Abstract
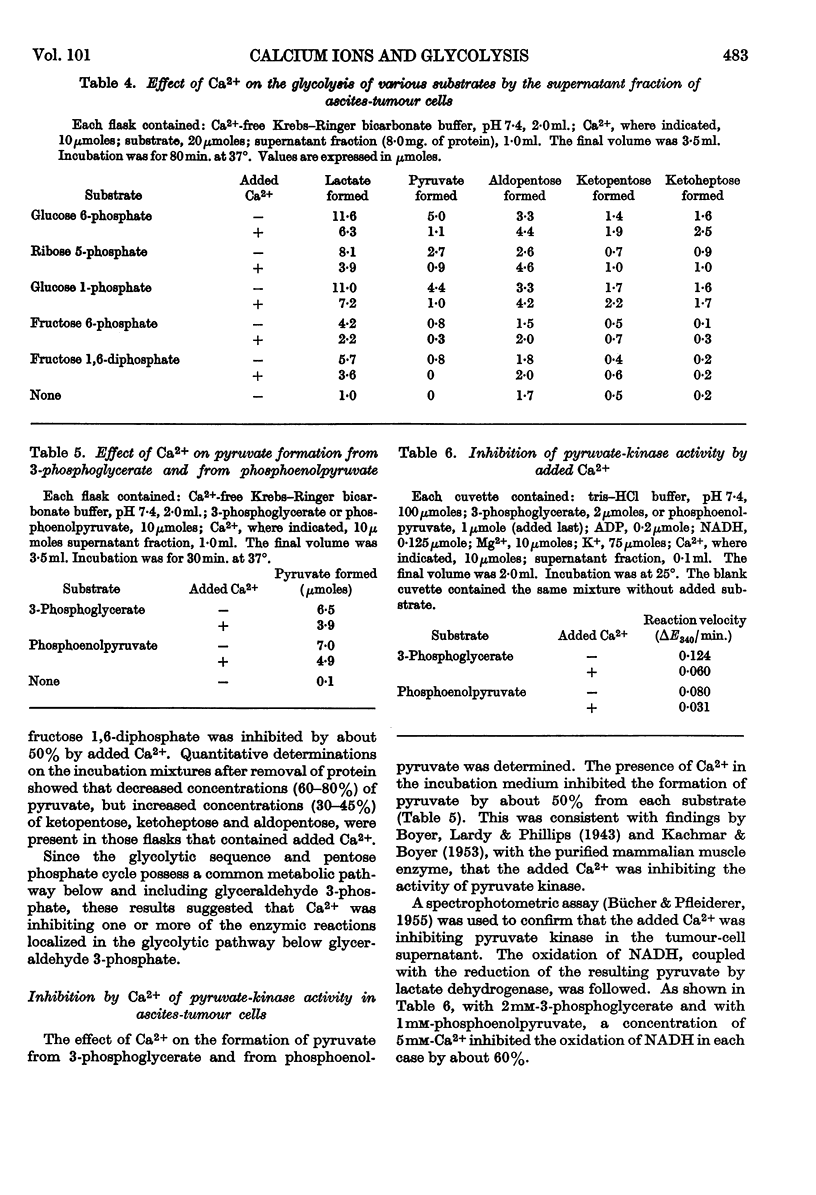
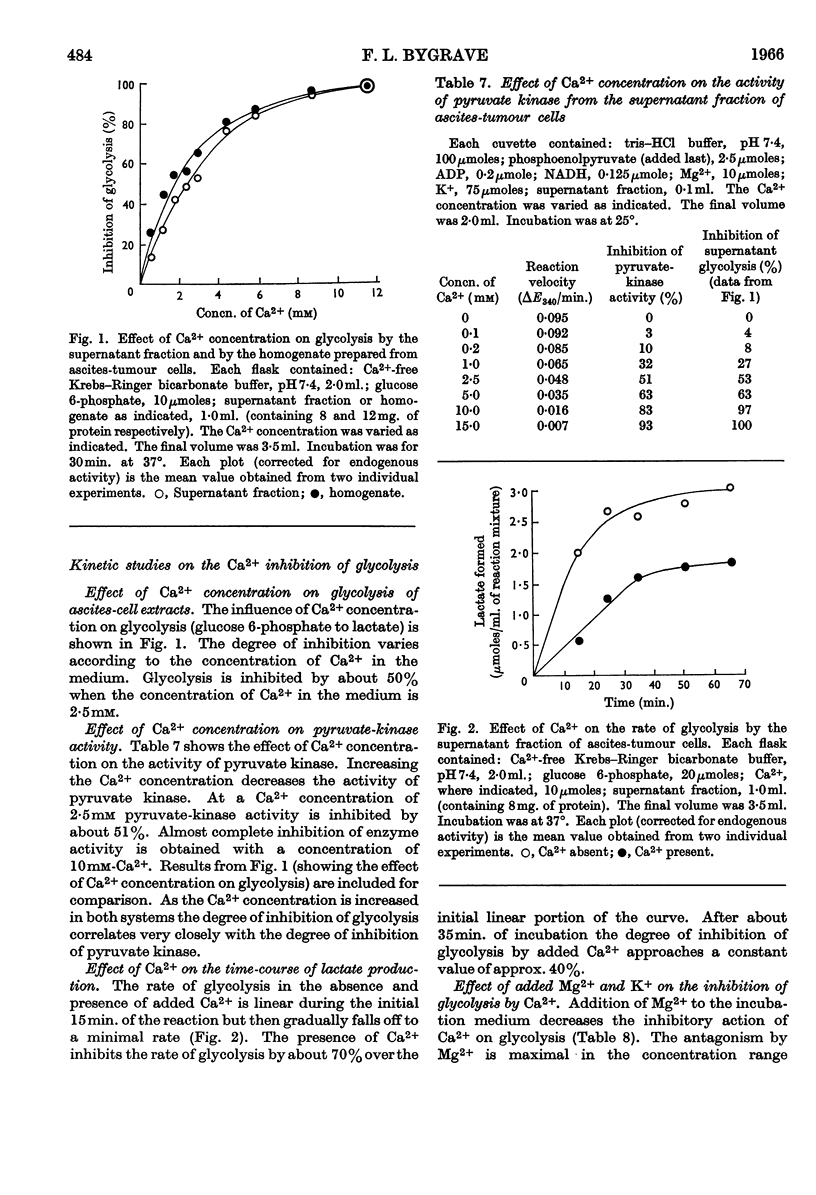
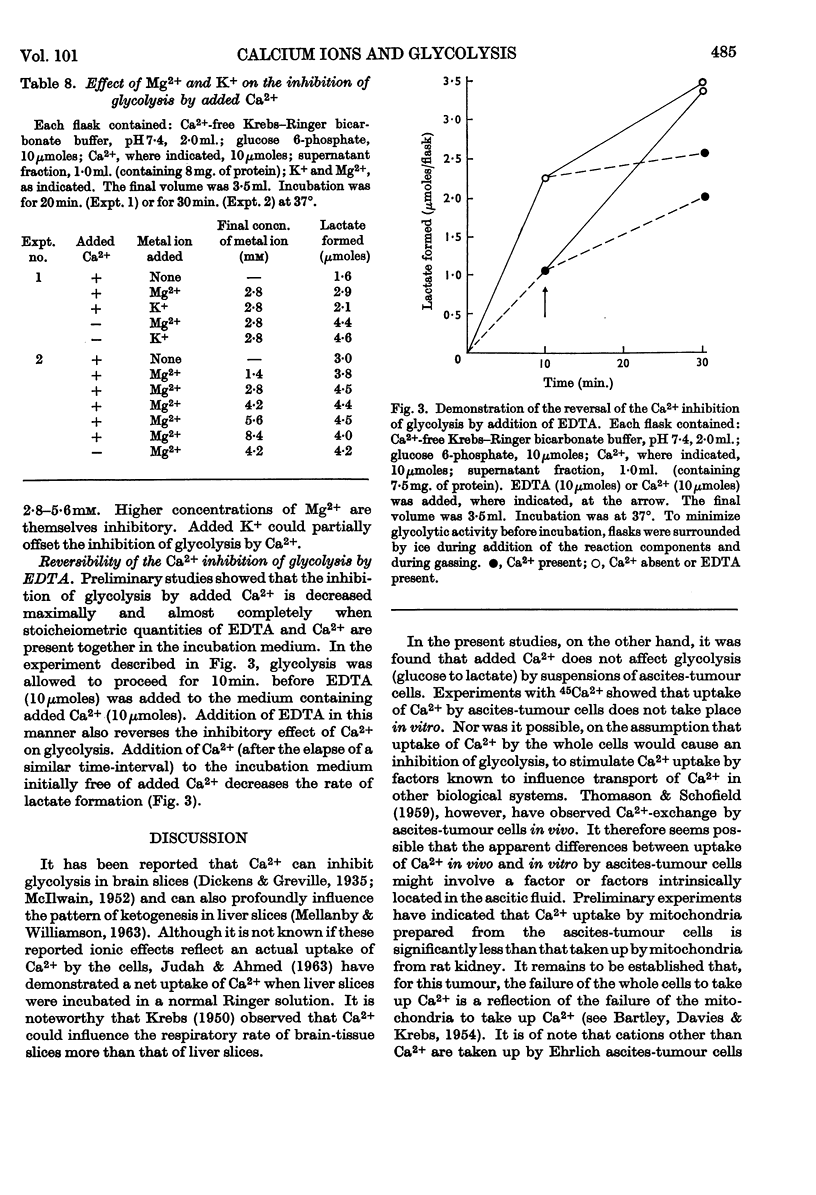
1. Added Ca2+ inhibited lactate formation from sugar phosphates by intact Ehrlich ascites-tumour cells. Lactate formation from glucose by these cells was unaffected by added Ca2+. 2. The Ca2+ inhibition of lactate formation by intact cells occurred in the extracellular medium. 3. Intact ascites-tumour cells did not take up Ca2+ in vitro. 4. Glycolysis of sugar phosphates by cell extracts as well as pyruvate formation from 3-phosphoglycerate and phosphoenolpyruvate was inhibited by Ca2+. 5. It was concluded that Ca2+ inhibited the pyruvate-kinase (EC 2.7.1.40) reaction. Further, Ca2+ inhibition of pyruvate kinase could be correlated with the overall inhibition of glycolysis. 6. Concentrations of Ca2+ usually present in Krebs–Ringer buffers, inhibited glycolysis and pyruvate-kinase activity by approx. 50%. 7. The inhibition of glycolysis by added Ca2+ could be partially reversed by K+ and completely reversed by Mg2+ or by stoicheiometric amounts of EDTA. 8. The hypothesis is advanced that the inability of tumour cells to take up Ca2+ is a factor contributing towards their high rate of glycolysis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHWELL G., DISCHE Z. Inhibition of the metabolism of nucleated red cells by intracellular ions and its relation to intracellular structural factors. Biochim Biophys Acta. 1950 Jan;4(1-3):276–292. doi: 10.1016/0006-3002(50)90034-5. [DOI] [PubMed] [Google Scholar]
- BARTLEY W., DAVIES R. E., KREBS H. A. Active transport in animal tissues and subcellular particles. Proc R Soc Lond B Biol Sci. 1954 Mar 25;142(907):187–196. doi: 10.1098/rspb.1954.0020. [DOI] [PubMed] [Google Scholar]
- BOSZORMENYI-NAGY I. Formation of phosphopyruvate from phosphoglycerate in hemolyzed human erythrocytes. J Biol Chem. 1955 Jan;212(1):495–499. [PubMed] [Google Scholar]
- COMAN D. R. Reduction in cellular adhesiveness upon contact with a carcinogen. Cancer Res. 1960 Sep;20:1202–1204. [PubMed] [Google Scholar]
- CRANE R. K., FIELD R. A., CORI C. F. Studies of tissue permeability. I. The penetration of sugars into the Ehrlich ascites tumor cells. J Biol Chem. 1957 Feb;224(2):649–662. [PubMed] [Google Scholar]
- DELUCA H. F., ENGSTROM G. W., RASMUSSEN H. The action of vitamin D and parathyroid hormone in vitro on calcium uptake and release by kidney mitochondria. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1604–1609. doi: 10.1073/pnas.48.9.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKENS F., WILLIAMSON D. H. Pentose phosphate isomerase and epimerase from animal tissues. Biochem J. 1956 Nov;64(3):567–578. doi: 10.1042/bj0640567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- Dickens F., Greville G. D. The metabolism of normal and tumour tissue: Neutral salt effects. Biochem J. 1935 Jun;29(6):1468–1483. doi: 10.1042/bj0291468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROBECKER H., KROMPHARDT H., MARIANI H., HEINZ E. UNTERSUCHUNGEN UEBER DEN ELEKTROLYTHAUSHALT DER EHRLICH-ASCITES-TUMORZELLE. Biochem Z. 1963 Jul 26;337:462–476. [PubMed] [Google Scholar]
- Geiger A. Glycolysis in cell-free extracts of brain. Biochem J. 1940 Apr;34(4):465–482. doi: 10.1042/bj0340465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEMPLING H. G. Potassium transport in the Ehrlich mouse ascites tumor cell: evidence for autoinhibition by external potassium. J Cell Comp Physiol. 1962 Dec;60:181–198. doi: 10.1002/jcp.1030600302. [DOI] [PubMed] [Google Scholar]
- KACHMAR J. F., BOYER P. D. Kinetic analysis of enzyme reactions. II. The potassium activation and calcium inhibition of pyruvic phosphoferase. J Biol Chem. 1953 Feb;200(2):669–682. [PubMed] [Google Scholar]
- KIT S., GRIFFIN A. C. Cellular metabolism and cancer: a review. Cancer Res. 1958 Jul;18(6):621–656. [PubMed] [Google Scholar]
- KREBS H. A. Body size and tissue respiration. Biochim Biophys Acta. 1950 Jan;4(1-3):249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MALMSTROM B. G. Metal-ion specificity in the activation of enolase. Arch Biochem Biophys. 1955 Oct;58(2):381–397. doi: 10.1016/0003-9861(55)90138-7. [DOI] [PubMed] [Google Scholar]
- MCILWAIN H. Phosphates of brain during in vitro metabolism: effects of oxygen, glucose, glutamate, glutamine, and calcium and potassium salts. Biochem J. 1952 Oct;52(2):289–295. doi: 10.1042/bj0520289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELLANBY J., WILLIAMSON D. H. THE EFFECT OF CALCIUM IONS ON KETONE-BODY PRODUCTION BY RAT-LIVER SLICES. Biochem J. 1963 Sep;88:440–444. doi: 10.1042/bj0880440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMASON D., SCHOFIELD R. Calcium exchanges between cells and environment. I. Ehrlich ascites tumour cells of mice, in vivo. Exp Cell Res. 1959 Feb;16(2):324–334. doi: 10.1016/0014-4827(59)90260-5. [DOI] [PubMed] [Google Scholar]
- WARBURG O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- WU R. Leakage of enzymes from ascites tumor cells. Cancer Res. 1959 Dec;19:1217–1222. [PubMed] [Google Scholar]
- WU R., RACKER E. Regulatory mechanisms in carbohydrate metabolism. III. Limiting factors in glycolysis of ascites tumor cells. J Biol Chem. 1959 May;234(5):1029–1035. [PubMed] [Google Scholar]


