Abstract
1. The ultrastructure of all the afferent fibres, or all the efferent fibres, was studied in selected nerves from chronically de-afferentated or de-efferentated cat hind limbs perfusion-fixed with glutaraldehyde.
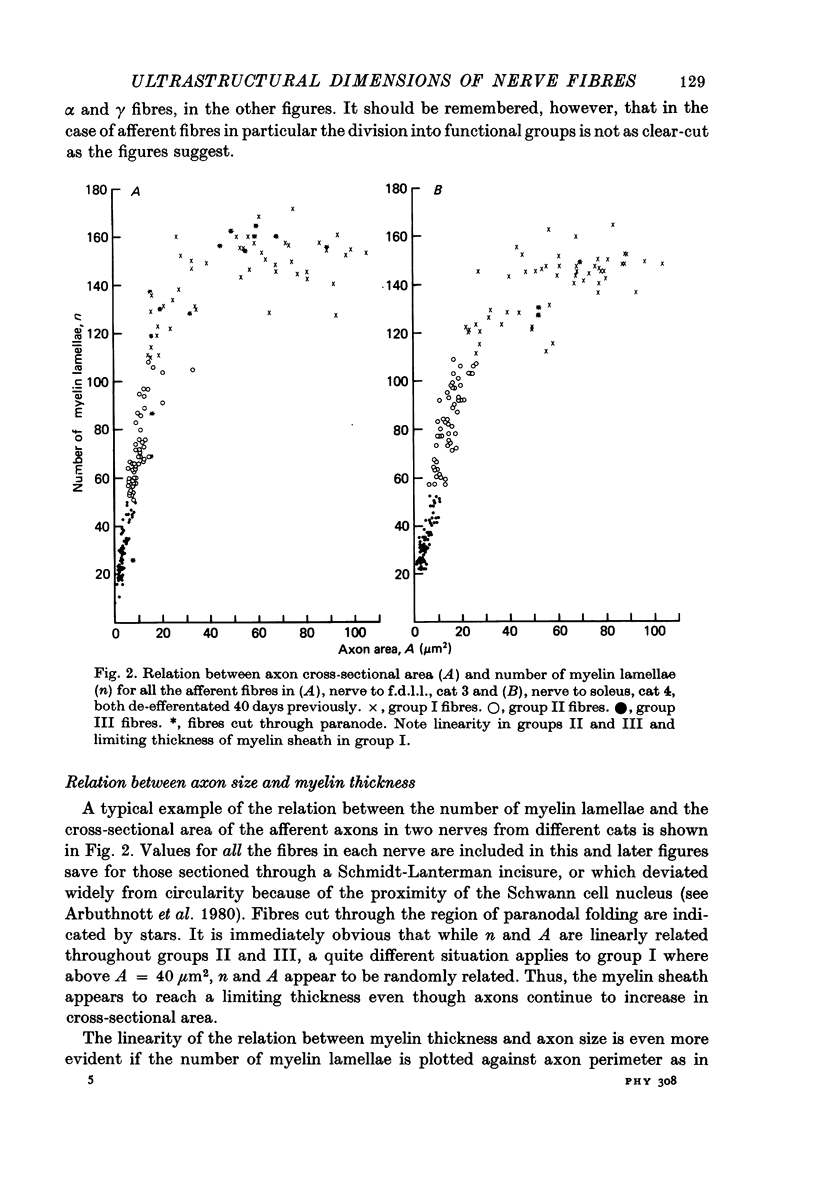
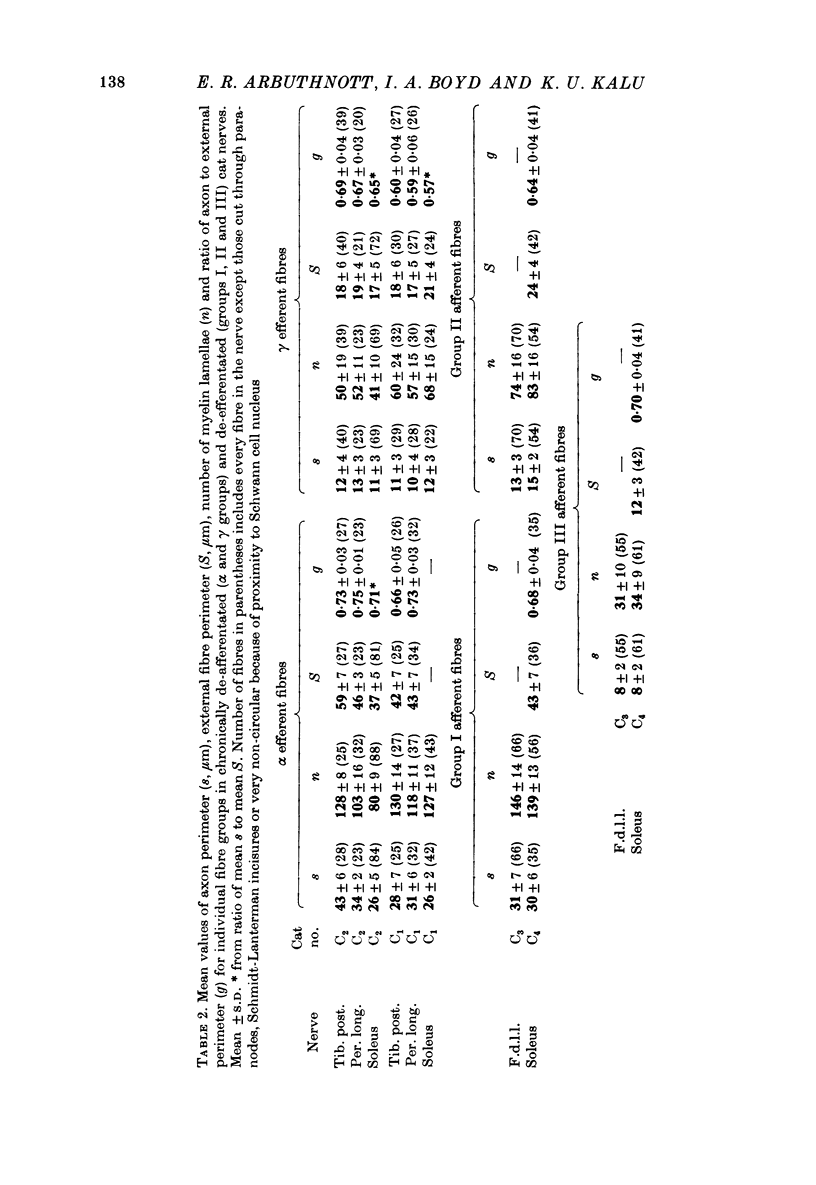
2. The following parameters were measured: number of lamellae in the myelin sheath (n), axon perimeter (s), external fibre perimeter (S), axon cross-sectional area (A). Fibres were allocated to afferent groups I, II, III or efferent groups α and γ according to the number of lamellae in the myelin sheath.
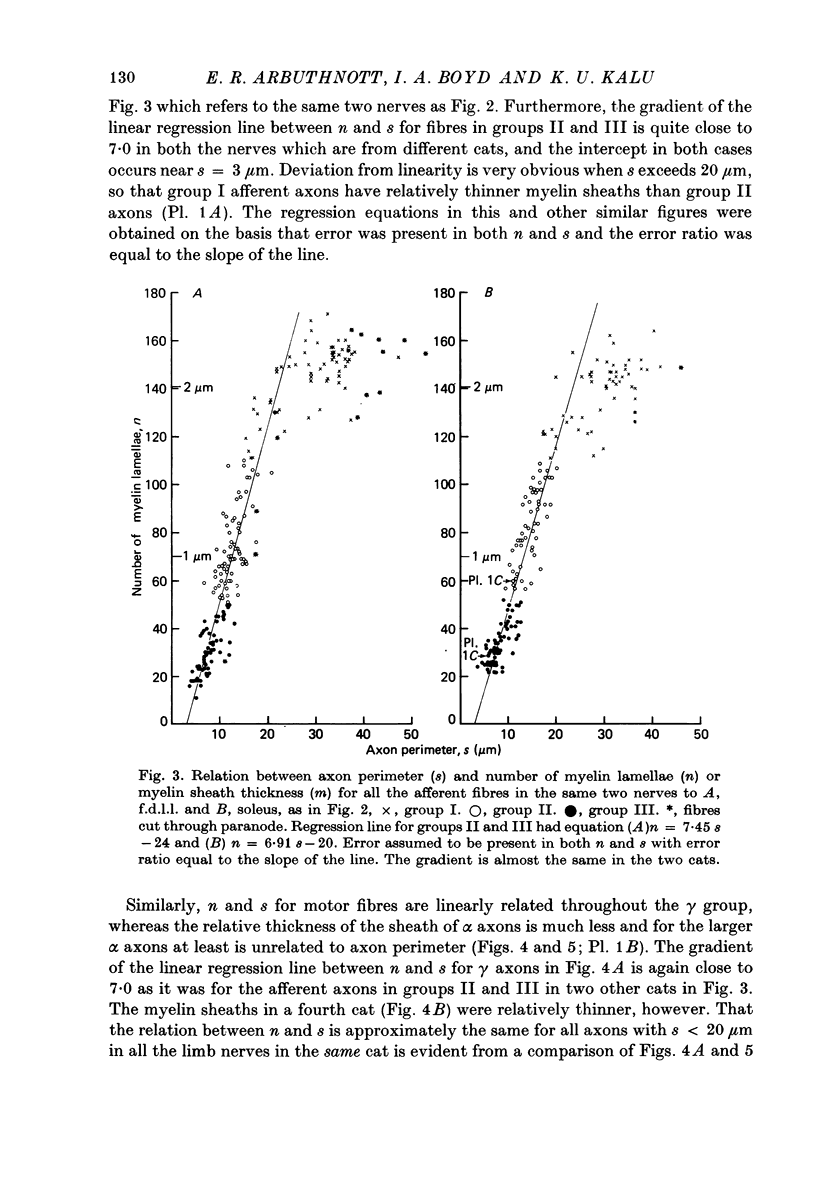
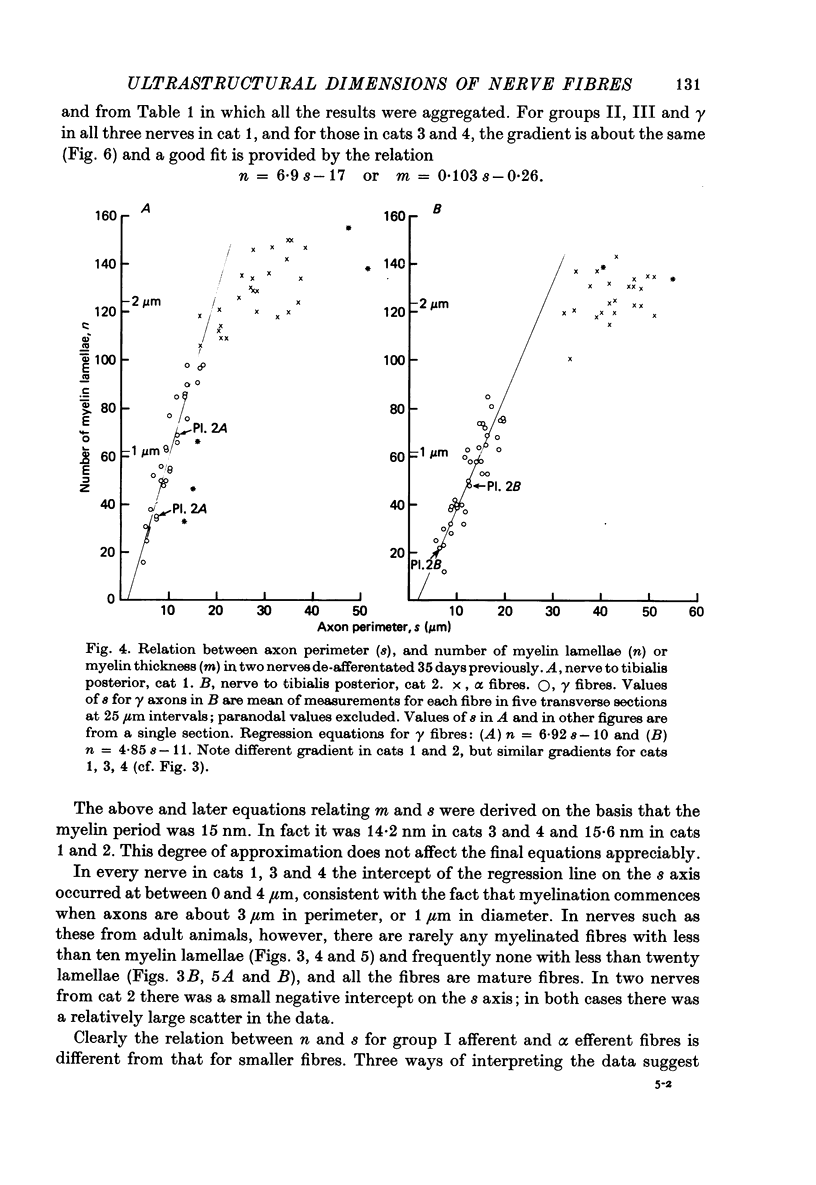
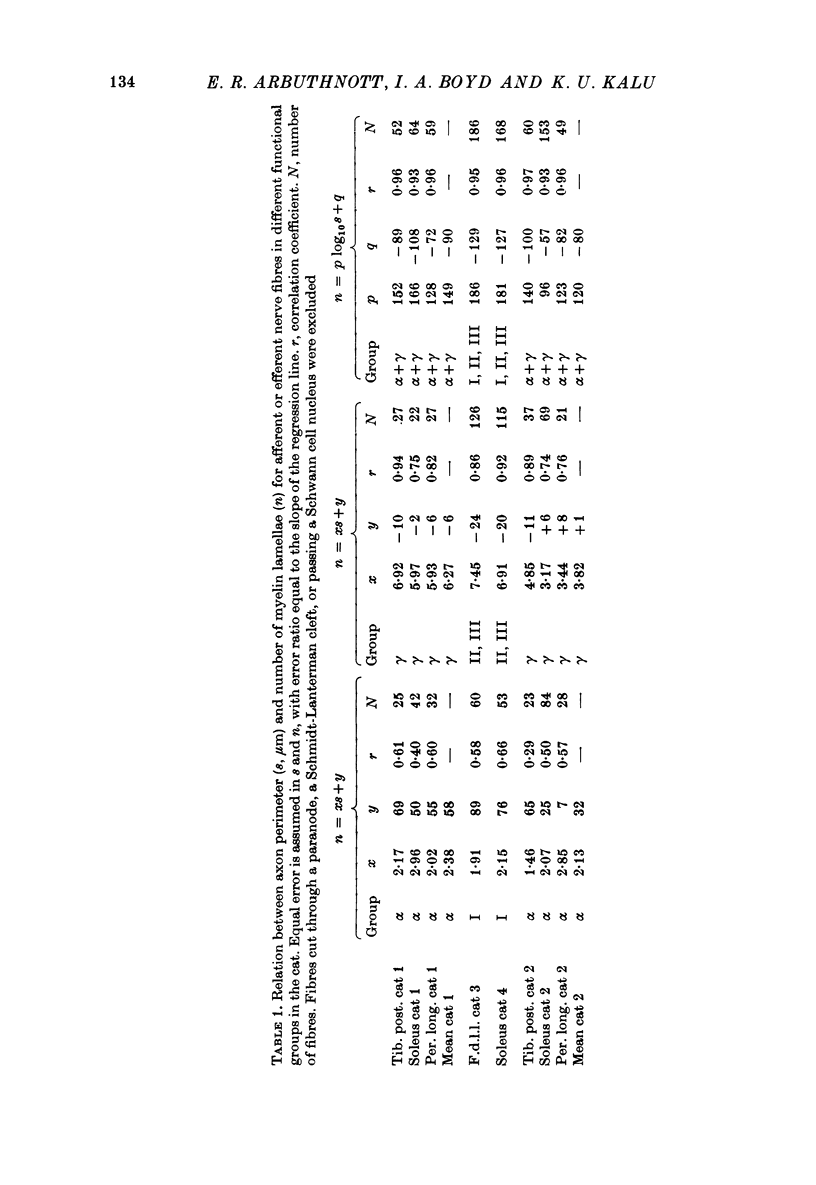
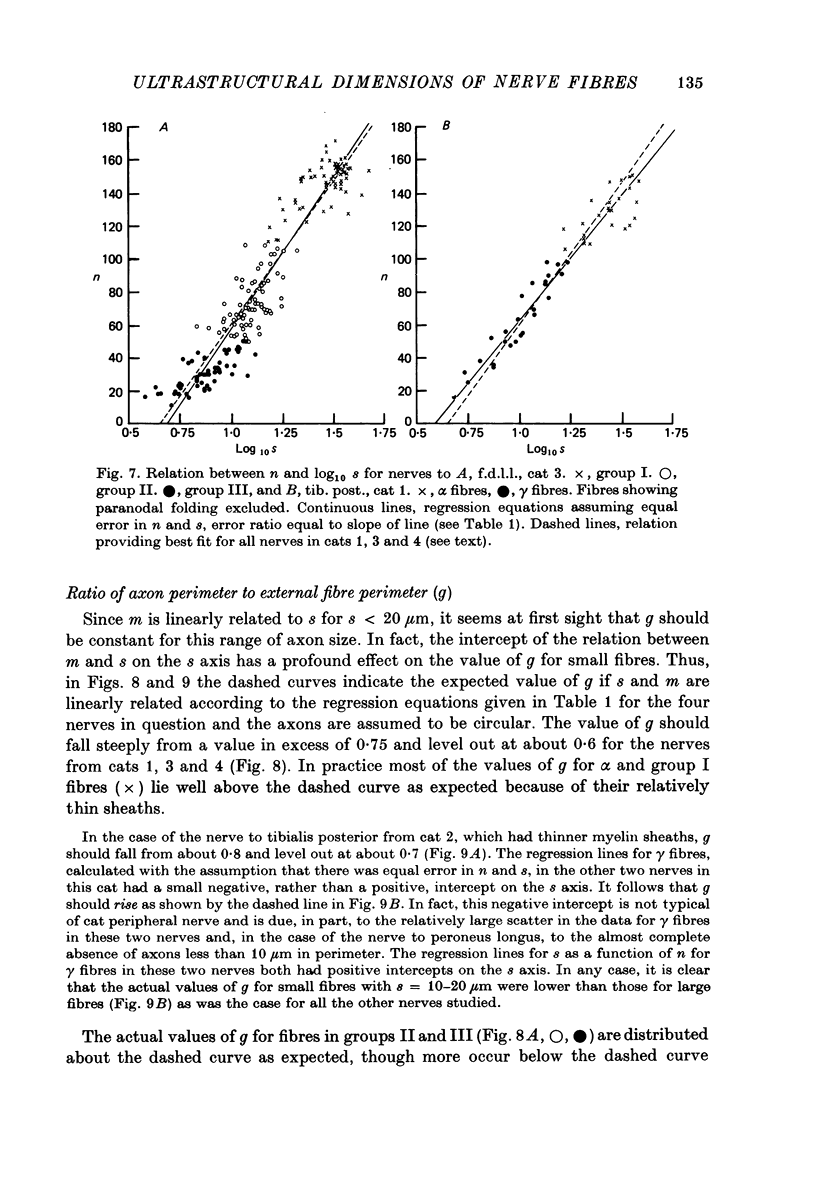
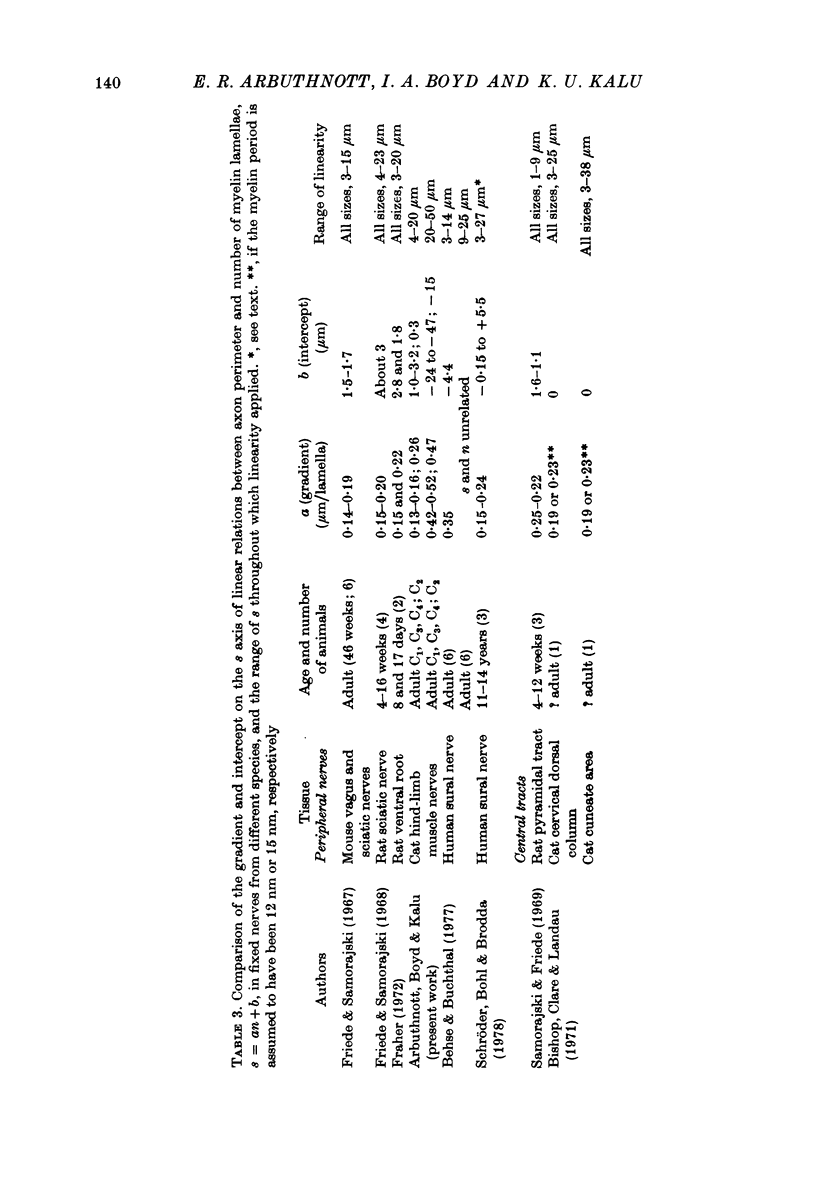
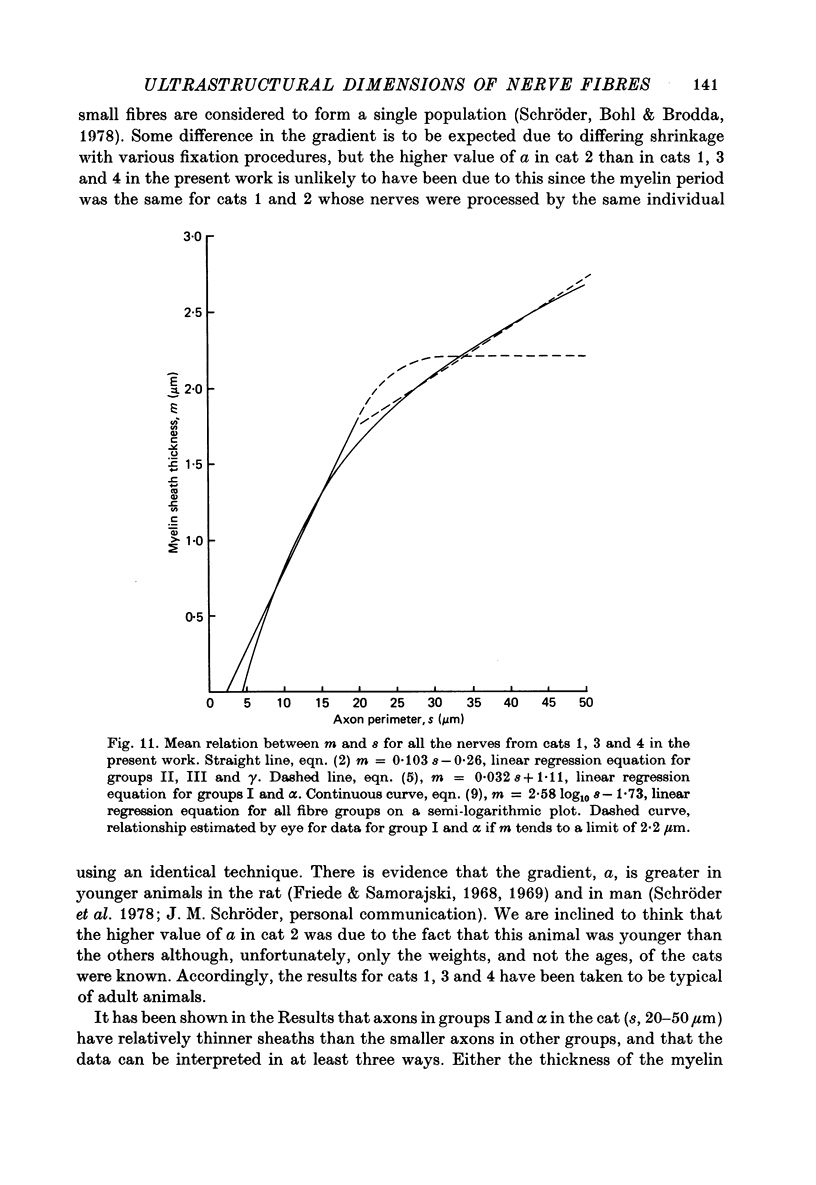
3. The thickness of the myelin sheath (m) was linearly related to axon perimeter within the range s = 4 μm to s = 20 μm (groups II, III and γ). The relation m = 0·103 s — 0·26 provided a good fit for all afferent and efferent axons in this range in several different anatomical muscle nerves in three cats. The myelin sheaths were thinner in a fourth, presumably younger, cat.
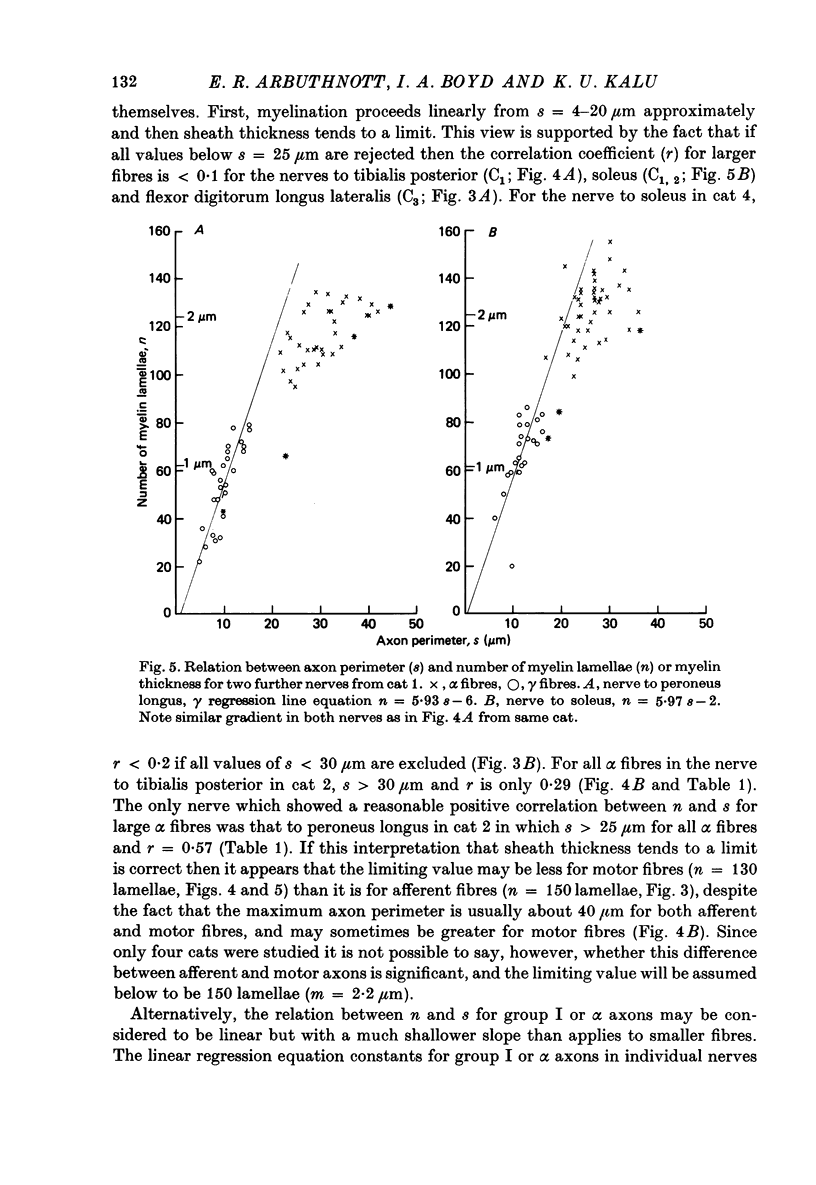
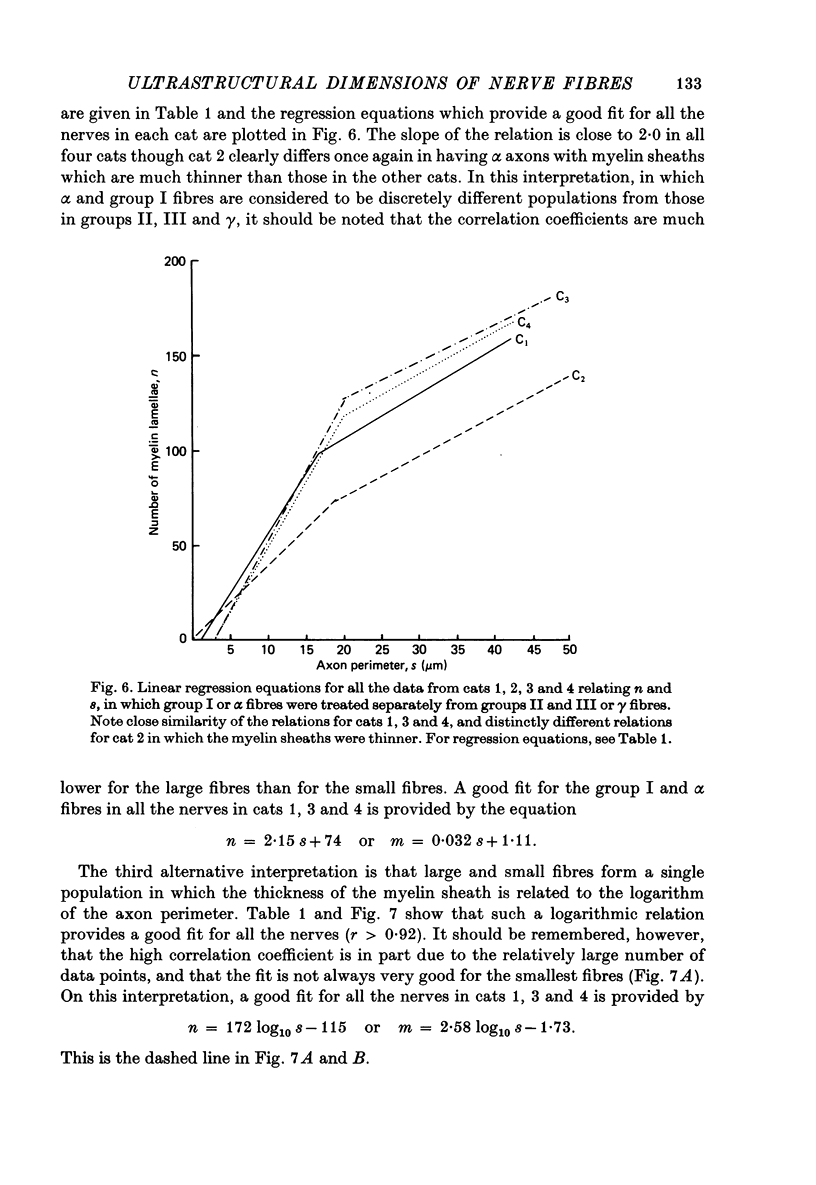
4. The myelin sheaths were relatively thinner for large fibres in groups I and α (s = 20-50 μm). The results are interpreted in one of three ways. Either m tends to a limit of about 2·2 μm, or m is linearly related to s such that for large fibres m = 0·032 s + 1·11.
5. Alternatively, m may be considered to be proportional to log10 s for all sizes of axon so that m = 2·58 log10 S — 1·73. The interpretation that there are two separate linear relations for large and small fibres is favoured.
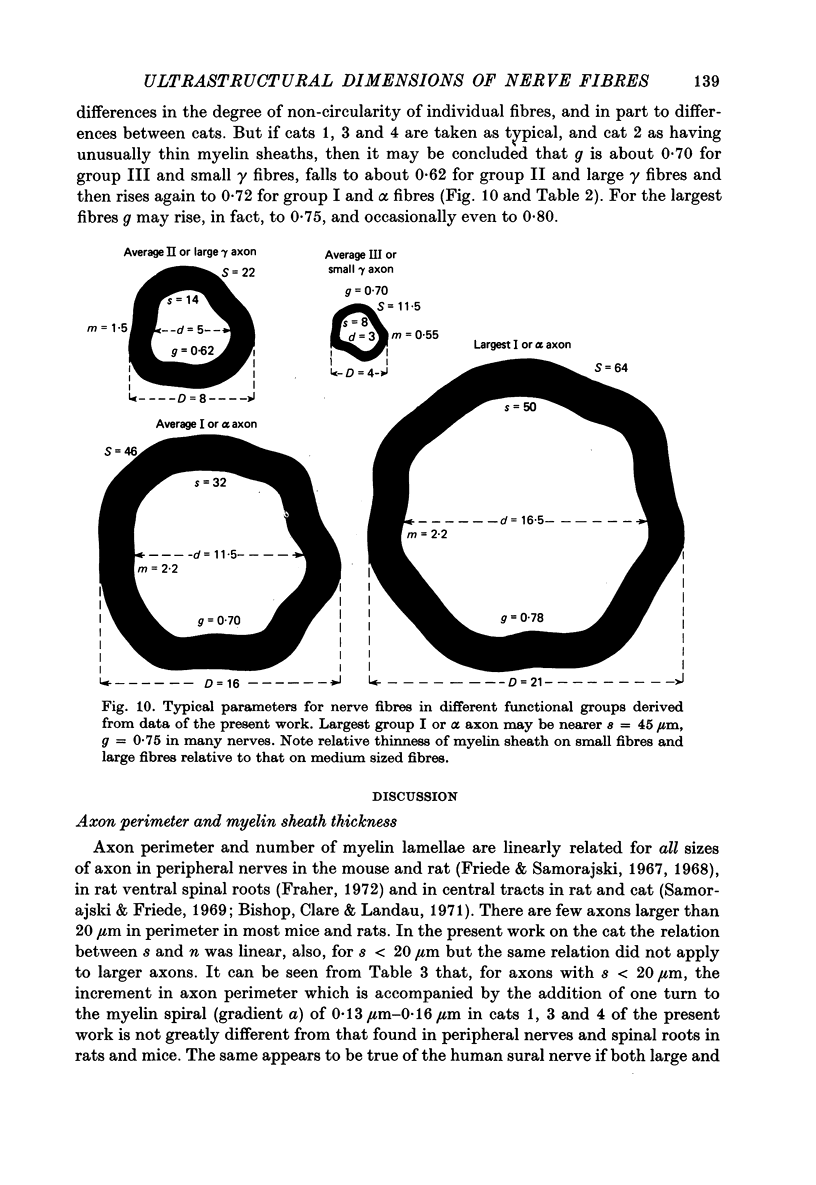
6. The ratio of axon to external fibre perimeter (g) falls from about 0·70 for group III and small γ fibres in the cat to about 0·62 for group II and large γ fibres and then rises again to 0·70, or even 0·75 for group I and α axons.
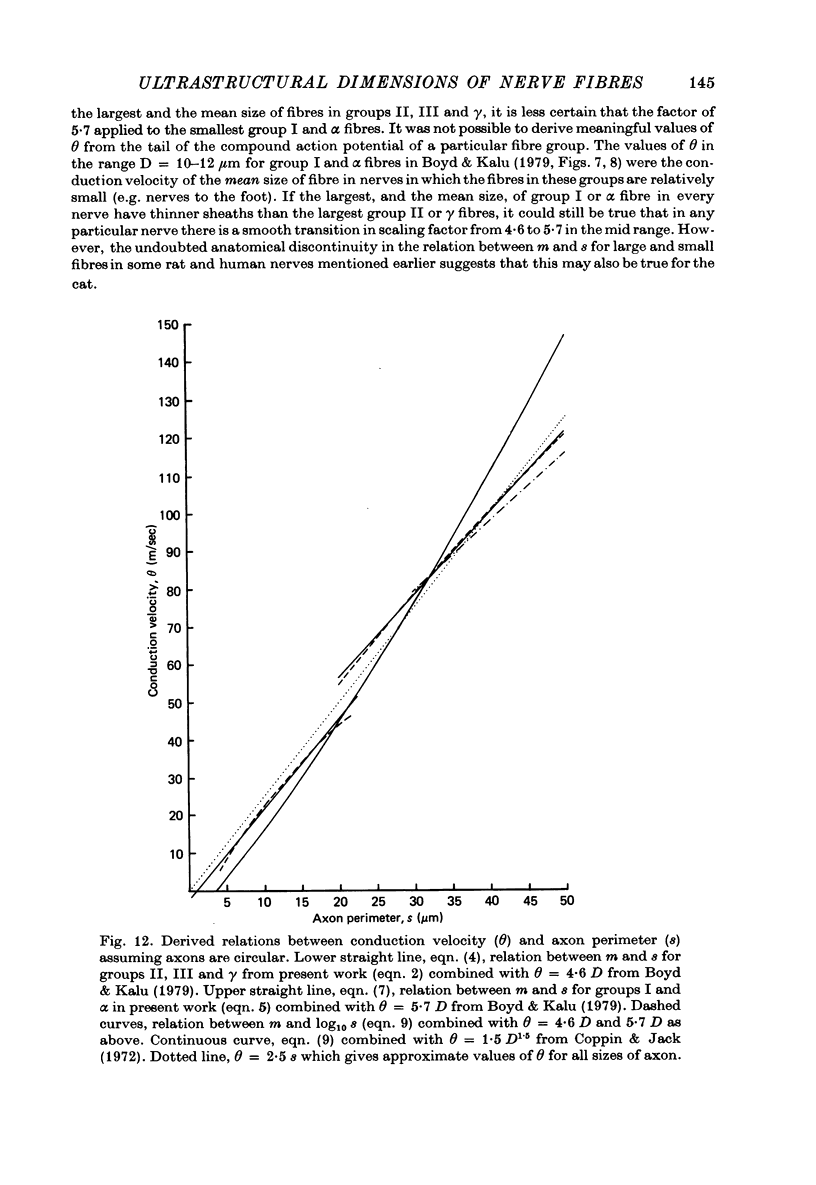
7. The above relations between m and s are combined with the observations of Boyd & Kalu (1979) that Θ = 5·7 D for groups I and α and Θ = 4·6 D for groups II, III and γ. It is shown that Θ = 2·5 s approximately for all sizes of axon (s from material fixed for electron microscopy) in rat, cat and man. The accuracy of this equation may be improved by deducting 3 m/sec in the case of small fibres. This conclusion is compatible with experimental observations of the relation between l and D (Hursh, 1939; Lubinska, 1960; Coppin, 1973) and between l and Θ (Coppin & Jack, 1972).
8. From the theoretical analyses of Rushton (1951) and others Θ should be proportional to the external dimensions of the fibre rather than to axon size. It is shown that the thinning of the myelin sheath ought to affect Θ substantially. Thus some other factors must compensate for the thinning of the sheath.
9. Small fibres are significantly more non-circular than large fibres. From the quantitative data of Arbuthnott et al. (1980) it is concluded that non-circularity may contribute to the fact that Θ ∝ s rather than Θ ∝ S, but cannot wholly account for it. Other possibilities considered are that axoplasmic resistivity or specific nodal conductance may differ for large and small fibres.
10. It is suggested that myelinated peripheral nerve fibres may fall into two distinct classes with different properties, one comprising groups I and α and the other groups II, III and γ. The conclusion predicted from theory may apply to each of these classes separately so that Θ = 2·0 S for the large-fibre class and Θ = 1·6 S for the small-fibre class.
Full text
PDF



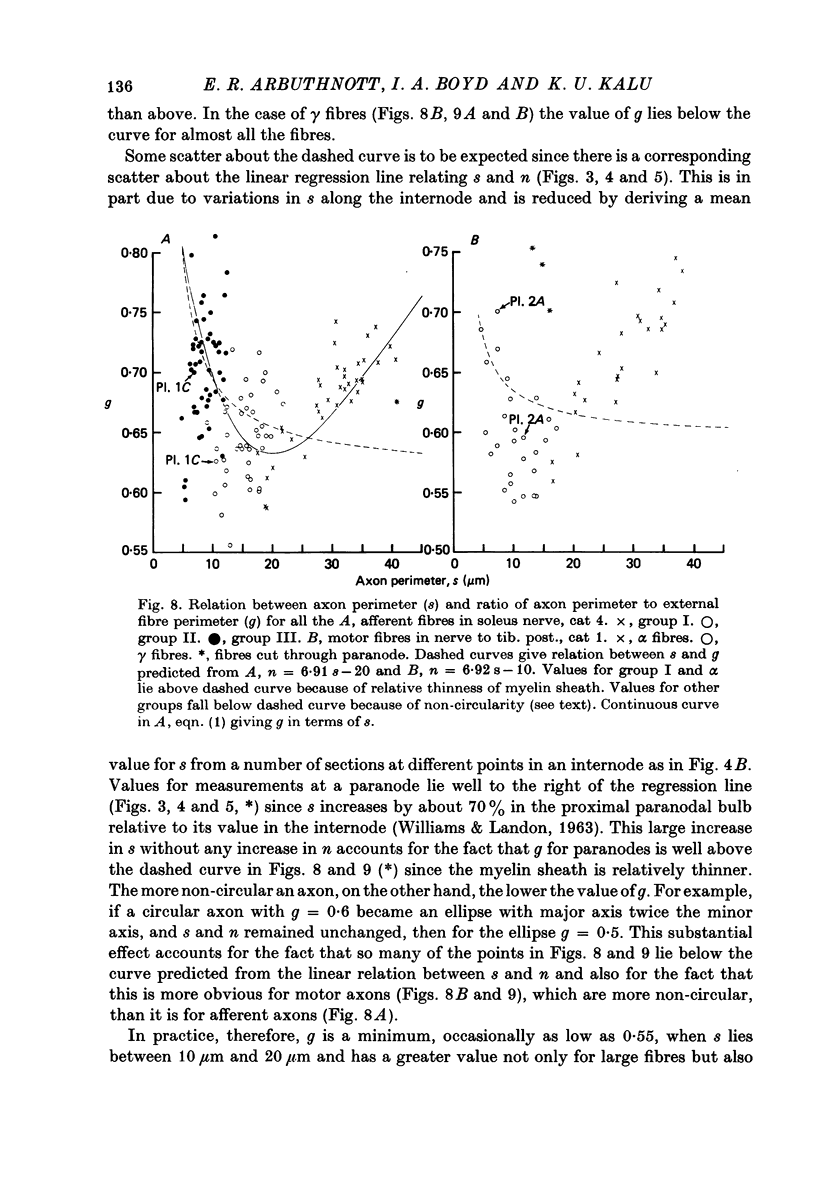







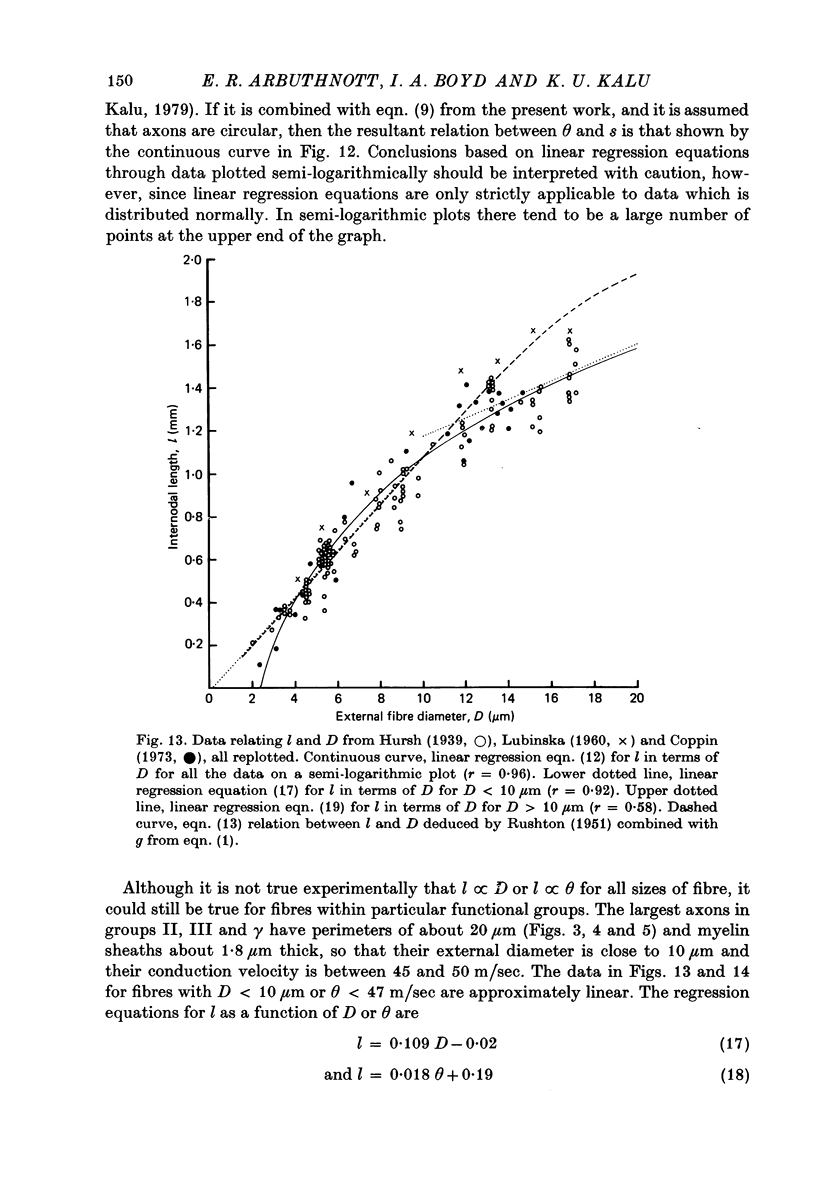
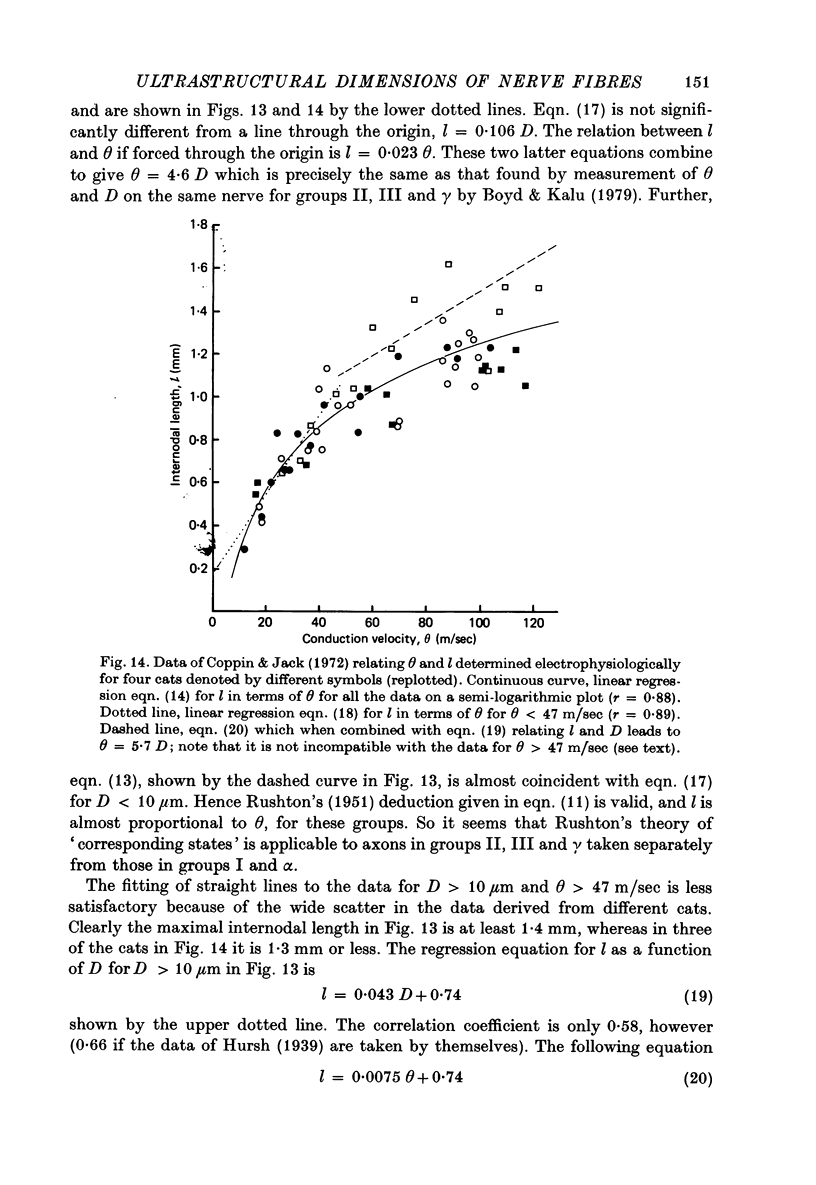








Images in this article
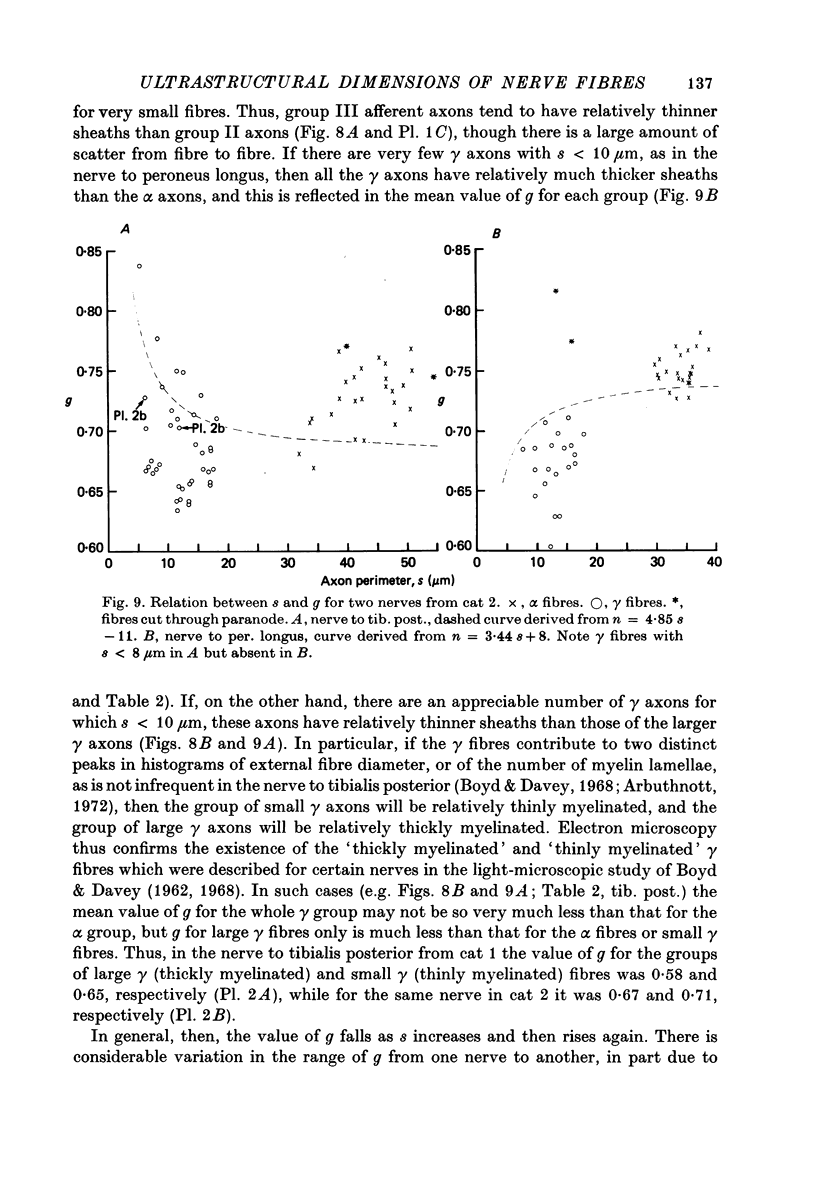
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew B. L., Leslie G. C., Thompson J. Distribution and properties of muscle spindles in the caudal segmental muscles of the rat together with some comparisons with hind limb muscle spindles. Q J Exp Physiol Cogn Med Sci. 1973 Jan;58(1):19–37. doi: 10.1113/expphysiol.1973.sp002188. [DOI] [PubMed] [Google Scholar]
- Arbuthnott E. R., Ballard K. J., Boyd I. A., Kalu K. U. Quantitative study of the non-circularity of myelinated peripheral nerve fibres in the cat. J Physiol. 1980 Nov;308:99–123. doi: 10.1113/jphysiol.1980.sp013464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott E. R. Routine collection of flat large-area sections for electron microscopy as applied to a detailed study of axon dimensions. J Microsc. 1974 Jul;101(Pt 2):219–221. doi: 10.1111/j.1365-2818.1974.tb03888.x. [DOI] [PubMed] [Google Scholar]
- Behse F., Buchthal F. Peroneal muscular atrophy (PMA) and related disorders. II. Histological findings in sural nerves. Brain. 1977 Mar;100(Pt 1):67–85. doi: 10.1093/brain/100.1.67. [DOI] [PubMed] [Google Scholar]
- Berthold C. H. A study on the fixation of large mature feline myelinated ventral lumbar spinal-root fibers. Acta Soc Med Ups. 1968;73(Suppl):1–36. [PubMed] [Google Scholar]
- Bishop G. H., Clare M. H., Landau W. M. The relation of axon sheath thickness to fiber size in the central nervous system of vertebrates. Int J Neurosci. 1971 Aug;2(2):69–77. doi: 10.3109/00207457109146994. [DOI] [PubMed] [Google Scholar]
- Boyd I. A., Kalu K. U. Scaling factor relating conduction velocity and diameter for myelinated afferent nerve fibres in the cat hind limb. J Physiol. 1979 Apr;289:277–297. doi: 10.1113/jphysiol.1979.sp012737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill M. H., Waxman S. G., Moore J. W., Joyner R. W. Conduction velocity and spike configuration in myelinated fibres: computed dependence on internode distance. J Neurol Neurosurg Psychiatry. 1977 Aug;40(8):769–774. doi: 10.1136/jnnp.40.8.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson R. T., Bishop Y., Hedley-Whyte E. T. A contribution to the electron microscopic morphometric analysis of peripheral nerve. J Comp Neurol. 1978 Mar 1;178(1):177–186. doi: 10.1002/cne.901780110. [DOI] [PubMed] [Google Scholar]
- Bronson R. T., Hedley-Whyte E. T. Morphometric analysis of the effects of exenteration and enucleation on the development of third and sixth cranial nerves in the rat. J Comp Neurol. 1977 Dec 1;176(3):315–329. doi: 10.1002/cne.901760302. [DOI] [PubMed] [Google Scholar]
- CRAGG B. G., THOMAS P. K. THE CONDUCTION VELOCITY OF REGENERATED PERIPHERAL NERVE FIBRES. J Physiol. 1964 May;171:164–175. doi: 10.1113/jphysiol.1964.sp007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter D. O., Hovey M. M., Bak A. F. Measurements of intracellular conductivity in Aplysia neurons: evidence for organization of water and ions. Ann N Y Acad Sci. 1973 Mar 30;204:502–533. doi: 10.1111/j.1749-6632.1973.tb30801.x. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Gutrecht J. A., Bastron J. A., Karnes W. E., Dale A. J. Histologic and teased-fiber measurements of sural nerve in disorders of lower motor and primary sensory neurons. Mayo Clin Proc. 1968 Feb;43(2):81–123. [PubMed] [Google Scholar]
- FitzHugh R. Dimensional analysis of nerve models. J Theor Biol. 1973 Aug 22;40(3):517–541. doi: 10.1016/0022-5193(73)90008-8. [DOI] [PubMed] [Google Scholar]
- Fraher J. P. A quantitative study of anterior root fibres during early myelination. J Anat. 1972 May;112(Pt 1):99–124. [PMC free article] [PubMed] [Google Scholar]
- Friede R. L., Samorajski T. Myelin formation in the sciatic nerve of the rat. A quantitative electron microscopic, histochemical and radioautographic study. J Neuropathol Exp Neurol. 1968 Oct;27(4):546–570. [PubMed] [Google Scholar]
- Friede R. L., Samorajski T. Relation between the number of myelin lamellae and axon circumference in fibers of vagus and sciatic nerves of mice. J Comp Neurol. 1967 Jul;130(3):223–231. doi: 10.1002/cne.901300304. [DOI] [PubMed] [Google Scholar]
- Goldman L., Albus J. S. Computation of impulse conduction in myelinated fibers; theoretical basis of the velocity-diameter relation. Biophys J. 1968 May;8(5):596–607. doi: 10.1016/S0006-3495(68)86510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Stämpfli R. Evidence for saltatory conduction in peripheral myelinated nerve fibres. J Physiol. 1949 May 15;108(3):315–339. [PMC free article] [PubMed] [Google Scholar]
- Jack J. J. Physiology of peripheral nerve fibres in relation to their size. Br J Anaesth. 1975 Feb;47 Suppl:173–182. [PubMed] [Google Scholar]
- MELLSTROEM A., SKOGLUND S. CALIBRE SPECTRA OF AFFERENT AND EFFERENT FIBRES IN MUSCLE NERVES OF THE ALBINO RAT'S HIND LIMB. Acta Morphol Neerl Scand. 1965;6:135–145. [PubMed] [Google Scholar]
- Moore J. W., Joyner R. W., Brill M. H., Waxman S. D., Najar-Joa M. Simulations of conduction in uniform myelinated fibers. Relative sensitivity to changes in nodal and internodal parameters. Biophys J. 1978 Feb;21(2):147–160. doi: 10.1016/S0006-3495(78)85515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. H. A theory of the effects of fibre size in medullated nerve. J Physiol. 1951 Sep;115(1):101–122. doi: 10.1113/jphysiol.1951.sp004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARZACHER H. G. Markscheidendicke und Achsenzylinderdurchmesser in peripheren menschlichen Nerven. Acta Anat (Basel) 1954;21(1):26–46. [PubMed] [Google Scholar]
- SUNDERLAND S., ROCHE A. F. Axon-myelin relationships in peripheral nerve fibres. Acta Anat (Basel) 1958;33(1-2):1–37. doi: 10.1159/000141338. [DOI] [PubMed] [Google Scholar]
- Samorajski T., Friede R. L. A quantitative electron microscopic study of myelination in the pyramidal tract of rat. J Comp Neurol. 1968 Nov;134(3):323–338. doi: 10.1002/cne.901340306. [DOI] [PubMed] [Google Scholar]
- Sanders F. K., Whitteridge D. Conduction velocity and myelin thickness in regenerating nerve fibres. J Physiol. 1946 Sep 18;105(2):152–174. [PMC free article] [PubMed] [Google Scholar]
- Schnepp P., Schnepp G. Faseranalytische Untersuchungen an peripheren Nerven bei Tieren verschiedener Grösse. II. Verhältnis Axondurchmesser-Gesamtdurchmesser und Internodallänge. Z Zellforsch Mikrosk Anat. 1971;119(1):99–114. [PubMed] [Google Scholar]
- Schröder J. M., Bohl J., Brodda K. Changes of the ratio between myelin thickness and axon diameter in the human developing sural nerve. Acta Neuropathol. 1978 Aug 7;43(1-2):169–178. doi: 10.1007/BF00685012. [DOI] [PubMed] [Google Scholar]
- Smith R. S., Koles Z. J. Myelinated nerve fibers: computed effect of myelin thickness on conduction velocity. Am J Physiol. 1970 Nov;219(5):1256–1258. doi: 10.1152/ajplegacy.1970.219.5.1256. [DOI] [PubMed] [Google Scholar]
- Thompson J. Parallel spindle systems in the small muscles of the rat tail. J Physiol. 1970 Dec;211(3):781–799. doi: 10.1113/jphysiol.1970.sp009304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomanek R. J., Tipton C. M. Influence of exercise and tenectomy on the morphology of a muscle nerve. Anat Rec. 1967 Sep;159(1):105–113. doi: 10.1002/ar.1091590114. [DOI] [PubMed] [Google Scholar]
- WILLIAMS P. L., LANDON D. N. Paranodal apparatus of peripheral myelinated nerve fibres of mammals. Nature. 1963 May 18;198:670–673. doi: 10.1038/198670a0. [DOI] [PubMed] [Google Scholar]
- Williams P. L., Wendell-Smith C. P. Some additional parametric variations between peripheral nerve fibre populations. J Anat. 1971 Sep;109(Pt 3):505–526. [PMC free article] [PubMed] [Google Scholar]