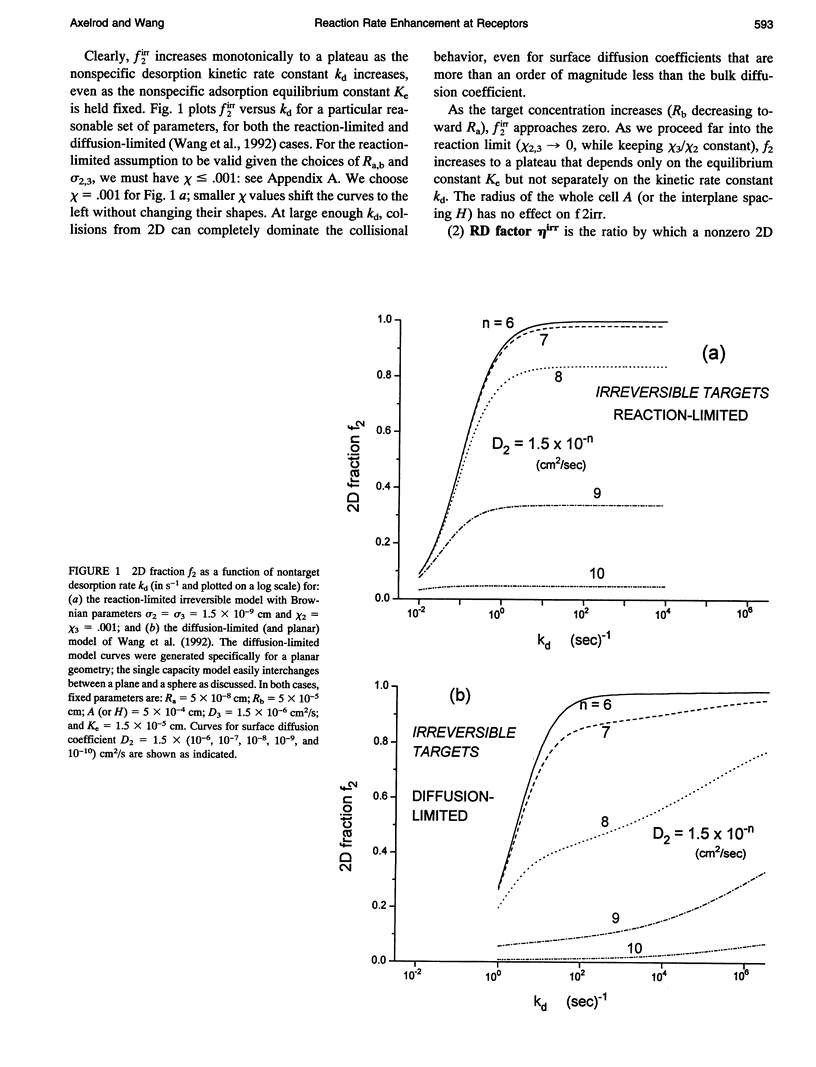
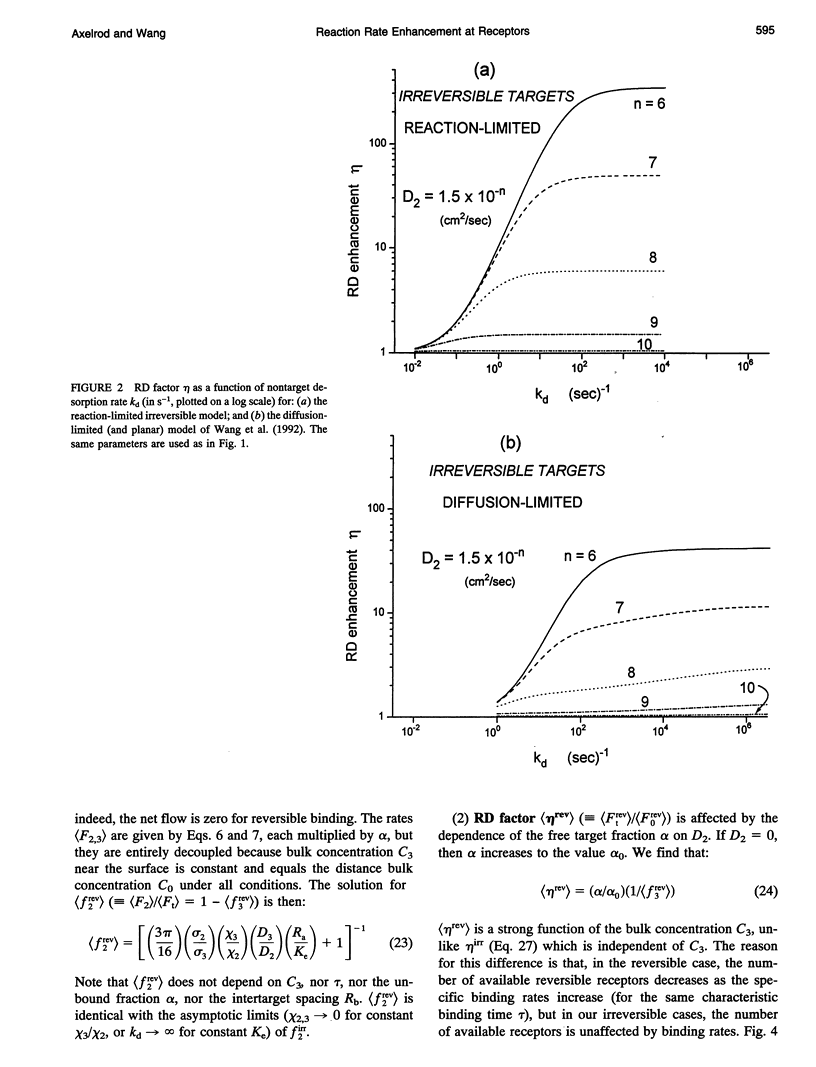
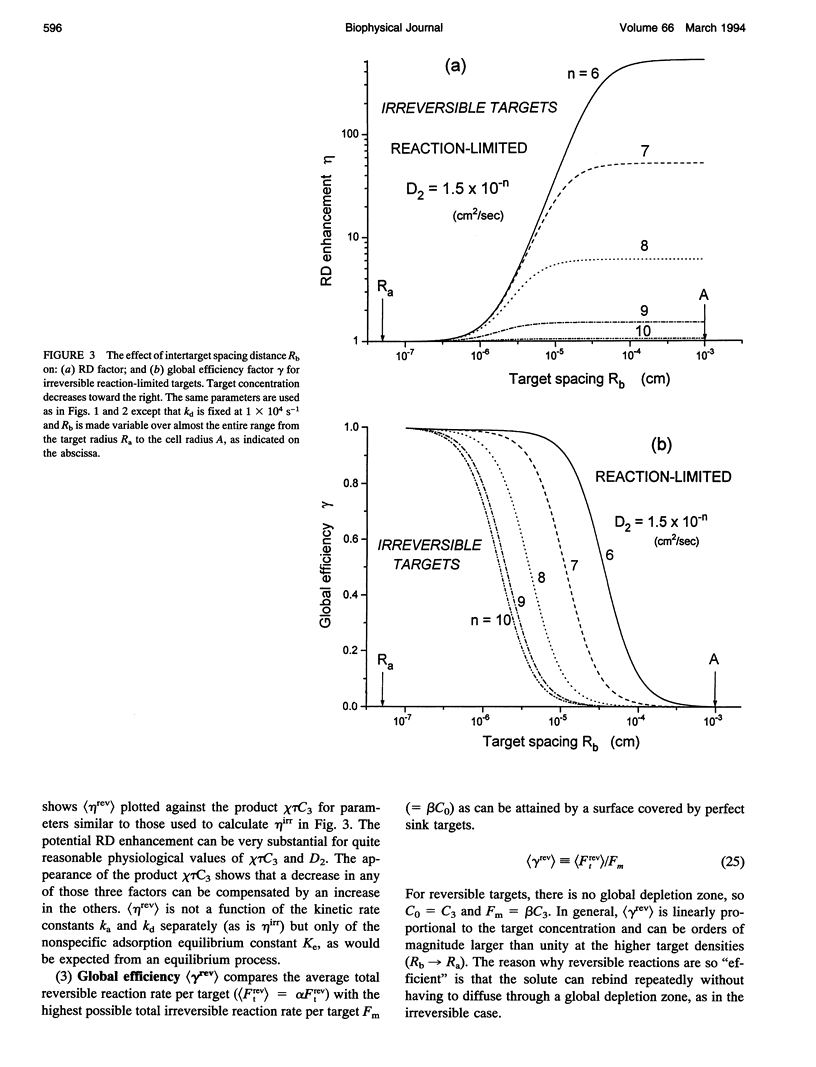
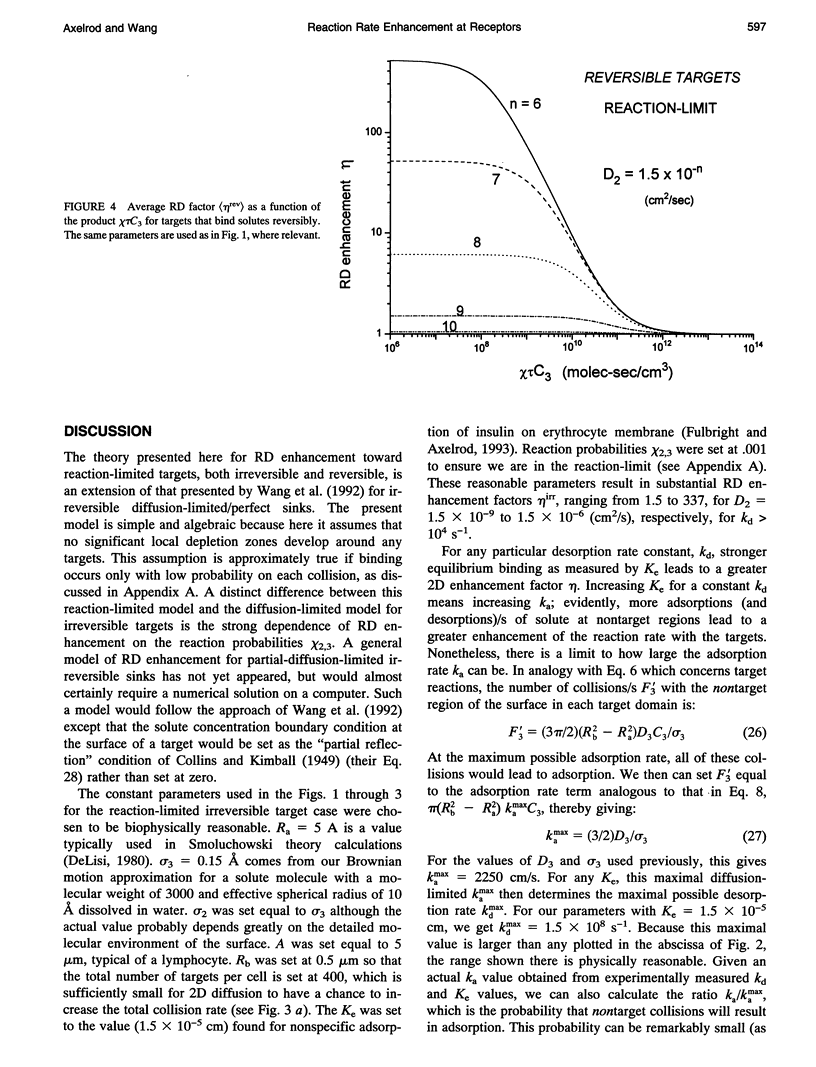
Abstract
It has been suggested for several years that reactions between ligands and cell surface receptors can be speeded up by nonspecific adsorption of the ligand to the cell surface followed by two-dimensional surface diffusion to the receptor, a mechanism referred to as "reduction-of-dimensionality" (RD) rate enhancement. Most of the theoretical treatments of this and related problems have assumed that the receptor is an irreversibly absorbing perfect sink. Such receptors induce a depletion zone of ligand probability density around themselves. The reaction rate in this case (called "diffusion-limited") is limited only by the time required for ligands to diffuse through this depletion zone. In some cases, however, the receptor may be far from "perfect" such that a collision with a ligand only rarely leads to binding. Receptors then do not create significant local depletion zones of ligand probability density, and the reaction rate becomes strongly affected by the (small) probability of reaction success per diffusive encounter (the "reaction-limited" case). This article presents a simple theory of RD rate enhancement for reaction-limited receptors that are either reversible or irreversible binders. In contrast to the diffusion-limited theories, the reaction-limited theory presented here: (a) differs quantitatively from diffusion-limited models; (b) is simple and algebraic in closed form; (c) exhibits significant rate enhancement in some realistic cases; (d) depends strongly on the actual Brownian rather than pure diffusive nature of the ligand's motion; (e) depends (for irreversibly binding receptors only) on the kinetic rates (not just equilibria) of reversible adsorption to nontarget regions, in contrast to some previous approximate theories of reduction of dimensionality; and (f) is applicable to actual ligand/receptor systems with binding success probabilities at the opposite extreme from the perfect sink/diffusion-limited models.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D. Lateral motion of membrane proteins and biological function. J Membr Biol. 1983;75(1):1–10. doi: 10.1007/BF01870794. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Purcell E. M. Physics of chemoreception. Biophys J. 1977 Nov;20(2):193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg O. G. Orientation constraints in diffusion-limited macromolecular association. The role of surface diffusion as a rate-enhancing mechanism. Biophys J. 1985 Jan;47(1):1–14. doi: 10.1016/S0006-3495(85)83870-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt T. P., Axelrod D. Total internal reflection/fluorescence photobleaching recovery study of serum albumin adsorption dynamics. Biophys J. 1981 Mar;33(3):455–467. doi: 10.1016/S0006-3495(81)84906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi C. Stochastic problems in information transfer across the plasma membrane. Bull Math Biol. 1983;45(4):467–482. doi: 10.1007/BF02459583. [DOI] [PubMed] [Google Scholar]
- DeLisi C. The biophysics of ligand-receptor interactions. Q Rev Biophys. 1980 May;13(2):201–230. doi: 10.1017/s0033583500001657. [DOI] [PubMed] [Google Scholar]
- McCloskey M. A., Poo M. M. Rates of membrane-associated reactions: reduction of dimensionality revisited. J Cell Biol. 1986 Jan;102(1):88–96. doi: 10.1083/jcb.102.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. G., Sarmiento J. G., Herbette L. G. Kinetics of binding of membrane-active drugs to receptor sites. Diffusion-limited rates for a membrane bilayer approach of 1,4-dihydropyridine calcium channel antagonists to their active site. Mol Pharmacol. 1985 Jun;27(6):612–623. [PubMed] [Google Scholar]
- Tan R. C., Truong T. N., McCammon J. A., Sussman J. L. Acetylcholinesterase: electrostatic steering increases the rate of ligand binding. Biochemistry. 1993 Jan 19;32(2):401–403. doi: 10.1021/bi00053a003. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., Burghardt T. P., Axelrod D. Measuring surface dynamics of biomolecules by total internal reflection fluorescence with photobleaching recovery or correlation spectroscopy. Biophys J. 1981 Mar;33(3):435–454. doi: 10.1016/S0006-3495(81)84905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Gou S. Y., Axelrod D. Reaction rate enhancement by surface diffusion of adsorbates. Biophys Chem. 1992 Jun;43(2):117–137. doi: 10.1016/0301-4622(92)80027-3. [DOI] [PubMed] [Google Scholar]
- Zwanzig R., Szabo A. Time dependent rate of diffusion-influenced ligand binding to receptors on cell surfaces. Biophys J. 1991 Sep;60(3):671–678. doi: 10.1016/S0006-3495(91)82096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


