Abstract
1. Studies were carried out to determine the cellular and subcellular site of biosynthesis of components of fraction I, an α-globulin fraction containing acidic glycoproteins isolated from guinea-pig serum. l-[U-14C]Leucine or -valine and d-[1-14C]glucosamine were used as precursors. 2. A lag of about 10min. occurred before appreciable label appeared in fraction I of serum after injection of leucine or glucosamine. Label in fraction I after 60min. labelling with glucosamine was present almost entirely in hexosamine and sialic acid. 3. Site of synthesis was investigated by studies in vivo up to 17min. after injection of precursor. Particulate subcellular fractions isolated from liver, spleen and kidney or homogenates of the latter two tissues were extracted with Lubrol. Extracts were allowed to react by double diffusion with antisera to fraction I or to subfractions isolated from it, and gels were subsequently subjected to radioautography. With either amino acid or glucosamine as precursor, only extracts of the microsome fraction of liver formed precipitin lines that were appreciably radioactive. 4. The role of the microsome fraction of liver in the synthesis of these glycoproteins was confirmed by immunological studies after incubation of liver slices with leucine or glucosamine. Incorporation of leucine was also investigated in a cell-free microsome system. 5. Material was also precipitated from certain Lubrol extracts of liver microsomes by direct addition of antiserum and its radioactivity measured. Degradation of material thus precipitated and use of heterologous immune systems showed that labelling of precipitin lines represented biosynthesis. 6. A study of extraction procedures suggested that the substances present in the microsome fraction of liver that react with specific antisera are associated with membranous structures. 7. Most or all precipitin lines formed by Lubrol extracts of liver microsomes interacted with precipitin lines given by guinea-pig serum or fraction I, immunological identity being apparent with some lines. The microsome-bound substances thus represent serum glycoproteins or precursors of them. 8. The distribution of label in various tissues and in the protein of subcellular fractions of liver after administration of [14C]glucosamine to the guinea pig was also studied. Some variation in results obtained with liver was found depending on the fractionation medium used.
Full text
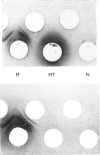
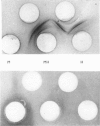
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATHINEOS E., THORNTON M., WINZLER R. J. Comparative antigenicity of native and "desialized" orosomucoid in rabbits. Proc Soc Exp Biol Med. 1962 Nov;111:353–356. doi: 10.3181/00379727-111-27790. [DOI] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKER S. A., WHITEHEAD P. H. SOME IMMUNOCHEMICAL STUDIES ON OROSOMUCOID. Clin Chim Acta. 1963 Nov;8:848–854. doi: 10.1016/0009-8981(63)90006-8. [DOI] [PubMed] [Google Scholar]
- Bruni C., Porter K. R. The Fine Structure of the Parenchymal Cell of the Normal Rat Liver: I. General Observations. Am J Pathol. 1965 May;46(5):691–755. [PMC free article] [PubMed] [Google Scholar]
- Cunningham W., Simkin J. L. Studies on glycopeptides deried from acidic glycoproteins of guinea-pig serum. Biochem J. 1966 May;99(2):434–442. doi: 10.1042/bj0990434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droz B. Elaboration de glycoproteines dans l'appareil de Golgi des cellules hépatiques chez le rat; étude radioautographique en microscopie électronique après injection de glactose-3H. C R Acad Sci Hebd Seances Acad Sci D. 1966 Apr 18;262(16):1766–1768. [PubMed] [Google Scholar]
- HELGELAND L. INCORPORATION OF RADIOACTIVE GLUCOSAMINE INTO SUBMICROSOMAL FRACTIONS ISOLATED FROM RAT LIVER. Biochim Biophys Acta. 1965 Mar 1;101:106–112. doi: 10.1016/0926-6534(65)90035-7. [DOI] [PubMed] [Google Scholar]
- HULTIN T., ARRHENIUS E., LOW H., MAGEE P. N. Toxic liver injury. Inhibition by dimethylnitrosamine of incorporation of labelled amino acids into proteins of rat-liver preparations in vitro. Biochem J. 1960 Jul;76:109–116. doi: 10.1042/bj0760109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHN P., WINZLER J., HOFFMAN R. C. Metabolism of D-glucosamine and N-acetyl-D-glucosamine in the intact rat. J Biol Chem. 1962 Feb;237:304–308. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARSH J. B., DRABKIN D. L. Metabolic channeling in experimental nephrosis. IV. Net synthesis of plasma albumin by liver slices from normal and nephrotic rats. J Biol Chem. 1958 Feb;230(2):1073–1081. [PubMed] [Google Scholar]
- MILLER L. L., BALE W. F. Synthesis of all plasma protein fractions except gamma globulins by the liver; the use of zone electrophoresis and lysine-epsilon-C14 to define the plasma proteins synthesized by the isolated perfused liver. J Exp Med. 1954 Feb;99(2):125–132. doi: 10.1084/jem.99.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLNAR J., ROBINSON G. B., WINZLER R. J. BIOSYNTHESIS OF GLYCOPROTEINS. IV. THE SUBCELLULAR SITES OF INCORPORATION OF GLUCOSAMINE-1-14-C INTO GLYCOPROTEIN RAT LIVER. J Biol Chem. 1965 May;240:1882–1888. [PubMed] [Google Scholar]
- MOLNAR J., ROBINSON G. B., WINZLER R. J. THE BIOSYNTHESIS OF GLYCOPROTEINS. 3. GLUCOSAMINE INTERMEDIATES IN PLASMA GLYCOPROTEIN SYNTHESIS IN LIVERS OF PUROMYCIN-TREATED RATS. J Biol Chem. 1964 Oct;239:3157–3162. [PubMed] [Google Scholar]
- MORGAN W. S., PERLMANN P., HULTIN T. Autoradiographic analysis on agar plates of antigens from subcellular fractions of rat liver slices. J Biophys Biochem Cytol. 1961 Jul;10:411–423. doi: 10.1083/jcb.10.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth R. A., Bekesi J. G., Sugden E., Bice S. The metabolism of plasma glycoproteins. I. Studies on the rate of incorporation of glucosamine-1-14C into protein-bound hexosamine and N-acetylneuraminic acid in the normal rat. J Biol Chem. 1965 Oct;240(10):3707–3713. [PubMed] [Google Scholar]
- O'Brien P. J., Canady M. R., Hall C. W., Neufeld E. F. Transfer of N-acetylneuraminic acid to incomplete glycoproteins associated with microsomes. Biochim Biophys Acta. 1966 Apr 25;117(2):331–341. doi: 10.1016/0304-4165(66)90084-5. [DOI] [PubMed] [Google Scholar]
- PETERS T., Jr The biosynthesis of rat serum albumin. II. Intracellular phenomena in the secretion of newly formed albumin. J Biol Chem. 1962 Apr;237:1186–1189. [PubMed] [Google Scholar]
- RICHMOND J. E. STUDIES ON THE METABOLISM OF PLASMA GLYCOPROTEINS. Biochemistry. 1963 Jul-Aug;2:676–683. doi: 10.1021/bi00904a010. [DOI] [PubMed] [Google Scholar]
- ROBINSON G. B., MOLNAR J., WINZLER R. J. BIOSYNTHESIS OF GLYCOPROTEINS. I. INCORPORATION OF GLUCOSAMINE-14C INTO LIVER AND PLASMA PROTEINS OF THE RAT. J Biol Chem. 1964 Apr;239:1134–1141. [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARCIONE E. J., BOHNE M., LEAHY M. THE SUBECELLULAR SITE OF HEXOSAMINE INCORPORATION INTO LIVER PROTEIN. Biochemistry. 1964 Dec;3:1973–1976. doi: 10.1021/bi00900a032. [DOI] [PubMed] [Google Scholar]
- SARCIONE E. J. Sunthesis of alphal-acid glycoprotein by the isolated perfused rat liver. Arch Biochem Biophys. 1963 Mar;100:516–519. doi: 10.1016/0003-9861(63)90120-6. [DOI] [PubMed] [Google Scholar]
- SARCIONE E. J. THE INITIAL SUBCELLULAR SITE OF INCORPORATION OF HEXOSES INTO LIVER PROTEIN. J Biol Chem. 1964 Jun;239:1686–1689. [PubMed] [Google Scholar]
- SCHALL H., TURBA F. RESISTENZ DER BIOSYNTHESE VON HAEMOGLOBINEN IN HUEHNER-RETICULOCYTEN GEGENUEBER ROENTGENBESTRAHLUNG. Biochem Z. 1963 Dec 3;339:224–232. [PubMed] [Google Scholar]
- SIMKIN J. L., SESHADRI H. S., SKINNER E. R. ACTION OF GLYCOSIDASES AND PERIODATE ON GUINEA PIG SERUM GLYCOPROTEINS. Nature. 1964 May 16;202:702–703. doi: 10.1038/202702b0. [DOI] [PubMed] [Google Scholar]
- SIMKIN J. L., WORK T. S. Protein synthesis in guinea-pig liver; incorporation of radioactive amino acids into proteins of the microsome fraction in vivo. Biochem J. 1957 Feb;65(2):307–315. doi: 10.1042/bj0650307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINOHARA H., SKY-PECK H. H. SOLUBLE RIBONUCLEIC ACID AND GLYCOPROTEIN BIOSYNTHESIS IN THE MOUSE LIVER. Biochim Biophys Acta. 1965 Mar 1;101:90–96. doi: 10.1016/0926-6534(65)90033-3. [DOI] [PubMed] [Google Scholar]
- Simkin J. L., Skinner E. R., Seshadri H. S. Studies on an acidic glycoprotein-containing fraction isolated from guinea-pig serum. Biochem J. 1964 Feb;90(2):316–330. doi: 10.1042/bj0900316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin J. L., Sutton C. R. The incorporation of radioactive amino acids into proteins of the microsome fraction of guinea-pig liver at very short time intervals after administration. Biochem J. 1960 Dec;77(3):489–492. doi: 10.1042/bj0770489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro R. G., Spiro M. J. Glycoprotein biosynthesis: studies on thyroglobulin. Characterization of a particulate precursor and radioisotope incorporation by thyroid slices and particle systems. J Biol Chem. 1966 Mar 25;241(6):1271–1282. [PubMed] [Google Scholar]
- Tsukada K., Lieberman I. Protein synthesis by liver polyribosomes after partial hepatectomy. Biochem Biophys Res Commun. 1965 Jun 9;19(6):702–707. doi: 10.1016/0006-291x(65)90314-1. [DOI] [PubMed] [Google Scholar]
- WEINFELD H., TUNIS M. The cleavage of human alpha1-acid glycoprotein by pepsin. J Biol Chem. 1960 Jun;235:1668–1672. [PubMed] [Google Scholar]