolution of SHV- and TEM-type ESBLs. Unfortunately there are very few epidemiologic data on the geographical spread of OXA-type ESBLs.
PER
The PER-type ESBLs share only around 25 to 27% homology with known TEM- and SHV-type ESBLs (29, 275). PER-1 β-lactamase efficiently hydrolyzes penicillins and cephalosporins and is susceptible to clavulanic acid inhibition. PER-1 was first detected in Pseudomonas aeruginosa (272), and later in Salmonella enterica serovar Typhimurium and Acinetobacter isolates as well (403-406). In Turkey, as many as 46% of nosocomial isolates of Acinetobacter spp. and 11% of Pseudomonas aeruginosa were found to produce PER-1 (405). PER-2, which shares 86% homology to PER-1, has been detected in S. enterica serovar Typhimurium, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Vibrio cholerae O1 El Tor (29, 305). PER-2 has only been found in South America thus far (29).
Although PER-1-producing organisms have been predominantly found in Turkey, a Pseudomonas aeruginosa outbreak in Italy occurred with no apparent contacts with Turkey (225). Worryingly, a Pseudomonas aeruginosa strain producing both PER-1 and the carbapenemase VIM-2 has been detected in Italy (115). The coexistence of these enzymes renders an organism resistant to virtually all β-lactam antibiotics. PER-1 has also been found in Proteus mirabilis and Alcaligenes faecalis in Italy (283, 300). Isolates of Pseudomonas aeruginosa producing PER-1 have been detected in France, Italy, and Belgium (87, 102, 282). Additionally, a high prevalence of PER-1 in Acinetobacter spp. from Korea has been noted (203, 434).
VEB-1, BES-1, and Other ESBLs
A variety of other β-lactamases which are plasmid-mediated or integron-associated class A enzymes have been recently discovered (45, 138, 240, 241, 320, 323, 327, 369). They are not simple point mutant derivatives of any known β-lactamases. They are remarkable for their geographic diversity. Novel chromosomally encoded ESBLs have also been described (34).
VEB-1 has greatest homology with PER-1 and PER-2 (38%) (323). It confers high-level resistance to ceftazidime, cefotaxime, and aztreonam, which is reversed by clavulanic acid. The gene encoding VEB-1 was found to be plasmid mediated; such plasmids also confer resistance to non-β-lactam antibiotics. The patient from whom the β-lactamase was originally described was a Vietnamese infant hospitalized in France (323). An identical β-lactamase has also been found in Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Enterobacter sakazakii, and Pseudomonas aeruginosa isolates in Thailand (142, 266, 397). Other VEB enzymes have also been detected in Kuwait and China (185, 325).
GES (76, 118, 320, 327, 410, 413, 416, 418), BES (45), TLA (8, 369), SFO (240), and IBC (133, 138, 191, 208, 241, 410-412) are other examples of non-TEM, non-SHV ESBLs and have been found in a wide range of geographic locations.
EPIDEMIOLOGY OF ESBL-PRODUCING ORGANISMS
Global Epidemiology
Europe.
ESBL-producing organisms were first detected in Europe. Although the initial reports were from Germany (197) and England (117), the vast majority of reports in the first decade after the discovery of ESBLs were from France (308, 373). The first large outbreak in France to be reported occurred in 1986 (62); 54 patients in three intensive care units were infected and spread of the infection to four other wards then occurred (62). The proliferation of ESBLs in France was quite dramatic. By the early 1990s, 25 to 35% of nosocomially acquired Klebsiella pneumoniae isolates in France were ESBL producing (239). However, in recent years, augmentation of infection control interventions has been accompanied by a decrease in incidence of ESBL-producing Klebsiella pneumoniae (7, 223). In northern France, the proportion of Klebsiella pneumoniae isolates which were ESBL-producing fell from 19.7% in 1996 to 7.9% in 2000 (7). It is noteworthy however that 30.2% of Enterobacter aerogenes isolates in 2000 were ESBL producers (7). It is also important to note that while the proportion of Klebsiella pneumoniae isolates which are ESBL-producing may be decreasing in some parts of Western Europe, a significant increase may be occurring in Eastern Europe (362). Outbreaks of infection with ESBL-producing organisms have now been reported from virtually every European country.
There is considerable geographical difference in the occurrence of ESBLs in European countries. Within countries, hospital-to-hospital variability in occurrence may also be marked (20). In a 1997-1998 survey of 433 isolates from 24 intensive care units in western and southern Europe, 25% of klebsiellae possessed ESBLs (20). A similar survey was performed by the same group in 1994; the overall proportion of klebsiellae which possessed ESBLs did not differ significantly between the two time periods, but the percentage of intensive care units which recorded ESBL-producing klebsiellae rose significantly from 74% to >90% (20, 221). Another large study from more than 100 European intensive care units found that the prevalence of ESBLs in klebsiellae ranged from as low as 3% in Sweden to as high as 34% in Portugal (158). A third study, which included both intensive care unit and non-intensive care unit isolates from 25 European hospitals, found that 21% of Klebsiella pneumoniae isolates had reduced susceptibility to ceftazidime (usually indicative of ESBL production, although it is acknowledged that other mechanisms of resistance may be responsible) (129). In Turkey, a survey of Klebsiella spp. from intensive care units from eight hospitals showed that 58% of 193 isolates harbored ESBLs (151).
North America.
First reports of ESBL-producing organisms in the United States occurred in 1988 (181). In 1989, significant infections with TEM-10-producing Klebsiella pneumoniae were noted in Chicago by Quinn and colleagues (331). Other early reports of outbreaks mainly described infections with TEM-type ESBLs (particularly TEM-10, TEM-12, and TEM-26) (246, 270, 337, 341-343, 358, 402). However, outbreaks with SHV-type ESBLs have also been described (177). CTX-M-type ESBLs have recently been described in the United States and Canada (50, 253, 313).
Assessment of the prevalence of ESBL-producing organisms in the United States has been hampered by reliance of statistics examining resistance of organisms to third-generation cephalosporins, where resistance is defined as an MIC of ≥32 μg/ml (ceftazidime) or ≥64 μg/ml (cefotaxime/ceftriaxone). Since many ESBL-producing organisms have MICs for third-generation cephalosporins between 2 and 16 μg/ml, the prevalence of ESBL-producing organisms in the United States may have been underestimated in the past. Moland and colleagues have shown that ESBL-producing isolates were found in 75% of 24 medical centers in the United States (254). In a survey of nearly 36,000 isolates from intensive care units in North America, nonsusceptibility of Klebsiella pneumoniae to third-generation cephalosporins averaged 13% (273). The percentage of isolates which were susceptible fell by 3% over the years 1994 to 2000 (273). National Nosocomial Infection Surveillance (NNIS) figures for the period January 1998 to June 2002 reveal that 6.1% of 6,101 Klebsiella pneumoniae isolates from 110 intensive care units were resistant to third-generation cephalosporins (268). In at least 10% of intensive care units, resistance rates exceeded 25%. In non-intensive care unit inpatient areas, 5.7% of 10,733 Klebsiella pneumoniae isolates were ceftazidime resistant (268). In outpatient areas, just 1.8% of 12,059 Klebsiella pneumoniae isolates and 0.4% of 71,448 Escherichia coli isolates were ceftazidime resistant (268).
South and Central America.
In 1988 and 1989 isolates of Klebsiella pneumoniae from Chile and Argentina were reported as harboring SHV-2 and SHV-5 (74). In retrospect, however a Klebsiella pneumoniae isolate kept lyophilized since 1982, during a preclinical study of cefotaxime in Buenos Aires, also proved to be a producer of an SHV-5 ESBL (J. M. Casellas, Abstracts of the International Congress of Chemotherapy, Birmingham, England, 1999). In 1989 an outbreak of multiresistant Salmonella enterica serovar Typhimurium infections occurred in 12 of 14 Argentinian provinces. From these isolates a new non-SHV, non-TEM ESBL named CTX-M-2 was identified (26, 28, 29, 333). Organisms with CTX-M-2 have spread throughout many parts of South America (333). Other CTX-M enzymes (CTX-M-8, -9, and -16) have been discovered in Brazil (44, 46). Curiously, TEM-type ESBLs have been very rarely reported from South America. As noted above, two novel non-TEM, non-SHV ESBLs have been recently reported from South America: GES-1, isolated from an infant previously hospitalized in French Guiana (320), and BES-1, from an ESBL-producing Serratia marcescens isolate from a hospital in Rio de Janeiro (45).
ESBLs have been found in 30 to 60% of klebsiellae from intensive care units in Brazil, Colombia, and Venezuela (245, 281, 306, 307, 349, 350). Reports of ESBL-producing organisms also exist from Central America and the Caribbean Islands (84, 114, 146, 370, 371).
Africa and the Middle East.
Several outbreaks of infections with ESBL-producing Klebsiella have been reported from South Africa (93, 188, 310, 367), but no national surveillance figures have been published. However, it has been reported that 36.1% of Klebsiella pneumoniae isolates collected in a single South African hospital in 1998 and 1999 were ESBL producers (33). ESBLs have also been documented in Israel, Saudi Arabia, and a variety of North African countries (3, 25, 35, 36, 47, 122, 247, 272). Outbreaks of Klebsiella infections with strains resistant to third-generation cephalosporins have been reported in Nigeria and Kenya without documentation of ESBL production (5, 264). A novel CTX-M enzyme (CTX-M-12) has been found in Kenya (190). Characterization of ESBLs from South Africa has revealed TEM and SHV types (especially SHV-2 and SHV-5) (160, 315). A nosocomial outbreak of infections with Pseudomonas aeruginosa, expressing GES-2 has been described in South Africa (326).
Australia.
The first ESBLs to be detected in Australia were isolated from a collection of gentamicin-resistant Klebsiella spp. collected between 1986 and 1988 from Perth (259). These were characterized as being of SHV derivation (260). In the last decade, ESBL-producing organisms have been detected in every state of Australia and in the Northern Territory (33, 121, 169, 348, 359). Outbreaks of infection have occurred in both adult and pediatric patients. Overall, it appears that the proportion of Klebsiella pneumoniae isolates which are ESBL producers in Australian hospitals is about 5% (33).
Asia.
In 1988, isolates of Klebsiella pneumoniae from China which contained SHV-2 were reported (181). Further reports of other SHV-2-producing organism in China occurred in 1994 (83). In reports comprising limited numbers of isolates collected in 1998 and 1999, 30.7% of Klebsiella pneumoniae isolates and 24.5% of Escherichia coli isolates were ESBL producers (33). In a major teaching hospital in Beijing, 27% of Escherichia coli and Klebsiella pneumoniae blood culture isolates collected from 1997 through 1999 were ESBL producers (116). Of isolates collected from Zhejiang Province, 34% of Escherichia coli isolates and 38.3% of Klebsiella pneumoniae isolates were ESBL producing (438).
National surveys have indicated the presence of ESBLs in 5 to 8% of Escherichia coli isolates from Korea, Japan, Malaysia, and Singapore but 12 to 24% in Thailand, Taiwan, the Philippines, and Indonesia. Rates of ESBL production by Klebsiella pneumoniae have been as low as 5% in Japan (215, 427) and 20 to 50% elsewhere in Asia. However, there are clearly differences from hospital to hospital: it has been reported that a quarter of all Klebsiella pneumoniae isolates from a hospital in Japan in 1998 and 1999 were ESBL producers (33).
ESBLs of the SHV-2, SHV-5, and SHV-12 lineage initially dominated in those studies in which genotypic characterization has been carried out (209). Newly described SHV-type ESBLs have recently been reported from Taiwan and Japan (82, 202). However, the appearance of CTX-M ESBLs in India (189, 318) and China (81, 414, 425), and more frequent reports of outbreaks of infection with CTX-M-type ESBLs in Japan (200, 226), Korea (284), and Taiwan (436), raise suspicions that these may indeed be the dominant ESBL types in Asia. Plasmid-mediated non-TEM, non-SHV ESBLs, showing homology to the chromosomal β-lactamases of Klebsiella oxytoca (Toho-1 and Toho-2), have been detected in Japan (173, 227). A new non-TEM, non-SHV ESBL (VEB-1) has been reported from Thailand and Vietnam (141, 142, 266, 323).
Molecular Epidemiology of Nosocomial Infections with ESBL-Producing Organisms
More than 50 studies (describing in total more than 3,000 patients) have been published in peer-reviewed medical literature utilizing molecular typing methods in the study of the epidemiology of nosocomial infections with ESBL-producing organisms (295). More than 75% of the studies have addressed ESBL-producing infections with Klebsiella pneumoniae. The predilection of ESBLs for Klebsiella pneumoniae has never been clearly explained. It should be noted that the parent enzyme of TEM-type ESBLs, TEM-1, is widespread in many other species. More relevant, given the frequent finding of SHV-type ESBLs in Klebsiella pneumoniae, may be the increased frequency of SHV-1 in Klebsiella pneumoniae versus other species. Almost all non-ESBL-producing Klebsiella pneumoniae isolates have chromosomally mediated SHV-1 β-lactamases (21). In contrast, fewer than 10% of ampicillin-resistant Escherichia coli isolates harbor SHV-1 (219).
Many ESBL genes are on large plasmids; even prior to the advent of ESBLs, large multiresistance plasmids were more common in klebsiellae than Escherichia coli (219). Of importance may be the well-noted adaptation of klebsiellae to the hospital environment. Klebsiellae survive longer than other enteric bacteria on hands and environmental surfaces, facilitating cross-infection within hospitals (75).
In 100% of the more than 50 studies previously mentioned, at least two patients were colonized or infected with genotypically similar strains, implying patient-to-patient transmission of the strain. A number of outbreaks have been decribed with dissemination of a single clone of genotypically identical organism (131, 132, 143, 273). Clones have been found to persist for more than 3 years (57).
However, in many hospitals a more complex molecular epidemiologic picture has emerged (20). Recent reports have described the clonal dissemination of at least five different ESBL-producing Klebsiella strains in the same unit at the same time (128). Additionally, members of a single epidemic strain may carry different plasmids (carrying different ESBL genes) (128). Furthermore, genotypically nonrelated strains may produce the same ESBL due to plasmid transfer from species to species (38, 128). Finally, although the same ESBL may be prevalent in a particular unit of a hospital, they may be mediated by different plasmids (53). This may imply independent evolution via the effects of antibiotic pressure, or plasmid transfer from organism to organism.
Intensive care units are often the epicenter of ESBL production in hospitals—in one large outbreak, more than 40% af all the hospital's ESBL-producing organisms were from patients in intensive care units (147). As was noted in the pre-ESBL era, neonatal intensive care units can also be a focus of infections with multiply resistant klebsiellae (6, 38, 121, 217, 348, 386, 387, 408). Intensive care units in tertiary referral hospitals may acquire patients already colonized with ESBL-producing organisms, thereby triggering an outbreak of infection (147, 363, 364).
Transfer of genotypically related ESBLs from hospital to hospital within a single city (40, 256, 351, 439), from city to city (439), and from country to country (128, 147, 365, 439) has been documented. A notable clone has been an SHV-4-producing, serotype K-25 isolate of Klebsiella pneumoniae which has spread to multiple hospitals in France and Belgium (439). Another notable dissemination has been of a TEM-24-producing Enterobacter aerogenes clone in France, Spain, and Belgium (68, 108, 119). Intercontinental transfer has also been described (365).
Although ESBL-producing organisms can be introduced into intensive care units, epidemics of infection from intensive care units to other parts of the hospital have been well documented to occur (37, 182, 363). Likewise, ESBLs may spontaneously evolve outside of the intensive care unit. Units noted to have been affected by outbreaks include neurosurgical (37), burns (339), renal (131), obstetrics and gynecology (132), hematology and oncology (163, 270), and geriatric units (149, 268). Nursing homes and chronic care facilities may also be a focus of infections with ESBL-producing organisms. In these settings, clonal spread has also been documented (54, 343, 419).
Risk Factors for Colonization and Infection with ESBL Producers
Numerous studies have used a case-control design with which to assess risk factors for colonization and infection with ESBL-producing organisms (16, 19, 40, 96, 103, 205, 222, 234, 291, 299, 311, 358, 419). Analysis of the results of these studies yelds a plethora of conflicting results, likely due to the differences in study populations, selection of cases, selection of controls, and sample size (286). Nevertheless, some generalizations can be made. Patients at high risk for developing colonization or infection with ESBL-producing organisms are often seriously ill patients with prolonged hospital stays and in whom invasive medical devices are present (urinary catheters, endotracheal tubes, central venous lines) for a prolonged duration. The median length of hospital stay prior to isolation of an ESBL producer has ranged from 11 to 67 days, depending on the study (19, 40, 96, 103, 104, 205, 419). In addition to those already mentioned, a myriad of other risk factors have been found in individual studies, including the presence of nasogastric tubes (19), gastrostomy or jejunostomy tubes (358, 419) and arterial lines (222, 299), administration of total parenteral nutrition (299), recent surgery (106), hemodialysis (96), decubitus ulcers (419), and poor nutritional status (234).
Heavy antibiotic use is also a risk factor for acquisition of an ESBL-producing organism (16, 205, 299). Several studies have found a relationship between third-generation cephalosporin use and acquisition of an ESBL-producing strain (16, 19, 116, 126, 164, 193, 196, 205, 210, 291, 302, 358). Other studies, which were underpowered to show statistical significance, showed trends towards such an association (the P values in all three studies were between 0.05 and 0.10) (103, 299, 419). Furthermore, a tight correlation has existed between ceftazidime use in individual wards within a hospital and prevalence of ceftazidime-resistant strains in those wards (341). In a survey of 15 different hospitals, an association existed between cephalosporin and aztreonam usage at each hospital and the isolation rate of ESBL-producing organisms at each hospital (329, 357).
Use of a variety of other antibiotic classes has been found to be associated with subsequent infections due to ESBL-producing organisms. These include quinolones (103, 205, 419), trimethoprim-sulfamethoxazole (103, 205, 419), aminoglycosides (19, 205), and metronidazole (205). Conversely, prior use of β-lactam/β-lactamase inhibitor combinations, penicillins, or carbapenems seems not to be associated with frequent infections with ESBL-producing organisms.
Nursing Homes and ESBL Producers
There is some evidence that nursing homes may serve as a portal of entry for ESBL-producing organisms into acute-care hospitals (54). Conversely, patients with hospital-acquired colonization or infection may return to their nursing home with ESBL carriage (39).
In a point prevalence study in the skilled care floor of a Chicago nursing home, 46% of residents were colonized with ESBL-producing organisms (all Escherichia coli) (419). These patients had been in the nursing home, without intercurrent hospitalization, for a mean of more than 6 months. Patients from this nursing home, as well as seven other nursing homes, served as a reservoir for introduction of ESBL-producing organisms into an acute-care hospital (419).
Within nursing homes, antibiotic use is a risk factor for colonization with ESBL-producing organisms. Antibiotic use is frequent in nursing homes; in one recent study, 38% of nursing home residents had taken a systemic antibiotic in the last month (375). Use of third-generation cephalosporins has been identified as a predisposing event in some (343), but not all studies (419). In contrast to the situation in acute-care hospitals, use of orally administered antibiotics (ciprofloxacin and/or trimethoprim-sulfamethoxazole may also be a risk for colonization with an ESBL-producing strain (419). Nursing home residents would appear to have several additional risk factors for infection with ESBL-producing organisms. They are prone to exposure to the microbial flora of other residents, especially if they are incontinent and require frequent contact with health care providers. It has been well documented that handwashing rates are low among nursing home personnel (111). Urinary catheterization and decubitus ulcers are frequent (375), and have been associated with colonization of non-ESBL-producing, antibiotic-resistant gram-negative bacilli (258, 368).
Community-Acquired Infections
A survey of more than 2,500 isolates of Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolated from nonhospitalized patients in France in 1993 revealed no truly community-acquired infections. Five out of 107 strains of Klebsiella pneumoniae produced ESBLs, but all were isolated from patients staying in a nursing home (145). However, in recent years there has been a wide variety of reports of true community-acquired infections with ESBL-producing organisms.
Several community-acquired pathogens that commonly cause diarrhea have been found to be ESBL producers. ESBL-producing Salmonella infections remain a concern in many parts of the world (3, 24, 26, 56, 135, 174, 201, 257, 303). Additionally, a Shigella flexneri islate from the stool of an Algerian child admitted to hospital with dysentery and harboring SHV-2 has been detected (130). SHV-11 has been detected in Shigella dysenteriae (1) as have a variety of TEM- and CTX-M-type ESBLs in Shigella sonnei (195, 284, 332). Finally, Vibrio cholerae isolates and Shiga toxin-producing Escherichia coli isolates which are ESBL producers have been found (172, 305).
In the last 3 years there have been several reports of true community-acquired infection or colonization with ESBL-producing Escherichia coli (42, 59, 88, 249, 261, 312, 345, 423). These reports have come from Spain, Israel, the United Kingdom, Canada, and Tanzania. Typically, patients have developed urinary tract infection with CTX-M-producing Escherichia coli. Some urinary tract infections have been associated with bacteremia. The majority of isolates have been resistant to commonly used first-line agents for urinary tract infection such as trimethoprim-sulfamethoxazole, ciprofloxacin, gentamicin, and ceftriaxone. Rodriguez-Bano, in Seville, Spain, performed a case-control study examining risk factors for ESBL-producing Escherichia coli infections in nonhospitalized patients and found that diabetes mellitus, prior quinolone use, recurrent urinary tract infections, prior hospital admissions, and older age were independent risk factors (345). Pitout, in Calgary, Canada, showed that 22 cases of ESBL-producing Escherichia coli infection occurred per year per 100,000 population greater than 65 years of age (312). The cause of this sudden upsurge in community-acquired infections with ESBL-producing organisms is not yet clear, but associations with foodstuffs, animal consumption of antibiotics, and frequent patient contact with health care facilities need to be explored.
INFECTION CONTROL IMPLICATIONS OF ESBL-PRODUCING ORGANISMS
Use of Typing Methods
Nosocomial bacterial infections are a major focus of concern for infection control programs. Such infections may occur as an outbreak (or epidemic) or may become established as a regular occurrence (endemic). It is important to be able to determine whether such nosocomial infections are caused by the same clone of organism (monoclonal or oligoclonal outbreaks) because this implies that the organisms are being passed horizontally by some means from patient to patient. This has important infection control implications in that some intervention should be introduced to prevent horizontal tranfer of organisms. Conversely, nosocomial infections with organisms of the same species which are not of the same clone (polyclonal outbreaks) may be due to selective pressure imposed by antibiotic use.
Before the advent of molecular biologic techniques to assess the genetic relationships between nosocomially acquired organisms, typing methods that assessed phenotypic differences between organisms were widely used. At least seven phenotypic methods could potentially be used to type Klebsiella pneumoniae isolates harboring ESBLs. These include biotyping (assessing the potential clonal relationship between organisms by way of observing common biochemical reactions, colonial morphology, or environmental tolerances) (18) and assessment of the antimicrobial susceptibility test pattern. Neither test has particularly good discriminatory power. Occasionally, stored isolates of organisms may lose transferrable genetic elements (for example, plasmids) which confer antibiotic resistance and appear to have a different antibiotic susceptibility pattern than when the isolate was examined fresh (242).
Serotyping is potentially useful in discriminating ESBL-producing klebsiellae. The klebsiellae typically express both lipopolysaccharide (O antigen) and capsule polysaccharide (K antigen) on the surface (159). Seventy-seven K antigen types form the basis of an internationally recognized capsule antigen scheme (280). The drawback of this method is the large number of serological cross-reactions that occur among the 77 capsule types. Thus, individual sera have to be absorbed with the cross-reacting K antigens. Moreover, the antisera are not commercially available and the typing procedure is cumbersome because of the time needed to perform the test. Finally the test is susceptible to subjectivity bacause of weak reactions that are not always easy to interpret (316). In contrast to the large number of capsular serotypes, only nine lipopolysaccharide O groups have been recognized. Since there are only nine O types compared with 77 K types, O typing is clearly less discriminatory than K typing. Furthermore, traditional methods of O typing are hampered by the heat stability of capsular polysaccharide (159). Recently, an inhibition enzyme-linked immunosorbent assay method has been developed which overcomes this technical problem (159). A number of studies have evaluated use of serotyping ESBL-producing klebsiellae using the K antigens (18, 32, 57, 62, 147, 153, 211, 363, 364, 439). No studies to date evaluated O types of ESBL-producing klebsiellae; however, a combination of K and O typing is likely to be a very discriminatory nonmolecular method of typing ESBL-producing klebsiellae.
Phage typing, bacteriocin typing, analytical isoelectric focusing, and multilocus enzyme electrophoresis are other methods which have been used to discriminate ESBL-producing strains (27, 175, 277, 316).
Since the vast majority of ESBLs are plasmid mediated, plasmid profile analysis has been applied to the epidemiologic study of ESBL-producing organisms. A simple method is to determine the number and size of the plasmids carried by the organism by preparing a plasmid extract and subjecting it to routine agarose gel electrophoresis. The reproducibility and discriminatory power of plasmid analysis can be improved by first digesting the plasmids with restriction enzymes and then performing agarose gel electrophoresis. This procedure and the analysis of the size and number of the resulting restriction fragments are referred to as restriction enzyme analysis of plasmids. A drawback in plasmid profile analysis is that plasmids may be lost after storage. It should be noted that plasmid extraction methods may yield different results if the efficiency of extraction is not optimal.
Most researchers use molecular methods to determine the relatedness of ESBL-producing organisms. Pulsed-field gel electrophoresis of chromosomal DNA is probably the most widely used method of genotyping ESBL-producing organisms (15, 17, 57, 68, 85, 113, 128, 132, 149, 207, 218, 222, 234, 235, 256, 268, 298, 328, 334, 341, 348, 351, 358, 374, 381, 408, 426, 431, 439). These references describe the restriction enzymes used for various organisms harboring ESBLs. Ribotyping, a southern blot analysis in which strains are characterized by the restriction fragment lengh polymorphisms associated with the ribosomal operons, is potentially very useful in typing ESBL-producing organisms, especially when automated ribotyping systems are used. Multiple variations of PCR have been applied to the typing of ESBL-producing organisms. These are randomly amplified polymorphic DNA, which is also known as arbitrarily primed PCR, and PCR based on repetitive chromosomal sequences. Of these, use of arbitrarily primed PCR has been by far the most popular method used to evaluate the genetic relatedness of ESBL-producing strains. The randomly amplified polymorphic method is based on the observation that short primers (around 10 base pairs), whose sequence is not directed to any known genetic locus, will regardless hybridize at random chromosomal sites with sufficient affinity to permit the initiation of polymerization. If two such sites are located within a few kilobases of each other on opposite DNA strands and in the proper orientation, amplification of the intervening fragment will occur. The number and locations of these random sites (and therefore the number and sizes of fragments) will vary among different strains of the same species (13).
Restriction site insertion PCR is a recently developed technique to detect mutations of the SHV genes to identify ESBLs. Restriction site insertion PCR uses amplification primers designed with one to three base mismatches near the 3′ end to engineer a desired restriction site. Chanawong et al. (80) demonstrated that the combination of PCR-restriction fragment length polymorphism and restriction site insertion PCR techniques can be readily applied to the epidemiological study of SHV β-lactamases. Using the combination of the techniques, eight different β-lactamases were reliably distinguished. Another useful tool for the detection of certain SHV variants is the combination of PCR-single strand conformational polymorphism and PCR-restriction fragment length polymorphism (79). Using PCR-single strand conformational polymorphism and PCR-restriction fragment length polymorphism with DdeI and NheI digestion, the genes encoding SHV-1, SHV-2a, SHV-3, SHV-4, SHV-5, SHV-11, and SHV-12 were distinguisable (79).
Ligase chain reaction is a recently developed technique also used to discriminate SHV variants. Ligase chain reaction uses a thermostable ligase and biotinylated primers. It can detect single base pair changes (194). Kim and Lee evaluated seven Escherichia coli strains known to produce different SHV enzymes and 46 clinical isolates, and found that ligase chain reaction typing simply and rapidly defined the SHV types (194).
A recently described method marries the sensitivity of PCR with fluorescently labeled probes. Randeggar and Haechler developed a technique using real-time PCR monitored with fluorescently labeled hybridization probes followed by melting curve analysis (335). Their technique was able to differentiate SHV variants in five well-characterized Escherichia coli strains and six clinical isolates, and to discriminate between non- ESBLs and ESBLs. It remains to be seen whether this technique, termed the SHV melting curve mutation detection method, will identify all SHV variants.
Modes of Spread of ESBL-Producing Organisms within Hospitals
How do ESBL-producing organisms spread, given the ample documentation of clonal outbreaks as described above? A common environmental source of ESBL-producing organisms has occasionally been discovered. Examples have included contamination of ultrasonography coupling gel (132), bronchoscopes (57), blood pressure cuffs (64), and glass thermometers (used in axillary measurement of temperature) (346). Cockroaches have been implicated as possible vectors of infection; in one recent study, ESBL-producing Klebsiella pneumoniae isolated from cockroaches was indistinguishable from that infecting patients (93). ESBL-producing organisms have been isolated from patients' soap (387), sink basins (166), and babies' baths (121), but the contribution of this environmental contamination to infection was impossible to determine.
Present evidence suggests that transient carriage on the hands of health care workers is a more important means of transfer from patient to patient. Hand carriage has been documented by most (121, 166, 348) but not all (339, 387) investigators who have sought it. In these instances, the hand isolates were genotypically identical to isolates which caused infection in patients. Hand carriage by health care workers is usually eliminated by washing with chlorhexidine or alcohol-based antiseptics. However, the authors know of one example of prolonged, persistent skin carriage in a nurse with chronic dermatitis. The use of artificial nails may also promote long-term carriage and has been associated with at least one outbreak (152). Gastrointestinal tract carriage has been documented in health care workers (131, 166, 378), but is astonishingly rare and seldom prolonged, except with ESBL-producing Salmonella species (157, 247).
The hands of health care workers are presumably colonized by contact with the skin of patients whose skin is colonized with the organism (131). It is important to recognize that many patients may have asymptomatic colonization with ESBL-producing organisms without signs of overt infection. These patients represent an important reservoir of organisms. For every patient with clinically significant infection with an ESBL-producing organism, at least one other patient exists in the same unit with gastrointestinal tract colonization with an ESBL producer (161, 222). In some hyperendemic intensive care units and transplants units, 30 to 70% of patients have gastrointestinal tract colonization with ESBL producers at any one time (150, 299, 378).
Since gastrointestinal tract colonization with klebsiellae occurs in every person, selective media must be used in order to assess the carriage of ESBL producers in stool. Examples of such media include Drigalski agar supplemented with cefotaxime 0.5 mg/liter (378), MacConkey agar supplemented with ceftazimide 4 mg/liter (299) and nutrient agar supplemented with ceftazimide 2 mg/liter, vancomycin 5 mg/liter, and amphotericin B 1,667 mg/liter (150). Prior gastrointestinal carriage of ESBL-producing Klebsiella pneumoniae is an independent variable associated with infection with ESBL-producing Klebsiella pneumoniae (298). At least 80% of patients with infection with ESBL-producing Klebsiella pneumoniae can be documented to have prior gastrointestinal tract carriage (222, 298). Patients who develop infection usually do so within weeks of acquiring gastrointestinal tract colonization (range, 0 to 90 days) (222, 298). There does not appear to be any variation in particular kinds of infection (bacteremia, pneumonia, etc.) and incidence of prior gastrointestinal tract colonization (298).
Infection Control When ESBL-Producing Organisms Have Not Been Endemic
In some hospitals, initial outbreaks of infection have been supplanted by endemicity of the ESBL-producing organisms (20, 223, 246, 334). This may lead to increased patient mortality when antibiotics inactive against ESBL producers are used (358). As a consequence, when a significant proportion of gram-negative isolates in a particular unit are ESBL producers, empirical therapy may change towards use of imipenem, quinolones, or β-lactam/β-lactamase inhibitor combinations. In some centers this has been associated with emergence of resistance in Pseudomonas aeruginosa, Acinetobacter baumanii, and in ESBL-producing organisms themselves (246, 334). Control of endemic ESBL producers is difficult, and may only be possible after significant nursing and medical reorganization, at substantial financial cost (223, 334).
Therefore, control of the initial outbreak of ESBL-producing organisms in a hospital or specialized unit of a hospital is of critical importance (Table 1).
TABLE 1.
Infection control interventions appropriate to controlling spread of ESBL-producing organisms within a hospital
| Identify patients infected with ESBL-producing organisms by use of appropriate detection methods in the clinical microbiology laboratory |
| Identify colonized patients by use of rectal swabs plated onto selective media |
| Perform molecular epidemiologic analysis of strains from infected or colonized patients (for example, by use of pulsed-field gel electrophoresis) |
| Institute contact isolation precautions, particularly if clonal spread is demonstrated |
| Institute controls on antibiotic use, particularly if numerous strain types are demonstrated |
The initial stages of the infection control program in a hospital or unit which has not previously been affected by ESBLs should therefore include (i) performance of rectal swabs to delineate patients colonized (but not infected) with ESBL producers, (ii) evaluation for the presence of a common environmental source of infection, (iii) a campaign to improve hand hygiene, and (iv) introduction of contact isolation for those patients found to be colonized or infected (294).
Although common environmental sources of infection have rarely been discovered, when they are recognized their impact on arresting an outbreak of infection with a multiresistant organism can be dramatic. Three examples of such an intervention have been described in the context of controlling outbreaks of infection with ESBL-producing organisms. Gaillot (132) found that contaminated gel used for ultrasonography was contaminated with ESBL-producing organisms. Replacement of this gel quickly curtailed the outbreak. Branger (57) found that a poorly maintained bronchoscope was colonized with ESBL-producing organisms and could be linked to respiratory tract infections with the same strain. Repair and proper maintenance of the bronchoscope stopped nosocomial transmission of the organism. Finally, Rogues (346) found colonization of four of 12 glass mercury thermometers with ESBL-producing Klebsiella pneumoniae and axillary colonization with the same strain in two patients. Disinfection of the thermometers curtailed the outbreak.
Contact isolation implies use of gloves and gowns when contacting the patient. Several studies have documented that this practice alone can lead to reduction in horizontal spread of ESBL-producing organisms. However, compliance with these precautions needs to be high in order to maximize the effectiveness of these precautions. Furthermore, we recommend that patients who have gastrointestinal tract colonization as well as those with frank infection should undergo contact isolation. It has been noted that standard methods of hand washing, screening for colonization, and patient isolation may not always be effective in controlling outbreaks of ESBL-producing organisms (230). Macrae and colleagues (230) were forced to close a ward tempoararily in order to adequately control an outbreak which had been unresponsive to conventional measures.
A number of groups have previously attempted selective digestive decontamination as a means of interrupting transmission of ESBL-producing organisms. Erythromycin-based therapies have not been effective (107, 110). However, three groups successfully used selective digestive decontamination with polymyxin, neomycin, and nalidixic acid (63), colistin and tobramycin (388), or norfloxacin (294) to interrupt outbreaks of infection with ESBL-producing infections that had not been completely controlled using traditional infection control measures. It should be noted that in many hospitals at least 15 to 30% of ESBL-producing organisms (20, 183, 206, 292, 435) are quinolone resistant and therefore unlikely to suppressed by use of norfloxacin prophylaxis. Additionally, multidrug-resistant isolates are unlikely to respond to selective digestive decontamination using aminoglycosides.
An alternative approach to digestive tract decolonization has been decolonization of the nasopharynx. A recent study has utilized a nasal spray with povidone-iodine as a means of decolonizing the upper respiratory tract (167). In this study (performed in a neurologic rehabilitation unit), only 1 of 10 patients had gastrointestinal carriage with an ESBL-producing organism but all had nasotracheal colonization. Upper airway decolonization led to management of an outbreak (167).
Infection Control When ESBL-Producing Organisms Are Already Endemic
In many hospitals, ESBL-producing organisms are already endemic. Is there any hope of controling ESBL producers in such a setting? A review of the literature reveals that some degree of control can be achieved. The importance of achieving control is underscored by the advent of carbapenem-resistant organisms in hospitals where carbapenem use is very high owing to endemicity of ESBL producers.
The methods used to achieve control over endemic ESBL producers have included close attention to practices that may lead to breakdowns in good infection control (223). This includes a review of procedures that lead to nurses, physicians and ancillary staff (e.g., radiography technicians) failing to use contact isolation precautions (223). Although infection control procedures continue to play a central role, changes in antibiotic policy may play an even greater role in this setting (352). Indeed in one institution, no effort was made to change infection control procedures (341). Instead, at this hospital, ceftazidime use decreased and piperacillin-tazobactam was introduced in the formulary. A number of authors have shown that ceftazidime restriction alone is insufficient to control continued infections with ESBL-producing organisms (334, 343, 387). Rahal et al. (334) were forced to withdraw cephalosporins as an entire class in order to exact control over endemic ESBL producers.
Some authors have suggested that use of β-lactam/β-lactamase inhibitor combinations, rather than cephalosporins, as workhorse empirical therapy for infections suspected as being due to gram-negative bacilli, may facilitate control of ESBL producers (296, 311, 341). The mechanism by which these drugs may reduce infections with ESBL producers is not certain. It should be noted, however, that many organisms now produce multiple β-lactamases (24, 53, 81, 366), which may reduce the effectiveness of β-lactam/β-lactamase inhibitor combinations. It remains to be shown whether a strategy based on the empiric use of carbapenems would be effective.
DETECTION OF ESBLs IN THE CLINICAL MICROBIOLOGY LABORATORY
Need for Clinical Microbiology Laboratories To Detect ESBL Production by Members of the Enterobacteriaceae
Many clinical microbiology laboratories make no effort to detect ESBL production by gram-negative bacilli, or are ineffective at doing so (389). In a 1998 survey of 369 American clinical microbiology laboratories, only 32% (117 of 369) reported performing tests to detect ESBL production by Enterobacteriaceae (77). A subsequent survey of laboratory personnel at 193 hospitals actively participating in the National Nosocomial Infections Surveillance system showed that only 98 (51%) correctly reported a test organism as an ESBL producer (155). In a study from Europe, just 36% of 91 ESBL-producing klebsiellae were reported by their original clinical laboratories as cefotaxime resistant (20).
ESBL detection originated because some ESBL-producing organisms appeared susceptible to cephalosporins using conventional breakpoints. How frequently are ESBL-producing organisms susceptible to cephalosporins? The answer to this question depends on which breakpoints are used. National differences are quite considerable. For example, susceptibility to cefotaxime may be reported for an organism with MICs ranging from ≤1 μg/ml to ≤8 μg/ml, depending on the country. Variation in reporting of resistance is from ≥2 μg/ml to ≥64 μg/ml. The most liberal interpretation of cephalosporin susceptibility has been that of the Clinical and Laboratory Standards Institute (CLSI, formerly National Committee for Clinical Laboratory Standards) (cephalosporin susceptibility indicated by MICs ≤ 8 μg/ml) (267) (Table 2). The CLSI breakpoints for susceptibility of members of the Enterobacteriaceae to extended-spectrum cephalosporins and aztreonam were developed in the early 1980s. At that time the clinical success rate of cephalosporin treatment for organisms with cephalosporin MICs of ≤8 μg/ml (that is, organisms with MICs in the susceptible range) was >95% (289). Unfortunately these breakpoints were developed at a time that was essentially prior to the advent of ESBLs.
TABLE 2.
Comparison of national MIC breakpoints for Enterobacteriaceae
| Country | MIC breakpointa (μg/ml)
|
|||
|---|---|---|---|---|
| Cefotaxime
|
Ceftazidime
|
|||
| S (≤) | R (≥) | S (≤) | R (≥) | |
| United States of America (CLSI) | 8 | 64 | 8 | 32 |
| United Kingdom | 1 | 2 | 2 | 4 |
| France | 4 | 32 | 4 | 32 |
| The Netherlands | 4 | 16 | 4 | 16 |
| Germany | 2 | 8 | 4 | 32 |
| Spain | 1 | 8 | 1 | 8 |
| Norway | 2 | 16 | 2 | 16 |
| Sweden | 4 | 32 | 4 | 16 |
S, susceptible; R, resistant.
In a review of studies which have evaluated collections of ESBL-producing organisms using standard CLSI disk diffusion or MIC breakpoints, 13 to 49% of isolates were cefotaxime susceptible, 36 to 79% ceftriaxone susceptible, 11 to 52% ceftazidime susceptible, and 10 to 67% aztreonam susceptible. Approximately 40% tested susceptible to at least one oxyimino β-lactam and 20% to all oxyimino β-lactams (91, 94, 165, 179, 289, 393, 409). The reasons for this apparent susceptibility to some cephalosporins is the result of various degrees of hydrolysis of cephalosporins by different β-lactamases and enhanced penetration through the bacterial outer membrane of some cephalosporins compared to others. Regardless, extended-spectrum cephalosporin MICs of 2 to 8 μg/ml are 4 to 8 dilutions higher than those seen in the same strain producing only the parent TEM-1, TEM-2, or SHV-1 β-lactamase (0.03 to 0.25 μg/ml) (308).
It has been well recognized for some time that poor outcome occurs when patients with serious infections due to ESBL-producing organisms are treated with cephalosporins to which the organism is frankly resistant. The failure rate in such patients has ranged from 42 to 100% (246, 358). Similar failure rates exist when cephalosporins are used to treat patients with serious infections due to ESBL producers which have cephalosporin MICs in the intermediate range and even with some MICs in the susceptible range (95, 164, 289). The failure rate when cephalosporins were used for serious infections (bacteremia, hospital-acquired pneumonia, peritonitis) with ESBL-producing organisms with MICs for the treating cephalosporin of 4 to 8 μg/ml exceeds 90% (95, 196, 289). The failure rate when MICs for the treating cephalosporin were ≤2 μg/ml is substantially lower (95, 196, 289).
Two opposing viewpoints have arisen in recognition of the poor outcome when patients with an infection due to an ESBL-producing organism are treated with a cephalosporin to which it appears susceptible in vitro. For many years, this has been regarded as the reason why ESBL detection in necessary. Others maintain, in this era of production of multiple different β-lactamase types by a single bacterial isolate and production of ESBLs by organisms that constituitively produce AmpC β-lactamases, that detection of ESBLs is too complex for clinical microbiology laboratories. These investigators maintain that alteration of cephalosporin breakpoints for Enterobacteriaceae by organizations such as the Clinical and Laboratory Standards Institute is a more appropriate endeavor than expanding efforts to detect ESBLs. The concept that MIC matters more than the presence or absence of an ESBL is consistent with unpublished stochastic models based on the pharmacokinetics/pharmacodynamics of cephalosporins versus the Enterobacteriaceae. An advantage of such a change would be that organisms such as Enterobacter spp. which are not currently considered in CLSI guidelines for ESBL detection would be covered.
There are a number of opposing arguments to this view. It has been proposed that a potential confounder to the observation that MIC is the key determinant of outcome is that severity of illness could have been greater in patients infected with organisms with higher MICs. It has also been stated that change in cephalosporin breakpoints would require substantial effort by antimicrobial susceptibility testing method manufacturers. Another viewpoint is that the inoculum effect is important for ESBL-producing organisms and that the MIC alone may give erroneous information. In vitro, the MICs of cephalosporins rise as the inoculum of ESBL-producing organisms increases (184, 244, 344, 391). For example, for a Klebsiella pneumoniae strain producing TEM-26, at an inoculum of 105 CFU/ml the cefotaxime MIC was 0.25 μg/ml, rising to 64 μg/ml when the inoculum rose to 107 CFU/ml (391). Some, but not all, animal studies have demonstrated that the inoculum effect may be clinically relevant. In animal models of infection, failure of cephalosporin therapy has been demonstrated despite levels of antibiotics in serum far exceeding the MIC of the antibiotic when tested at the conventional inoculum of 105 CFU/ml (127, 344).
Those in favor of changing cephalosporin breakpoints dispute that the inoculum effect is important (231). For example, ESBL production did not have an impact upon the time above MIC necessary for cefepime efficacy in a murine thigh infection model (D. Andes and W. A. Craig, Program and Abstracts of the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, abstract A-1099, 2001).
A final argument in favor of ongoing efforts aimed at ESBL detection is the infection control significance of detecting plasmid-mediated multidrug resistance. There are epidemologic implications for the detection of ESBL-producing organisms in that this resistance issue may not be as apparent if organisms are simply reported as intermediate or resistant to individual cephalosporins. As noted above, outbreaks of ESBL-producing organisms can be abruptly halted using appropriate infection control interventions. Endemic transmission of ESBL producers can also be curtailed using infection control measures and antibiotic management interventions. Detection of ESBL production in organisms from samples such as urine may be important because this represents an epidemiologic marker of colonization (and therefore the potential for transfer of such organisms to other patients).
At the time of writing, it is still recommended by the CLSI that clinical microbiology laboratories perform specialized tests for detection of ESBLs. For this reason, ESBL detection methods are discussed in some detail below. It is uncertain whether cephalosporin breakpoints will change. Our opinion is that change in cephalosporin breakpoints will be necessary: existing data suggest that there is a high chance of clinical failure if the MIC of the cephalosporin used in treatment is 4 to 8 μg/ml, regardless of the dosing regimen used. We would still favor additional use of ESBL detection methods. There are two reasons for this. First, there is value of the knowledge of presence of ESBLs in terms of aiding infection control. Second, in the presence of high-inoculum infections (for example, intra-abdominal abscess, some cases of pneumonia) or infections at sites in which drug penetration may be poor (for example, meningitis, endocarditis, or osteomyelitis), physicians should avoid cephalosporins if an ESBL-producing organism is present.
CLSI Recommended Methods for ESBL Detection
Screening for ESBL producers. (i) Disk diffusion methods.
The CLSI has proposed disk diffusion methods for screening for ESBL production by klebsiellae, Escherichia coli, and Proteus mirabilis. Laboratories using disk diffusion methods for antibiotic susceptibility testing can screen for ESBL production by noting specific zone diameters which indicate a high level of suspicion for ESBL production (267). Cefpodoxime, ceftazidime, aztreonam, cefotaxime, or ceftriaxone is used. However, the use of more than one of these agents for screening improves the sensitivity of detection. If any of the zone diameters indicate suspicion for ESBL production, phenotypic confirmatory tests should be used to ascertain the diagnosis.
Mention should be made of the use of cefpodoxime as a screening antibiotic since this antibiotic is not widely used in inpatient facilities. In 1995, Thomson noted that cefpodoxime susceptibility by disk diffusion reliably discriminated between ESBL-producing and non-ESBL-producing Klebsiella pneumoniae and Escherichia coli (394). The CLSI initially recommended a zone diameter of ≤22 mm for a 10-μg cefpodoxime disk as a suitable screening test for ESBL production. Unfortunately, the cefpodoxime screening test using a zone diameter of ≤22 mm lacks specificity when used to screen Escherichia coli isolates for ESBL production (390). Therefore, the CLSI now recommends a change in the cefpodoxime screening breakpoint to ≤17 mm (267); that is, isolates with a cefpodoxime zone diameter of ≤17 mm should undergo phenotypic confirmatory tests for ESBL production.
(ii) Screening by dilution antimicrobial susceptibility tests.
The CLSI has proposed dilution methods for screening for ESBL production by klebsiellae and Escherichia coli. Ceftazidime, aztreonam, cefotaxime, or ceftriaxone can be used at a screening concentration of 1 μg/ml (267). Growth at this screening antibiotic concentration (that is, MIC of the cephalosporin of ≥2 μg/ml) is suspicious of ESBL production and is an indication for the organism to be tested by a phenotypic confirmatory test.
The originally proposed screening criterion for cefpodoxime was that isolates which were potential ESBL producers had cefpodoxime MIC of ≥2 μg/ml. However, in a study of the mechanisms of decreased susceptibility of Escherichia coli to cefpodoxime, Oliver et al. (279) found that none of 59 strains with cefpodoxime MICs of 2 or 4 μg/ml produced ESBLs. The most common mechanism of reduced susceptibility to cefpodoxime was production of the TEM-1 β-lactamase associated with the loss or alteration of a major porin protein. Other strains lacked production of TEM-1 but had porin changes, sometimes coupled with modest elevation in production of the AmpC chromosomal β-lactamase. Finally, some of the isolates produced the OXA-30 β-lactamase (279). There are neither reports of clinical failure nor of outbreaks of infection with ESBL-negative strains of Escherichia coli with cefpodoxime MICs ≥ 2 μg/ml. Therefore a more clinically useful screening test for cefpodoxime is to use a cefpodoxime MIC of ≥8 μg/ml, as a trigger to perform phenotypic confirmatory tests for ESBL production (267).
Phenotypic Confirmatory Tests for ESBL Production
Cephalosporin/clavulanate combination disks.
The CLSI advocates use of cefotaxime (30 μg) or ceftazidime disks (30 μg) with or without clavulanate (10 μg) for phenotypic confirmation of the presence of ESBLs (267) in klebsiellae and Escherichia coli. Disks for use in phenotypic confirmatory tests are available from several suppliers (Becton Dickinson, Oxoid, and MAST). (Prior to the combination disks becoming available, it was recommended that clavulanic acid solution be applied to the cephalosporin disks within one hour before they are applied to the agar plates.) The CLSI recommends that the disk tests be performed with confluent growth on Mueller-Hinton agar. A difference of ≥5 mm between the zone diameters of either of the cephalosporin disks and their respective cephalosporin/clavulanate disk is taken to be phenotypic confirmation of ESBL production (267).
In an evaluation of 139 Klebsiella pneumoniae isolates that met the National Committee for Clinical Laboratory Standards screening criteria for potential ESBL production, Steward and colleagues (379) found that 84% (117 of 139) met the criteria for a positive phenotypic confirmatory test. Of the 117 isolates, 104 (89%) met the criteria for a positive phenotypic confirmatory test with both ceftazidime and cefotaxime, 11 (9%) with ceftazidime only and 2 (2%) with cefotaxime only. It should be noted that all of these isolates were from the United States. It should be emphasized that both cefotaxime and ceftazidime with and without clavulanate should be used. One reason for this is that the use of ceftazidime alone has resulted in the inability to detect CTX-M-producing organisms (58).
Broth microdilution.
Phenotypic confirmatory testing can also be performed by broth microdilution assays using ceftazidime (0.25 to 128 μg/ml), ceftazidime plus clavulanic acid (0.25/4 to 128/4 μg/ml), cefotaxime (0.25 to 64 μg/ml), and cefotaxime plus clavulanic acid (0.25/4 to 64/4 μg/ml). Again it should be emphasized that both ceftazidime and cefotaxime should be used (330). Broth microdilution is performed using standard methods. Phenotypic confirmation is considered as a ≥3-twofold-serial-dilution decrease in MIC of either cephalosporin in the presence of clavulanic acid compared to its MIC when tested alone.
Hadziyannis (154) evaluated a set of 12 isolates with well-characterized β-lactamases using this method. Five isolates with known ESBLs and five without ESBLs were correctly identified. There was one false-positive, a Klebsiella pneumoniae isolate lacking ESBLs but hyperproducing SHV-1 with a ceftazidime MIC of 4 μg/ml reducing to ≤0.025 μg/ml with ceftazidime/clavulanate. One Klebsiella oxytoca isolate which hyperproduced the chromosomal K1 β-lactamase and was resistant to aztreonam was negative for ESBL production using the criteria above.
Steward and colleagues (379), in their analysis of 139 Klebsiella pneumoniae isolates with ceftazidime or cefotaxime MICs of ≥2 μg/ml, found that the definition for a positive phenotypic confirmatory test using broth microdilution was met in 114 (82%) of isolates. Of the 114 isolates, 108 (95%) met the criteria for a positive phenotypic confirmatory test with both ceftazidime and cefotaxime and 6 (5%) with ceftazidime only. For five of the 139 isolates, the results were indeterminate because no ratio of ceftazidime to ceftazidime/clavulanic acid MICs was calculable. Such a situation arises when the ceftazidime MIC was above the highest dilution tested and the ceftazidime plus clavulanic acid MICs were 64 μg/ml or greater. Testing cefepime and cefepime plus clavulanic acid (a test not specified in CLSI guidelines), or utilizing CLSI ESBL disk diffusion confirmatory tests, may sometimes determine whether a clavulanic acid effect truly occurs in such isolates (379).
Steward and colleagues have devised a useful algorithm for ESBL testing using phenotypic methods (379). They suggest using cefoxitin susceptibility in isolates with positive screening tests but negative confirmatory tests as a means of deducing the mechanism of resistance. Cefoxitin resistant isolates may produce AmpC-type enzymes or possess porin changes, although it must be recognized that these can coexist with ESBL production.
Quality control when performing screening and phenotypic confirmatory tests.
Quality control recommendations are that simultaneous testing with a non-ESBL-producing organism (Escherichia coli ATCC 25922) and an ESBL-producing organism (Klebsiella pneumoniae ATCC 700603) also be performed. Control limits for these organisms are widely available (267). The ESBL control organism, Klebsiella pneumoniae K6 (ATCC 700603), has been recently characterized; it produces SHV-18 and has lost the OmpK35 and OmpK37 porins (336).
Implications of positive phenotypic confirmatory tests.
According to CLSI guidelines, isolates which have a positive phenotypic confirmatory test should be reported as resistant to all cephalosporins (except the cephamycins, cefoxitin, and cefotetan) and aztreonam, regardless of the MIC of that particular cephalosporin. For example, an isolate with a ceftazidime MIC of >256 μg/ml, cefotaxime MIC of 4 μg/ml and cefepime MIC of 2 μg/ml, which has a positive phenotypic confirmatory test should be reported as resistant to ceftazidime, cefotaxime and cefepime, when CLSI guidelines are followed. Penicillins (for example, piperacillin or ticarcillin) are reported as resistant regardless of MIC, but β-lactam/β-lactamase inhibitor combinations (for example, ticarcillin/clavulanate or piperacillin-tazobactam) are reported as susceptible if MICs or zone diameters are within the appropriate range.
False positives and false negatives obtained with phenotypic confirmatory tests.
The phenotypic confirmatory tests are highly sensitive and specific compared to genotypic confirmatory tests. However, there are a number of instances whereby the phenotypic confirmatory tests may be falsely positive or negative.
Klebsiella pneumoniae or Escherichia coli isolates which lack ESBLs but which hyperproduce SHV-1 may give false-positive confirmatory tests. Such isolates can have ceftazidime MICs as high as 32 μg/ml (252, 304, 340). Rice and colleagues (340) characterized one such organism where a single base pair change in the promoter sequence resulted in increased production of chromosomally encoded SHV-1. Additionally, outer membrane protein (OMP) analysis revealed a decrease in the quantity of a minor 45-kDa outer membrane protein. There are limited clinical data to determine whether cephalosporins can be successfully used in patients with such non-ESBL-producing organisms. Wu and colleagues described an outbreak in a pediatric ward in which Klebsiella pneumoniae isolates without ESBLs had positive confirmatory tests (424). The bacteria were subsequently shown to produce TEM-1 and SHV-1 and be deficient in the outer membrane protein OmpK35. Eight bacteremic patients were successfully treated with third-generation cephalosporins, for which MICs were 1 μg/ml or lower (424).
There are now numerous reports in which Klebsiella pneumoniae isolates have been found to harbor plasmid-mediated AmpC-type β-lactamases. Some of these organisms have been found to harbor both AmpC-type β-lactamases and ESBLs (401). The coexistence of both enzyme types in the same strain results in elevated cephalosporin MICs but may result in false negative tests for the detection of ESBLs. The likely explanation is that AmpC-type β-lactamases resist inhibition by clavulanate and hence obscure the synergistic effect of clavulanate and cephalosporins against ESBLs. The relative amount of each β-lactamase required to obscure the presence of the other is not known.
Queenan and colleagues evaluated the performance of broth microdilution screening and confirmatory tests at inocula within 0.5 log unit of the standard inoculum (330). False negative results occurred with both screening and confirmatory tests when lower inocula were used.
Commercially Available Methods for ESBL Detection
Etest for ESBLs.
AB Biodisk (Solna, Sweden) produces plastic drug-impregnated strips, one end of which contains a gradient of ceftazidime (MIC test range 0.5 to 32 μg/ml) and the other with a gradient of ceftazidime plus a constant concentration of clavulanate (4 μg/ml). Similar strips containing cefotaxime and cefotaxime/clavulanate are now available. These strips are useful for both screening and phenotypic confirmation of ESBL production. The reported sensitivity of the method as a phenotypic confirmatory test for ESBLs is 87 to 100% (61, 91, 165, 409) and the specificity is 95 to 100%. The sensitivity and specificity of the method depend on the ratio of MICs of the cephalosporin versus cephalosporin/clavulanate combination used -the manufacturer currently recommends a ≥8-fold reduction in cephalosporin MICs in the presence of clavulanate. Occasionally the MIC of the cephalosporin alone is difficult to read because the inhibition zone is distorted by the clavulanic acid diffusing from the opposite ends of the strip. In these cases, some prefer to measure the MIC of the cephalosporin alone using a separate conventional strip containing only ceftazidime or cefotaxime.
The availability of cefotaxime as well as ceftazidime strips improves the ability to detect ESBL types which preferentially hydrolyze cefotaxime such as CTX-M-type enzymes.
Vitek ESBL cards.
Laboratories using conventional Vitek cards risk incorrectly reporting ESBL-producing organisms as susceptible to cephalosporins when MICs are ≤8 μg/ml (380). A specific card which includes tests for ESBL production has now been FDA approved. The Vitek ESBL test (bioMerieux Vitek, Hazelton, Missouri) utilizes cefotaxime and ceftazidime, alone (at 0.5 μg/ml), and in combination with clavulanic acid (4 μg/ml). Inoculation of the cards is identical to that performed for regular Vitek cards. Analysis of all wells is performed automatically once the growth control well has reached a set threshold (4 to 15 h of incubation). A predetermined reduction in the growth of the cefotaxime or ceftazidime wells containing clavulanic acid, compared with the level of growth in the well with the cephalosporin alone, indicates a positive result. Sensitivity and specificity of the method exceed 90% (355).
The principal advantage of the Vitek ESBL card is that it can be easily integrated into the workflow of laboratories already using the Vitek system. False-negative results have been observed in Klebsiella pneumoniae isolates producing both an ESBL and an AmpC-type β-lactamase (401). Given that neither the sensitivity nor specificity of the test is 100%, some laboratories perform additional phenotypic confirmatory tests in isolates that lack cephalosporin susceptibility but are negative by the Vitek ESBL test or in isolates called positive by the Vitek ESBL test (248). The reliability of the test to detect ESBLs in species other than Klebsiella pneumoniae, Klebsiella oxytoca, and Escherichia coli is unknown.
The Vitek 2 system has been evaluated for its ability to detect ESBL production. Leverstein-van Hall and colleagues found that a high percentage of indeterminate test results occurred (212), but Livermore and colleagues found that overall agreement with reference methods exceeded 90% (220).
MicroScan panels.
Dade Behring MicroScan (Sacramento, Calif.) produces dehydrated panels for microdilution antibiotic susceptibility testing. In a study of the MicroScan Gram-Negative Urine MIC 7 and Gram-Negative MIC Plus 2 panels, read by the Walkaway system, the panels reliably indicated the presence of ESBL production (255). In the field, MicroScan conventional panel users reliably report ESBL producers as ceftazidime resistant but are less likely to report cefotaxime or ceftriaxone as resistant (380). Use of MicroScan panels in conjunction with the Wider system for determination of antibiotic susceptibilities in a series of 100 clinical Enterobacteriaceae isolates with characterized resistance mechanisms has been studied (69). (The Wider system is a newly developed computer-assisted image-processing device which can analyze commercial bacterial identification and susceptibility testing panels.) In comparison with reference broth microdilution results, the Wider system had a 0.8% very major error rate, 2.0% major error rate, and 3.5% minor error rate (69).
MicroScan panels which contain combinations of ceftazidime or cefotaxime plus β-lactamase inhibitors have received Food and Drug Administration approval and in studies of large numbers of ESBL-producing isolates they have appeared highly reliable (199, 383, 395).
BD Phoenix Automated Microbiology System.
Becton Dickinson Biosciences (Sparks, Md) have introduced a short-incubation system for bacterial identification and susceptibility testing, known as BD Phoenix (212, 356, 384). The Phoenix ESBL test uses growth response to cefpodoxime, ceftazidime, ceftriaxone and cefotaxime, with or without clavulanic acid, to detect the production of ESBLs. The test algorithm has been delineated by Sanguinetti et al. (356). Results are usually available within 6 hours.
Three European studies have assessed the BD Phoenix ESBL detection method in evaluations of its ability to detect a variety of ESBL types (predominantly isolates producing SHV-2, SHV-5 or SHV-12) (212, 356, 384). The only CTX-M-type ESBL observed in isolates in these studies was CTX-M-1. The BD Phoenix ESBL detection method detected ESBL production in greater than 90% of strains genotypically confirmed to produce ESBLs. The method correctly detected ESBL production by Enterobacter, Proteus, and Citrobacter spp., in addition to klebsiellae and Escherichia coli (356).
Other Methods for ESBL Detection
Cephalosporin/clavulanate combination disks on Iso-Sensitest agar.
The British Society for Antimicrobial Chemotherapy has recommended the disk-diffusion method for phenotypic confirmation of ESBL presence using ceftazidime/clavulanate and cefotaxime/clavulanate combination disks, although recommends that the test be performed with semiconfluent growth on Iso-Sensitest agar (rather than confluent growth on Mueller-Hinton agar). Using this method, M'Zali et al. (265) compared the zone diameters of each combination compared with those of the cephalosporin alone, and calculated a ratio of cephalosporin/clavulanate zone size divided by cephalosporin zone size. A ratio of 1.5 or greater was taken to signify the presence of ESBL activity. Using this method, the sensitivity of the test for detecting ESBLs was 86% using the disks containing ceftazidime and 65.5% for the disks containing cefotaxime. If results from both antibiotics were taken into consideration, the sensitivity increased to 93%. The test did not detect ESBL production by strains producing SHV-6.
Carter et al. (73) have evaluated a cefpodoxime/clavulanate combination disk and compared zone sizes with those obtained with cefpodoxime alone. 180 known ESBL producers gave a zone size at least 5 mm larger with the combination disks (mean difference 11.6 mm), while none of 50 ESBL negative control isolates gave a difference in zone diameter of more than 1 mm.
Double-disk diffusion test.
In the late 1980s, French investigators (62, 182) described a disk diffusion test in which synergy between cefotaxime and clavulanate was detected by placing a disk of amoxicillin/clavulanate (20 μg/10 μg, respectively) and a disk of cefotaxime (30 μg), 30 mm apart (center to center) on an inoculated agar plate. A clear extension of the edge of the cefotaxime inhibition zone toward the disk containing clavulanate was interpreted as synergy, indicating the presence of an ESBL; 30-μg antibiotic disks of ceftazidime, aztreonam, and ceftriaxone are also placed on the plate, 30 mm (center to center) from the amoxicillin/clavulanate disk, since sometimes this keyhole effect is not observed with cefotaxime but is with other β-lactam antibiotics containing the oxyimino group.
Evaluation of the double-disk diffusion test against strains which have been genotypically confirmed to be ESBL producers or non producers have revealed sensitivities of the method ranging from 79% to 97% and specificities ranging from 94% to 100% (31, 61, 165, 229, 393, 409). False-negative results have been observed with isolates harboring SHV-2 (31, 393), SHV-3 (165), and TEM-12 (409). In isolates which are suspicious for harboring ESBLs but are negative using the standard distance of 30 mm between disks, the test should be repeated using closer (for example, 20 mm) or more distant (for example, 40 mm) spacing (165, 393).
A major advantage of the double-disk diffusion test is that is technically simple. However, the interpretation of the test is quite subjective. Sensitivity may be reduced when ESBL activity is very low, leading to wide zones of inhibition around the cephalosporin and aztreonam disks. This has been noted for Proteus mirabilis (409). The test, like other inhibitor-based tests, may give an erroneous impression of the presence of ESBLs in organisms such as Stenotrophomonas maltophilia (262). A falsely positive test occurs for this organism because aztreonam is not a substrate for the L2 metalloenzymes and clavulanic acid inhibits other β-lactamases produced by Stenotrophomonas maltophilia (262).
Agar supplemented with clavulanate.
Ho et al. (165) described a method by which Mueller-Hinton agar was supplemented with 4 μg/ml of clavulanate. Antibiotic disks containing ceftazidime (30 μg), cefotaxime (30 μg), ceftriaxone (30 μg), and aztreonam (30 μg) were placed on the clavulanate containing agar and on regular clavulanate free Mueller-Hinton agar plates. A difference in β-lactam zone width of ≥10 mm on the two media was considered positive for ESBL production. The difference in ceftazidime zone sizes was 93 to 96% sensitive and 100% specific in the hands of the originators of the test (165), but somewhat less so according to other investigators (31). A major drawback of the method is the need to freshly prepare clavulanate containing plates. The potency of clavulanic acid begins to decrease after 72 h.
Disk replacement method.
Casals and Pringler (Mediterranean Congress of Chemotherapy, Barcelona, Spain, 20 to 25 May 1990) described the following method. Three amoxicillin/clavulanate disks are applied to a Mueller-Hinton plate inoculated with the test organism. After 1 hour at room temperature, these antibiotic disks are removed and replaced on the same spot by disks containing cefotaxime, ceftazidime and aztreonam. Control disks of these three antibiotics are simultaneously placed at least 30 mm from these locations. A positive test is indicated by a zone increase of ≥5 mm for the disks which have replaced the amoxicillin/clavulanate disks compared to the control disks.
A modification of this test, in which 6-mm sterile paper disks inoculated with 20 μl of clavulanic acid (200 μg/ml) were used instead of the amoxicillin-clavulanic acid disks has been described (359). The results of this method are comparable to the double-disk synergy test (359). Unfortunately the method is not suitable for busy clinical microbiology laboratories given the need for a second step one hour after the initial plate inoculation.
Three-dimensional test.
The three-dimensional test gives phenotypic evidence of ESBL-induced inactivation of extended-spectrum cephalosporins or aztreonam without relying on demonstration of inactivation of the β-lactamases by a β-lactamase inhibitor. In this test, developed by Thomson (393), the surface of the susceptibility plate is inoculated by standard methods for disk diffusion testing, but additionally a circular slit is cut in the agar concentric with the margin of the plate. A heavy inoculum of the test organism (109 to 1010 CFU of cells) is pipetted into the slit. β-Lactam-impregnated disks are then placed on the surface of the agar 3 mm outside of the inoculated circular slit. β-Lactamase-induced inactivation of each test antibiotic is detected by inspection of the margin of the zone of inhibition in the vicinity of its intersection with the circular three-dimensional inoculation. The presence of β-lactamase-induced drug inactivation is visualized as a distortion or discontinuity in the usually circular inhibition zone or the production of discrete colonies in the vicinity of the inoculated slit.
A modification of the test involves removing a cylindrical plug of agar (diameter, 4 mm), 2 mm from the antibiotic disks (393, 409). This cup is then filled with a milky suspension of the test organism. The modified test is interpreted as positive if the inhibition zone around the β-lactam antibiotic disk is distorted in such a way that growth of the test organism appears within the zone, behind the cup, and fully reaching the cup.
Disadvantages of the test include the need for an additional indirect test if inhibition zones are small or absent. The indirect test is performed by inoculating the surface of the agar with a fully susceptible strain, such as Escherichia coli ATCC 25922, and then inoculating the circular cut in the agar with the suspension of the test organism. This precludes simultaneous determination of antibiotic susceptibilities but does permit investigation of the β-lactamases of organisms for which inhibition zones are very small.
If the indirect test is used, the three-dimensional test is more sensitive than the double-disk diffusion test (393, 409), but if the indirect test is not used, sensitivity declines (31).
Difficult Organisms
Klebsiella oxytoca.
Most strains of Klebsiella oxytoca produce a chromosomally mediated β-lactamase (K1) that is in the same group, 2be, as plasmid-mediated ESBLs. Like the plasmid-mediated ESBLs, K1 hydrolyzes penicillins, third-generation cephalosporins and aztreonam and is inhibited by clavulanic acid. Mutational hyperproduction of the chromosomal K1 enzyme produces a characteristic antibiogram with susceptibility to ceftazidime, but resistance to piperacillin, cefuroxime, and aztreonam. Cefotaxime MICs are usually 0.12 to 2 μg/ml and ceftriaxone MICs are in the range of 0.5 to 32 μg/ml (136). Although the majority of Klebsiella oxytoca isolates produce only low levels of the K1 β-lactamase, hyperproduction of the enzyme is seen in 10 to 20% of clinical isolates (221).
Klebsiella oxytoca isolates producing TEM or SHV-type ESBLs may be distinguished from isolates hyperproducing K1 since the former β-lactamases usually have ceftazidime MICs ≥2 μg/ml (or equivalent zone diameters), while K1 hyperproducers do not. Klebsiella oxytoca strains hyperproducing the K1 β-lactamase will usually be recorded as positive on the Vitek ESBL test, but fewer than 20% of strains will appear positive using the double-disk diffusion test (355). Klebsiella oxytoca strains with a TEM or SHV-type ESBL will usually be positive on the Vitek ESBL test and the double-disk diffusion test (355). A little over 50% of Klebsiella oxytoca strains hyperproducing K1 will have cefpodoxime zone sizes of ≤17 mm (73).
Enterobacteriaceae other than Escherichia coli and Klebsiella spp.
CLSI screening and confirmatory tests apply only to Escherichia coli, Klebsiella spp., and Proteus mirabilis. They probably apply quite well to Salmonella spp. An evaluation of the use of CLSI methods for Enterobacteriaceae other than Escherichia coli and Klebsiella spp. collected from hospitals in the United States from 1996 to 1999 found that while approximately half of these isolates were screen positive for ESBL production, only 2% showed any clavulanic acid effect (360). Thus, it was not considered warranted, by the authors, to extend ESBL detection tests to other Enterobacteriaceae (360). A similar situation probably exists with nonfermentative bacteria such as Pseudomonas aeruginosa or Acinetobacter spp.
Of note is that there have been numerous reports of both Enterobacter cloacae and Enterobacter aerogenes harboring ESBLs, in addition to chromosomal AmpC-type β-lactamases (41, 48, 68, 108, 119, 134, 213, 214, 232, 271, 314, 356). Could it be clinically important to determine which of these Enterobacter isolates produce both enzyme types? A casual inspection of the antibiotic susceptibility patterns of organisms producing ESBLs versus those producing only AmpC-type β-lactamases would suggest that detection of the type of enzyme is not important since the therapeutic options are similarly limited. However, cefepime MICs appear to be higher in ESBL-producing versus non-ESBL-producing strains of Enterobacter cloacae (148). Many cefepime-resistant Enterobacter strains are found to be ESBL producers (108, 267). However, between 40 and 50% of ESBL-producing strains had cefepime MICs in the susceptible range (≤8 μg/ml) (148, 267); it is not certain if clinical failure with this drug might be higher in ESBL-producing versus non-ESBL-producing Enterobacter cloacae. In addition to this therapeutic concern is the epidemiologic significance of hidden ESBL production in Enterobacter spp. The presence of ESBLs may imply the possibility of plasmid transmission among different species in addition to patient to patient transmission of ESBL-producing strains (407). A clonal strain of ESBL-producing Enterobacter aerogenes has caused numerous infections in France, and has raised important infection control issues (48).
As noted above, the CLSI has not published guidelines for detection of ESBLs in any organisms other than Escherichia coli, klebsiellae, or Proteus mirabilis, and the sensitivity and specificity of many ESBL detection methods in this genus are not known. In a study of Greek Enterobacter isolates, the Vitek ESBL detection test was positive for fewer than 10% of strains producing both an ESBL and an AmpC-type enzyme (398). The conventional double-disk synergy test was positive only for 16% of strains. Closer application of the disks (20 mm instead of 30 mm) increased the sensitivity of detection to 71%. In a study of SHV-7-producing Enterobacter cloacae isolates from Philadelphia, the conventional double-disk synergy test was positive for only 5 of 14 isolates when ceftazidime was used as the cephalosporin substrate (214).
Why are these tests less reliable in detecting ESBLs in Enterobacter spp. than in klebsiellae and Escherichia coli? In organisms which produce ESBLs but not AmpC, clavulanate will inhibit the activity of ESBL, leading to enhancement of the zone of inhibition in the area between the amoxicillin/clavulanate disk and any of the third-generation cephalosporin disks. However, in organisms which produce both ESBLs and AmpC, clavulanate may induce hyperproduction of the AmpC β-lactamase, leading to hydrolysis of the third-generation cephalosporin, masking any synergy arising from inhibition of the ESBL.
Modification of the conventional double-disk diffusion test in which 30-μg cefepime (or cefpirome) disks were placed at distances of 30 or 20 mm (center to center) from a disk containing 20 μg amoxicillin plus 10 μg clavulanate has been used to detect ESBLs in Enterobacter spp. (148, 214, 398, 407). Since cefepime is less subject to hydrolysis by AmpC β-lactamases than third-generation cephalosporins, the issue of induction by clavulanate is less relevant; therefore, enhancement of the zone of inhibition in the area between the amoxicillin/clavulanate disk and the cefepime disk may still be observed. The sensitivity of this test in detecting ESBLs in Enterobacter spp. was 61% with disk spacing of 30 mm and 90% with disk spacing of 20 mm (398). The specificity of such a procedure was 92% at 30 mm and 97% at 20 mm. Levison and colleagues found that all 14 ESBL-producing Enterobacter cloacae isolates had a positive double-disk test when amoxicillin/clavulanate and cefepime disks were 20 mm apart (edge to edge) (214). An additional test showing synergy between ceftazidime and imipenem has been utilized in the detection of a novel class A enzyme, IBC-1, in Enterobacter cloacae isolates (191).
Thomson (395) has published an alternative approach for the detection of ESBLs in Enterobacter spp. In this approach, a decrease in ceftriaxone MICs of ≥8-fold in the presence of 8 μg/ml sulbactam was highly predictive of ESBL production (395). However, this study evaluated just 16 strains and did not include strains with CTX-M-type ESBLs.
TREATMENT AND OUTCOME OF INFECTIONS WITH ESBL-PRODUCING ORGANISMS
In Vitro Studies
Given the ability of ESBL-producing organisms to hydrolyze many β-lactam antibiotics, it is not surprising that antibiotic choice for infections with such organisms is seriously reduced. Furthermore, however, the plasmids bearing the genes encoding ESBLs frequently also carry genes encoding resistance to aminoglycosides and trimethioprim/sulfamethoxazole. There have been increasing reports of plasmid-encoded decrease in susceptibility to quinolones, frequently in association with plasmid-mediated cephalosporin resistance (178, 233, 238, 415). Even when plasmid-encoded decrease in quinolone susceptibility is not present, there is a strong association between quinolone resistance and ESBL production (20, 60, 206, 292). The reason for this association is not well understood.
Martinez-Martinez and colleagues have performed a careful analysis of mechanisms of quinolone resistance in Klebsiella pneumoniae isolates of clinical origin (236). These authors found that porin loss was observed only in those Klebsiella pneumoniae strains producing an ESBL. A significant number of these porin deficient strains also showed active efflux of quinolones. However, in the absence of topoisomerase changes, porin loss and active efflux did not have a significant effect upon quinolone resistance (236). It must be noted that patients with infections with ESBL producers and patients with quinolone-resistant isolates frequently share heavy antibiotic use of both extended-spectrum cephalosporins and quinolones (206, 292). Lautenbach and colleagues (206) additionally found that long-term-care facility residence was a significant risk factor for quinolone resistance in ESBL-producing Klebsiella pneumoniae and Escherichia coli.
β-Lactam/β-lactamase inhibitor combinations are usually active against organisms possessing a single ESBL. As has been noted previously, many organisms now produce multiple ESBLs (24, 53, 81, 366), which may reduce the effectiveness of β-lactam/β-lactamase inhibitor combinations. In vitro resistance of ESBL-producing isolates to such combinations is increasing; in a study of isolates from 35 intensive care units in Europe, the percentage of ESBL-producing isolates resistant to piperacillin-tazobactam rose from 31% in 1994 to 63% in 1997-1998 (20).
In vitro, the carbapenems (including imipenem, meropenem, and ertapenem) have the most consistent activity against ESBL-producing organisms, given their stability to hydrolysis by ESBLs. Cephamycins are also stable to the hydrolytic effects of ESBLs. Unfortunately, ESBL-producing organisms may lose outer membrane proteins leading to resistance to the cephamycins not related to β-lactamase production.
Antibiotic Choice for Serious Infections
As noted above, third-generation cephalosporins are poor choices for the treatment of serious infections due to ESBL-producing organisms. Failure rates are high when MICs of the cephalosporins are elevated (for example, 4 or 8 μg/ml) but still within the susceptible range (95, 196, 289).
Stochastic modeling suggests that cefepime at 2 g every 12 h may have a high probability of achieving pharmacokinetic/pharmacodynamic targets which have previously been correlated with clinical success (11). However, published clinical experience with the use of cefepime for the treatment of ESBL-producing organisms has been quite limited (289, 290). In a randomized trial of cefepime versus imipenem for nosocomial pneumonia, clinical response for infections with ESBL-producing organisms was seen in 100% (10 of 10) patients treated with imipenem but only 69% (9 of 13) patients treated with cefepime (440).
In common with other extended-spectrum cephalosporins, MICs for cefepime rise substantially when the inoculum of infecting organisms rises (30, 184, 392). Cefepime resistance may be more frequent in strains which produce the CTX-M-type ESBLs (436). Cefepime should not be used as first-line therapy against ESBL-producing organisms; if it is used (for example, against organisms with a cefepime MIC of <2 μg/ml), it should be used in high dosage (at least 2 g twice a day). In vitro synergy may be achievable between cefepime and amikacin (123, 287).
There are few published reports of the use of cephamycins (for example, cefoxitin and cephamycin) in the treatment of ESBL producers (55, 285, 374). In one of these reports, selection of porin resistant mutants occurred during therapy, resulting in cefoxitin resistance and relapse of infection. In addition, combined cephamycin and carbapenem resistance in Klebsiella pneumoniae has been observed in the setting of widespread cephamycin use in response to an outbreak of infection with ESBL-producing organisms (55). Therefore, we are reluctant to recommend cephamycins as first-line therapy for ESBL-producing organisms, despite their good in vitro activity.
β-Lactam/β-lactamase inhibitor combinations are subject to rising MICs as inoculum rises (392). Additionally, it is well known that ESBL-producing organisms may continue to harbor parent enyzmes (for example, SHV-1 or TEM-1). Hyperproduction of these non-ESBL-producing β-lactamases (4) or the combination of β-lactamase production and porin loss can also lead to a reduction in activity of β-lactamase inhibitors. Some animal studies have shown β-lactam/β-lactamase inhibitor combinations to be less effective than carbapenems against ESBL-producing organisms (187). Stochastic modeling suggests that some dosing regimens of piperacillin-tazobactam may not have a high probability of achieving pharmacokinetic/pharmacodynamic targets which have previously been correlated with clinical success (11). Published clinical experience with β-lactam/β-lactamase inhibitor combinations in the treatment of serious infections due to ESBL producers is limited to just a few patients (65, 95, 124, 293, 299, 310), and for this reason we do not regard β-lactam/β-lactamase inhibitor combinations as suitable first-line therapy for serious infections with ESBL-producing organisms.
Quinolones may be regarded as the treatment of choice for complicated urinary tract infections due to ESBL-producing organisms, if there is not in vitro resistance to quinolones (Table 3). Unfortunately, increasing in vitro resistance of ESBL producers to quinolones will limit the role of these antibiotics in the future. In general, the newer quinolones are unlikely to provide great additional benefits over ciprofloxacin. Three observational clinical studies have assessed the relative merits of quinolones and carbapenems for serious infections due to ESBL-producing organisms (125, 186, 290). Two of these studies found that carbapenems were superior to quinolones (125, 290), whereas one of the studies found that they were equivalent in effectiveness (186). It is possible that suboptimal dosing of quinolones in the presence of strains with elevated quinolone MICs (yet remaining in the susceptible range) may account for these differences.
TABLE 3.
Recommended treatment for infections with ESBL-producing organisms
| Infection type | Therapy of choice | Second-line therapy |
|---|---|---|
| Urinary tract infection | Quinolonea | Amoxicillin/clavualante |
| Bacteremia | Carbapenem | Quinolonea |
| Hospital-acquired pneumonia | Carbapenem | Quinolonea |
| Intra-abdominal infection | Carbapenem | Quinolonea (plus metronidazole) |
| Meningitis | Meropenem | Intrathecal polymyxin B |
If the organism is quinolone susceptible.
Two studies have suggested that synergy may occur when ciprofloxacin is added to β-lactam antibiotics in vitro against ESBL-producing strains. Addition of ciprofloxacin to imipenem and to the combination of cefotaxime and sulbactam has been found to be synergistic (14). The antimicrobial combinations of ciprofloxacin plus either cefpirome or cefepime resulted in a 4 log decrease in ESBL-producing Klebsiella pneumoniae (123). There are no publications on the clinical use of these combinations.
Carbapenems should be regarded as the drugs of choice for serious infections with ESBL-producing organisms. The basis for this statement is not just the almost uniform susceptibility in vitro of these compounds but also increasingly extensive clinical experience (65, 116, 125, 186, 246, 287, 289-291, 420, 421, 440). The choice between imipenem and meropenem is difficult. Published experience is greatest with imipenem, but MICs are slightly lower for meropenem. In nosocomial meningitis, meropenem should be regarded as the drug of choice. Intrathecal polymyxin B should also be considered, along with removal of neurosurgical hardware in cases of CSF shunt infections (361). Ertapenem shares the good in vitro activity of the other carbapenems (176). The ability to use ertapenem once daily makes it potentially useful in serious infections with ESBL producers in nursing home residents or patients continuing parenteral therapy out of hospital. However there is no published clinical experience on use of this drug for infections with ESBL-producing organisms.
There is no evidence that combination therapy with a carbapenem and antibiotics of other classes is superior to use of a carbapenem alone (116, 290). Synergy has been exhibited in some but not all studies (297, 347).
Carbapenem-Resistant Klebsiella pneumoniae Isolates
Carbapenem resistant, ESBL-producing isolates remain exceedingly rare. Neither ESBLs nor plasmid-mediated AmpC β-lactamases are capable of hydrolyzing carbapenems to any great degree. However, in an experimental investigation of the roles of β-lactamases and porins in the activities of carbapenems against Klebsiella pneumoniae, Martinez-Martinez et al. (237) found that carbapenem resistance could be achieved by the combination of porin loss and the presence of the plasmid-mediated β-lactamases. In studies which have investigated clinical isolates of Klebsiella pneumoniae exhibiting carbapenem resistance, the combination of porin loss and β-lactamase production has resulted in carbapenem resistance. The relevant β-lactamases have included previously characterized ESBLs (SHV-2) (228) and AmpC-type enzymes (ACT-1 and CMY-4) (55, 72).
A second documented mechanism for carbapenem resistance is the presence of a β-lactamase capable of hydrolysis of carbapenems. Worryingly, such carbapenemases have been found to be plasmid-mediated. This is a great concern given the propensity for klebsiellae to host plasmids. Two types of carbapenemases have thus far been detected. The first are Bush-Jacoby-Medeiros group 3 enzymes. These metalloenzymes were originally found in Klebsiella pneumoniae isolates in Japan in 1994. This IMP-1 enzyme has now been found in a Klebsiella pneumoniae isolate in Singapore, where a combination with porin loss contributed to high-level carbapenem resistance (198). A recent report from Taiwan has described a Klebsiella pneumoniae isolate with a novel IMP-type carbapenemase (IMP-7), which was encoded on a plasmid also harboring genes encoding TEM-1 and the ESBL SHV-12 (430). Several other examples of IMP and VIM metalloenzyme producing klebsiellae have been reported (89, 137, 139, 216, 224, 250).
The second carbapenemase detected in Klebsiella pneumoniae was the novel Bush-Jacoby-Medeiros group 2f β-lactamase, coined KPC-1 (432). The amino acid sequence of this β-lactamase showed 45% homology to the Sme-1 carbapenemase of Serratia marcescens. Although the strain also harbored an ESBL, SHV-29, the carbapenemase was also responsible for resistance to extended-spectrum cephalosporins and aztreonam. Related carbapenemases, KPC-2 and KPC-3, have also been reported, especially from New York City (52, 168, 251, 376, 422, 433).
A third potential mechanism for carbapenem resistance is a change in the affinity of penicillin-binding proteins for carbapenems. Thus, far, such a mechanism has not been described in carbapenem-resistant, ESBL-producing strains.
The epidemiology of carbapenem-resistant Klebsiella pneumoniae isolates has yet to be studied in great detail (2). Eight patients with carbapenem resistance mediated by the combination of porin loss and the ACT-1 enzyme were identified in a single intensive care unit -all eight had previously been treated with imipenem. In two instances, sequential isolates of imipenem susceptible and imipenem resistant isolates had identical pulsed-field gel electrophoresis patterns indicating emergence of resistance during imipenem therapy. Three distinct clones of organisms were identified, and no personnel or environmental sources of the infection were identified. Since no other antibiotic options were available, six of the eight patients died from the infection. The outbreak was halted by reinforcement of infection control, despite increasing use of imipenem. Tigecycline or polymyxins may be considered in treatment of carbapenem-resistant klebsiellae.
Outcome of Serious ESBL- and Non-ESBL-Producing Infections
The case-control studies noted in the section on risk factors for ESBL production were reviewed for differences in outcome between the two groups. A number of these studies gave data on outcome (103, 104, 205, 311, 358). In each of these studies, there was no significant difference between mortality in patients colonized or infected with ESBL producers (range 19 to 30%) and those who did not get colonized or infected. In one study (358), mortality (42%) was higher in patients bacteremic with ESBL producers who did not receive appropriate antibiotic therapy. Additionally, duration of hospital stay and hospital charges may be higher in patients infected with ESBL-producing organisms than with non-ESBL-producing organisms of the same species (12, 205). Analysis of data from the Brooklyn Antibiotic Resistance Task Force showed that patients with infection due to ESBL-producing Klebsiella pneumoniae had a median length of hospital stay postinfection of 29 days compared to 11 days in those with infection due to non-ESBL-producing Klebsiella pneumoniae (12).
CONCLUSIONS
ESBLs have evolved greatly over the last 20 years. Their presence, plus the potential for plasmid-mediated quinolone and carbapanem resistance, will be sure to create significant therapeutic problems in the future. It is unlikely that many new antibiotic options will be available in the next 5 to 10 years to tackle such multiresistant infections. Enhanced infection control, coupled with antibiotic stewardship programs, therefore plays an important role in limiting the spread of ESBL-producing organisms. As previously stated, there is no doubt that the ESBLs will become increasingly complex and diverse in the future. This will create increasing challenges for those creating guidelines for detection of ESBLs in the clinical microbiology laboratory. Alteration of antibiotic susceptibility breakpoints may become necessary but need to be carefully considered in combination with pharmacokinetic, pharmacodynamic, and clinical data.
Acknowledgments
The Veterans Affairs Merit Review Program and the National Institutes of Health (R01 AI063517-01) supported R.A.B. The Centers for Disease Control (grant RS1/CCR322404) supported D.L.P.
REFERENCES
- 1.Ahamed, J., and M. Kundu. 1999. Molecular characterization of the SHV-11 beta-lactamase of Shigella dysenteriae. Antimicrob. Agents Chemother. 43:2081-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad, M., C. Urban, N. Mariano, P. A. Bradford, E. Calcagni, S. J. Projan, K. Bush, and J. J. Rahal. 1999. Clinical characteristics and molecular epidemiology associated with imipenem-resistant Klebsiella pneumoniae. Clin. Infect. Dis. 29:352-355. [DOI] [PubMed] [Google Scholar]
- 3.AitMhand, R., A. Soukri, N. Moustaoui, H. Amarouch, N. ElMdaghri, D. Sirot, and M. Benbachir. 2002. Plasmid-mediated TEM-3 extended-spectrum beta-lactamase production in Salmonella typhimuium in Casablanca. J. Antimicrob. Chemother. 49:169-172. [DOI] [PubMed] [Google Scholar]
- 4.Akhan, S., F. Coskunkan, O. Tansel, and H. Vahaboglu. 2001. Conjugative resistance to tazobactam plus piperacillin among extended-spectrum beta-lactamase-producing nosocomial Klebsiella pneumoniae. Scand. J. Infect. Dis. 33:512-515. [DOI] [PubMed] [Google Scholar]
- 5.Akindele, J. A., and I. O. Rotilu. 1997. Outbreak of neonatal Klebsiella septicaemia: a review of antimicrobial sensitivities. Afr. J. Med. Med. Sci. 26:51-53. [PubMed] [Google Scholar]
- 6.Aktas, E., N. Yigit, H. Yazgi, and A. Ayyildiz. 2002. Detection of antimicrobial resistance and extended-spectrum beta-lactamase production in Klebsiella pneumoniae strains from infected neonates. J. Int. Med. Res. 30:445-448. [DOI] [PubMed] [Google Scholar]
- 7.Albertini, M. T., C. Benoit, L. Berardi, Y. Berrouane, A. Boisivon, P. Cahen, C. Cattoen, Y. Costa, P. Darchis, E. Deliere, D. Demontrond, F. Eb, F. Golliot, G. Grise, A. Harel, J. L. Koeck, M. P. Lepennec, C. Malbrunot, M. Marcollin, S. Maugat, M. Nouvellon, B. Pangon, S. Ricouart, M. Roussel-Delvallez, A. Vachee, A. Carbonne, L. Marty, and V. Jarlier. 2002. Surveillance of methicillin-resistant Staphylococcus aureus (MRSA) and Enterobacteriaceae producing extended-spectrum beta-lactamase (ESBLE) in Northern France: a five-year multicentre incidence study. J. Hosp. Infect. 52:107-113. [DOI] [PubMed] [Google Scholar]
- 8.Alcantar-Curiel, D., J. C. Tinoco, C. Gayosso, A. Carlos, C. Daza, M. C. Perez-Prado, L. Salcido, J. I. Santos, and C. M. Alpuche-Aranda. 2004. Nosocomial bacteremia and urinary tract infections caused by extended-spectrum beta-lactamase-producing Klebsiella pneumoniae with plasmids carrying both SHV-5 and TLA-1 genes. Clin. Infect. Dis. 38:1067-1074. [DOI] [PubMed] [Google Scholar]
- 9.Alobwede, I., F. H. M'Zali, D. M. Livermore, J. Heritage, N. Todd, and P. M. Hawkey. 2003. CTX-M extended-spectrum beta-lactamase arrives in the UK. J. Antimicrob. Chemother. 51:470-471. [DOI] [PubMed] [Google Scholar]
- 10.Ambler, R. P., A. F. Coulson, J. M. Frere, J. M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A beta-lactamases. Biochem. J. 276:269-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambrose, P. G., S. M. Bhavnani, and R. N. Jones. 2003. Pharmacokinetics-pharmacodynamics of cefepime and piperacillin-tazobactam against Escherichia coli and Klebsiella pneumoniae strains producing extended-spectrum beta-lactamases: report from the ARREST program. Antimicrob. Agents Chemother. 47:1643-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anonymous. 2002. The cost of antibiotic resistance: effect of resistance among Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudmonas aeruginosa on length of hospital stay. Infect. Control Hosp. Epidemiol. 23:106-108. [DOI] [PubMed] [Google Scholar]
- 13.Arbeit, R. D. 1999. Laboratory procedures for the epidemiologic analysis of microorganisms. .In P. R. Murray (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 14.Archambaud, M., E. Labau, D. Clave, and C. Suc. 1989. Bactericidal effect of cefotaxime-sulbactam and imipenem combined with gentamicin and/or ciprofloxacin against CTX-1 producing Klebsiella pneumoniae. Pathol. Biol. (Paris) 37:534-539. [PubMed] [Google Scholar]
- 15.Ardanuy, C., J. Linares, M. A. Dominguez, S. Hernandez-Alles, V. J. Benedi, and L. Martinez-Martinez. 1998. Outer membrane profiles of clonally related Klebsiella pneumoniae isolates from clinical samples and activities of cephalosporins and carbapenems. Antimicrob. Agents Chemother. 42:1636-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ariffin, H., P. Navaratnam, M. Mohamed, A. Arasu, W. A. Abdullah, C. L. Lee, and L. H. Peng. 2000. Ceftazidime-resistant Klebsiella pneumoniae bloodstream infection in children with febrile neutropenia. Int. J. Infect. Dis. 4:21-25. [DOI] [PubMed] [Google Scholar]
- 17.Arlet, G., M. Rouveau, I. Casin, P. J. Bouvet, P. H. Lagrange, and A. Philippon. 1994. Molecular epidemiology of Klebsiella pneumoniae strains that produce SHV-4 beta-lactamase and which were isolated in 14 French hospitals. J. Clin. Microbiol. 32:2553-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arlet, G., M. J. Sanson-le Pors, M. Rouveau, G. Fournier, O. Marie, B. Schlemmer, and A. Philippon. 1990. Outbreak of nosocomial infections due to Klebsiella pneumoniae producing SHV-4 beta-lactamase. Eur. J. Clin. Microbiol. Infect. Dis. 9:797-803. [DOI] [PubMed] [Google Scholar]
- 19.Asensio, A., A. Oliver, P. Gonzalez-Diego, F. Baquero, J. C. Perez-Diaz, P. Ros, J. Cobo, M. Palacios, D. Lasheras, and R. Canton. 2000. Outbreak of a multiresistant Klebsiella pneumoniae strain in an intensive care unit: antibiotic use as risk factor for colonization and infection. Clin. Infect. Dis. 30:55-60. [DOI] [PubMed] [Google Scholar]
- 20.Babini, G. S., and D. M. Livermore. 2000. Antimicrobial resistance amongst Klebsiella spp. collected from intensive care units in Southern and Western Europe in 1997-1998. J. Antimicrob. Chemother. 45:183-189. [DOI] [PubMed] [Google Scholar]
- 21.Babini, G. S., and D. M. Livermore. 2000. Are SHV beta-lactamases universal in Klebsiella pneumoniae? Antimicrob. Agents Chemother. 44:2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baraniak, A., J. Fiett, W. Hryniewicz, P. Nordmann, and M. Gniadkowski. 2002. Ceftazidime-hydrolysing CTX-M-15 extended-spectrum beta-lactamase (ESBL) in Poland. J. Antimicrob. Chemother. 50:393-396. [DOI] [PubMed] [Google Scholar]
- 23.Baraniak, A., J. Fiett, A. Sulikowska, W. Hryniewicz, and M. Gniadkowski. 2002. Countrywide spread of CTX-M-3 extended-spectrum beta-lactamase-producing microorganisms of the family Enterobacteriaceae in Poland. Antimicrob. Agents Chemother. 46:151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baraniak, A., E. Sadowy, W. Hryniewicz, and M. Gniadkowski. 2002. Two different extended-spectrum beta-lactamases (ESBLs) in one of the first ESBL-producing Salmonella isolates in Poland. J. Clin. Microbiol. 40:1095-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barguellil, F., C. Burucoa, A. Amor, J. L. Fauchere, and C. Fendri. 1995. In vivo acquisition of extended-spectrum beta-lactamase in Salmonella enteritidis during antimicrobial therapy. Eur. J. Clin. Microbiol. Infect. Dis. 14:703-706. [DOI] [PubMed] [Google Scholar]
- 26.Bauernfeind, A., J. M. Casellas, M. Goldberg, M. Holley, R. Jungwirth, P. Mangold, T. Rohnisch, S. Schweighart, and R. Wilhelm. 1992. A new plasmidic cefotaximase from patients infected with Salmonella typhimurium. Infection 20:158-163. [DOI] [PubMed] [Google Scholar]
- 27.Bauernfeind, A., E. Rosenthal, E. Eberlein, M. Holley, and S. Schweighart. 1993. Spread of Klebsiella pneumoniae producing SHV-5 beta-lactamase among hospitalized patients. Infection 21:18-22. [DOI] [PubMed] [Google Scholar]
- 28.Bauernfeind, A., I. Stemplinger, R. Jungwirth, S. Ernst, and J. M. Casellas. 1996. Sequences of beta-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other beta-lactamases. Antimicrob. Agents Chemother. 40:509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauernfeind, A., I. Stemplinger, R. Jungwirth, P. Mangold, S. Amann, E. Akalin, O. Ang, C. Bal, and J. M. Casellas. 1996. Characterization of beta-lactamase gene blaPER-2, which encodes an extended-spectrum class A beta-lactamase. Antimicrob. Agents Chemother. 40:616-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bedenic, B., N. Beader, and Z. Zagar. 2001. Effect of inoculum size on the antibacterial activity of cefpirome and cefepime against Klebsiella pneumoniae strains producing SHV extended-spectrum beta-lactamases. Clin. Microbiol. Infect. 7:626-635. [DOI] [PubMed] [Google Scholar]
- 31.Bedenic, B., C. Randegger, A. Boras, and H. Haechler. 2001. Comparison of five different methods for detection of SHV extended-spectrum beta-lactamases. J. Chemother. 13:24-33. [DOI] [PubMed] [Google Scholar]
- 32.Bedenic, B., C. C. Randegger, E. Stobberingh, and H. Hachler. 2001. Molecular epidemiology of extended-spectrum beta-lactamases from Klebsiella pneumoniae strains isolated in Zagreb, Croatia. Eur. J. Clin. Microbiol. Infect. Dis. 20:505-508. [DOI] [PubMed] [Google Scholar]
- 33.Bell, J. M., J. D. Turnidge, A. C. Gales, M. A. Pfaller, and R. N. Jones. 2002. Prevalence of extended spectrum beta-lactamase (ESBL)-producing clinical isolates in the Asia-Pacific region and South Africa: regional results from SENTRY Antimicrobial Surveillance Program (1998-99). Diagn. Microbiol. Infect. Dis. 42:193-198. [DOI] [PubMed] [Google Scholar]
- 34.Bellais, S., L. Poirel, N. Fortineau, J. W. Decousser, and P. Nordmann. 2001. Biochemical-genetic characterization of the chromosomally encoded extended-spectrum class A beta-lactamase from Rahnella aquatilis. Antimicrob. Agents Chemother. 45:2965-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben Redjeb, S., H. Ben Yaghlane, A. Boujnah, A. Philippon, and R. Labia. 1988. Synergy between clavulanic acid and newer beta-lactams on nine clinical isolates of Klebsiella pneumoniae, Escherichia coli and Salmonella typhimurium resistant to third-generation cephalosporins. J. Antimicrob. Chemother. 21:263-266. [DOI] [PubMed] [Google Scholar]
- 36.Ben Redjeb, S., G. Fournier, C. Mabilat, A. Ben Hassen, and A. Philippon. 1990. Two novel transferable extended-spectrum beta-lactamases from Klebsiella pneumoniae in Tunisia. FEMS Microbiol. Lett. 55:33-38. [DOI] [PubMed] [Google Scholar]
- 37.Bermudes, H., C. Arpin, F. Jude, Z. el-Harrif, C. Bebear, and C. Quentin. 1997. Molecular epidemiology of an outbreak due to extended-spectrum beta-lactamase-producing enterobacteria in a French hospital. Eur. J. Clin. Microbiol. Infect. Dis. 16:523-529. [DOI] [PubMed] [Google Scholar]
- 38.Bingen, E. H., P. Desjardins, G. Arlet, F. Bourgeois, P. Mariani-Kurkdjian, N. Y. Lambert-Zechovsky, E. Denamur, A. Philippon, and J. Elion. 1993. Molecular epidemiology of plasmid spread among extended broad-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates in a pediatric hospital. J. Clin. Microbiol. 31:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bird, J., R. Browning, R. P. Hobson, F. M. MacKenzie, J. Brand, and I. M. Gould. 1998. Multiply-resistant Klebsiella pneumoniae: failure of spread in community-based elderly care facilities. J. Hosp. Infect. 40:243-247. [DOI] [PubMed] [Google Scholar]
- 40.Bisson, G., N. O. Fishman, J. B. Patel, P. H. Edelstein, and E. Lautenbach. 2002. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species: risk factors for colonization and impact of antimicrobial formulary interventions on colonization prevalence. Infect. Control Hosp. Epidemiol. 23:254-260. [DOI] [PubMed] [Google Scholar]
- 41.Blahova, J., K. Kralikova, V. Krcmery, Sr., and V. Schafer. 1999. Extended-spectrum beta-lactamase producing strains of Enterobacter cloacae transferring resistance to cefotaxime and ceftazidime. J. Chemother. 11:97-102. [DOI] [PubMed] [Google Scholar]
- 42.Blomberg, B., R. Jureen, K. P. Manji, B. S. Tamim, D. S. Mwakagile, W. K. Urassa, M. Fataki, V. Msangi, M. G. Tellevik, S. Y. Maselle, and N. Langeland. 2005. High rate of fatal cases of pediatric septicemia caused by gram-negative bacteria with extended-spectrum beta-lactamases in Dar es Salaam, Tanzania. J. Clin. Microbiol. 43:745-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonnet, R. 2004. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnet, R., C. Dutour, J. L. Sampaio, C. Chanal, D. Sirot, R. Labia, C. De Champs, and J. Sirot. 2001. Novel cefotaximase (CTX-M-16) with increased catalytic efficiency due to substitution Asp-240->Gly. Antimicrob. Agents Chemother. 45:2269-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonnet, R., J. L. Sampaio, C. Chanal, D. Sirot, C. De Champs, J. L. Viallard, R. Labia, and J. Sirot. 2000. A novel class A extended-spectrum beta-lactamase (BES-1) in Serratia marcescens isolated in Brazil. Antimicrob. Agents Chemother. 44:3061-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonnet, R., J. L. Sampaio, R. Labia, C. De Champs, D. Sirot, C. Chanal, and J. Sirot. 2000. A novel CTX-M beta-lactamase (CTX-M-8) in cefotaxime-resistant Enterobacteriaceae isolated in Brazil. Antimicrob. Agents Chemother. 44:1936-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borer, A., J. Gilad, G. Menashe, N. Peled, K. Riesenberg, and F. Schlaeffer. 2002. Extended-spectrum beta-lactamase-producing Enterobacteriaceae strains in community-acquired bacteremia in southern Israel. Med. Sci. Monit. 8:CR44-47. [PubMed] [Google Scholar]
- 48.Bosi, C., A. Davin-Regli, C. Bornet, M. Mallea, J. M. Pages, and C. Bollet. 1999. Most Enterobacter aerogenes strains in France belong to a prevalent clone. J. Clin. Microbiol. 37:2165-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bou, G., M. Cartelle, M. Tomas, D. Canle, F. Molina, R. Moure, J. M. Eiros, and A. Guerrero. 2002. Identification and broad dissemination of the CTX-M-14 beta-lactamase in different Escherichia coli strains in the northwest area of Spain. J. Clin. Microbiol. 40:4030-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyd, D. A., S. Tyler, S. Christianson, A. McGeer, M. P. Muller, B. M. Willey, E. Bryce, M. Gardam, P. Nordmann, and M. R. Mulvey. 2004. Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob. Agents Chemother. 48:3758-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bradford, P. A. 2001. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bradford, P. A., S. Bratu, C. Urban, M. Visalli, N. Mariano, D. Landman, J. J. Rahal, S. Brooks, S. Cebular, and J. Quale. 2004. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 beta-lactamases in New York City. Clin. Infect. Dis. 39:55-60. [DOI] [PubMed] [Google Scholar]
- 53.Bradford, P. A., C. E. Cherubin, V. Idemyor, B. A. Rasmussen, and K. Bush. 1994. Multiply resistant Klebsiella pneumoniae strains from two Chicago hospitals: identification of the extended-spectrum TEM-12 and TEM-10 ceftazidime-hydrolyzing beta-lactamases in a single isolate. Antimicrob. Agents Chemother. 38:761-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bradford, P. A., C. Urban, A. Jaiswal, N. Mariano, B. A. Rasmussen, S. J. Projan, J. J. Rahal, and K. Bush. 1995. SHV-7, a novel cefotaxime-hydrolyzing beta-lactamase, identified in Escherichia coli isolates from hospitalized nursing home patients. Antimicrob. Agents Chemother. 39:899-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bradford, P. A., C. Urban, N. Mariano, S. J. Projan, J. J. Rahal, and K. Bush. 1997. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC beta-lactamase, and the foss of an outer membrane protein. Antimicrob. Agents Chemother. 41:563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bradford, P. A., Y. Yang, D. Sahm, I. Grope, D. Gardovska, and G. Storch. 1998. CTX-M-5, a novel cefotaxime-hydrolyzing beta-lactamase from an outbreak of Salmonella typhimurium in Latvia. Antimicrob. Agents Chemother. 42:1980-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Branger, C., B. Bruneau, A. L. Lesimple, P. J. Bouvet, P. Berry, J. Sevali-Garcia, and N. Lambert-Zechovsky. 1997. Epidemiological typing of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates responsible for five outbreaks in a university hospital. J. Hosp. Infect. 36:23-36. [DOI] [PubMed] [Google Scholar]
- 58.Brenwald, N. P., G. Jevons, J. M. Andrews, J. H. Xiong, P. M. Hawkey, and R. Wise. 2003. An outbreak of a CTX-M-type beta-lactamase-producing Klebsiella pneumoniae: the importance of using cefpodoxime to detect extended-spectrum beta-lactamases. J. Antimicrob. Chemother. 51:195-196. [DOI] [PubMed] [Google Scholar]
- 59.Brigante, G., F. Luzzaro, M. Perilli, G. Lombardi, A. Coli, G. M. Rossolini, G. Amicosante, and A. Toniolo. 2005. Evolution of CTX-M-type beta-lactamases in isolates of Escherichia coli infecting hospital and community patients. Int. J. Antimicrob. Agents 25:157-162. [DOI] [PubMed] [Google Scholar]
- 60.Brisse, S., D. Milatovic, A. C. Fluit, J. Verhoef, and F. J. Schmitz. 2000. Epidemiology of quinolone resistance of Klebsiella pneumoniae and Klebsiella oxytoca in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 19:64-68. [DOI] [PubMed] [Google Scholar]
- 61.Brown, D. F., J. Andrews, A. King, and A. P. MacGowan. 2000. Detection of extended-spectrum beta-lactamases with Etest and double-disc potentiation methods. J. Antimicrob. Chemother. 46:327-328. [DOI] [PubMed] [Google Scholar]
- 62.Brun-Buisson, C., P. Legrand, A. Philippon, F. Montravers, M. Ansquer, and J. Duval. 1987. Transferable enzymatic resistance to third-generation cephalosporins during nosocomial outbreak of multiresistant Klebsiella pneumoniae. Lancet ii:302-306. [DOI] [PubMed] [Google Scholar]
- 63.Brun-Buisson, C., P. Legrand, A. Rauss, C. Richard, F. Montravers, M. Besbes, J. L. Meakins, C. J. Soussy, and F. Lemaire. 1989. Intestinal decontamination for control of nosocomial multiresistant gram-negative bacilli. Study of an outbreak in an intensive care unit. Ann. Intern. Med. 110:873-881. [DOI] [PubMed] [Google Scholar]
- 64.Bureau-Chalot, F., L. Drieux, C. Pierrat-Solans, D. Forte, C. de Champs, and O. Bajolet. 2004. Blood pressure cuffs as potential reservoirs of extended-spectrum beta-lactamase VEB-1-producing isolates of Acinetobacter baumannii. J. Hosp. Infect. 58:91-92. [DOI] [PubMed] [Google Scholar]
- 65.Burgess, D. S., R. G. Hall, Jr., J. S. Lewis, Jr., J. H. Jorgensen, and J. E. Patterson. 2003. Clinical and microbiologic analysis of a hospital's extended-spectrum beta-lactamase-producing isolates over a 2-year period. Pharmacotherapy 23:1232-1237. [DOI] [PubMed] [Google Scholar]
- 66.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bush, K., C. Macalintal, B. A. Rasmussen, V. J. Lee, and Y. Yang. 1993. Kinetic interactions of tazobactam with beta-lactamases from all major structural classes. Antimicrob. Agents Chemother. 37:851-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Canton, R., A. Oliver, T. M. Coque, C. Varela Mdel, J. C. Perez-Diaz, and F. Baquero. 2002. Epidemiology of extended-spectrum beta-lactamase-producing Enterobacter isolates in a Spanish hospital during a 12-year period. J. Clin. Microbiol. 40:1237-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Canton, R., M. Perez-Vazquez, A. Oliver, B. Sanchez Del Saz, M. O. Gutierrez, M. Martinez-Ferrer, and F. Baquero. 2000. Evaluation of the Wider system, a new computer-assisted image-processing device for bacterial identification and susceptibility testing. J. Clin. Microbiol. 38:1339-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao, V., T. Lambert, and P. Courvalin. 2002. ColE1-like plasmid pIP843 of Klebsiella pneumoniae encoding extended-spectrum beta-lactamase CTX-M-17. Antimicrob. Agents Chemother. 46:1212-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao, V., T. Lambert, D. Q. Nhu, H. K. Loan, N. K. Hoang, G. Arlet, and P. Courvalin. 2002. Distribution of extended-spectrum beta-lactamases in clinical isolates of Enterobacteriaceae in Vietnam. Antimicrob. Agents Chemother. 46:3739-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao, V. T., G. Arlet, B. M. Ericsson, A. Tammelin, P. Courvalin, and T. Lambert. 2000. Emergence of imipenem resistance in Klebsiella pneumoniae owing to combination of plasmid-mediated CMY-4 and permeability alteration. J. Antimicrob. Chemother. 46:895-900. [DOI] [PubMed] [Google Scholar]
- 73.Carter, M. W., K. J. Oakton, M. Warner, and D. M. Livermore. 2000. Detection of extended-spectrum beta-lactamases in klebsiellae with the Oxoid combination disk method. J. Clin. Microbiol. 38:4228-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Casellas, J. M., and M. Goldberg. 1989. Incidence of strains producing extended spectrum beta-lactamases in Argentina. Infection 17:434-436. [DOI] [PubMed] [Google Scholar]
- 75.Casewell, M. W., and I. Phillips. 1981. Aspects of the plasmid-mediated antibiotic resistance and epidemiology of Klebsiella species. Am. J. Med. 70:459-462. [DOI] [PubMed] [Google Scholar]
- 76.Castanheira, M., R. E. Mendes, T. R. Walsh, A. C. Gales, and R. N. Jones. 2004. Emergence of the extended-spectrum beta-lactamase GES-1 in a Pseudomonas aeruginosa strain from Brazil: report from the SENTRY antimicrobial surveillance program. Antimicrob. Agents Chemother. 48:2344-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Centers for Disease Control. 2000. Laboratory capacity to detect antimicrobial resistance, 1998. Morb. Mortal. Wkly. Rep. 48:1167-1171. [PubMed] [Google Scholar]
- 78.Chaibi, E. B., D. Sirot, G. Paul, and R. Labia. 1999. Inhibitor-resistant TEM beta-lactamases: phenotypic, genetic and biochemical characteristics. J. Antimicrob. Chemother. 43:447-458. [DOI] [PubMed] [Google Scholar]
- 79.Chanawong, A., F. H. M'Zali, J. Heritage, A. Lulitanond, and P. M. Hawkey. 2000. Characterisation of extended-spectrum beta-lactamases of the SHV family using a combination of PCR-single strand conformational polymorphism (PCR-SSCP) and PCR-restriction fragment length polymorphism (PCR-RFLP). FEMS Microbiol. Lett. 184:85-89. [DOI] [PubMed] [Google Scholar]
- 80.Chanawong, A., F. H. M'Zali, J. Heritage, A. Lulitanond, and P. M. Hawkey. 2001. Discrimination of SHV beta-lactamase genes by restriction site insertion-PCR. Antimicrob. Agents Chemother. 45:2110-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chanawong, A., F. H. M'Zali, J. Heritage, J. H. Xiong, and P. M. Hawkey. 2002. Three cefotaximases, CTX-M-9, CTX-M-13, and CTX-M-14, among Enterobacteriaceae in the People's Republic of China. Antimicrob. Agents Chemother. 46:630-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang, F. Y., L. K. Siu, C. P. Fung, M. H. Huang, and M. Ho. 2001. Diversity of SHV and TEM beta-lactamases in Klebsiella pneumoniae: gene evolution in northern Taiwan and two novel beta-lactamases, SHV-25 and SHV-26. Antimicrob. Agents Chemother. 45:2407-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng, Y., Y. Li, and M. Chen. 1994. A plasmid-mediated SHV type extended-spectrum beta-lactamase in Beijing isolate of Enterobacter gergoviae. Wei Sheng Wu Xue Bao 34:106-112. [PubMed] [Google Scholar]
- 84.Cherian, B. P., N. Singh, W. Charles, and P. Prabhakar. 1999. Extended-spectrum beta-lactamase-producing Salmonella enteritidis in Trinidad and Tobago. Emerg. Infect. Dis. 5:181-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chetoui, H., E. Delhalle, P. Melin, A. Sabri, P. Thonart, and P. De Mol. 1999. Epidemiological typing of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates by pulsed-field gel electrophoresis and antibiotic susceptibility patterns. Res. Microbiol. 150:265-272. [DOI] [PubMed] [Google Scholar]
- 86.Chow, J. W., M. J. Fine, D. M. Shlaes, J. P. Quinn, D. C. Hooper, M. P. Johnson, R. Ramphal, M. M. Wagener, D. K. Miyashiro, and V. L. Yu. 1991. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann. Intern. Med. 115:585-590. [DOI] [PubMed] [Google Scholar]
- 87.Claeys, G., G. Verschraegen, T. de Baere, and M. Vaneechoutte. 2000. PER-1 beta-lactamase-producing Pseudomonas aeruginosa in an intensive care unit. J. Antimicrob. Chemother. 45:924-925. [DOI] [PubMed] [Google Scholar]
- 88.Colodner, R., W. Rock, B. Chazan, N. Keller, N. Guy, W. Sakran, and R. Raz. 2004. Risk factors for the development of extended-spectrum beta-lactamase-producing bacteria in nonhospitalized patients. Eur. J. Clin. Microbiol. Infect. Dis. 23:163-167. [DOI] [PubMed] [Google Scholar]
- 89.Conceicao, T., A. Brizio, A. Duarte, and R. Barros. 2005. First isolation of bla(VIM-2) in Klebsiella oxytoca clinical isolates from Portugal. Antimicrob. Agents Chemother. 49:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Coque, T. M., A. Oliver, J. C. Perez-Diaz, F. Baquero, and R. Canton. 2002. Genes encoding TEM-4, SHV-2, and CTX-M-10 extended-spectrum beta-lactamases are carried by multiple Klebsiella pneumoniae clones in a single hospital (Madrid, 1989 to 2000). Antimicrob. Agents Chemother. 46:500-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cormican, M. G., S. A. Marshall, and R. N. Jones. 1996. Detection of extended-spectrum beta-lactamase (ESBL)-producing strains by the Etest ESBL screen. J. Clin. Microbiol. 34:1880-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cosgrove, S. E., K. S. Kaye, G. M. Eliopoulous, and Y. Carmeli. 2002. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch. Intern. Med. 162:185-190. [DOI] [PubMed] [Google Scholar]
- 93.Cotton, M. F., E. Wasserman, C. H. Pieper, D. C. Theron, D. van Tubbergh, G. Campbell, F. C. Fang, and J. Barnes. 2000. Invasive disease due to extended spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal unit: the possible role of cockroaches. J. Hosp. Infect. 44:13-17. [DOI] [PubMed] [Google Scholar]
- 94.Coudron, P. E., E. S. Moland, and C. C. Sanders. 1997. Occurrence and detection of extended-spectrum beta-lactamases in members of the family Enterobacteriaceae at a veterans medical center: seek and you may find. J. Clin. Microbiol. 35:2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crowley, B. D. 2001. Extended-spectrum beta-lactamases in blood culture isolates of. Klebsiella pneumoniae: seek and you may find! J. Antimicrob. Chemother. 47:728-729. [DOI] [PubMed] [Google Scholar]
- 96.D'Agata, E., L. Venkataraman, P. DeGirolami, L. Weigel, M. Samore, and F. Tenover. 1998. The molecular and clinical epidemiology of enterobacteriaceae-producing extended-spectrum beta-lactamase in a tertiary care hospital. J. Infect. 36:279-285. [DOI] [PubMed] [Google Scholar]
- 97.Danel, F., L. M. Hall, B. Duke, D. Gur, and D. M. Livermore. 1999. OXA-17, a further extended-spectrum variant of OXA-10 beta-lactamase, isolated from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:1362-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Danel, F., L. M. Hall, D. Gur, and D. M. Livermore. 1995. OXA-14, another extended-spectrum variant of OXA-10 (PSE-2) beta-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 39:1881-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Danel, F., L. M. Hall, D. Gur, and D. M. Livermore. 1997. OXA-15, an extended-spectrum variant of OXA-2 beta-lactamase, isolated from a Pseudomonas aeruginosa strain. Antimicrob. Agents Chemother. 41:785-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Danel, F., L. M. Hall, D. Gur, and D. M. Livermore. 1998. OXA-16, a further extended-spectrum variant of OXA-10 beta-lactamase, from two Pseudomonas aeruginosa isolates. Antimicrob. Agents Chemother. 42:3117-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Datta, N., and P. Kontomichalou. 1965. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature 208:239-241. [DOI] [PubMed] [Google Scholar]
- 102.De Champs, C., L. Poirel, R. Bonnet, D. Sirot, C. Chanal, J. Sirot, and P. Nordmann. 2002. Prospective survey of beta-lactamases produced by ceftazidime-resistant Pseudomonas aeruginosa isolated in a French hospital in 2000. Antimicrob. Agents Chemother. 46:3031-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De Champs, C., D. Rouby, D. Guelon, J. Sirot, D. Sirot, D. Beytout, and J. M. Gourgand. 1991. A case-control study of an outbreak of infections caused by Klebsiella pneumoniae strains producing CTX-1 (TEM-3) beta-lactamase. J. Hosp. Infect. 18:5-13. [DOI] [PubMed] [Google Scholar]
- 104.De Champs, C., M. P. Sauvant, C. Chanal, D. Sirot, N. Gazuy, R. Malhuret, J. C. Baguet, and J. Sirot. 1989. Prospective survey of colonization and infection caused by expanded-spectrum-beta-lactamase-producing members of the family Enterobacteriaceae in an intensive care unit. J. Clin. Microbiol. 27:2887-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Champs, C., D. Sirot, C. Chanal, R. Bonnet, and J. Sirot. 2000. A 1998 survey of extended-spectrum beta-lactamases in Enterobacteriaceae in France. The French Study Group. Antimicrob. Agents Chemother. 44:3177-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Champs, C., D. Sirot, C. Chanal, M. C. Poupart, M. P. Dumas, and J. Sirot. 1991. Concomitant dissemination of three extended-spectrum beta-lactamases among different Enterobacteriaceae isolated in a French hospital. J. Antimicrob. Chemother. 27:441-457. [DOI] [PubMed] [Google Scholar]
- 107.de Champs, C. L., D. P. Guelon, R. M. Garnier, M. C. Poupart, O. Y. Mansoor, F. L. Dissait, and J. L. Sirot. 1993. Selective digestive decontamination by erythromycin-base in a polyvalent intensive care unit. Intensive Care Med. 19:191-196. [DOI] [PubMed] [Google Scholar]
- 108.De Gheldre, Y., M. J. Struelens, Y. Glupczynski, P. De Mol, N. Maes, C. Nonhoff, H. Chetoui, C. Sion, O. Ronveaux, and M. Vaneechoutte. 2001. National epidemiologic surveys of Enterobacter aerogenes in Belgian hospitals from 1996 to 1998. J. Clin. Microbiol. 39:889-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Decousser, J. W., L. Poirel, and P. Nordmann. 2001. Characterization of a chromosomally encoded extended-spectrum class A beta-lactamase from Kluyvera cryocrescens. Antimicrob. Agents Chemother. 45:3595-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Decre, D., B. Gachot, J. C. Lucet, G. Arlet, E. Bergogne-Berezin, and B. Regnier. 1998. Clinical and bacteriologic epidemiology of extended-spectrum beta-lactamase-producing strains of Klebsiella pneumoniae in a medical intensive care unit. Clin. Infect. Dis. 27:834-844. [DOI] [PubMed] [Google Scholar]
- 111.Denman, S. J., and J. R. Burton. 1992. Fluid intake and urinary tract infection in the elderly. JAMA 267:2245-2249. [DOI] [PubMed] [Google Scholar]
- 112.Di Conza, J., J. A. Ayala, P. Power, M. Mollerach, and G. Gutkind. 2002. Novel class 1 integron (InS21) carrying blaCTX-M-2 in Salmonella enterica serovar infantis. Antimicrob. Agents Chemother. 46:2257-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Didier, M. E., and D. G. Maki. 1997. Epidemiology of infection caused by multiresistant ESBL-producing Klebsiella (abstract). Infect. Control Hosp. Epidemiol. 18:35. [Google Scholar]
- 114.Diekema, D. J., M. A. Pfaller, R. N. Jones, G. V. Doern, P. L. Winokur, A. C. Gales, H. S. Sader, K. Kugler, and M. Beach. 1999. Survey of bloodstream infections due to gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin. Infect. Dis. 29:595-607. [DOI] [PubMed] [Google Scholar]
- 115.Docquier, J. D., F. Luzzaro, G. Amicosante, A. Toniolo, and G. M. Rossolini. 2001. Multidrug-resistant Pseudomonas aeruginosa producing PER-1 extended-spectrum serine-beta-lactamase and VIM-2 metallo-beta-lactamase. Emerg. Infect. Dis. 7:910-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Du, B., Y. Long, H. Liu, D. Chen, D. Liu, Y. Xu, and X. Xie. 2002. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae bloodstream infection: risk factors and clinical outcome. Intensive Care Med. 28:1718-1723. [DOI] [PubMed] [Google Scholar]
- 117.Du Bois, S. K., M. S. Marriott, and S. G. Amyes. 1995. TEM- and SHV-derived extended-spectrum beta-lactamases: relationship between selection, structure and function. J. Antimicrob. Chemother. 35:7-22. [DOI] [PubMed] [Google Scholar]
- 118.Dubois, V., L. Poirel, C. Marie, C. Arpin, P. Nordmann, and C. Quentin. 2002. Molecular characterization of a novel class 1 integron containing bla(GES-1) and a fused product of aac3-Ib/aac6′-Ib' gene cassettes in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 46:638-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dumarche, P., C. De Champs, D. Sirot, C. Chanal, R. Bonnet, and J. Sirot. 2002. TEM Derivative-Producing Enterobacter aerogenes Strains: Dissemination of a Prevalent Clone. Antimicrob. Agents Chemother. 46:1128-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dutour, C., R. Bonnet, H. Marchandin, M. Boyer, C. Chanal, D. Sirot, and J. Sirot. 2002. CTX-M-1, CTX-M-3, and CTX-M-14 beta-lactamases from Enterobacteriaceae isolated in France. Antimicrob. Agents Chemother. 46:534-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eisen, D., E. G. Russell, M. Tymms, E. J. Roper, M. L. Grayson, and J. Turnidge. 1995. Random amplified polymorphic DNA and plasmid analyses used in investigation of an outbreak of multiresistant Klebsiella pneumoniae. J. Clin. Microbiol. 33:713-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.El-Karsh, T., A. F. Tawfik, F. Al-Shammary, S. Al-Salah, A. M. Kambal, and A. M. Shibl. 1995. Antimicrobial resistance and prevalence of extended spectrum beta-lactamase among clinical isolates of gram-negative bacteria in Riyadh. J. Chemother. 7:509-514. [DOI] [PubMed] [Google Scholar]
- 123.Elkhaili, H., N. Kamili, L. Linger, D. Leveque, D. Pompei, H. Monteil, and F. Jehl. 1997. In vitro time-kill curves of cefepime and cefpirome combined with amikacin, gentamicin or ciprofloxacin against Klebsiella pneumoniae producing extended-spectrum beta-lactamase. Chemotherapy 43:245-253. [DOI] [PubMed] [Google Scholar]
- 124.Emery, C. L., and L. A. Weymouth. 1997. Detection and clinical significance of extended-spectrum beta-lactamases in a tertiary-care medical center. J. Clin. Microbiol. 35:2061-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Endimiani, A., F. Luzzaro, M. Perilli, G. Lombardi, A. Coli, A. Tamborini, G. Amicosante, and A. Toniolo. 2004. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum beta-lactamase: treatment outcome of patients receiving imipenem or ciprofloxacin. Clin. Infect. Dis. 38:243-251. [DOI] [PubMed] [Google Scholar]
- 126.Eveillard, M., J. L. Schmit, and F. Eb. 2002. Antimicrobial use prior to the acquisition of multiresistant bacteria. Infect. Control Hosp. Epidemiol. 23:155-158. [DOI] [PubMed] [Google Scholar]
- 127.Fantin, B., B. Pangon, G. Potel, F. Caron, E. Vallee, J. M. Vallois, J. Mohler, A. Bure, A. Philippon, and C. Carbon. 1990. Activity of sulbactam in combination with ceftriaxone in vitro and in experimental endocarditis caused by Escherichia coli producing SHV-2-like beta-lactamase. Antimicrob. Agents Chemother. 34:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fiett, J., A. Palucha, B. Miaczynska, M. Stankiewicz, H. Przondo-Mordarska, W. Hryniewicz, and M. Gniadkowski. 2000. A novel complex mutant beta-lactamase, TEM-68, identified in a Klebsiella pneumoniae isolate from an outbreak of extended-spectrum beta-lactamase-producing klebsiellae. Antimicrob. Agents Chemother. 44:1499-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fluit, A. C., M. E. Jones, F. J. Schmitz, J. Acar, R. Gupta, and J. Verhoef. 2000. Antimicrobial susceptibility and frequency of occurrence of clinical blood isolates in Europe from the SENTRY antimicrobial surveillance program, 1997 and 1998. Clin. Infect. Dis. 30:454-460. [DOI] [PubMed] [Google Scholar]
- 130.Fortineau, N., T. Naas, O. Gaillot, and P. Nordmann. 2001. SHV-type extended-spectrum beta-lactamase in a Shigella flexneri clinical isolate. J. Antimicrob. Chemother. 47:685-688. [DOI] [PubMed] [Google Scholar]
- 131.French, G. L., K. P. Shannon, and N. Simmons. 1996. Hospital outbreak of Klebsiella pneumoniae resistant to broad-spectrum cephalosporins and beta-lactam-beta-lactamase inhibitor combinations by hyperproduction of SHV-5 beta-lactamase. J. Clin. Microbiol. 34:358-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gaillot, O., C. Maruejouls, E. Abachin, F. Lecuru, G. Arlet, M. Simonet, and P. Berche. 1998. Nosocomial outbreak of Klebsiella pneumoniae producing SHV-5 extended-spectrum beta-lactamase, originating from a contaminated ultrasonography coupling gel. J. Clin. Microbiol. 36:1357-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Galani, I., M. Souli, Z. Chryssouli, D. Katsala, and H. Giamarellou. 2004. First identification of an Escherichia coli clinical isolate producing both metallo-beta-lactamase VIM-2 and extended-spectrum beta-lactamase IBC-1. Clin. Microbiol. Infect. 10:757-760. [DOI] [PubMed] [Google Scholar]
- 134.Galdbart, J. O., F. Lemann, D. Ainouz, P. Feron, N. Lambert-Zechovsky, and C. Branger. 2000. TEM-24 extended-spectrum beta-lactamase-producing Enterobacter aerogenes: long-term clonal dissemination in French hospitals. Clin. Microbiol. Infect. 6:316-323. [DOI] [PubMed] [Google Scholar]
- 135.Gazouli, M., S. V. Sidorenko, E. Tzelepi, N. S. Kozlova, D. P. Gladin, and L. S. Tzouvelekis. 1998. A plasmid-mediated beta-lactamase conferring resistance to cefotaxime in a Salmonella typhimurium clone found in St Petersburg, Russia. J. Antimicrob. Chemother. 41:119-121. [DOI] [PubMed] [Google Scholar]
- 136.Gheorghiu, R., M. Yuan, L. M. Hall, and D. M. Livermore. 1997. Bases of variation in resistance to beta-lactams in Klebsiella oxytoca isolates hyperproducing K1 beta-lactamase. J. Antimicrob. Chemother. 40:533-541. [DOI] [PubMed] [Google Scholar]
- 137.Giakkoupi, P., L. S. Tzouvelekis, G. L. Daikos, V. Miriagou, G. Petrikkos, N. J. Legakis, and A. C. Vatopoulos. 2005. Discrepancies and interpretation problems in susceptibility testing of VIM-1-producing Klebsiella pneumoniae isolates. J. Clin. Microbiol. 43:494-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Giakkoupi, P., L. S. Tzouvelekis, A. Tsakris, V. Loukova, D. Sofianou, and E. Tzelepi. 2000. IBC-1, a novel integron-associated class A beta-lactamase with extended-spectrum properties produced by an Enterobacter cloacae clinical strain. Antimicrob. Agents Chemother. 44:2247-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Giakkoupi, P., A. Xanthaki, M. Kanelopoulou, A. Vlahaki, V. Miriagou, S. Kontou, E. Papafraggas, H. Malamou-Lada, L. S. Tzouvelekis, N. J. Legakis, and A. C. Vatopoulos. 2003. VIM-1 Metallo-beta-lactamase-producing Klebsiella pneumoniae strains in Greek hospitals. J. Clin. Microbiol. 41:3893-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gierczynski, R., J. Szych, A. Cieslik, W. Rastawicki, and M. Jagielski. 2003. The occurrence of the first two CTX-M-3 and TEM-1 producing isolates of Salmonella enterica serovar Oranienburg in Poland. Int. J. Antimicrob. Agents 21:497-499. [DOI] [PubMed] [Google Scholar]
- 141.Girlich, D., T. Naas, A. Leelaporn, L. Poirel, M. Fennewald, and P. Nordmann. 2002. Nosocomial spread of the integron-located VEB-1-like cassette encoding an extended-pectrum beta-lactamase in Pseudomonas aeruginosa in Thailand. Clin. Infect. Dis. 34:603-611. [DOI] [PubMed] [Google Scholar]
- 142.Girlich, D., L. Poirel, A. Leelaporn, A. Karim, C. Tribuddharat, M. Fennewald, and P. Nordmann. 2001. Molecular epidemiology of the integron-located VEB-1 extended-spectrum beta-lactamase in nosocomial enterobacterial isolates in Bangkok, Thailand. J. Clin. Microbiol. 39:175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gniadkowski, M., I. Schneider, R. Jungwirth, W. Hryniewicz, and A. Bauernfeind. 1998. Ceftazidime-resistant Enterobacteriaceae isolates from three Polish hospitals: identification of three novel TEM- and SHV-5-type extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 42:514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gniadkowski, M., I. Schneider, A. Palucha, R. Jungwirth, B. Mikiewicz, and A. Bauernfeind. 1998. Cefotaxime-resistant Enterobacteriaceae isolates from a hospital in Warsaw, Poland: identification of a new CTX-M-3 cefotaxime-hydrolyzing beta-lactamase that is closely related to the CTX-M-1/MEN-1 enzyme. Antimicrob. Agents Chemother. 42:827-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Goldstein, F. W., Y. Pean, and J. Gertner. 1995. Resistance to ceftriaxone and other beta-lactams in bacteria isolated in the community. The Vigil'Roc Study Group. Antimicrob. Agents Chemother. 39:2516-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gonzalez-Vertiz, A., D. Alcantar-Curiel, M. Cuauhtli, C. Daza, C. Gayosso, G. Solache, C. Horta, F. Mejia, J. I. Santos, and C. Alpuche-Aranda. 2001. Multiresistant extended-spectrum beta-lactamase-producing Klebsiella pneumoniae causing an outbreak of nosocomial bloodstream infection. Infect. Control Hosp. Epidemiol. 22:723-725. [DOI] [PubMed] [Google Scholar]
- 147.Gori, A., F. Espinasse, A. Deplano, C. Nonhoff, M. H. Nicolas, and M. J. Struelens. 1996. Comparison of pulsed-field gel electrophoresis and randomly amplified DNA polymorphism analysis for typing extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae. J. Clin. Microbiol. 34:2448-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gottlieb, T., and C. Wolfson. 2000. Comparison of the MICs of cefepime for extended-spectrum beta-lactamase-producing and non-extended-spectrum beta-lactamase-producing strains of Enterobacter cloacae. J. Antimicrob. Chemother. 46:330-331. [DOI] [PubMed] [Google Scholar]
- 149.Gouby, A., C. Neuwirth, G. Bourg, N. Bouziges, M. J. Carles-Nurit, E. Despaux, and M. Ramuz. 1994. Epidemiological study by pulsed-field gel electrophoresis of an outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a geriatric hospital. J. Clin. Microbiol. 32:301-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Green, M., and K. Barbadora. 1998. Recovery of ceftazidime-resistant Klebsiella pneumoniae from pediatric liver and intestinal transplant recipients. Pediatr. Transplant. 2:224-230. [PubMed] [Google Scholar]
- 151.Gunseren, F., L. Mamikoglu, S. Ozturk, M. Yucesoy, K. Biberoglu, N. Yulug, M. Doganay, B. Sumerkan, S. Kocagoz, S. Unal, S. Cetin, S. Calangu, I. Koksal, H. Leblebicioglu, and M. Gunaydin. 1999. A surveillance study of antimicrobial resistance of gram-negative bacteria isolated from intensive care units in eight hospitals in Turkey. J. Antimicrob. Chemother. 43:373-378. [DOI] [PubMed] [Google Scholar]
- 152.Gupta, A., P. Della-Latta, B. Todd, P. San Gabriel, J. Haas, F. Wu, D. Rubenstein, and L. Saiman. 2004. Outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit linked to artificial nails. Infect. Control Hosp. Epidemiol. 25:210-215. [DOI] [PubMed] [Google Scholar]
- 153.Gur, D., T. L. Pitt, L. M. Hall, H. E. Akalin, and D. M. Livermore. 1992. Diversity of klebsiellae with extended-spectrum beta-lactamases at a Turkish university hospital. J. Hosp. Infect. 22:163-167. [DOI] [PubMed] [Google Scholar]
- 154.Hadziyannis, E., M. Tuohy, L. Thomas, G. W. Procop, J. A. Washington, and G. S. Hall. 2000. Screening and confirmatory testing for extended spectrum beta-lactamases (ESBL) in Escherichia coli, Klebsiella pneumoniae, and Klebsiella oxytoca clinical isolates. Diagn. Microbiol. Infect. Dis. 36:113-117. [DOI] [PubMed] [Google Scholar]
- 155.Hageman, J. C., S. K. Fridkin, J. M. Mohammed, C. D. Steward, R. P. Gaynes, and F. C. Tenover. 2003. Antimicrobial proficiency testing of National Nosocomial Infections Surveillance System hospital laboratories. Infect. Control Hosp. Epidemiol. 24:356-361. [DOI] [PubMed] [Google Scholar]
- 156.Hall, L. M., D. M. Livermore, D. Gur, M. Akova, and H. E. Akalin. 1993. OXA-11, an extended-spectrum variant of OXA-10 (PSE-2) beta-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 37:1637-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hammami, A., G. Arlet, S. Ben Redjeb, F. Grimont, A. Ben Hassen, A. Rekik, and A. Philippon. 1991. Nosocomial outbreak of acute gastroenteritis in a neonatal intensive care unit in Tunisia caused by multiply drug resistant Salmonella wien producing SHV-2 beta-lactamase. Eur. J. Clin. Microbiol. Infect. Dis. 10:641-646. [DOI] [PubMed] [Google Scholar]
- 158.Hanberger, H., J. A. Garcia-Rodriguez, M. Gobernado, H. Goossens, L. E. Nilsson, and M. J. Struelens. 1999. Antibiotic susceptibility among aerobic gram-negative bacilli in intensive care units in 5 European countries. French and Portuguese ICU Study Groups. JAMA 281:67-71. [DOI] [PubMed] [Google Scholar]
- 159.Hansen, D. S., F. Mestre, S. Alberti, S. Hernandez-Alles, D. Alvarez, A. Domenech-Sanchez, J. Gil, S. Merino, J. M. Tomas, and V. J. Benedi. 1999. Klebsiella pneumoniae lipopolysaccharide O typing: revision of prototype strains and O-group distribution among clinical isolates from different sources and countries. J. Clin. Microbiol. 37:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hanson, N. D., E. Smith Moland, and J. D. Pitout. 2001. Enzymatic characterization of TEM-63, a TEM-type extended spectrum beta-lactamase expressed in three different genera of Enterobacteriaceae from South Africa. Diagn. Microbiol. Infect. Dis. 40:199-201. [DOI] [PubMed] [Google Scholar]
- 161.Harris, A. D., L. Nemoy, J. A. Johnson, A. Martin-Carnahan, D. L. Smith, H. Standiford, and E. N. Perencevich. 2004. Co-carriage rates of vancomycin-resistant Enterococcus and extended-spectrum beta-lactamase-producing bacteria among a cohort of intensive care unit patients: implications for an active surveillance program. Infect. Control Hosp. Epidemiol. 25:105-108. [DOI] [PubMed] [Google Scholar]
- 162.Heritage, J., F. H. M'Zali, D. Gascoyne-Binzi, and P. M. Hawkey. 1999. Evolution and spread of SHV extended-spectrum beta-lactamases in gram-negative bacteria. J. Antimicrob. Chemother. 44:309-318. [DOI] [PubMed] [Google Scholar]
- 163.Hibbert-Rogers, L. C., J. Heritage, D. M. Gascoyne-Binzi, P. M. Hawkey, N. Todd, I. J. Lewis, and C. Bailey. 1995. Molecular epidemiology of ceftazidime resistant Enterobacteriaceae from patients on a paediatric oncology ward. J. Antimicrob. Chemother. 36:65-82. [DOI] [PubMed] [Google Scholar]
- 164.Ho, P. L., W. M. Chan, K. W. Tsang, S. S. Wong, and K. Young. 2002. Bacteremia caused by Escherichia coli producing extended-spectrum beta-lactamase: a case-control study of risk factors and outcomes. Scand. J. Infect. Dis. 34:567-573. [DOI] [PubMed] [Google Scholar]
- 165.Ho, P. L., K. H. Chow, K. Y. Yuen, W. S. Ng, and P. Y. Chau. 1998. Comparison of a novel, inhibitor-potentiated disc-diffusion test with other methods for the detection of extended-spectrum beta-lactamases in Escherichia coli and Klebsiella pneumoniae. J. Antimicrob. Chemother. 42:49-54. [DOI] [PubMed] [Google Scholar]
- 166.Hobson, R. P., F. M. MacKenzie, and I. M. Gould. 1996. An outbreak of multiply-resistant Klebsiella pneumoniae in the Grampian region of Scotland. J. Hosp. Infect. 33:249-262. [DOI] [PubMed] [Google Scholar]
- 167.Hollander, R., M. Ebke, H. Barck, and E. von Pritzbuer. 2001. Asymptomatic carriage of Klebsiella pneumoniae producing extended-spectrum beta-lactamase by patients in a neurological early rehabilitation unit: management of an outbreak. J. Hosp. Infect. 48:207-213. [DOI] [PubMed] [Google Scholar]
- 168.Hossain, A., M. J. Ferraro, R. M. Pino, R. B. Dew, 3rd, E. S. Moland, T. J. Lockhart, K. S. Thomson, R. V. Goering, and N. D. Hanson. 2004. Plasmid-mediated carbapenem-hydrolyzing enzyme KPC-2 in an Enterobacter sp. Antimicrob. Agents Chemother. 48:4438-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Howard, C., A. van Daal, G. Kelly, J. Schooneveldt, G. Nimmo, and P. M. Giffard. 2002. Identification and minisequencing-based discrimination of SHV beta-lactamases in nosocomial infection-associated Klebsiella pneumoniae in Brisbane, Australia. Antimicrob. Agents Chemother. 46:659-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huang, Z. M., P. H. Mao, Y. Chen, L. Wu, and J. Wu. 2004. Study on the molecular epidemiology of SHV type beta-lactamase-encoding genes of multiple-drug-resistant Acinetobacter baumannii. Zhonghua Liu Xing Bing Xue Za Zhi. 25:425-427. [PubMed] [Google Scholar]
- 171.Humeniuk, C., G. Arlet, V. Gautier, P. Grimont, R. Labia, and A. Philippon. 2002. Beta-lactamases of Kluyvera ascorbata, probable progenitors of some plasmid-encoded CTX-M types. Antimicrob. Agents Chemother. 46:3045-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ishii, Y., S. Kimura, J. Alba, K. Shiroto, M. Otsuka, N. Hashizume, K. Tamura, and K. Yamaguchi. 2005. Extended-spectrum β-lactamase-producing shiga toxin gene (stx1)-positive Escherichia coli O26:H11: a new concern. J. Clin. Microbiol. 43:1072-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ishii, Y., A. Ohno, H. Taguchi, S. Imajo, M. Ishiguro, and H. Matsuzawa. 1995. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A beta-lactamase isolated from Escherichia coli. Antimicrob. Agents Chemother. 39:2269-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Issack, M. I., K. P. Shannon, S. A. Qureshi, and G. L. French. 1995. Extended-spectrum beta-lactamase in Salmonella spp. J. Hosp. Infect. 30:319-321. [DOI] [PubMed] [Google Scholar]
- 175.Jackson, R. M., M. L. Heginbothom, and J. T. Magee. 1997. Epidemiological typing of Klebsiella pneumoniae by pyrolysis mass spectrometry. Zentralbl. Bakteriol. 285:252-257. [DOI] [PubMed] [Google Scholar]
- 176.Jacoby, G., P. Han, and J. Tran. 1997. Comparative in vitro activities of carbapenem L-749,345 and other antimicrobials against multiresistant gram-negative clinical pathogens. Antimicrob. Agents Chemother. 41:1830-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jacoby, G. A. 1997. Extended-spectrum beta-lactamases and other enzymes providing resistance to oxyimino-beta-lactams. Infect. Dis. Clin. N. Am. 11:875-887. [DOI] [PubMed] [Google Scholar]
- 178.Jacoby, G. A., N. Chow, and K. B. Waites. 2003. Prevalence of plasmid-mediated quinolone resistance. Antimicrob. Agents Chemother. 47:559-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jacoby, G. A., and P. Han. 1996. Detection of extended-spectrum beta-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli. J. Clin. Microbiol. 34:908-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jacoby, G. A., and A. A. Medeiros. 1991. More extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 35:1697-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jacoby, G. A., A. A. Medeiros, T. F. O'Brien, M. E. Pinto, and H. Jiang. 1988. Broad-spectrum, transmissible beta-lactamases. N. Engl. J. Med. 319:723-724. [DOI] [PubMed] [Google Scholar]
- 182.Jarlier, V., M. H. Nicolas, G. Fournier, and A. Philippon. 1988. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867-878. [DOI] [PubMed] [Google Scholar]
- 183.Jean, S. S., L. J. Teng, P. R. Hsueh, S. W. Ho, and K. T. Luh. 2002. Antimicrobial susceptibilities among clinical isolates of extended-spectrum cephalosporin-resistant Gram-negative bacteria in a Taiwanese University Hospital. J. Antimicrob. Chemother. 49:69-76. [DOI] [PubMed] [Google Scholar]
- 184.Jett, B. D., D. J. Ritchie, R. Reichley, T. C. Bailey, and D. F. Sahm. 1995. In vitro activities of various beta-lactam antimicrobial agents against clinical isolates of Escherichia coli and Klebsiella spp. resistant to oxyimino cephalosporins. Antimicrob. Agents Chemother. 39:1187-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jiang, X., Y. Ni, Y. Jiang, F. Yuan, L. Han, M. Li, H. Liu, L. Yang, and Y. Lu. 2005. Outbreak of infection caused by Enterobacter cloacae producing the novel VEB-3 beta-lactamase in China. J. Clin. Microbiol. 43:826-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kang, C. I., S. H. Kim, W. B. Park, K. D. Lee, H. B. Kim, E. C. Kim, M. D. Oh, and K. W. Choe. 2004. Bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for mortality and treatment outcome, with special emphasis on antimicrobial therapy. Antimicrob. Agents Chemother. 48:4574-4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Karadenizli, A., B. Mutlu, E. Okay, F. Kolayli, and H. Vahaboglu. 2001. Piperacillin with and without tazobactam against extended-spectrum beta-lactamase-producing Pseudomonas aeruginosa in a rat thigh abscess model. Chemotherapy 47:292-296. [DOI] [PubMed] [Google Scholar]
- 188.Karas, J. A., D. G. Pillay, D. Muckart, and A. W. Sturm. 1996. Treatment failure due to extended spectrum beta-lactamase. J. Antimicrob. Chemother. 37:203-204. [DOI] [PubMed] [Google Scholar]
- 189.Karim, A., L. Poirel, S. Nagarajan, and P. Nordmann. 2001. Plasmid-mediated extended-spectrum beta-lactamase (CTX-M-3 like) from India and gene association with insertion sequence ISEcp1. FEMS Microbiol. Lett. 201:237-241. [DOI] [PubMed] [Google Scholar]
- 190.Kariuki, S., J. E. Corkill, G. Revathi, R. Musoke, and C. A. Hart. 2001. Molecular characterization of a novel plasmid-encoded cefotaximase (CTX-M-12) found in clinical Klebsiella pneumoniae isolates from Kenya. Antimicrob. Agents Chemother. 45:2141-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kartali, G., E. Tzelepi, S. Pournaras, C. Kontopoulou, F. Kontos, D. Sofianou, A. N. Maniatis, and A. Tsakris. 2002. Outbreak of infections caused by Enterobacter cloacae producing the integron-associated beta-lactamase IBC-1 in a neonatal intensive care unit of a Greek hospital. Antimicrob. Agents Chemother. 46:1577-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kaye, K. S., S. Cosgrove, A. Harris, G. M. Eliopoulos, and Y. Carmeli. 2001. Risk factors for emergence of resistance to broad-spectrum cephalosporins among Enterobacter spp. Antimicrob. Agents Chemother. 45:2628-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim, B. N., J. H. Woo, M. N. Kim, J. Ryu, and Y. S. Kim. 2002. Clinical implications of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae bacteraemia. J. Hosp. Infect. 52:99-106. [DOI] [PubMed] [Google Scholar]
- 194.Kim, J., and H. J. Lee. 2000. Rapid discriminatory detection of genes coding for SHV beta-lactamases by ligase chain reaction. Antimicrob. Agents Chemother. 44:1860-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kim, S., J. Kim, Y. Kang, Y. Park, and B. Lee. 2004. Occurrence of extended-spectrum beta-lactamases in members of the genus Shigella in the Republic of Korea. J. Clin. Microbiol. 42:5264-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kim, Y. K., H. Pai, H. J. Lee, S. E. Park, E. H. Choi, J. Kim, J. H. Kim, and E. C. Kim. 2002. Bloodstream infections by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob. Agents Chemother. 46:1481-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Knothe, H., P. Shah, V. Krcmery, M. Antal, and S. Mitsuhashi. 1983. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 11:315-317. [DOI] [PubMed] [Google Scholar]
- 198.Koh, T. H., L. H. Sng, G. S. Babini, N. Woodford, D. M. Livermore, and L. M. Hall. 2001. Carbapenem-resistant Klebsiella pnuemoniae in Singapore producing IMP-1 beta-lactamase and lacking an outer membrane protein. Antimicrob. Agents Chemother. 45:1939-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Komatsu, M., M. Aihara, K. Shimakawa, M. Iwasaki, Y. Nagasaka, S. Fukuda, S. Matsuo, and Y. Iwatani. 2003. Evaluation of MicroScan ESBL confirmation panel for Enterobacteriaceae-producing, extended-spectrum beta-lactamases isolated in Japan. Diagn. Microbiol. Infect. Dis. 46:125-130. [DOI] [PubMed] [Google Scholar]
- 200.Komatsu, M., N. Ikeda, M. Aihara, Y. Nakamachi, S. Kinoshita, K. Yamasaki, and K. Shimakawa. 2001. Hospital outbreak of MEN-1-derived extended spectrum beta-lactamase-producing Klebsiella pneumoniae. J. Infect. Chemother. 7:94-101. [DOI] [PubMed] [Google Scholar]
- 201.Kruger, T., D. Szabo, K. H. Keddy, K. Deeley, J. W. Marsh, A. M. Hujer, R. A. Bonomo, and D. L. Paterson. 2004. Infections with nontyphoidal Salmonella species producing TEM-63 or a novel TEM enzyme, TEM-131, in South Africa. Antimicrob. Agents Chemother. 48:4263-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kurokawa, H., T. Yagi, N. Shibata, K. Shibayama, K. Kamachi, and Y. Arakawa. 2000. A new SHV-derived extended-spectrum beta-lactamase (SHV-24) that hydrolyzes ceftazidime through a single-amino-acid substitution (D179G) in the-loop. Antimicrob. Agents Chemother. 44:1725-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kwon, N. Y., J. D. Kim, and H. J. Pai. 2002. The resistance mechanisms of b-lactam antimicrobials in clinical isolates of Acinetobacter baumannii. Korean J. Intern. Med. 17:94-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Labia, R. 1999. Analysis of the bla(toho) gene coding for Toho-2 beta-lactamase. Antimicrob. Agents Chemother. 43:2576-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lautenbach, E., J. B. Patel, W. B. Bilker, P. H. Edelstein, and N. O. Fishman. 2001. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin. Infect. Dis. 32:1162-1171. [DOI] [PubMed] [Google Scholar]
- 206.Lautenbach, E., B. L. Strom, W. B. Bilker, J. B. Patel, P. H. Edelstein, and N. O. Fishman. 2001. Epidemiological investigation of fluoroquinolone resistance in infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Clin. Infect. Dis. 33:1288-1294. [DOI] [PubMed] [Google Scholar]
- 207.Lebessi, E., H. Dellagrammaticas, P. T. Tassios, L. S. Tzouvelekis, S. Ioannidou, M. Foustoukou, and N. J. Legakis. 2002. Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit in the high-prevalence area of Athens, Greece. J. Clin. Microbiol. 40:799-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lebessi, E., G. Stamos, M. Foustoukou, S. Vourli, N. J. Legakis, and L. S. Tzouvelekis. 2003. Performance of methods for detection of extended-spectrum beta-lactamases applied to clinical enterobacterial strains producing IBC-type beta-lactamases. J. Clin. Microbiol. 41:912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lee, S. H., J. Y. Kim, S. H. Shin, Y. J. An, Y. W. Choi, Y. C. Jung, H. I. Jung, E. S. Sohn, S. H. Jeong, and K. J. Lee. 2003. Dissemination of SHV-12 and characterization of new AmpC-type beta-lactamase genes among clinical isolates of Enterobacter Species in Korea. J. Clin. Microbiol. 41:2477-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lee, S. O., E. S. Lee, S. Y. Park, S. Y. Kim, Y. H. Seo, and Y. K. Cho. 2004. Reduced use of third-generation cephalosporins decreases the acquisition of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. Infect. Control Hosp. Epidemiol. 25:832-837. [DOI] [PubMed] [Google Scholar]
- 211.Legakis, N. J., L. S. Tzouvelekis, G. Hatzoudis, E. Tzelepi, A. Gourkou, T. L. Pitt, and A. C. Vatopoulos. 1995. Klebsiella pneumoniae infections in Greek hospitals. Dissemination of plasmids encoding an SHV-5 type beta-lactamase. J. Hosp. Infect. 31:177-187. [DOI] [PubMed] [Google Scholar]
- 212.Leverstein-van Hall, M. A., A. C. Fluit, A. Paauw, A. T. Box, S. Brisse, and J. Verhoef. 2002. Evaluation of the Etest ESBL and the BD Phoenix, VITEK 1, and VITEK 2 automated instruments for detection of extended-spectrum beta-lactamases in multiresistant Escherichia coli and Klebsiella spp. J. Clin. Microbiol. 40:3703-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Levison, M. E. 2002. Plasmid-mediated extended-spectrum beta-lactamases in organisms other than Klebsiella pneumoniae and Escherichia coli: a hidden reservoir of transferable resistance genes. Curr. Infect. Dis. Rep. 4:181-183. [DOI] [PubMed] [Google Scholar]
- 214.Levison, M. E., Y. V. Mailapur, S. K. Pradhan, G. A. Jacoby, P. Adams, C. L. Emery, P. L. May, and P. G. Pitsakis. 2002. Regional occurrence of plasmid-mediated SHV-7, an extended-spectrum beta-lactamase, in Enterobacter cloacae in Philadelphia Teaching Hospitals. Clin. Infect. Dis. 35:1551-1554. [DOI] [PubMed] [Google Scholar]
- 215.Lewis, M. T., K. Yamaguchi, D. J. Biedenbach, and R. N. Jones. 1999. In vitro evaluation of cefepime and other broad-spectrum beta-lactams in 22 medical centers in Japan: a phase II trial comparing two annual organism samples. The Japan Antimicrobial Resistance Study Group. Diagn. Microbiol. Infect. Dis. 35:307-315. [DOI] [PubMed] [Google Scholar]
- 216.Lincopan, N., J. A. McCulloch, C. Reinert, V. C. Cassettari, A. C. Gales, and E. M. Mamizuka. 2005. First isolation of metallo-beta-lactamase-producing multiresistant Klebsiella pneumoniae from a patient in Brazil. J. Clin. Microbiol. 43:516-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Linkin, D. R., N. O. Fishman, J. B. Patel, J. D. Merrill, and E. Lautenbach. 2004. Risk factors for extended-spectrum beta-lactamase-producing Enterobacteriaceae in a neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 25:781-783. [DOI] [PubMed] [Google Scholar]
- 218.Liu, P. Y., J. C. Tung, S. C. Ke, and S. L. Chen. 1998. Molecular epidemiology of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates in a district hospital in Taiwan. J. Clin. Microbiol. 36:2759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Livermore, D. M. 1995. Beta-lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Livermore, D. M., M. Struelens, J. Amorim, F. Baquero, J. Bille, R. Canton, S. Henning, S. Gatermann, A. Marchese, H. Mittermayer, C. Nonhoff, K. J. Oakton, F. Praplan, H. Ramos, G. C. Schito, J. Van Eldere, J. Verhaegen, J. Verhoef, and M. R. Visser. 2002. Multicentre evaluation of the VITEK 2 Advanced Expert System for interpretive reading of antimicrobial resistance tests. J. Antimicrob. Chemother. 49:289-300. [DOI] [PubMed] [Google Scholar]
- 221.Livermore, D. M., and M. Yuan. 1996. Antibiotic resistance and production of extended-spectrum beta-lactamases amongst Klebsiella spp. from intensive care units in Europe. J. Antimicrob. Chemother. 38:409-424. [DOI] [PubMed] [Google Scholar]
- 222.Lucet, J. C., S. Chevret, D. Decre, D. Vanjak, A. Macrez, J. P. Bedos, M. Wolff, and B. Regnier. 1996. Outbreak of multiply resistant enterobacteriaceae in an intensive care unit: epidemiology and risk factors for acquisition. Clin. Infect. Dis. 22:430-436. [DOI] [PubMed] [Google Scholar]
- 223.Lucet, J. C., D. Decre, A. Fichelle, M. L. Joly-Guillou, M. Pernet, C. Deblangy, M. J. Kosmann, and B. Regnier. 1999. Control of a prolonged outbreak of extended-spectrum beta-lactamase-producing enterobacteriaceae in a university hospital. Clin. Infect. Dis. 29:1411-1418. [DOI] [PubMed] [Google Scholar]
- 224.Luzzaro, F., J. D. Docquier, C. Colinon, A. Endimiani, G. Lombardi, G. Amicosante, G. M. Rossolini, and A. Toniolo. 2004. Emergence in Klebsiella pneumoniae and Enterobacter cloacae clinical isolates of the VIM-4 metallo-beta-lactamase encoded by a conjugative plasmid. Antimicrob. Agents Chemother. 48:648-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Luzzaro, F., E. Mantengoli, M. Perilli, G. Lombardi, V. Orlandi, A. Orsatti, G. Amicosante, G. M. Rossolini, and A. Toniolo. 2001. Dynamics of a nosocomial outbreak of multidrug-resistant Pseudomonas aeruginosa producing the PER-1 extended-spectrum beta-lactamase. J. Clin. Microbiol. 39:1865-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ma, L., Y. Ishii, F. Y. Chang, K. Yamaguchi, M. Ho, and L. K. Siu. 2002. CTX-M-14, a plasmid-mediated CTX-M type extended-spectrum beta-lactamase isolated from Escherichia coli. Antimicrob. Agents Chemother. 46:1985-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ma, L., Y. Ishii, M. Ishiguro, H. Matsuzawa, and K. Yamaguchi. 1998. Cloning and sequencing of the gene encoding Toho-2, a class A beta-lactamase preferentially inhibited by tazobactam. Antimicrob. Agents Chemother. 42:1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.MacKenzie, F. M., K. J. Forbes, T. Dorai-John, S. G. Amyes, and I. M. Gould. Emergence of a carbapenem-resistant Klebsiella pneumoniae. Lancet 350:783, 1997. [DOI] [PubMed] [Google Scholar]
- 229.MacKenzie, F. M., C. A. Miller, and I. M. Gould. 2002. Comparison of screening methods for TEM- and SHV-derived extended-spectrum beta-lactamase detection. Clin. Microbiol. Infect. 8:715-724. [DOI] [PubMed] [Google Scholar]
- 230.Macrae, M. B., K. P. Shannon, D. M. Rayner, A. M. Kaiser, P. N. Hoffman, and G. L. French. 2001. A simultaneous outbreak on a neonatal unit of two strains of multiply antibiotic resistant Klebsiella pneumoniae controllable only by ward closure. J. Hosp. Infect. 49:183-192. [DOI] [PubMed] [Google Scholar]
- 231.Maglio, D., C. Ong, M. A. Banevicius, Q. Geng, C. H. Nightingale, and D. P. Nicolau. 2004. Determination of the in vivo pharmacodynamic profile of cefepime against extended-spectrum-beta-lactamase-producing Escherichia coli at various inocula. Antimicrob. Agents Chemother. 48:1941-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mammeri, H., G. Laurans, M. Eveillard, S. Castelain, and F. Eb. 2001. Coexistence of SHV-4- and TEM-24-producing Enterobacter aerogenes strains before a large outbreak of TEM-24-producing strains in a French hospital. J. Clin. Microbiol. 39:2184-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mammeri, H., M. Van De Loo, L. Poirel, L. Martinez-Martinez, and P. Nordmann. 2005. Emergence of plasmid-mediated quinolone resistance in Escherichia coli in Europe. Antimicrob. Agents Chemother. 49:71-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mangeney, N., P. Niel, G. Paul, E. Faubert, S. Hue, C. Dupeyron, F. Louarn, and G. Leluan. 2000. A 5-year epidemiological study of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates in a medium- and long-stay neurological unit. J. Appl. Microbiol. 88:504-511. [DOI] [PubMed] [Google Scholar]
- 235.Martinez-Aguilar, G., C. M. Alpuche-Aranda, C. Anaya, D. Alcantar-Curiel, C. Gayosso, C. Daza, C. Mijares, J. C. Tinoco, and J. I. Santos. 2001. Outbreak of nosocomial sepsis and pneumonia in a newborn intensive care unit by multiresistant extended-spectrum beta-lactamase-producing Klebsiella pneumoniae: high impact on mortality. Infect. Control Hosp. Epidemiol. 22:725-728. [DOI] [PubMed] [Google Scholar]
- 236.Martinez-Martinez, L., A. Pascual, C. Conejo Mdel, I. Garcia, P. Joyanes, A. Domenech-Sanchez, and V. J. Benedi. 2002. Energy-dependent accumulation of norfloxacin and porin expression in clinical isolates of Klebsiella pneumoniae and relationship to extended-spectrum beta-lactamase production. Antimicrob. Agents Chemother. 46:3926-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Martinez-Martinez, L., A. Pascual, S. Hernandez-Alles, D. Alvarez-Diaz, A. I. Suarez, J. Tran, V. J. Benedi, and G. A. Jacoby. 1999. Roles of beta-lactamases and porins in activities of carbapenems and cephalosporins against Klebsiella pneumoniae. Antimicrob. Agents Chemother. 43:1669-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Martinez-Martinez, L., A. Pascual, and G. A. Jacoby. 1998. Quinolone resistance from a transferable plasmid. Lancet 351:797-799. [DOI] [PubMed] [Google Scholar]
- 239.Marty, L., and V. Jarlier. 1998. Surveillance of multiresistant bacteria: justification, role of the laboratory, indicators, and recent French data. Pathol. Biol. (Paris) 46:217-226. [PubMed] [Google Scholar]
- 240.Matsumoto, Y., and M. Inoue. 1999. Characterization of SFO-1, a plasmid-mediated inducible class A beta-lactamase from Enterobacter cloacae. Antimicrob. Agents Chemother. 43:307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mavroidi, A., E. Tzelepi, A. Tsakris, V. Miriagou, D. Sofianou, and L. S. Tzouvelekis. 2001. An integron-associated beta-lactamase (IBC-2) from Pseudomonas aeruginosa is a variant of the extended-spectrum beta-lactamase IBC-1. J. Antimicrob. Chemother. 48:627-630. [DOI] [PubMed] [Google Scholar]
- 242.Mayer, L. W. 1988. Use of plasmid profiles in epidemiologic surveillance of disease outbreaks and in tracing the transmission of antibiotic resistance. Clin. Microbiol. Rev. 1:228-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Medeiros, A. A. 1997. Evolution and dissemination of beta-lactamases accelerated by generations of beta-lactam antibiotics. Clin. Infect. Dis. 24 Suppl. 1:S19-45. [DOI] [PubMed] [Google Scholar]
- 244.Medeiros, A. A., and J. Crellin. 1997. Comparative susceptibility of clinical isolates producing extended spectrum beta-lactamases to ceftibuten: effect of large inocula. Pediatr. Infect. Dis. J. 16:S49-55. [DOI] [PubMed] [Google Scholar]
- 245.Mendes, C., A. Hsiung, C. Kiffer, C. Oplustil, S. Sinto, I. Mimica, and C. Zoccoli. 2000. Evaluation of the in vitro activity of 9 antimicrobials against bacterial strains isolated from patients in intensive care units in Brazil: MYSTIC Antimicrobial Surveillance Program. Braz. J. Infect. Dis. 4:236-244. [PubMed] [Google Scholar]
- 246.Meyer, K. S., C. Urban, J. A. Eagan, B. J. Berger, and J. J. Rahal. 1993. Nosocomial outbreak of Klebsiella infection resistant to late-generation cephalosporins. Ann. Intern. Med. 119:353-358. [DOI] [PubMed] [Google Scholar]
- 247.Mhand, R. A., N. Brahimi, N. Moustaoui, N. El Mdaghri, H. Amarouch, F. Grimont, E. Bingen, and M. Benbachir. 1999. Characterization of extended-spectrum beta-lactamase-producing Salmonella typhimurium by phenotypic and genotypic typing methods. J. Clin. Microbiol. 37:3769-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Midolo, P. D., D. Matthews, C. D. Fernandez, and T. G. Kerr. 2002. Detection of extended spectrum beta-lactamases in the routine clinical microbiology laboratory. Pathology 34:362-364. [DOI] [PubMed] [Google Scholar]
- 249.Mirelis, B., F. Navarro, E. Miro, R. J. Mesa, P. Coll, and G. Prats. 2003. Community transmission of extended-spectrum beta-lactamase. Emerg. Infect. Dis. 9:1024-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Miriagou, V., E. Tzelepi, G. L. Daikos, P. T. Tassios, and L. S. Tzouvelekis. 2005. Panresistance in VIM-1-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 55:810-811. [DOI] [PubMed] [Google Scholar]
- 251.Miriagou, V., L. S. Tzouvelekis, S. Rossiter, E. Tzelepi, F. J. Angulo, and J. M. Whichard. 2003. Imipenem resistance in a Salmonella clinical strain due to plasmid-mediated class A carbapenemase KPC-2. Antimicrob. Agents Chemother. 47:1297-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Miro, E., M. del Cuerpo, F. Navarro, M. Sabate, B. Mirelis, and G. Prats. 1998. Emergence of clinical Escherichia coli isolates with decreased susceptibility to ceftazidime and synergic effect with co-amoxiclav due to SHV-1 hyperproduction. J. Antimicrob. Chemother. 42:535-538. [DOI] [PubMed] [Google Scholar]
- 253.Moland, E. S., J. A. Black, A. Hossain, N. D. Hanson, K. S. Thomson, and S. Pottumarthy. 2003. Discovery of CTX-M-like extended-Spectrum beta-lactamases in Escherichia coli isolates from five U.S. states. Antimicrob. Agents Chemother. 47:2382-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Moland, E. S., J. A. Black, J. Ourada, M. D. Reisbig, N. D. Hanson, and K. S. Thomson. 2002. Occurrence of newer beta-lactamases in Klebsiella pneumoniae isolates from 24 U.S. hospitals. Antimicrob. Agents Chemother. 46:3837-3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Moland, E. S., C. C. Sanders, and K. S. Thomson. 1998. Can. results obtained with commercially available MicroScan microdilution panels serve as an indicator of beta-lactamase production among Escherichia coli and Klebsiella isolates with hidden resistance to expanded-spectrum cephalosporins and aztreonam? J. Clin. Microbiol. 36:2575-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Monnet, D. L., J. W. Biddle, J. R. Edwards, D. H. Culver, J. S. Tolson, W. J. Martone, F. C. Tenover, and R. P. Gaynes. 1997. Evidence of interhospital transmission of extended-spectrum beta-lactam-resistant Klebsiella pneumoniae in the United States, 1986 to 1993. The National Nosocomial Infections Surveillance System. Infect. Control Hosp. Epidemiol. 18:492-498. [DOI] [PubMed] [Google Scholar]
- 257.Morosini, M. I., R. Canton, J. Martinez-Beltran, M. C. Negri, J. C. Perez-Diaz, F. Baquero, and J. Blazquez. 1995. New extended-spectrum TEM-type beta-lactamase from Salmonella enterica subsp. enterica isolated in a nosocomial outbreak. Antimicrob. Agents Chemother. 39:458-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Muder, R. R., C. Brennen, S. D. Drenning, J. E. Stout, and M. M. Wagener. 1997. Multiply antibiotic-resistant gram-negative bacilli in a long-term-care facility: a case-control study of patient risk factors and prior antibiotic use. Infect. Control Hosp. Epidemiol. 18:809-813. [PubMed] [Google Scholar]
- 259.Mulgrave, L. 1990. Extended broad-spectrum beta-lactamases in Australia. Med. J. Aust. 152:444-445. [DOI] [PubMed] [Google Scholar]
- 260.Mulgrave, L., and P. V. Attwood. 1993. Characterization of an SHV-5 related extended broad-spectrum beta-lactamase in Enterobacteriaceae from Western Australia. Pathology 25:71-75. [DOI] [PubMed] [Google Scholar]
- 261.Munday, C. J., G. M. Whitehead, N. J. Todd, M. Campbell, and P. M. Hawkey. 2004. Predominance and genetic diversity of community- and hospital-acquired CTX-M extended-spectrum beta-lactamases in York, UK. J. Antimicrob. Chemother. 54:628-633. [DOI] [PubMed] [Google Scholar]
- 262.Munoz Bellido, J. L., and J. A. Garcia-Rodriguez. 1998. Aztreonam-clavulanic acid synergy does not mean extended-spectrum beta-lactamase in Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 41:493-494. [DOI] [PubMed] [Google Scholar]
- 263.Mushtaq, S., N. Woodford, N. Potz, and D. M. Livermore. 2003. Detection of CTX-M-15 extended-spectrum beta-lactamase in the United Kingdom. J. Antimicrob. Chemother. 52:528-529. [DOI] [PubMed] [Google Scholar]
- 264.Musoke, R. N., and G. Revathi. 2000. Emergence of multidrug-resistant gram-negative organisms in a neonatal unit and the therapeutic implications. J. Trop. Pediatr. 46:86-91. [DOI] [PubMed] [Google Scholar]
- 265.M'Zali, F. H., A. Chanawong, K. G. Kerr, D. Birkenhead, and P. M. Hawkey. 2000. Detection of extended-spectrum beta-lactamases in members of the family enterobacteriaceae: comparison of the MAST DD test, the double disc and the Etest ESBL. J. Antimicrob. Chemother. 45:881-885. [DOI] [PubMed] [Google Scholar]
- 266.Naas, T., L. Poirel, A. Karim, and P. Nordmann. 1999. Molecular characterization of In50, a class 1 integron encoding the gene for the extended-spectrum beta-lactamase VEB-1 in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 176:411-419. [DOI] [PubMed] [Google Scholar]
- 267.National Committee for Clinical Laboratory Standards. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement (M100-S15). National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 268.National Nosocomial Infections Surveillance. 2002. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 to June 2002, issued August 2002. Am. J. Infect. Control 30:458-475. [DOI] [PubMed] [Google Scholar]
- 269.Naumiuk, L., A. Samet, and E. Dziemaszkiewicz. 2001. Cefepime in vitro activity against derepressed extended-spectrum beta-lactamase (ESBL)-producing and non-ESBL-producing Enterobacter cloacae by a disc diffusion method. J. Antimicrob. Chemother. 48:321-322. [DOI] [PubMed] [Google Scholar]
- 270.Naumovski, L., J. P. Quinn, D. Miyashiro, M. Patel, K. Bush, S. B. Singer, D. Graves, T. Palzkill, and A. M. Arvin. 1992. Outbreak of ceftazidime resistance due to a novel extended-spectrum beta-lactamase in isolates from cancer patients. Antimicrob. Agents Chemother. 36:1991-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Navon-Venezia, S., O. Hammer-Munz, D. Schwartz, D. Turner, B. Kuzmenko, and Y. Carmeli. 2003. Occurrence and phenotypic characteristics of extended-spectrum beta-lactamases among members of the family Enterobacteriaceae at the Tel-Aviv Medical Center (Israel) and evaluation of diagnostic tests. J. Clin. Microbiol. 41:155-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Neuhauser, M. M., R. A. Weinstein, R. Rydman, L. H. Danziger, G. Karam, and J. P. Quinn. 2003. Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA 289:885-888. [DOI] [PubMed] [Google Scholar]
- 273.Neuwirth, C., S. Madec, E. Siebor, A. Pechinot, J. M. Duez, M. Pruneaux, M. Fouchereau-Peron, A. Kazmierczak, and R. Labia. 2001. TEM-89 beta-lactamase produced by a Proteus mirabilis clinical isolate: new complex mutant (CMT 3) with mutations in both TEM-59 (IRT-17) and TEM-3. Antimicrob. Agents Chemother. 45:3591-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Neuwirth, C., E. Siebor, J. Lopez, A. Pechinot, and A. Kazmierczak. 1996. Outbreak of TEM-24-producing Enterobacter aerogenes in an intensive care unit and dissemination of the extended-spectrum beta-lactamase to other members of the family enterobacteriaceae. J. Clin. Microbiol. 34:76-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Nordmann, P., and T. Naas. 1994. Sequence analysis of PER-1 extended-spectrum beta-lactamase from Pseudomonas aeruginosa and comparison with class A beta-lactamases. Antimicrob. Agents Chemother. 38:104-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Nordmann, P., E. Ronco, T. Naas, C. Duport, Y. Michel-Briand, and R. Labia. 1993. Characterization of a novel extended-spectrum beta-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 37:962-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Nouvellon, M., J. L. Pons, D. Sirot, M. L. Combe, and J. F. Lemeland. 1994. Clonal outbreaks of extended-spectrum beta-lactamase-producing strains of Klebsiella pneumoniae demonstrated by antibiotic susceptibility testing, beta-lactamase typing, and multilocus enzyme electrophoresis. J. Clin. Microbiol. 32:2625-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Oliver, A., J. C. Perez-Diaz, T. M. Coque, F. Baquero, and R. Canton. 2001. Nucleotide sequence and characterization of a novel cefotaxime-hydrolyzing beta-lactamase (CTX-M-10) isolated in Spain. Antimicrob. Agents Chemother. 45:616-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Oliver, A., L. M. Weigel, J. K. Rasheed, J. E. McGowan Juniorperiod, Jr., P. Raney, and F. C. Tenover. 2002. Mechanisms of decreased susceptibility to cefpodoxime in Escherichia coli. Antimicrob. Agents Chemother. 46:3829-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Orskov, I., F. Orskov, B. Jann, and K. Jann. 1977. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol. Rev. 41:667-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Otman, J., E. D. Cavassin, M. E. Perugini, and M. C. Vidotto. 2002. An outbreak of extended-spectrum beta-lactamase-producing Klebsiella species in a neonatal intensive care unit in Brazil. Infect. Control Hosp. Epidemiol. 23:8-9. [DOI] [PubMed] [Google Scholar]
- 282.Pagani, L., E. Mantengoli, R. Migliavacca, E. Nucleo, S. Pollini, M. Spalla, R. Daturi, E. Romero, and G. M. Rossolini. 2004. Multifocal detection of multidrug-resistant Pseudomonas aeruginosa producing the PER-1 extended-spectrum beta-lactamase in Northern Italy. J. Clin. Microbiol. 42:2523-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Pagani, L., R. Migliavacca, L. Pallecchi, C. Matti, E. Giacobone, G. Amicosante, E. Romero, and G. M. Rossolini. 2002. Emerging extended-spectrum beta-lactamases in Proteus mirabilis. J. Clin. Microbiol. 40:1549-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Pai, H., E. H. Choi, H. J. Lee, J. Y. Hong, and G. A. Jacoby. 2001. Identification of CTX-M-14 extended-spectrum beta-lactamase in clinical isolates of Shigella sonnei, Escherichia coli, and Klebsiella pneumoniae in Korea. J. Clin. Microbiol. 39:3747-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Pangon, B., C. Bizet, A. Bure, F. Pichon, A. Philippon, B. Regnier, and L. Gutmann. 1989. In vivo selection of a cephamycin-resistant, porin-deficient mutant of Klebsiella pneumoniae producing a TEM-3 beta-lactamase. J. Infect. Dis. 159:1005-1006. [DOI] [PubMed] [Google Scholar]
- 286.Paterson, D. L. 2002. Looking for risk factors for the acquisition of antibiotic resistance: a 21st-century approach. Clin. Infect. Dis. 34:1564-1567. [DOI] [PubMed] [Google Scholar]
- 287.Paterson, D. L. 2000. Recommendation for treatment of severe infections caused by Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs). Clin. Microbiol. Infect. 6:460-463. [DOI] [PubMed] [Google Scholar]
- 288.Paterson, D. L., K. M. Hujer, A. M. Hujer, B. Yeiser, M. D. Bonomo, L. B. Rice, and R. A. Bonomo. 2003. Extended-spectrum beta-lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: Dominance and widespread prevalence of SHV- and CTX-M-type beta-lactamases. Antimicrob. Agents Chemother. 47:3554-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Paterson, D. L., W. C. Ko, A. Von Gottberg, J. M. Casellas, L. Mulazimoglu, K. P. Klugman, R. A. Bonomo, L. B. Rice, J. G. McCormack, and V. L. Yu. 2001. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J. Clin. Microbiol. 39:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Paterson, D. L., W. C. Ko, A. Von Gottberg, S. Mohapatra, J. M. Casellas, H. Goossens, L. Mulazimoglu, G. Trenholme, K. P. Klugman, R. A. Bonomo, L. B. Rice, M. M. Wagener, J. G. McCormack, and V. L. Yu. 2004. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin. Infect. Dis. 39:31-37. [DOI] [PubMed] [Google Scholar]
- 291.Paterson, D. L., W. C. Ko, A. Von Gottberg, S. Mohapatra, J. M. Casellas, H. Goossens, L. Mulazimoglu, G. Trenholme, K. P. Klugman, R. A. Bonomo, L. B. Rice, M. M. Wagener, J. G. McCormack, and V. L. Yu. 2004. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann. Intern. Med. 140:26-32. [DOI] [PubMed] [Google Scholar]
- 292.Paterson, D. L., L. Mulazimoglu, J. M. Casellas, W. C. Ko, H. Goossens, A. Von Gottberg, S. Mohapatra, G. M. Trenholme, K. P. Klugman, J. G. McCormack, and V. L. Yu. 2000. Epidemiology of ciprofloxacin resistance and its relationship to extended-spectrum beta-lactamase production in Klebsiella pneumoniae isolates causing bacteremia. Clin. Infect. Dis. 30:473-478. [DOI] [PubMed] [Google Scholar]
- 293.Paterson, D. L., N. Singh, T. Gayowski, and I. R. Marino. 1999. Fatal infection due to extended-spectrum beta-lactamase-producing Escherichia coli: implications for antibiotic choice for spontaneous bacterial peritonitis. Clin. Infect. Dis. 28:683-684. [DOI] [PubMed] [Google Scholar]
- 294.Paterson, D. L., N. Singh, J. D. Rihs, C. Squier, B. L. Rihs, and R. R. Muder. 2001. Control of an outbreak of infection due to extended-spectrum beta-lactamase-producing Escherichia coli in a liver transplantation unit. Clin. Infect. Dis. 33:126-128. [DOI] [PubMed] [Google Scholar]
- 295.Paterson, D. L., and V. L. Yu. 1999. Extended-spectrum beta-lactamases: a call for improved detection and control. Clin. Infect. Dis. 29:1419-1422. [DOI] [PubMed] [Google Scholar]
- 296.Patterson, J. E., T. C. Hardin, C. A. Kelly, R. C. Garcia, and J. H. Jorgensen. 2000. Association of antibiotic utilization measures and control of multiple-drug resistance in Klebsiella pneumoniae. Infect. Control Hosp. Epidemiol. 21:455-458. [DOI] [PubMed] [Google Scholar]
- 297.Pattharachayakul, S., M. M. Neuhauser, J. P. Quinn, and S. L. Pendland. 2003. Extended-spectrum beta-lactamase (ESBL)-producing Klebsiella pneumoniae: activity of single versus combination agents. J. Antimicrob. Chemother. 51:737-739. [DOI] [PubMed] [Google Scholar]
- 298.Pena, C., M. Pujol, C. Ardanuy, A. Ricart, R. Pallares, J. Linares, J. Ariza, and F. Gudiol. 1998. Epidemiology and successful control of a large outbreak due to Klebsiella pneumoniae producing extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 42:53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Pena, C., M. Pujol, A. Ricart, C. Ardanuy, J. Ayats, J. Linares, F. Garrigosa, J. Ariza, and F. Gudiol. 1997. Risk factors for faecal carriage of Klebsiella pneumoniae producing extended spectrum beta-lactamase (ESBL-KP) in the intensive care unit. J. Hosp. Infect. 35:9-16. [DOI] [PubMed] [Google Scholar]
- 300.Pereira, M., M. Perilli, E. Mantengoli, F. Luzzaro, A. Toniolo, G. M. Rossolini, and G. Amicosante. 2000. PER-1 extended-spectrum beta-lactamase production in an Alcaligenes faecalis clinical isolate resistant to expanded-spectrum cephalosporins and monobactams from a hospital in northern Italy. Microb. Drug Resist. 6:85-90. [DOI] [PubMed] [Google Scholar]
- 301.Perilli, M., A. Felici, N. Franceschini, A. De Santis, L. Pagani, F. Luzzaro, A. Oratore, G. M. Rossolini, J. R. Knox, and G. Amicosante. 1997. Characterization of a new TEM-derived beta-lactamase produced in a Serratia marcescens strain. Antimicrob. Agents Chemother. 41:2374-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Pessoa-Silva, C. L., B. Meurer Moreira, V. Camara Almeida, B. Flannery, M. C. Almeida Lins, J. L. Mello Sampaio, L. Martins Teixeira, L. E. Vaz Miranda, L. W. Riley, and J. L. Gerberding. 2003. Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit: risk factors for infection and colonization. J. Hosp. Infect. 53:198-206. [DOI] [PubMed] [Google Scholar]
- 303.Pessoa-Silva, C. L., C. M. Toscano, B. M. Moreira, A. L. Santos, A. C. Frota, C. A. Solari, E. L. Amorim, G. Carvalho Mda, L. M. Teixeira, and W. R. Jarvis. 2002. Infection due to extended-spectrum beta-lactamase-producing Salmonella enterica subsp. enterica serotype infantis in a neonatal unit. J. Pediatr. 141:381-387. [DOI] [PubMed] [Google Scholar]
- 304.Petit, A., H. Ben Yaghlane-Bouslama, L. Sofer, and R. Labia. 1992. Does high level production of SHV-type penicillinase confer resistance to ceftazidime in Enterobacteriaceae? FEMS Microbiol. Lett. 71:89-94. [DOI] [PubMed] [Google Scholar]
- 305.Petroni, A., A. Corso, R. Melano, M. L. Cacace, A. M. Bru, A. Rossi, and M. Galas. 2002. Plasmidic extended-spectrum beta-lactamases in Vibrio cholerae O1 El Tor isolates in Argentina. Antimicrob. Agents Chemother. 46:1462-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Pfaller, M. A., R. N. Jones, and G. V. Doern. 1999. Multicenter evaluation of the antimicrobial activity for six broad-spectrum beta-lactams in Venezuela: comparison of data from 1997 and 1998 using the Etest method. Venezuelan Antimicrobial Resistance Study Group. Diagn. Microbiol. Infect. Dis. 35:153-158. [DOI] [PubMed] [Google Scholar]
- 307.Pfaller, M. A., R. N. Jones, G. V. Doern, and J. C. Salazar. 1999. Multicenter evaluation of antimicrobial resistance to six broad-spectrum beta-lactams in Colombia: comparison of data from 1997 and 1998 using the Etest method. The Colombian Antimicrobial Resistance Study Group. Diagn. Microbiol. Infect. Dis. 35:235-241. [DOI] [PubMed] [Google Scholar]
- 308.Philippon, A., R. Labia, and G. Jacoby. 1989. Extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 33:1131-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Philippon, L. N., T. Naas, A. T. Bouthors, V. Barakett, and P. Nordmann. 1997. OXA-18, a class D clavulanic acid-inhibited extended-spectrum beta-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 41:2188-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Pillay, T., D. G. Pillay, M. Adhikari, and A. W. Sturm. 1998. Piperacillin/tazobactam in the treatment of Klebsiella pneumoniae infections in neonates. Am. J. Perinatol. 15:47-51. [DOI] [PubMed] [Google Scholar]
- 311.Piroth, L., H. Aube, J. M. Doise, and M. Vincent-Martin. 1998. Spread of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae: are beta-lactamase inhibitors of therapeutic value? Clin. Infect. Dis. 27:76-80. [DOI] [PubMed] [Google Scholar]
- 312.Pitout, J. D., N. D. Hanson, D. L. Church, and K. B. Laupland. 2004. Population-based laboratory surveillance for Escherichia coli-producing extended-spectrum beta-lactamases: importance of community isolates with blaCTX-M genes. Clin. Infect. Dis. 38:1736-1741. [DOI] [PubMed] [Google Scholar]
- 313.Pitout, J. D., A. Hossain, and N. D. Hanson. 2004. Phenotypic and molecular detection of CTX-M-beta-lactamases produced by Escherichia coli and Klebsiella spp. J. Clin. Microbiol. 42:5715-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Pitout, J. D., K. S. Thomson, N. D. Hanson, A. F. Ehrhardt, P. Coudron, and C. C. Sanders. 1998. Plasmid-mediated resistance to expanded-spectrum cephalosporins among Enterobacter aerogenes strains. Antimicrob. Agents Chemother. 42:596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Pitout, J. D., K. S. Thomson, N. D. Hanson, A. F. Ehrhardt, E. S. Moland, and C. C. Sanders. 1998. β-Lactamases responsible for resistance to expanded-spectrum cephalosporins in Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis isolates recovered in South Africa. Antimicrob. Agents Chemother. 42:1350-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Podschun, R., and U. Ullmann. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11:589-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Poirel, L., D. Girlich, T. Naas, and P. Nordmann. 2001. OXA-28, an extended-spectrum variant of OXA-10 beta-lactamase from Pseudomonas aeruginosa and its plasmid- and integron-located gene. Antimicrob. Agents Chemother. 45:447-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Poirel, L., M. Gniadkowski, and P. Nordmann. 2002. Biochemical analysis of the ceftazidime-hydrolysing extended-spectrum beta-lactamase CTX-M-15 and of its structurally related beta-lactamase CTX-M-3. J. Antimicrob. Chemother. 50:1031-1034. [DOI] [PubMed] [Google Scholar]
- 319.Poirel, L., P. Kampfer, and P. Nordmann. 2002. Chromosome-encoded Ambler class A beta-lactamase of Kluyvera georgiana, a probable progenitor of a subgroup of CTX-M extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 46:4038-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Poirel, L., I. Le Thomas, T. Naas, A. Karim, and P. Nordmann. 2000. Biochemical sequence analyses of GES-1, a novel class A extended-spectrum beta-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 44:622-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Poirel, L., E. Lebessi, M. Castro, C. Fevre, M. Foustoukou, and P. Nordmann. 2004. Nosocomial outbreak of extended-spectrum beta-lactamase SHV-5-producing isolates of Pseudomonas aeruginosa in Athens, Greece. Antimicrob. Agents Chemother. 48:2277-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Poirel, L., H. Mammeri, and P. Nordmann. 2004. TEM-121, a novel complex mutant of TEM-type beta-lactamase from Enterobacter aerogenes. Antimicrob. Agents Chemother. 48:4528-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Poirel, L., T. Naas, M. Guibert, E. B. Chaibi, R. Labia, and P. Nordmann. 1999. Molecular and biochemical characterization of VEB-1, a novel class A extended-spectrum beta-lactamase encoded by an Escherichia coli integron gene. Antimicrob. Agents Chemother. 43:573-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Poirel, L., T. Naas, I. Le Thomas, A. Karim, E. Bingen, and P. Nordmann. 2001. CTX-M-type extended-spectrum beta-lactamase that hydrolyzes ceftazidime through a single amino acid substitution in the omega loop. Antimicrob. Agents Chemother. 45:3355-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Poirel, L., V. O. Rotimi, E. M. Mokaddas, A. Karim, and P. Nordmann. 2001. VEB-1-like extended-spectrum beta-lactamases in Pseudomonas aeruginosa, Kuwait. Emerg. Infect. Dis. 7:468-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Poirel, L., G. F. Weldhagen, C. De Champs, and P. Nordmann. 2002. A nosocomial outbreak of Pseudomonas aeruginosa isolates expressing the extended-spectrum beta-lactamase GES-2 in South Africa. J. Antimicrob. Chemother. 49:561-565. [DOI] [PubMed] [Google Scholar]
- 327.Poirel, L., G. F. Weldhagen, T. Naas, C. De Champs, M. G. Dove, and P. Nordmann. 2001. GES-2, a class A beta-lactamase from Pseudomonas aeruginosa with increased hydrolysis of imipenem. Antimicrob. Agents Chemother. 45:2598-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Prodinger, W. M., M. Fille, A. Bauernfeind, I. Stemplinger, S. Amann, B. Pfausler, C. Lass-Florl, and M. P. Dierich. 1996. Molecular epidemiology of Klebsiella pneumoniae producing SHV-5 beta-lactamase: parallel outbreaks due to multiple plasmid transfer. J. Clin. Microbiol. 34:564-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Quale, J. M., D. Landman, P. A. Bradford, M. Visalli, J. Ravishankar, C. Flores, D. Mayorga, K. Vangala, and A. Adedeji. 2002. Molecular epidemiology of a citywide outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae infection. Clin. Infect. Dis. 35:834-841. [DOI] [PubMed] [Google Scholar]
- 330.Queenan, A. M., B. Foleno, C. Gownley, E. Wira, and K. Bush. 2004. Effects of inoculum and beta-lactamase activity in AmpC- and extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae clinical isolates tested by using National Committee for Clinical Laboratory Standards ESBL methodology. J. Clin. Microbiol. 42:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Quinn, J. P., D. Miyashiro, D. Sahm, R. Flamm, and K. Bush. 1989. Novel plasmid-mediated beta-lactamase (TEM-10) conferring selective resistance to ceftazidime and aztreonam in clinical isolates of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 33:1451-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Radice, M., C. Gonzealez, P. Power, M. C. Vidal, and G. Gutkind. 2001. Third-generation cephalosporin resistance in Shigella sonnei, Argentina. Emerg. Infect. Dis. 7:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Radice, M., P. Power, J. Di Conza, and G. Gutkind. 2002. Early dissemination of CTX-M-derived enzymes in South America. Antimicrob. Agents Chemother. 46:602-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Rahal, J. J., C. Urban, D. Horn, K. Freeman, S. Segal-Maurer, J. Maurer, N. Mariano, S. Marks, J. M. Burns, D. Dominick, and M. Lim. 1998. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 280:1233-1237. [DOI] [PubMed] [Google Scholar]
- 335.Randegger, C. C., and H. Hachler. 2001. Real-time PCR and melting curve analysis for reliable and rapid detection of SHV extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 45:1730-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Rasheed, J. K., G. J. Anderson, H. Yigit, A. M. Queenan, A. Domenech-Sanchez, J. M. Swenson, J. W. Biddle, M. J. Ferraro, G. A. Jacoby, and F. C. Tenover. 2000. Characterization of the extended-spectrum beta-lactamase reference strain, Klebsiella pneumoniae K6 (ATCC 700603), which produces the novel enzyme SHV-18. Antimicrob. Agents Chemother. 44:2382-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Rasmussen, B. A., P. A. Bradford, J. P. Quinn, J. Wiener, R. A. Weinstein, and K. Bush. 1993. Genetically diverse ceftazidime-resistant isolates from a single center: biochemical and genetic characterization of TEM-10 beta-lactamases encoded by different nucleotide sequences. Antimicrob. Agents Chemother. 37:1989-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Rasmussen, B. A., and K. Bush. 1997. Carbapenem-hydrolyzing beta-lactamases. Antimicrob. Agents Chemother. 41:223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Revathi, G., K. P. Shannon, P. D. Stapleton, B. K. Jain, and G. L. French. 1998. An outbreak of extended-spectrum, beta-lactamase-producing Salmonella senftenberg in a burns ward. J. Hosp. Infect. 40:295-302. [DOI] [PubMed] [Google Scholar]
- 340.Rice, L. B., L. L. Carias, A. M. Hujer, M. Bonafede, R. Hutton, C. Hoyen, and R. A. Bonomo. 2000. High-level expression of chromosomally encoded SHV-1 beta-lactamase and an outer membrane protein change confer resistance to ceftazidime and piperacillin-tazobactam in a clinical isolate of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 44:362-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Rice, L. B., E. C. Eckstein, J. DeVente, and D. M. Shlaes. 1996. Ceftazidime-resistant Klebsiella pneumoniae isolates recovered at the Cleveland Department of Veterans Affairs Medical Center. Clin. Infect. Dis. 23:118-124. [DOI] [PubMed] [Google Scholar]
- 342.Rice, L. B., S. H. Marshall, L. L. Carias, L. Sutton, and G. A. Jacoby. 1993. Sequences of MGH-1, YOU-1, and YOU-2 extended-spectrum beta-lactamase genes. Antimicrob. Agents Chemother. 37:2760-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Rice, L. B., S. H. Willey, G. A. Papanicolaou, A. A. Medeiros, G. M. Eliopoulos, R. C. Moellering, Jr., and G. A. Jacoby. 1990. Outbreak of ceftazidime resistance caused by extended-spectrum beta-lactamases at a Massachusetts chronic-care facility. Antimicrob. Agents Chemother. 34:2193-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Rice, L. B., J. D. Yao, K. Klimm, G. M. Eliopoulos, and R. C. Moellering, Jr. 1991. Efficacy of different beta-lactams against an extended-spectrum beta-lactamase-producing Klebsiella pneumoniae strain in the rat intra-abdominal abscess model. Antimicrob. Agents Chemother. 35:1243-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Rodriguez-Bano, J., M. D. Navarro, L. Romero, L. Martinez-Martinez, M. A. Muniain, E. J. Perea, R. Perez-Cano, and A. Pascual. 2004. Epidemiology and clinical features of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in nonhospitalized patients. J. Clin. Microbiol. 42:1089-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Rogues, A. M., G. Boulard, A. Allery, C. Arpin, C. Quesnel, C. Quentin, C. Bebear, J. C. Labadie, and J. P. Gachie. 2000. Thermometers as a vehicle for transmission of extended-spectrum-beta-lactamase producing Klebisiella pneumoniae. J. Hosp. Infect. 45:76-77. [DOI] [PubMed] [Google Scholar]
- 347.Roussel-Delvallez, M., D. Sirot, Y. Berrouane, M. Goffart, B. Gourde, F. Wallet, and R. J. Courcol. 1995. Bactericidal effect of beta-lactams and amikacin alone or in association against Klebsiella pneumoniae producing extended spectrum beta-lactamase. J. Antimicrob. Chemother. 36:241-246. [DOI] [PubMed] [Google Scholar]
- 348.Royle, J., S. Halasz, G. Eagles, G. Gilbert, D. Dalton, P. Jelfs, and D. Isaacs. 1999. Outbreak of extended spectrum beta lactamase producing Klebsiella pneumoniae in a neonatal unit. Arch. Dis. Child Fetal Neonatal Ed. 80:F64-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Sader, H. S., A. C. Gales, T. D. Granacher, M. A. Pfaller, and R. N. Jones. 2000. Prevalence of antimicrobial resistance among respiratory tract isolates in Latin America: results from SENTRY antimicrobial surveillance program (1997-98). Braz. J. Infect. Dis. 4:245-254. [PubMed] [Google Scholar]
- 350.Sader, H. S., R. N. Jones, A. C. Gales, P. Winokur, K. C. Kugler, M. A. Pfaller, and G. V. Doern. 1998. Antimicrobial susceptibility patterns for pathogens isolated from patients in Latin American medical centers with a diagnosis of pneumonia: analysis of results from the SENTRY Antimicrobial Surveillance Program (1997). SENTRY Latin America Study Group. Diagn. Microbiol. Infect. Dis. 32:289-301. [DOI] [PubMed] [Google Scholar]
- 351.Sader, H. S., M. A. Pfaller, and R. N. Jones. 1994. Prevalence of important pathogens and the antimicrobial activity of parenteral drugs at numerous medical centers in the United States. II. Study of the intra- and interlaboratory dissemination of extended-spectrum beta-lactamase-producing Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 20:203-208. [DOI] [PubMed] [Google Scholar]
- 352.Safdar, N., and D. G. Maki. 2002. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann. Intern. Med. 136:834-844. [DOI] [PubMed] [Google Scholar]
- 353.Saladin, M., V. T. Cao, T. Lambert, J. L. Donay, J. L. Herrmann, Z. Ould-Hocine, C. Verdet, F. Delisle, A. Philippon, and G. Arlet. 2002. Diversity of CTX-M beta-lactamases and their promoter regions from Enterobacteriaceae isolated in three Parisian hospitals. FEMS Microbiol. Lett. 209:161-168. [DOI] [PubMed] [Google Scholar]
- 354.Sanders, C. C. 1996. In vitro activity of fourth generation cephalosporins against enterobacteriaceae producing extended-spectrum beta-lactamases. J. Chemother. 8(Suppl. 2):57-62. [PubMed] [Google Scholar]
- 355.Sanders, C. C., A. L. Barry, J. A. Washington, C. Shubert, E. S. Moland, M. M. Traczewski, C. Knapp, and R. Mulder. 1996. Detection of extended-spectrum-beta-lactamase-producing members of the family Enterobacteriaceae with Vitek ESBL test. J. Clin. Microbiol. 34:2997-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Sanguinetti, M., B. Posteraro, T. Spanu, D. Ciccaglione, L. Romano, B. Fiori, G. Nicoletti, S. Zanetti, and G. Fadda. 2003. Characterization of clinical isolates of Enterobacteriaceae from Italy by the BD Phoenix extended-spectrum beta-lactamase detection method. J. Clin. Microbiol. 41:1463-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Saurina, G., J. M. Quale, V. M. Manikal, E. Oydna, and D. Landman. 2000. Antimicrobial resistance in Enterobacteriaceae in Brooklyn, N.Y.: epidemiology and relation to antibiotic usage patterns. J. Antimicrob. Chemother. 45:895-898. [DOI] [PubMed] [Google Scholar]
- 358.Schiappa, D. A., M. K. Hayden, M. G. Matushek, F. N. Hashemi, J. Sullivan, K. Y. Smith, D. Miyashiro, J. P. Quinn, R. A. Weinstein, and G. M. Trenholme. 1996. Ceftazidime-resistant Klebsiella pneumoniae and Escherichia coli bloodstream infection: a case-control and molecular epidemiologic investigation. J. Infect. Dis. 174:529-536. [DOI] [PubMed] [Google Scholar]
- 359.Schooneveldt, J. M., G. R. Nimmo, and P. Giffard. 1998. Detection and characterisation of extended spectrum beta-lactamases in Klebsiella pneumoniae causing nosocomial infection. Pathology 30:164-168. [DOI] [PubMed] [Google Scholar]
- 360.Schwaber, M. J., P. M. Raney, J. K. Rasheed, J. W. Biddle, P. Williams, J. E. McGowan, Jr., and F. C. Tenover. 2004. Utility of National Committee for Clinical Laboratory Standards guidelines for identifying extended-spectrum beta-lactamases in non-Escherichia coli and non-Klebsiella spp. of Enterobacteriaceae. J. Clin. Microbiol. 42:294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Segal-Maurer, S., N. Mariano, A. Qavi, C. Urban, and J. J. Rahal, Jr. 1999. Successful treatment of ceftazidime-resistant Klebsiella pneumoniae ventriculitis with intravenous meropenem and intraventricular polymyxin B: case report and review. Clin. Infect. Dis. 28:1134-1138. [DOI] [PubMed] [Google Scholar]
- 362.Sekowska, A., G. Janicka, C. Klyszejko, M. Wojda, M. Wroblewski, and M. Szymankiewicz. 2002. Resistance of Klebsiella pneumoniae strains producing and not producing ESBL (extended-spectrum beta-lactamase) type enzymes to selected non-beta-lactam antibiotics. Med. Sci. Monit. 8:BR100-104. [PubMed] [Google Scholar]
- 363.Shannon, K., K. Fung, P. Stapleton, R. Anthony, E. Power, and G. French. 1998. A hospital outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae investigated by RAPD typing and analysis of the genetics and mechanisms of resistance. J. Hosp. Infect. 39:291-300. [DOI] [PubMed] [Google Scholar]
- 364.Shannon, K., P. Stapleton, X. Xiang, A. Johnson, H. Beattie, F. El Bakri, B. Cookson, and G. French. 1998. Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae strains causing nosocomial outbreaks of infection in the United Kingdom. J. Clin. Microbiol. 36:3105-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Shannon, K. P., A. King, I. Phillips, M. H. Nicolas, and A. Philippon. 1990. Importance of organisms producing broad-spectrum SHV-group beta-lactamases into the United Kingdom. J. Antimicrob. Chemother. 25:343-351. [DOI] [PubMed] [Google Scholar]
- 366.Shen, D., P. Winokur, and R. N. Jones. 2001. Characterization of extended spectrum beta-lactamase-producing Klebsiella pneumoniae from Beijing, China. Int. J. Antimicrob. Agents 18:185-188. [DOI] [PubMed] [Google Scholar]
- 367.Shipton, S. E., M. F. Cotton, G. Wessels, and E. Wasserman. 2001. Nosocomial endocarditis due to extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a child. S. Afr. Med. J. 91:321-322. [PubMed] [Google Scholar]
- 368.Shlaes, D. M., M. H. Lehman, C. A. Currie-McCumber, C. H. Kim, and R. Floyd. 1986. Prevalence of colonization with antibiotic resistant gram-negative bacilli in a nursing home care unit: the importance of cross-colonization as documented by plasmid analysis. Infect. Control. 7:538-545. [DOI] [PubMed] [Google Scholar]
- 369.Silva, J., C. Aguilar, G. Ayala, M. A. Estrada, U. Garza-Ramos, R. Lara-Lemus, and L. Ledezma. 2000. TLA-1: a new plasmid-mediated extended-spectrum beta-lactamase from Escherichia coli. Antimicrob. Agents Chemother. 44:997-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Silva, J., C. Aguilar, Z. Becerra, F. Lopez-Antunano, and R. Garcia. 1999. Extended-spectrum beta-lactamases in clinical isolates of enterobacteria in Mexico. Microb. Drug Resist. 5:189-193. [DOI] [PubMed] [Google Scholar]
- 371.Silva, J., R. Gatica, C. Aguilar, Z. Becerra, U. Garza-Ramos, M. Velazquez, G. Miranda, B. Leanos, F. Solorzano, and G. Echaniz. 2001. Outbreak of infection with extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a Mexican hospital. J. Clin. Microbiol. 39:3193-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Sirot, D., C. Recule, E. B. Chaibi, L. Bret, J. Croize, C. Chanal-Claris, R. Labia, and J. Sirot. 1997. A complex mutant of TEM-1 beta-lactamase with mutations encountered in both IRT-4 and extended-spectrum TEM-15, produced by an Escherichia coli clinical isolate. Antimicrob. Agents Chemother. 41:1322-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Sirot, D., J. Sirot, R. Labia, A. Morand, P. Courvalin, A. Darfeuille-Michaud, R. Perroux, and R. Cluzel. 1987. Transferable resistance to third-generation cephalosporins in clinical isolates of Klebsiella pneumoniae: identification of CTX-1, a novel beta-lactamase. J. Antimicrob. Chemother. 20:323-334. [DOI] [PubMed] [Google Scholar]
- 374.Siu, L. K., P. L. Lu, P. R. Hsueh, F. M. Lin, S. C. Chang, K. T. Luh, M. Ho, and C. Y. Lee. 1999. Bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a pediatric oncology ward: clinical features and identification of different plasmids carrying both SHV-5 and TEM-1 genes. J. Clin. Microbiol. 37:4020-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Smith, P. W., C. W. Seip, S. C. Schaefer, and C. Bell-Dixon. 2000. Microbiologic survey of long-term care facilities. Am. J. Infect. Control. 28:8-13. [DOI] [PubMed] [Google Scholar]
- 376.Smith Moland, E., N. D. Hanson, V. L. Herrera, J. A. Black, T. J. Lockhart, A. Hossain, J. A. Johnson, R. V. Goering, and K. S. Thomson. 2003. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J. Antimicrob. Chemother. 51:711-714. [DOI] [PubMed] [Google Scholar]
- 377.Sougakoff, W., S. Goussard, G. Gerbaud, and P. Courvalin. 1988. Plasmid-mediated resistance to third-generation cephalosporins caused by point mutations in TEM-type penicillinase genes. Rev. Infect. Dis. 10:879-884. [DOI] [PubMed] [Google Scholar]
- 378.Soulier, A., F. Barbut, J. M. Ollivier, J. C. Petit, and A. Lienhart. 1995. Decreased transmission of Enterobacteriaceae with extended-spectrum beta-lactamases in an intensive care unit by nursing reorganization. J. Hosp. Infect. 31:89-97. [DOI] [PubMed] [Google Scholar]
- 379.Steward, C. D., J. K. Rasheed, S. K. Hubert, J. W. Biddle, P. M. Raney, G. J. Anderson, P. P. Williams, K. L. Brittain, A. Oliver, J. E. McGowan, Jr., and F. C. Tenover. 2001. Characterization of clinical isolates of Klebsiella pneumoniae from 19 laboratories using the National Committee for Clinical Laboratory Standards extended-spectrum beta-lactamase detection methods. J. Clin. Microbiol. 39:2864-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Steward, C. D., D. Wallace, S. K. Hubert, R. Lawton, S. K. Fridkin, R. P. Gaynes, J. E. McGowan, Jr., and F. C. Tenover. 2000. Ability of laboratories to detect emerging antimicrobial resistance in nosocomial pathogens: a survey of project ICARE laboratories. Diagn. Microbiol. Infect. Dis. 38:59-67. [DOI] [PubMed] [Google Scholar]
- 381.Stewart, B. A., and M. P. Lessing. 1999. A hospital outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. J. Hosp. Infect. 41:71-72. [DOI] [PubMed] [Google Scholar]
- 382.Sturenburg, E., A. Kuhn, D. Mack, and R. Laufs. 2004. A novel extended-spectrum beta-lactamase CTX-M-23 with a P167T substitution in the active-site omega loop associated with ceftazidime resistance. J. Antimicrob. Chemother. 54:406-409. [DOI] [PubMed] [Google Scholar]
- 383.Sturenburg, E., M. Lang, M. A. Horstkotte, R. Laufs, and D. Mack. 2004. Evaluation of the MicroScan ESBL plus confirmation panel for detection of extended-spectrum beta-lactamases in clinical isolates of oxyimino-cephalosporin-resistant Gram-negative bacteria. J. Antimicrob. Chemother. 54:870-875. [DOI] [PubMed] [Google Scholar]
- 384.Sturenburg, E., I. Sobottka, H. H. Feucht, D. Mack, and R. Laufs. 2003. Comparison of BDPhoenix and VITEK2 automated antimicrobial susceptibility test systems for extended-spectrum beta-lactamase detection in Escherichia coli and Klebsiella species clinical isolates. Diagn. Microbiol. Infect. Dis. 45:29-34. [DOI] [PubMed] [Google Scholar]
- 385.Sykes, R. B., and K. Bush. 1982. Physiology, biochemistry and inactivation of beta-lactamases, p. 155-207. In R. B. Morin and M. Gorman (ed.), The chemistry and biology of beta-lactam antibiotics, vol. 3. Academic Press, London, England. [Google Scholar]
- 386.Szabo, D., I. Barcs, and F. Rozgonyi. 1997. Extended-spectrum beta-lactamases: an actual problem of hospital microbiology (review). Acta Microbiol. Immunol. Hung. 44:309-325. [PubMed] [Google Scholar]
- 387.Szabo, D., Z. Filetoth, J. Szentandrassy, M. Nemedi, E. Toth, C. Jeney, G. Kispal, and F. Rozgonyi. 1999. Molecular epidemiology of a cluster of cases due to Klebsiella pneumoniae producing SHV-5 extended-spectrum beta-lactamase in the premature intensive care unit of a Hungarian hospital. J. Clin. Microbiol. 37:4167-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Taylor, M. E., and B. A. Oppenheim. 1991. Selective decontamination of the gastrointestinal tract as an infection control measure. J. Hosp. Infect. 17:271-278. [DOI] [PubMed] [Google Scholar]
- 389.Tenover, F. C., M. J. Mohammed, T. S. Gorton, and Z. F. Dembek. 1999. Detection and reporting of organisms producing extended-spectrum beta-lactamases: survey of laboratories in Connecticut. J. Clin. Microbiol. 37:4065-4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Tenover, F. C., P. M. Raney, P. P. Williams, J. K. Rasheed, J. W. Biddle, A. Oliver, S. K. Fridkin, L. Jevitt, and J. E. McGowan, Jr. 2003. Evaluation of the National Committee for Clinical Laboratory Standards extended-spectrum beta-lactamase confirmation methods for Escherichia coli with isolates collected during Project ICARE. J. Clin. Microbiol. 41:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Thauvin-Eliopoulos, C., M. F. Tripodi, R. C. Moellering, Jr., and G. M. Eliopoulos. 1997. Efficacies of piperacillin-tazobactam and cefepime in rats with experimental intra-abdominal abscesses due to an extended-spectrum beta-lactamase-producing strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 41:1053-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Thomson, K. S., and E. S. Moland. 2001. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 45:3548-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Thomson, K. S., and C. C. Sanders. 1992. Detection of extended-spectrum beta-lactamases in members of the family Enterobacteriaceae: comparison of the double-disk and three-dimensional tests. Antimicrob. Agents Chemother. 36:1877-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Thomson, K. S., and C. C. Sanders. 1997. A simple and reliable method to screen isolates of Escherichia coli and Klebsiella pneumoniae for the production of TEM- and SHV-derived extended-spectrum beta-lactamases. Clin. Microbiol. Infect. 3:549-554. [DOI] [PubMed] [Google Scholar]
- 395.Thomson, K. S., C. C. Sanders, and E. S. Moland. 1999. Use of microdilution panels with and without beta-lactamase inhibitors as a phenotypic test for beta-lactamase production among Escherichia coli, Klebsiella spp., Enterobacter spp., Citrobacter freundii, and Serratia marcescens. Antimicrob. Agents Chemother. 43:1393-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Toleman, M. A., K. Rolston, R. N. Jones, and T. R. Walsh. 2003. Molecular and biochemical characterization of OXA-45, an extended-spectrum class 2d′ beta-lactamase in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 47:2859-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Tribuddharat, C., and M. Fennewald. 1999. Integron-mediated rifampin resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:960-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Tzelepi, E., P. Giakkoupi, D. Sofianou, V. Loukova, A. Kemeroglou, and A. Tsakris. 2000. Detection of extended-spectrum beta-lactamases in clinical isolates of Enterobacter cloacae and Enterobacter aerogenes. J. Clin. Microbiol. 38:542-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Tzouvelekis, L. S., and R. A. Bonomo. 1999. SHV-type beta-lactamases. Curr. Pharm. Des. 5:847-864. [PubMed] [Google Scholar]
- 400.Tzouvelekis, L. S., E. Tzelepi, P. T. Tassios, and N. J. Legakis. 2000. CTX-M-type beta-lactamases: an emerging group of extended-spectrum enzymes. Int. J. Antimicrob. Agents 14:137-142. [DOI] [PubMed] [Google Scholar]
- 401.Tzouvelekis, L. S., A. C. Vatopoulos, G. Katsanis, and E. Tzelepi. 1999. Rare case of failure by an automated system to detect extended-spectrum beta-lactamase in a cephalosporin-resistant Klebsiella pneumoniae isolate. J. Clin. Microbiol. 37:2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Urban, C., K. S. Meyer, N. Mariano, J. J. Rahal, R. Flamm, B. A. Rasmussen, and K. Bush. 1994. Identification of TEM-26 beta-lactamase responsible for a major outbreak of ceftazidime-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 38:392-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Vahaboglu, H., F. Coskunkan, O. Tansel, R. Ozturk, N. Sahin, I. Koksal, B. Kocazeybek, M. Tatman-Otkun, H. Leblebicioglu, M. A. Ozinel, H. Akalin, S. Kocagoz, and V. Korten. 2001. Clinical importance of extended-spectrum beta-lactamase (PER-1-type)-producing Acinetobacter spp. and Pseudomonas aeruginosa strains. J. Med. Microbiol. 50:642-645. [DOI] [PubMed] [Google Scholar]
- 404.Vahaboglu, H., L. M. Hall, L. Mulazimoglu, S. Dodanli, I. Yildirim, and D. M. Livermore. 1995. Resistance to extended-spectrum cephalosporins, caused by PER-1 beta-lactamase, in Salmonella typhimurium from Istanbul, Turkey. J. Med. Microbiol. 43:294-299. [DOI] [PubMed] [Google Scholar]
- 405.Vahaboglu, H., R. Ozturk, G. Aygun, F. Coskunkan, A. Yaman, A. Kaygusuz, H. Leblebicioglu, I. Balik, K. Aydin, and M. Otkun. 1997. Widespread detection of PER-1-type extended-spectrum beta-lactamases among nosocomial Acinetobacter and Pseudomonas aeruginosa isolates in Turkey: a nationwide multicenter study. Antimicrob. Agents Chemother. 41:2265-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Vahaboglu, H., S. Saribas, H. Akbal, R. Ozturk, and A. Yucel. 1998. Activities of cefepime and five other antibiotics against nosocomial PER-1-type and/or OXA-10-type beta-lactamase-producing Pseudomonas aeruginosa and Acinetobacter spp. J. Antimicrob. Chemother. 42:269-270. [PubMed] [Google Scholar]
- 407.Varela, C., A. Oliver, T. M. Coque, F. Baquero, and R. Canton. 2001. Prevalence of extended-spectrum beta-lactamases in group-1 beta-lactamase-producing isolates. Clin. Microbiol. Infect. 7:278-282. [DOI] [PubMed] [Google Scholar]
- 408.Venezia, R. A., F. J. Scarano, K. E. Preston, L. M. Steele, T. P. Root, R. Limberger, W. Archinal, and M. A. Kacica. 1995. Molecular epidemiology of an SHV-5 extended-spectrum beta-lactamase in enterobacteriaceae isolated from infants in a neonatal intensive care unit. Clin. Infect. Dis. 21:915-923. [DOI] [PubMed] [Google Scholar]
- 409.Vercauteren, E., P. Descheemaeker, M. Ieven, C. C. Sanders, and H. Goossens. 1997. Comparison of screening methods for detection of extended-spectrum beta-lactamases and their prevalence among blood isolates of Escherichia coli and Klebsiella spp. in a Belgian teaching hospital. J. Clin. Microbiol. 35:2191-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Vourli, S., P. Giakkoupi, V. Miriagou, E. Tzelepi, A. C. Vatopoulos, and L. S. Tzouvelekis. 2004. Novel GES/IBC extended-spectrum beta-lactamase variants with carbapenemase activity in clinical enterobacteria. FEMS Microbiol. Lett. 234:209-213. [DOI] [PubMed] [Google Scholar]
- 411.Vourli, S., L. S. Tzouvelekis, E. Tzelepi, G. Kartali, C. Kontopoulou, and D. Sofianou. 2003. Characterization of Klebsiella pneumoniae clinical strains producing a rare extended-spectrum beta-lactamase (IBC-1). Int. J. Antimicrob. Agents 21:495-497. [DOI] [PubMed] [Google Scholar]
- 412.Vourli, S., L. S. Tzouvelekis, E. Tzelepi, E. Lebessi, N. J. Legakis, and V. Miriagou. 2003. Characterization of In111, a class 1 integron that carries the extended-spectrum beta-lactamase gene blaIBC-1. FEMS Microbiol. Lett. 225:149-153. [DOI] [PubMed] [Google Scholar]
- 413.Wachino, J., Y. Doi, K. Yamane, N. Shibata, T. Yagi, T. Kubota, H. Ito, and Y. Arakawa. 2004. Nosocomial spread of ceftazidime-resistant Klebsiella pneumoniae strains producing a novel class a beta-lactamase, GES-3, in a neonatal intensive care unit in Japan. Antimicrob. Agents Chemother. 48:1960-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413a.Walsh, T. R., M. A. Toleman, L. Poirel, and P. Nordmann. 2005. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Wang, H., S. Kelkar, W. Wu, M. Chen, and J. P. Quinn. 2003. Clinical Isolates of Enterobacteriaceae Producing Extended-Spectrum beta-Lactamases: Prevalence of CTX-M-3 at a Hospital in China. Antimicrob. Agents Chemother. 47:790-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Wang, M., D. F. Sahm, G. A. Jacoby, and D. C. Hooper. 2004. Emerging plasmid-mediated quinolone resistance associated with the qnr gene in Klebsiella pneumoniae clinical isolates in the United States. Antimicrob. Agents Chemother. 48:1295-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Weldhagen, G. F. 2004. Sequence-selective recognition of extended-spectrum beta-lactamase GES-2 by a competitive, peptide nucleic acid-based multiplex PCR assay. Antimicrob. Agents Chemother. 48:3402-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Weldhagen, G. F., L. Poirel, and P. Nordmann. 2003. Ambler class A extended-spectrum beta-lactamases in Pseudomonas aeruginosa: novel developments and clinical impact. Antimicrob. Agents Chemother. 47:2385-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Weldhagen, G. F., and A. Prinsloo. 2004. Molecular detection of GES-2 extended spectrum beta-lactamase producing Pseudomonas aeruginosa in Pretoria, South Africa. Int. J. Antimicrob. Agents 24:35-38. [DOI] [PubMed] [Google Scholar]
- 419.Wiener, J., J. P. Quinn, P. A. Bradford, R. V. Goering, C. Nathan, K. Bush, and R. A. Weinstein. 1999. Multiple antibiotic-resistant Klebsiella and Escherichia coli in nursing homes. JAMA 281:517-523. [DOI] [PubMed] [Google Scholar]
- 420.Wong-Beringer, A. 2001. Therapeutic challenges associated with extended-spectrum, beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Pharmacotherapy. 21:583-592. [DOI] [PubMed] [Google Scholar]
- 421.Wong-Beringer, A., J. Hindler, M. Loeloff, A. M. Queenan, N. Lee, D. A. Pegues, J. P. Quinn, and K. Bush. 2002. Molecular correlation for the treatment outcomes in bloodstream infections caused by Escherichia coli and Klebsiella pneumoniae with reduced susceptibility to ceftazidime. Clin. Infect. Dis. 34:135-146. [DOI] [PubMed] [Google Scholar]
- 422.Woodford, N., P. M. Tierno, Jr., K. Young, L. Tysall, M. F. Palepou, E. Ward, R. E. Painter, D. F. Suber, D. Shungu, L. L. Silver, K. Inglima, J. Kornblum, and D. M. Livermore. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York Medical Center. Antimicrob. Agents Chemother. 48:4793-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Woodford, N., M. E. Ward, M. E. Kaufmann, J. Turton, E. J. Fagan, D. James, A. P. Johnson, R. Pike, M. Warner, T. Cheasty, A. Pearson, S. Harry, J. B. Leach, A. Loughrey, J. A. Lowes, R. E. Warren, and D. M. Livermore. 2004. Community and hospital spread of Escherichia coli producing CTX-M extended-spectrum beta-lactamases in the UK. J. Antimicrob. Chemother. 54:735-743. [DOI] [PubMed] [Google Scholar]
- 424.Wu, T. L., L. K. Siu, L. H. Su, T. L. Lauderdale, F. M. Lin, H. S. Leu, T. Y. Lin, and M. Ho. 2001. Outer membrane protein change combined with co-existing TEM-1 and SHV-1 beta-lactamases lead to false identification of ESBL-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 47:755-761. [DOI] [PubMed] [Google Scholar]
- 425.Xiong, Z., D. Zhu, F. Wang, Y. Zhang, R. Okamoto, and M. Inoue. 2002. Investigation of extended-spectrum beta-lactamase in klebsiellae pneumoniae and Escherichia coli from China. Diagn. Microbiol. Infect. Dis. 44:195-200. [DOI] [PubMed] [Google Scholar]
- 426.Yagi, T., H. Kurokawa, K. Senda, S. Ichiyama, H. Ito, S. Ohsuka, K. Shibayama, K. Shimokata, N. Kato, M. Ohta, and Y. Arakawa. 1997. Nosocomial spread of cephem-resistant Escherichia coli strains carrying multiple Toho-1-like beta-lactamase genes. Antimicrob. Agents Chemother. 41:2606-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Yamaguchi, K., D. Mathai, D. J. Biedenbach, M. T. Lewis, A. C. Gales, and R. N. Jones. 1999. Evaluation of the in vitro activity of six broad-spectrum beta-lactam antimicrobial agents tested against over 2,000 clinical isolates from 22 medical centers in Japan. Japan Antimicrobial Resistance Study Group. Diagn. Microbiol. Infect. Dis. 34:123-134. [DOI] [PubMed] [Google Scholar]
- 428.Yamasaki, K., M. Komatsu, T. Yamashita, K. Shimakawa, T. Ura, H. Nishio, K. Satoh, R. Washidu, S. Kinoshita, and M. Aihara. 2003. Production of CTX-M-3 extended-spectrum beta-lactamase and IMP-1 metallo beta-lactamase by five Gram-negative bacilli: survey of clinical isolates from seven laboratories collected in 1998 and 2000, in the Kinki region of Japan. J. Antimicrob. Chemother. 51:631-638. [DOI] [PubMed] [Google Scholar]
- 429.Yan, J. J., W. C. Ko, S. H. Tsai, H. M. Wu, Y. T. Jin, and J. J. Wu. 2000. Dissemination of CTX-M-3 and CMY-2 beta-lactamases among clinical isolates of Escherichia coli in southern Taiwan. J. Clin. Microbiol. 38:4320-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Yan, J. J., W. C. Ko, S. H. Tsai, H. M. Wu, and J. J. Wu. 2001. Outbreak of infection with multidrug-resistant Klebsiella pneumoniae carrying bla(IMP-8) in a university medical center in Taiwan. J. Clin. Microbiol. 39:4433-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Yang, Y., N. Bhachech, P. A. Bradford, B. D. Jett, D. F. Sahm, and K. Bush. 1998. Ceftazidime-resistant Klebsiella pneumoniae and Escherichia coli isolates producing TEM-10 and TEM-43 beta-lactamases from St. Louis, Missouri. Antimicrob. Agents Chemother. 42:1671-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Yigit, H., A. M. Queenan, G. J. Anderson, A. Domenech-Sanchez, J. W. Biddle, C. D. Steward, S. Alberti, K. Bush, and F. C. Tenover. 2001. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45:1151-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Yigit, H., A. M. Queenan, J. K. Rasheed, J. W. Biddle, A. Domenech-Sanchez, S. Alberti, K. Bush, and F. C. Tenover. 2003. Carbapenem-resistant strain of Klebsiella oxytoca harboring carbapenem-hydrolyzing beta-lactamase KPC-2. Antimicrob. Agents Chemother. 47:3881-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Yong, D., J. H. Shin, S. Kim, Y. Lim, J. H. Yum, K. Lee, Y. Chong, and A. Bauernfeind. 2003. High prevalence of PER-1 extended-spectrum beta-lactamase-producing Acinetobacter spp. in Korea. Antimicrob. Agents Chemother. 47:1749-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Yu, W. L., R. N. Jones, R. J. Hollis, S. A. Messer, D. J. Biedenbach, L. M. Deshpande, and M. A. Pfaller. 2002. Molecular epidemiology of extended-spectrum beta-lactamase-producing, fluoroquinolone-resistant isolates of Klebsiella pneumoniae in Taiwan. J. Clin. Microbiol. 40:4666-4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Yu, W. L., M. A. Pfaller, P. L. Winokur, and R. N. Jones. 2002. Cefepime MIC as a predictor of the extended-spectrum beta-lactamase type in Klebsiella pneumoniae, Taiwan. Emerg. Infect. Dis. 8:522-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Yu, W. L., P. L. Winokur, D. L. Von Stein, M. A. Pfaller, J. H. Wang, and R. N. Jones. 2002. First description of Klebsiella pneumoniae harboring CTX-M beta-lactamases (CTX-M-14 and CTX-M-3) in Taiwan. Antimicrob. Agents Chemother. 46:1098-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Yu, Y., W. Zhou, Y. Chen, Y. Ding, and Y. Ma. 2002. Epidemiological and antibiotic resistant study on extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Zhejiang Province. Chin Med. J. (Engl.) 115:1479-1482. [PubMed] [Google Scholar]
- 439.Yuan, M., H. Aucken, L. M. Hall, T. L. Pitt, and D. M. Livermore. 1998. Epidemiological typing of klebsiellae with extended-spectrum beta-lactamases from European intensive care units. J. Antimicrob. Chemother. 41:527-539. [DOI] [PubMed] [Google Scholar]
- 440.Zanetti, G., F. Bally, G. Greub, J. Garbino, T. Kinge, D. Lew, J. A. Romand, J. Bille, D. Aymon, L. Stratchounski, L. Krawczyk, E. Rubinstein, M. D. Schaller, R. Chiolero, M. P. Glauser, and A. Cometta. 2003. Cefepime versus imipenem-cilastatin for treatment of nosocomial pneumonia in intensive care unit patients: a multicenter, evaluator-blind, prospective, randomized study. Antimicrob. Agents Chemother. 47:3442-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]