Abstract
In yeast and mammals, amino acid motifs in the cytosolic tails of transmembrane domains play a role in protein trafficking by facilitating export from the endoplasmic reticulum (ER). However, little is known about ER export signals of membrane proteins in plants. Therefore, we investigated the role of diacidic motifs in the ER export of Golgi-localized membrane proteins. We show that diacidic motifs perform a significant function in the export of transmembrane proteins to the Golgi apparatus, as mutations of these signals impede the efficient anterograde transport of multispanning, type II, and type I proteins. Furthermore, we demonstrate that diacidic motifs instigate the export of proteins that reside in the ER due to the lengths of their transmembrane domains. However, not all of the diacidic motifs in the cytosolic tails of the proteins studied were equally important in ER export. Transport of Golgi proteins was disrupted only by mutagenesis of specific diacidic signals, suggesting that the protein environment of these signals affects their function. Our findings indicate that diacidic ER export motifs are present and functional in plant membrane proteins and that they are dominant over transmembrane domain length in determining the export of proteins from the ER in plant cells.
INTRODUCTION
It has been shown in plants that membrane-bound and soluble proteins destined for the early secretory pathway can maintain their localizations by cycling between the endoplasmic reticulum (ER) and the cis-Golgi (Denecke et al., 1992; Brandizzi et al., 2002b; reviewed in Hanton et al., 2005). Retrograde Golgi–ER transport of soluble proteins is achieved by C-terminal H/KDEL signals that are recognized by the receptor ER retention defective 2 (ERD2) (Denecke et al., 1992; Lee et al., 1993), whereas transmembrane proteins are selected for transport to the ER by a dilysine motif at the C terminus of the cytosolic tail that interacts with components of the COPI coat (Benghezal et al., 2000; Contreras et al., 2004a). Anterograde ER–Golgi transport of soluble proteins occurs via a passive bulk-flow mechanism (Denecke et al., 1990; Phillipson et al., 2001), but the selectivity of the ER export of transmembrane proteins is subject to considerable debate. It is generally assumed that transmembrane proteins are exported via a COPII-mediated mechanism similar to that of soluble proteins (Andreeva et al., 2000; Takeuchi et al., 2000; Phillipson et al., 2001; daSilva et al., 2004), although a Golgi-independent route for the sorting of α-TIP has also been suggested (Hofte and Chrispeels, 1992; Gomez and Chrispeels, 1993; Jiang and Rogers, 1998). It has been shown that the transmembrane domain alone is sufficient to dictate protein export from the ER in plants (Hofte and Chrispeels, 1992; Brandizzi et al., 2002a). In particular, it has been shown that the length of the transmembrane domain plays a role in determining the destination of type I proteins along the plant secretory pathway, whereby increasing the transmembrane domain length from 17 to 20 amino acids was sufficient to instigate the export of an ER-retained protein (Brandizzi et al., 2002a). However, it is not yet known whether specific signals within the transmembrane domain of plant proteins influence ER–Golgi transport. A Tyr residue that is present in the transmembrane domain of CASP (for CCAAT displacement protein alternatively spliced product), a type II protein, plays a prominent role in ER–Golgi transport in mammalian cells (Gillingham et al., 2002) but was shown not to have the same function in plant cells (Renna et al., 2005).
In yeast and mammals, several classes of ER export signals have been identified in the cytosolic domains of transmembrane proteins (Kappeler et al., 1997; Nishimura and Balch, 1997; Giraudo and Maccioni, 2003). The cytoplasmic sorting motifs are usually divided into two groups, the dihydrophobic and diacidic signals (reviewed in Barlowe, 2003), although a new group of dibasic signals was recently proposed (Giraudo and Maccioni, 2003). Dihydrophobic export signals generally contain two large hydrophobic or aromatic amino acid residues adjacent to one another and are usually found toward the C terminus of the protein (Fiedler and Rothman, 1997; Kappeler et al., 1997; Dominguez et al., 1998; Nakamura et al., 1998; Otte and Barlowe, 2002; Sato and Nakano, 2002). Diacidic motifs consist of two acidic amino acid residues, separated by any other amino acid, usually denoted DXE or Asp-X-Glu (Nishimura and Balch, 1997; Nishimura et al., 1999; Sevier et al., 2000; Ma et al., 2001; Votsmeier and Gallwitz, 2001), although DXD (Asp-X-Asp) motifs have also been shown to be functional (Malkus et al., 2002; Wang et al., 2004). In yeast, these motifs are thought to interact directly with Sec24, a component of the COPII coat (Votsmeier and Gallwitz, 2001). However, Miller et al. (2003) have shown that the use of a mutated Sec24 affects the packaging of only certain proteins bearing diacidic motifs, indicating that other factors must dictate the mechanism of export. For example, an additional Tyr residue adjacent to the diacidic motif may also be involved in the export of certain proteins in mammalian cells (Sevier et al., 2000), and an expanded version of the signal is important for the export of potassium channels from the ER (Ma et al., 2001). In addition to these data on single export signals, a study of the interactions between the COPII coat and SNARE proteins resulted in the identification of two independent export signals on a single protein (Mossessova et al., 2003). These may act as backup systems to ensure the efficient export of this protein, although the three other SNAREs featured in the study appear to possess only one export signal each. Furthermore, it has been suggested that multiple signals may be needed for efficient export of the Can1p Arg permease (Malkus et al., 2002), the Erv41–Erv46 complex (Otte and Barlowe, 2002), and an ATP binding cassette transporter protein, Yor1p (Epping and Moye-Rowley, 2002).
In contrast with the multitude of cytosolic ER export signals identified and characterized in transmembrane proteins from mammals and yeast, current knowledge of this topic in plants is extremely limited. It remains unclear whether export signals in the cytosolic tails of transmembrane proteins regulate ER–Golgi transport in plants or whether the export is exclusively dependent on a bulk-flow mechanism dictated by transmembrane domain length. The latter hypothesis seems unlikely because of the need for tight control of protein movement from the ER to distal compartments. Furthermore, evidence has been presented for membrane protein–mediated recruitment of Sar1p to ER export sites (daSilva et al., 2004), suggesting that the cytosolic tails of plant membrane proteins may contain signals that attract COPII components. Contreras et al. (2004b) have identified a dihydrophobic signal in the cytosolic domain of a type I membrane-spanning plant p24 protein and have demonstrated its ability to interact with Arabidopsis thaliana Sec23, a COPII coat component, in vitro, although this is only possible when a dilysine motif adjacent to the dihydrophobic motif is mutated. It is thought that both signals cooperate to recruit the COPI coat (Contreras et al., 2004a), but only the dihydrophobic motif is able to interact with Arabidopsis Sec23. The biological relevance of this signal has yet to be shown in live cells, but its occurrence indicates that protein motifs may play a role in the ER export of transmembrane proteins in plants. The recent discovery that the mutation of a basic motif in the cytoplasmic domain of a type II membrane-spanning prolyl hydroxylase inhibits its transport to the Golgi apparatus in BY-2 cells (Yuasa et al., 2005) adds to the argument for the existence of active export signals in plants. To date, however, the characterization of diacidic motifs in plant transmembrane proteins has remained elusive.
In this work, we investigated whether the ER export of different types of transmembrane proteins occurs purely by passive bulk flow of membrane or whether diacidic signals in the cytosolic tail domains are required for efficient transport. To do so, we identified diacidic ER export motifs in a multispanning and a type II transmembrane protein and demonstrated in vivo through point mutagenesis and live cell imaging that these sequences play a significant role in the ER export of both types of proteins. Moreover, we have shown the relevance of these motifs in type I membrane proteins by the transplantation of a cytosolic domain containing diacidic motifs to an ER-restricted type I protein, with subsequent site-directed mutagenesis of these signals. Our data show that diacidic motifs can influence the export of different classes of transmembrane proteins from the ER in plant cells, indicating the conservation of mechanisms for protein transport at the ER–Golgi interface among kingdoms.
RESULTS
To identify diacidic motifs (two acidic amino acid residues separated by another amino acid) (Nishimura and Balch, 1997; Nishimura et al., 1999; Sevier et al., 2000; Votsmeier and Gallwitz, 2001; Malkus et al., 2002) in proteins that are exported from the ER, we first screened existing Golgi marker proteins. We found that the multispanning nucleotide sugar transporter GONST1 (Baldwin et al., 2001; Handford et al., 2004) and the type II Golgi membrane protein CASP (Renna et al., 2005) contained diacidic motifs in their cytosolic domains (Figure 1A; see Figure 4A below).
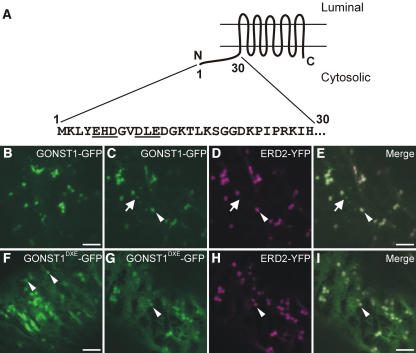
Figure 1.
A Diacidic Motif in the N-Terminal Cytosolic Domain of GONST1 Influences Its ER Export.
(A) Scheme of the topology of GONST1, with the sequence of the 30 N-terminal amino acids. Note the EHD and DLE motifs (underlined).
(B) GONST1-GFP localizes to punctate structures in tobacco leaf epidermal cells.
(C) to (E) The larger GONST1-labeled structures colocalize with ERD2-YFP (arrowheads), whereas the smaller structures are not labeled by ERD2-YFP (arrows).
(F) Mutation of DLE to GLA in GONST1 results in impaired ER export. Transport of GONST1DXE-GFP out of the ER is reduced compared with that of the wild-type protein. Note the increase in ER staining, although some punctate structures remain visible (arrowheads).
(G) to (I) Coexpression of GONST1DXE-GFP (G) with ERD2-YFP (H) confirms that the larger punctate structures labeled by GONST1DXE-GFP are Golgi bodies (arrowheads). Note the colocalization of the two fluorochromes on dots in the merged image (I).
Bars = 5 μm.
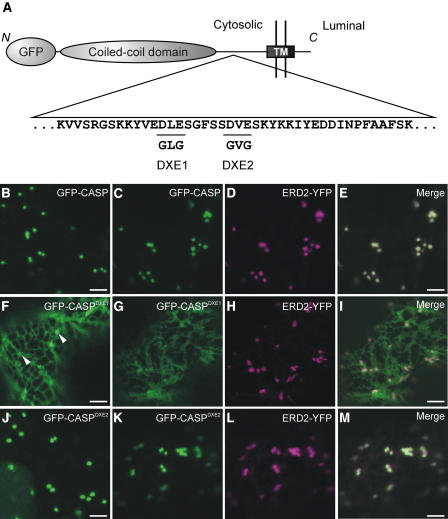
Figure 4.
The Cytosolic Domain of CASP Contains Potential Diacidic ER Export Signals.
(A) Scheme of an N-terminal fusion of GFP to the Golgi matrix protein CASP. The GFP moiety and coiled-coil domain are shown as gray ellipses, and the transmembrane domain (TM) is shown as a black rectangle. The N terminus of the protein is cytosolic, whereas the C terminus is found in the lumen of the secretory pathway, giving the protein a type II topology (Renna et al., 2005). Part of the amino acid sequence of the cytosolic domain of CASP is shown (Renna et al., 2005). This domain contains two DXE motifs (underlined), which were mutated independently to GLG (DXE1) and GVG (DXE2).
(B) to (D) GFP-CASP localizes to the Golgi apparatus ([B] and [C]) (Renna et al., 2005), as demonstrated by its colocalization with ERD2-YFP (D).
(E) Merged image of (C) and (D).
(F) to (I) Mutation of DLE to GLG (DXE1) results in the redistribution of a large proportion of the fluorescence to the ER ([F] and [G]), although some punctate structures are visible ([F], arrowheads). Coexpression of ERD2-YFP (H) confirms that these structures are Golgi bodies through colocalization (I).
(J) to (M) Mutation of DVE to GVG (DXE2) has no obvious effect on ER–Golgi trafficking of the protein ([J] and [K]). Golgi bodies alone remain visible, confirmed by the colocalization of ERD2-YFP (L) with GFP-CASPDXE2 (M).
Bars = 5 μm.
A Diacidic Signal Is Required for Efficient ER Export of the Multispanning Protein GONST1
GONST1 is a GDP-mannose transporter that accumulates primarily at the Golgi apparatus in tobacco (Nicotiana tabacum) leaves but also at other, smaller punctate structures of unknown nature (Figures 1C to 1E, arrows; see Supplemental Figure 1 online) (daSilva et al., 2004; Handford et al., 2004). It contains 10 transmembrane domains (Baldwin et al., 2001) and is oriented in such a way that the first 30 amino acids at the N terminus are found in the cytosol (Figure 1A). We found that there are two potential diacidic motifs (EHD and DLE) in this sequence (Figure 1A, underlined). We initially mutated the DXE motif to GXA, effectively neutralizing the diacidic motif. We hypothesized that the mutation of this putative signal would reduce the ability of the protein to exit the ER and travel to the Golgi apparatus. To test this notion, we generated a fusion of the mutant GONST1 to the green fluorescent protein (GONST1DXE-GFP) and expressed it in tobacco leaf epidermal cells. Analysis by confocal laser scanning microscopy showed increased levels of ER staining (Figure 1F), supporting our hypothesis. However, some punctate structures of different sizes were also visible (Figure 1F, arrowheads; see Supplemental Figures 2 and 5 online). To ascertain the identities of these structures, we coexpressed GONST1DXE-GFP with ERD2-YFP (for yellow fluorescent protein), an ER and Golgi marker (Brandizzi et al., 2002b). The larger punctate structures observed for GONST1DXE-GFP (Figure 1G, arrowhead) colocalized with the Golgi bodies labeled by ERD2-YFP (Figures 1G to 1I), whereas smaller structures visible in some cells were independent of the structures labeled by ERD2-YFP (see Supplemental Figure 2 online) but were the same as the small structures labeled by wild-type GONST1-fluorescent protein fusions (see Supplemental Figure 5 online) (Handford et al., 2004). These data indicate that GONST1DXE-GFP is able to be transported to the Golgi apparatus and beyond, albeit to a lesser extent than the wild-type protein.
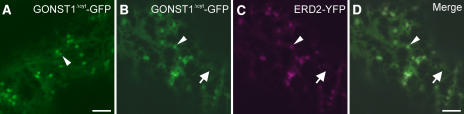
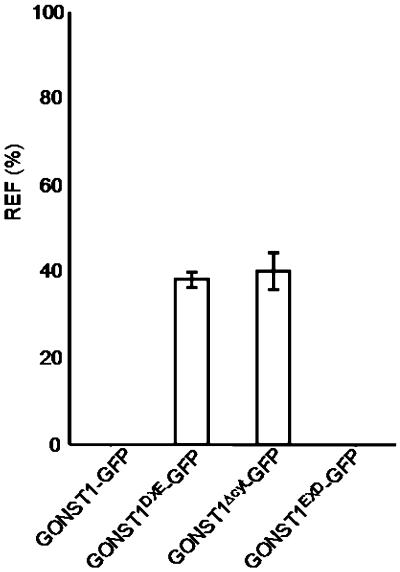
Because GONST1DXE-GFP can be transported to the Golgi apparatus in spite of the mutation of its DXE motif, we speculated that there might be other active export signals in the protein. These could continue to operate in the absence of the DXE motif, resulting in reduced efficiency but not complete abolition of transport, as observed in Figures 1F to 1I. The EXD motif in the N-terminal region of GONST1 (Figure 1A, underlined) was a possible candidate for this putative additional export signal, but the protein was transported to the Golgi and post-Golgi structures with no increase in ER staining when this motif was mutated (see Supplemental Figures 3 and 6 online), unlike the altered localization of GONST1DXE-GFP. This finding suggested that other, less easily identifiable export signals might be present in the protein. Therefore, we decided to delete the entire N-terminal cytosolic region of GONST1, which is the longest cytosolic domain in the protein. This deletion would rule out the possibility that any signals in the N-terminal domain could permit the protein to be exported from the ER to the Golgi apparatus. The deletion mutant was fused to GFP (GONST1Δcyt-GFP) and expressed in tobacco leaf epidermal cells (Figure 2A). Increased levels of ER staining were observed, the ER having a sheet-like appearance that was probably attributable to the accumulation of large amounts of the fusion protein in that organelle (Lee et al., 2002). Despite this accrual of protein in the ER, punctate structures still remained clearly visible (Figure 2A, arrowhead). Coexpression of GONST1Δcyt-GFP with ERD2-YFP verified that these punctate structures were Golgi bodies (Figures 2B to 2D). In some cells, smaller punctate structures were also visible (Figures 2B to 2D, arrows; see Supplemental Figure 4 online), indicating that this mutant was also able to be exported from the ER to post-Golgi destinations, despite the deletion of the N-terminal domain containing the clearly identifiable diacidic motifs. To confirm these qualitative observations, we quantified the fluorescence of the ER relative to that of the Golgi for GONST1-GFP and each of the mutants used. Figure 3 shows that the relative ER fluorescence is similar for both GONST1DXE-GFP and GONST1Δcyt-GFP. This finding suggests that the DXE motif is the only active ER export signal in the N-terminal cytosolic region of GONST1, which is supported by the fact that no ER fluorescence was observed for GONSTEXD-GFP. Our data indicate that the diacidic DXE motif of GONST1 influences the export of this protein from the ER, although it appears that additional mechanisms are also in place to ensure its transport to the Golgi apparatus.
Figure 2.
Deletion of the N-Terminal Cytosolic Domain of GONST1 Does Not Abolish Its ER Export.
(A) Expression of GONST1Δcyt-GFP in tobacco leaf epidermal cells shows increased ER staining compared with that in the wild-type protein (see Figures 1B to 1E), although some punctate structures remain visible (arrowhead), similar to the result observed with GONST1DXE-GFP (see Figures 1F to 1I).
(B) to (D) The larger punctate structures labeled by GONST1Δcyt-GFP (B) are Golgi bodies, as confirmed by coexpression of ERD2-YFP (C). Note the colocalization of the two fluorochromes on large dots (arrowhead) but not on small dots (arrow) in the merged image (D).
Bars = 5 μm.
Figure 3.
Quantification of ER Fluorescence for GONST1-GFP and Its Mutants.
The fluorescence of the ER relative to that of the Golgi was quantified for each of the GONST1-GFP derivatives. The relative ER fluorescence (REF) is given as a percentage of the ratio between fluorescence intensity values (arbitrary units) measured in the ER and the sum of intensity values for the ER and Golgi (see Methods). Error bars represent sd for 21 measurements.
CASP, a Type II Protein, Contains Multiple Diacidic Motifs
Having established that a diacidic signal is present and functional in the ER export of GONST1, a multispanning protein, we next wanted to test whether similar signals in other types of proteins would have a comparable effect. Previous studies have shown only that a type I protein contains a dihydrophobic signal (Contreras et al., 2004b) and that a type II protein contains a dibasic signal (Yuasa et al., 2005). Finding a diacidic ER export motif in a type II protein would rule out the possibility that different types of proteins contain specific types of sorting signals.
CASP is a type II Golgi matrix protein with a short C-terminal luminal domain and an extensive N-terminal cytosolic coiled-coil domain (Figure 4A) (Renna et al., 2005). It is a member of the membrane-spanning golgin family of proteins, all of which have similar domain structures (Misumi et al., 2001; Gillingham et al., 2002; Gillingham and Munro, 2003). It has been shown that some mammalian golgins specifically require the 100 amino acids between the membrane-bound domain and the coiled-coil region to localize at the Golgi (Misumi et al., 2001). Moreover, a truncated form of CASP containing only the luminal and transmembrane domains plus 78 amino acids of the cytosolic domain is efficiently transported to the Golgi (Renna et al., 2005), indicating that if any export signals were present, they would most likely be found in this segment of the cytosolic domain of CASP. We found two DXE motifs that could be important for ER–Golgi transport within this region (Figure 4A, underlined and annotated). We then mutated the acidic residues of each potential motif to Gly and expressed fluorescent fusions of the wild-type protein or each mutant in tobacco leaf cells alone or in the presence of ERD2-YFP (Figures 4B to 4M). ERD2 was used as an ER–Golgi marker rather than wild-type CASP, because the mammalian homolog of CASP has been shown to form dimers (Gillingham et al., 2002). Dimerization of a mutant form of CASP with the wild-type protein might permit its export from the ER in spite of the mutation of putative export signals. Each fusion was expressed in the absence of ERD2-YFP to verify that its localization was unaffected by the presence of the marker, as expression of ERD2-GFP has been shown to induce the formation of ER export sites (daSilva et al., 2004).
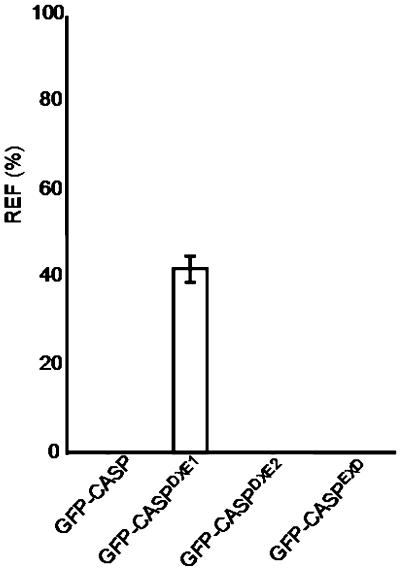
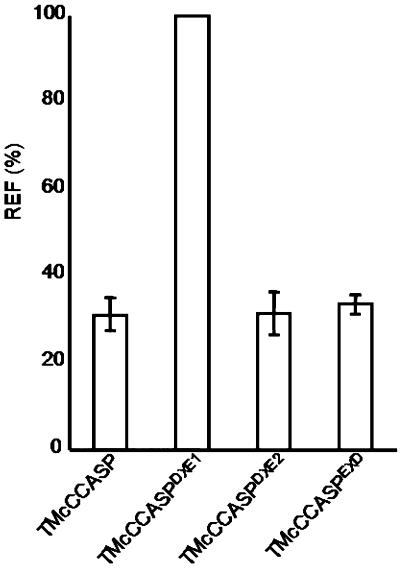
When we expressed GFP-CASP in tobacco leaf epidermal cells alone, we verified that it labeled punctate structures (Figure 4B). Coexpression of GFP-CASP with ERD2-YFP confirmed that these structures were Golgi bodies (Figures 4C to 4E), in agreement with the data presented by Renna et al. (2005). Mutation of DXE1 to GXG (GFP-CASPDXE1) resulted in the redistribution of the protein to the ER, although some punctate structures remained visible (Figure 4F, arrowheads). Coexpression of ERD2-YFP and GFP-CASPDXE1 showed that these punctate structures were Golgi bodies (Figures 4G to 4I). In contrast with these data, mutation of DXE2 to GXG (GFP-CASPDXE2) had no apparent effect on the localization of the fusion (Figures 4J to 4M). Mutation of an EXD motif present in the same region of CASP gave similar results (see Supplemental Figure 7 online), whereas quantification of the ER fluorescence for each construct shows that only GFP-CASPDXE1 exhibited redistribution to the ER (Figure 5). Our results demonstrate that a diacidic motif influences the transport of a type II membrane protein, similar to the multispanning protein GONST1. In addition, only one DXE motif functions in ER export, despite the fact that three diacidic motifs are found within the same region of the protein.
Figure 5.
Quantification of ER Fluorescence for GFP-CASP and Its Mutants.
The fluorescence of the ER relative to that of the Golgi was quantified for each of the GFP-CASP derivatives. The relative ER fluorescence (REF) is given as a percentage as described for Figure 3. Error bars represent sd for 21 measurements.
Generation of a Synthetic Type I Reporter Protein
We next wanted to investigate whether diacidic motifs would also function in type I transmembrane proteins. However, none of the Golgi-localized type I proteins that have been described to date contains a DXE motif. Furthermore, the importance of the length of the transmembrane domain in the transport of type I proteins in the secretory pathway (Brandizzi et al., 2002a) meant that we could not use the transmembrane domain of a Golgi-localized protein in our study, because the transmembrane domain might carry the protein to the Golgi independently of the export signals. Therefore, we chose to create a type I reporter protein using the 17–amino acid transmembrane domain (TM17) used in the study by Brandizzi et al. (2002a), which was shown to be localized only in the ER. TM17 is a shortened version of the transmembrane domain of the human LAMP1 protein. The use of this transmembrane domain gave the additional advantage of showing whether an export motif was dominant over the length of the transmembrane domain or vice versa. To facilitate biochemical assays, we fused a form of GFP carrying a signal peptide for entry into the ER and an N-glycosylation site (Batoko et al., 2000) to the N terminus of the TM17 transmembrane domain (Figure 6A, spNGFP-TM17). We then fused this sequence, with the stop codon deleted to allow read-through, to the N terminus of the 78 amino acids of the cytosolic domain of CASP directly adjacent to the transmembrane domain, thereby generating a DNA sequence encoding a protein termed TMcCCASP (Figure 6A). We used only the 78 amino acids on the cytoplasmic side of the transmembrane domain because CASP has an extensive coiled-coil domain that does not appear to be involved in ER export and that might become misfolded if transplanted to a type I protein. The sequence used encompasses the region of interest containing the export signals characterized previously and corresponds to the cytosolic domain of the truncated form of CASP used by Renna et al. (2005). Because of the opposite topologies of type I and type II proteins, the orientation of the tail domain with respect to the transmembrane domain was reversed; in other words, the TM17 domain was fused at the N terminus of the tail domain rather than at the C terminus (Figure 6A). This would provide additional information regarding the functionality of the signals when placed in a different environment.
Figure 6.
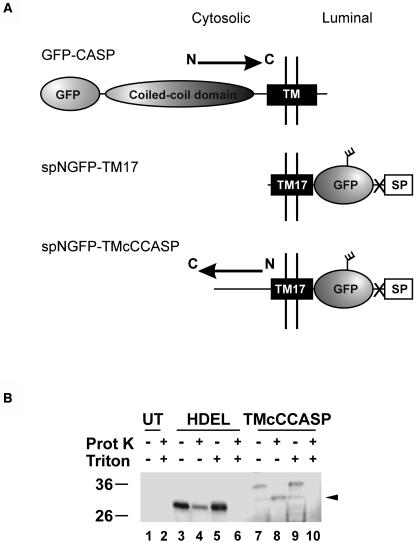
A Synthetic Cargo Protein for Studying the Properties of the CASP Cytosolic Tail.
(A) Comparison of the topologies and domains of GFP-CASP, spNGFP-TM17, and spNGFP-TMcCCASP. The glycosylation site on GFP is represented by a short branch, and the transmembrane domain is shown as a black rectangle for each protein. The X represents the cleavage site for the signal peptide. The reverse orientation of the CASP tail in TMcCCASP is denoted by an arrow indicating the N-terminal to C-terminal direction.
(B) Proteinase K treatment of microsomes containing either spNGFP-HDEL or TMcCCASP. Note that the major band detected for TMcCCASP (lane 7) exhibits a shift to a lower molecular mass on treatment with proteinase K (lane 8, arrowhead), indicating that the GFP moiety of the protein is found in the lumen of the secretory pathway. spNGFP-HDEL does not show the same shift in molecular mass on treatment with the proteinase because it is contained within the lumen of the ER (cf. lanes 3 and 4). Both proteins become undetectable when the membranes are disrupted with Triton X-100 (lanes 6 and 10). UT, untransformed sample.
TMcCCASP Adopts a Type I Orientation
To ensure that TMcCCASP was correctly oriented with the GFP moiety in the lumen of the secretory pathway and the CASP C terminus in the cytoplasm, we examined its topology by means of a proteinase K protection assay on a microsomal fraction isolated from protoplasts by osmotic shock (Denecke et al., 1991; Brandizzi et al., 2003; Renna et al., 2005). The fusion protein was then detected through protein gel blotting with anti-GFP serum (Figure 6B). We used spNGFP-HDEL, a soluble ER-retained form of GFP bearing a glycosylation site (Batoko et al., 2000; daSilva et al., 2005), as a control to confirm that the microsomes remained intact throughout the treatment. If, as predicted, the GFP portion of the TMcCCASP fusion was located within the microsomal lumen, it would be inaccessible to proteinase K and would be detectable with anti-GFP serum. However, because the 78–amino acid tail fragment from CASP is predicted to be in the cytosol, it would be accessible to the proteinase and would be digested, giving a protein with a lower molecular weight. The difference in molecular weight between the full-length and partially digested proteins would be detectable on a protein gel blot. Figure 6B shows that treatment of microsomes containing TMcCCASP with proteinase K alone resulted in the detection of a truncated form of the protein (Figure 6B, arrowhead). We interpreted this to mean that the predicted cytoplasmic domain had been exposed to and digested by the proteinase, as the GFP moiety was still detectable. A band of similar molecular weight to this truncated form was detected, albeit faintly, in samples lacking proteinase K (Figure 6B, lanes 7 and 9). Because these specific degradation bands are not visible after whole cell extraction with buffers containing the protease inhibitor phenylmethylsulfonyl fluoride (see Figure 9 below), it seems likely that this was the result of rupturing the lytic vacuole during the initial osmotic shock and releasing active proteases. Partial rupture of the microsomes was verified by the reduced detection of the luminal cargo spNGFP-HDEL on treatment with proteinase K. To establish that the N-terminal part of the fusion could be digested by proteases, we treated the microsomes with 1% (v/v) Triton X-100 to dissolve the membranes (Figure 6B, lanes 9 and 10). In the absence of proteinase K, the full-length fusion was detected, but on treatment with the protease, no signal was detectable, indicating that the entire protein had been digested. Disruption of the microsomal membranes with the detergent was demonstrated by the complete digestion of the control cargo spNGFP-HDEL. We conclude from these data that the orientation of TMcCCASP is as shown in Figure 6A, with a luminal N terminus and a cytosolic C terminus, and that TMcCCASP therefore adopts a type I topology.
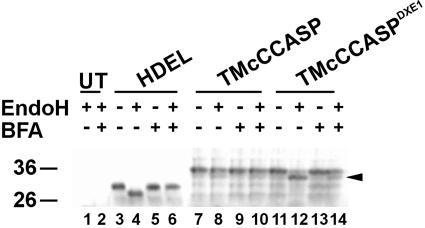
Figure 9.
TMcCCASPDXE1 Does Not Travel to the Same Endomembrane Compartment as TMcCCASP.
EndoH treatment of protoplasts expressing spNGFP-HDEL, TMcCCASP, or TMcCCASPDXE1. Although the glycan on TMcCCASP is resistant to digestion by EndoH (lane 8), that on TMcCCASPDXE1 remains sensitive, resulting in a shift in molecular weight (arrowhead, lane 12). Treatment of the protoplasts with 20 μg/mL BFA to redistribute the Golgi enzymes responsible for glycan modification to the ER resulted in the glycan on TMcCCASPDXE1 becoming resistant to the endoglycosidase, in the same manner as spNGFP-HDEL (lanes 6 and 14). UT, untransformed sample.
Export Signals Are Dominant Over Transmembrane Domain Length in Determining the Localization of Type I Proteins
We next wanted to check the intracellular localization of TMcCCASP compared with that of spNGFP-TM17. spNGFP-TM17 fluorescence was restricted to the ER (Figure 7A), as reported for the protein lacking the glycosylation site (Brandizzi et al., 2002a). These data confirm that the 17–amino acid transmembrane domain is sufficient to retain a type I protein in the ER. When expressed in tobacco leaf epidermal cells, TMcCCASP localized mainly at punctate structures (Figure 7B) with some weak ER staining (Figure 7B, arrowheads), in contrast with the exclusively ER-localized spNGFP-TM17. Coexpression of TMcCCASP with ERD2-YFP confirmed that the punctate structures were Golgi bodies (Figures 7C to 7E). It seems likely that the presence of the 17–amino acid transmembrane domain causes a slight inhibition of ER export, resulting in the low levels of ER staining observed, but it does not prevent the delivery of the protein to the Golgi apparatus. This finding indicates that the truncated cytosolic domain of CASP is capable of initiating the transport of a type I protein with a 17–amino acid transmembrane domain from the ER to the Golgi.
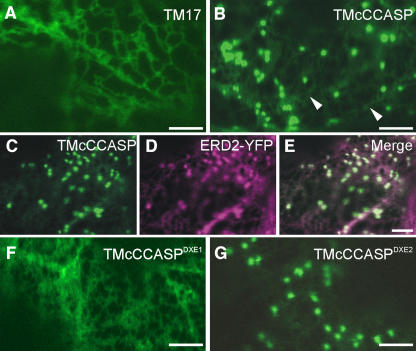
Figure 7.
Export Signals Can Override Transmembrane Domain Length in Determining Destinations within the Secretory Pathway.
(A) spNGFP-TM17 is found exclusively in the ER, as a result of its short transmembrane domain.
(B) TMcCCASP localizes mainly to punctate structures, although some ER localization is also visible (arrowheads).
(C) to (E) Coexpression of TMcCCASP (C) with ERD2-YFP (D) demonstrates that the punctate structures labeled by TMcCCASP are Golgi bodies. Note the colocalization shown in the merged image (E).
(F) TMcCCASPDXE1 is retained in the ER, in contrast with TMcCCASP.
(G) Export of TMcCCASPDXE2 from the ER is apparently unaffected by the mutation.
Bars = 5 μm.
Having established that TMcCCASP was capable of exiting the ER, we then wanted to confirm that its observed Golgi localization was attributable to the presence of a DXE motif acting as an ER export signal, because it was possible that the cytosolic fusion extended the transmembrane domain sufficiently to allow it to travel to the Golgi apparatus independently of any signals. Correspondingly, we mutated the two DXE motifs to GXG and expressed these mutants in tobacco leaves (Figures 7F and 7G). Mutation of DXE1 to GXG (TMcCCASPDXE1) resulted in complete ER localization, unlike the DXE1 mutation of CASP (Figures 4F to 4I). This demonstrates that the presence of a functional ER export motif is essential for the transport of TMcCCASP to the Golgi. No apparent defect in transport was seen on mutation of DXE2 to GXG (TMcCCASPDXE2), corresponding to the data obtained with the full-length CASP protein. The reversed orientation of the tail did not affect the functionality of the DXE1 motif, and mutagenesis of the EXD motif, which in its new orientation in TMcCCASP became a DXE motif, did not affect the ability of the protein to travel to the Golgi apparatus (see Supplemental Figure 8 online). These data were supported by quantification of the relative levels of ER and Golgi fluorescence, which showed that similar levels of ER staining were observed for all of the TMcCCASP derivatives except TMcCCASPDXE1, which was localized solely in the ER (Figure 8). This finding indicates that neither the direction of the motif nor its precise distance from the transmembrane domain is specifically required for export from the ER.
Figure 8.
Quantification of ER Fluorescence for TMcCCASP and Its Mutants.
The fluorescence of the ER relative to that of the Golgi was quantified for each of the TMcCCASP derivatives. The relative ER fluorescence (REF) is given as a percentage as described for Figure 3. Error bars represent sd for 21 measurements.
To confirm biochemically the ER retention of TMcCCASPDXE1 compared with TMcCCASP, we performed an endoglycosidase H (EndoH) digest on extracts expressing each of the two fusion proteins, or spNGFP-HDEL as a control, on the basis that the N-linked glycan attached to the GFP moiety would be modified to an EndoH-resistant form if the protein traveled beyond the cis-Golgi. Figure 9 shows that the N-linked glycan on TMcCCASP was resistant to digestion by EndoH (lane 8), indicating that it has been modified by enzymes in the medial and/or trans-Golgi compartments. By contrast, the glycan on TMcCCASPDXE1 remained sensitive to the glycosidase and therefore exhibited a shift in molecular weight (Figure 9, lane 12, arrowhead). This result indicates that the mutant does not reach the same Golgi compartment as the wild-type protein. However, to verify that the glycan on TMcCCASPDXE1 was accessible for modification to a complex form, we performed the same experiment in the presence of the drug brefeldin A (BFA), which causes the redistribution of Golgi-resident enzymes to the ER (Driouich et al., 1993; Crofts et al., 1999; Ritzenthaler et al., 2002). BFA treatment resulted in the glycan on TMcCCASPDXE1 acquiring resistance to EndoH (Figure 9, lane 14), indicating that it was susceptible to modification when the enzymes responsible were present in the same endomembrane compartment and confirming that it does not travel to a compartment containing these enzymes under normal circumstances.
This result does not exclude the possibility that, like HDEL-tagged proteins, TMcCCASPDXE1 travels to the cis-Golgi and quickly recycles to the ER, as EndoH-sensitive glycans are also found on proteins that are known to cycle between the ER and the Golgi (Figure 6, HDEL) (Crofts et al., 1999). However, it does show that the mutant form of the fusion does not travel to the same compartment as the wild-type form, demonstrating that the proper transport of the protein is dependent on the presence of a functional DXE motif. Moreover, Golgi bodies were never observed, even at high levels of expression of the mutant, whereas in conditions of overexpression of spNGFP-HDEL, Golgi bodies can become visible (data not shown). These data indicate that mutation of the DXE motif results in the inability of the protein to exit the ER.
DISCUSSION
The mechanisms and machinery involved in the transport of proteins between the ER and the Golgi apparatus in plants are only now beginning to be identified (reviewed in Hanton et al., 2005). Mechanisms for retrograde Golgi–ER transport of soluble and transmembrane proteins, and for ER export of soluble proteins, have been characterized (Denecke et al., 1990, 1992; Benghezal et al., 2000; Phillipson et al., 2001; Contreras et al., 2004a). However, the means by which transmembrane proteins leave the ER are less well understood. It has been demonstrated that the length of the transmembrane domain influences the destination of type I proteins in the plant secretory pathway (Hofte and Chrispeels, 1992; Brandizzi et al., 2002a), but nothing is known about the influence of the transmembrane domain on the transport of type II and multispanning proteins. Furthermore, until recently, no evidence had been presented for the existence of specific motifs regulating the export of proteins from the ER in plants. Contreras et al. (2004b) have suggested that dihydrophobic motifs can interact with plant COPII coat components in vitro, whereas Yuasa et al. (2005) have shown that mutation of a dibasic signal in a prolyl hydroxylase impairs its export from the ER. These motifs may be involved in the recruitment of COPII coat components that would facilitate the transport of membrane proteins from the ER, as has been shown in other systems (Campbell and Schekman, 1997; Aridor et al., 1998, 1999, 2001; Kuehn et al., 1998; Nishimura et al., 1999; Votsmeier and Gallwitz, 2001; Malkus et al., 2002; Giraudo and Maccioni, 2003; Miller et al., 2003; Mossessova et al., 2003; Wang et al., 2004). Despite these recent advances, it remains uncertain whether diacidic motifs contribute to the regulation of the ER export of transmembrane proteins in plants. Here, we have shown that the presence of a functional diacidic (DXE) motif in certain plant transmembrane proteins influences their export from the ER to the Golgi apparatus. Our data demonstrate that the DXE motif is functional in type I, type II, and multispanning proteins and that its functionality is unaffected by changes in orientation or position relative to the transmembrane domain.
Factors Other Than Diacidic Motifs Play a Role in the ER–Golgi Transport of GONST1 and CASP
We have shown that the transport to the Golgi apparatus of mutant proteins bearing mutagenized specific DXE motifs, GONST1DXE-GFP and GFP-CASPDXE1, was noticeably inhibited compared with that of the wild-type protein. However, Golgi bodies were clearly visible for both mutant proteins, and the transport of the Golgi marker ST-mRFP (Saint-Jore et al., 2002; Renna et al., 2005) was unaffected by the expression of these mutants (see Supplemental Figures 9 and 10 online). This indicates that factors other than the DXE motif influence the anterograde transport of these proteins from the ER. Deletion of the N-terminal cytosolic domain of GONST1 did not completely abolish its export from the ER but resulted in a similar level of inhibition of transport to that of the DXE mutant (Figure 3). This finding indicates that the DXE motif may be the only active signal in the N-terminal cytosolic region, but it does not exclude the possibility that signals for ER export may exist in other parts of the protein, such as the C terminus or the four cytosolic loops connecting the transmembrane domains. Information mediating the targeting of GONST1 to the smaller structures observed in tobacco leaf cells also appears to be contained in regions of the protein other than the N terminus, as these structures remained visible when either GONST1DXE-GFP or GONST1Δcyt-GFP was expressed. This may contribute to the continued transport of the mutants of GONST1 to the Golgi apparatus. Similarly, the sequence of the portion of CASP shown in Figure 4A may contain export signals of a nondiacidic nature, which could influence the export of the protein from the ER. The fact that mutation of the EXD motif had no effect on the transport of the protein to the Golgi apparatus (Figure 8; see Supplemental Figure 7 online), corresponding to data on an EXE motif in yeast presented by Votsmeier and Gallwitz (2001) and our data on the EXD motif of GONST1 (see Supplemental Figure 6 online), indicates that factors other than diacidic motifs may be involved in the ER export of these proteins. Evidence for the existence of cytosolic ER export signals that cannot be classified into any of the established groups (Barlowe, 2003; Giraudo and Maccioni, 2003) has been presented for yeast and mammalian cells (Stockklausner et al., 2001; Mossessova et al., 2003; Miranda et al., 2004; Paulhe et al., 2004), and the possibility cannot be ruled out that a similar situation exists in plant cells.
Export signals may also exist within the transmembrane domain of the protein. In mammalian cells, a conserved Tyr residue in the transmembrane domain of CASP is necessary for export of the protein to the Golgi apparatus to occur (Gillingham et al., 2002). In plants, mutation of this residue does not impair the ER export of CASP (Renna et al., 2005), but the Tyr residue may be capable of mediating the residual ER–Golgi transport observed when the DXE1 motif is mutated. The generation of a form of CASP bearing both mutations would answer this question. Another possibility for the continued transport of CASP to the Golgi apparatus is its potential for dimerization. The human homolog of CASP is known to form homodimers, likely through its coiled-coil domain (Gillingham et al., 2002). A tobacco homolog of CASP may exist, in which case it is possible that the Arabidopsis CASP used in this study could form dimers with this protein, although dimerization of plant CASP has yet to be demonstrated. Interactions between AtCASPDXE1 and a wild-type endogenous CASP molecule might result in the transport of the mutant protein to the Golgi apparatus because of the presence of export signals in the endogenous protein. The length of the transmembrane domain must also be considered; although it has been shown that the length of the transmembrane domain of type I proteins in plants is a significant factor in their destinations within the secretory pathway (Brandizzi et al., 2002a), no similar studies have been published for multispanning or type II proteins. It is possible that transmembrane domain length plays a role in transporting these types of proteins through the secretory pathway, which could explain the residual transport of the mutants of GONST1 and CASP to the Golgi apparatus, as both of these proteins have transmembrane domains of 19 amino acids or more, the length required for type I proteins to leave the ER (Brandizzi et al., 2002a).
We also wanted to know whether DXE motifs were able to influence the export of a type I protein from the ER. We developed a synthetic type I membrane-spanning cargo molecule that provided an in vivo method to investigate the transport properties of different sorting motifs. This fusion protein eliminates many of the variables listed above for GONST1 and CASP, which could have an effect on ER export. This protein consists of a form of GFP carrying a signal peptide for entry into the secretory pathway and an N-glycosylation signal (Batoko et al., 2000), fused to a 17–amino acid transmembrane domain (Brandizzi et al., 2002a) and the cytosolic domain of CASP (Renna et al., 2005), which carries several potential ER export signals. The GFP moiety of the fusion can be detected through fluorescence microscopy or protein gel blot analysis, whereas the presence of the N-glycosylation site makes it possible to carry out biochemical transport studies using EndoH treatment. The influence of the length of the transmembrane domain on the transport of type I proteins was shown by Brandizzi et al. (2002a), when they demonstrated that a type I membrane-spanning protein with a 17–amino acid transmembrane domain is retained in the ER. We were able to use this model cargo protein to show that the presence of a functional ER export motif in the cytosolic tail domain could overcome transmembrane-mediated ER localization and that the length of the transmembrane domain was therefore not limiting for the export of the protein from the ER when active ER export motifs were present. Weak ER staining was observed for each of the TMcCCASP fusions, unlike for wild-type CASP, indicating that the short transmembrane domain might impair ER export of the fusions to a minor extent. This finding suggests that the role of the transmembrane domain length in ER–Golgi transport becomes apparent only when export motifs are removed or made nonfunctional. Of the two DXE motifs investigated in the cytosolic domain of CASP, only one appears to be required for ER–Golgi transport whether in the wild-type protein or in the reporter fusion system. We also demonstrated that the orientation of the cytosolic tail had no effect on the functionality of the diacidic signals, perhaps because Sec24 appears to exhibit different binding modes dependent on the motif to which it is bound (Mossessova et al., 2003). However, it cannot be ruled out that the rate of transport of mutants that have no apparent effect is reduced compared with that of the wild-type protein, as our data reflect the steady state distribution of the proteins. The distance of the signal from the transmembrane domain and its orientation do not appear to be involved in determining the functionality of a diacidic motif. Therefore, we propose that other factors, such as the nature of the surrounding residues, may determine the prevalence of one signal over another in the same domain.
METHODS
Molecular Cloning
Standard molecular techniques were used as described by Sambrook et al. (1989). The fluorescent proteins used in this study were based on fusions with either mGFP5 (Haseloff et al., 1997) or EYFP (Clontech). The spectral properties of mGFP5 allow efficient spectral separation from YFP (Brandizzi et al., 2002a).
GONST1 (Baldwin et al., 2001) was amplified by PCR and subcloned upstream of a fluorescent protein sequence using the unique XbaI and SalI sites of the binary vector pVKH18En6. Each of the mutants was subcloned in a similar manner. The truncated GONST1 mutant was created by PCR amplification of the DNA encoding the entire gene but lacking the first 30 amino acids. To generate the DXE or EXD mutants for GONST1 or CASP, we performed site-directed mutagenesis by altering the relevant codons to encode Gly or Ala. For CASP (Renna et al., 2005), the PCR products were fused in frame downstream of a fluorescent protein sequence using the unique BamHI and SacI sites of pVKH18En6. For TMcCCASP, the TM17 transmembrane domain was amplified by PCR and the stop codon mutated to allow read-through. This PCR product was inserted into pVKH18En6 downstream of spNGFP using unique BamHI and SacI sites. The cytosolic domain of CASP was amplified by PCR and inserted into the spNGFP-TM17 vector using SacI sites. The correct orientation of the fragment was detected by PCR screening. The fidelity of each plasmid was ensured by sequencing. The primer sequences used for the cloning and mutagenesis described above are available upon request.
Expression in Plant Cells
The confocal images produced for this work were obtained from transient transformation of tobacco (Nicotiana tabacum cv Petit Havana) leaves. Four-week-old tobacco greenhouse plants grown at 25°C were used for Agrobacterium tumefaciens (strain GV3101)–mediated transient expression (Batoko et al., 2000). The bacterial OD600 used for plant transformation was 0.1 for all cargo molecules except TMcCCASP and its mutants, for which OD600 = 0.2 was used, and spNGFP-HDEL, for which OD600 = 0.03 was used.
Sampling and Imaging
Transformed leaves were analyzed 48 h after infection of the lower epidermis. Confocal imaging was performed using an upright Zeiss LSM 510 META confocal microscope and a ×63 water-immersion objective. For imaging expression of either GFP constructs or YFP constructs or both, we used imaging settings as described by Brandizzi et al. (2002a) with a 1- to 3-μm optical slice. Appropriate controls were used to exclude the possibility of energy transfer between fluorochromes and crosstalk. Postacquisition image processing was done with CorelDraw 9 and Adobe Photoshop 6.0 software. Relative ER fluorescence was calculated by measuring the fluorescence intensity values (arbitrary units) using ImageJ software in a circle of diameter 25 pixels centered on a Golgi body (G) and in a circle of the same dimensions adjacent to the Golgi body, covering a region containing the ER (E). Relative ER fluorescence (REF) was then calculated as a percentage of the total fluorescence of both areas: REF = (E/(G + E)) × 100. Measurements were performed on images acquired with nonsaturating settings and maximum pinhole aperture.
Proteinase K Protection and Protein Gel Blot Analysis
For proteinase K protection assays, we isolated protoplasts as described by Phillipson et al. (2001), except that the protoplasts were isolated from transiently transformed tobacco leaves. The microsomal fraction was prepared by osmotic shock (Denecke et al., 1991; Brandizzi et al., 2003; Renna et al., 2005) in GFP extraction buffer (0.2 M NaCl, 0.1 M Tris, pH 7.8, and 1 mM EDTA, pH 8.0) and incubated on ice for 10 min. The resulting microsomal preparation was then incubated on ice with or without 0.3 mg/mL proteinase K (New England Biolabs) and with or without 1% (v/v) Triton X-100 for 30 min. After incubation, the samples were boiled for 10 min to inactivate the proteinase. One percent (v/v) Triton X-100 was added to samples that had not previously been treated with the detergent to release proteins from the membranes. Extracts were diluted 50:50 with 2× SDS loading buffer (Crofts et al., 1999), and the samples were analyzed by protein gel blotting. Equal volumes of all extracts were loaded on SDS–polyacrylamide gels, transferred to nitrocellulose membranes by electroblotting, and blocked with PBS supplemented with 0.05% Tween 20 and 1% milk powder for 1 h. The filter was then incubated in PBS supplemented with 1% BSA and 0.02% sodium azide with anti-GFP serum from rabbit (Molecular Probes) at a dilution of 1:1000 overnight. Further steps were performed as described by Crofts et al. (1999). The anti-GFP serum is known to recognize all GFP variants (Molecular Probes).
EndoH Treatment
Protoplasts were isolated from transiently transformed leaves and incubated for 6 h in the presence or absence of 20 μg/mL BFA (Sigma-Aldrich; stock 10 mg/mL in DMSO). Extraction and EndoH (New England Biolabs) treatments were then performed as described by Crofts et al. (1999). Samples were diluted 50:50 with 2× SDS loading buffer and analyzed by protein gel blotting as described above.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers BAB01114 and CAC69066.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. GONST1-GFP Is Transported to the Golgi and to Smaller, Non-Golgi Structures.
Supplemental Figure 2. GONST1-GFPDXE Is Able to Be Transported to the Golgi and to Smaller, Non-Golgi Structures.
Supplemental Figure 3. GONST1-GFPEXD Labels the Golgi and Smaller, Non-Golgi Structures.
Supplemental Figure 4. GONST1-GFPΔcyt Is Partially Retained in the ER but Is Also Transported to the Golgi Apparatus and Small Non-Golgi Structures.
Supplemental Figure 5. GONST1DXE-GFP Is Transported to the Same Small Dots as Wild-Type GONST1.
Supplemental Figure 6. The EXD Motif in the N Terminus of GONST1 Does Not Regulate Its ER Export.
Supplemental Figure 7. Mutation of the EXD Motif in CASP Has No Effect on Its Transport out of the ER.
Supplemental Figure 8. The EXD Motif Does Not Function as an Export Signal in TMcCCASP.
Supplemental Figure 9. Expression of ER-Retained Mutants of GONST1 Does Not Alter the Golgi Localization of ST-mRFP.
Supplemental Figure 10. Expression of ER-Retained Mutants of CASP and TMcCCASP Does Not Alter the Golgi Localization of ST-mRFP.
Supplementary Material
Acknowledgments
This work was supported by grants awarded to F.B. from the University of Saskatchewan, the Canada Foundation for Innovation, and the Canada Research Chair (CRC) fund. S.L.H. is indebted to the Department of Biology, University of Saskatchewan, for a postdoctoral fellowship and to CRC funding to F.B. L.R. is supported by a University of Saskatchewan New Faculty Award, L.E.B. by CRC funding to F.B., and L.C. by Government of Canada Award International Council for Canadian Studies. G.S. is jointly supported by a Graduate College Studies Award and CRC funding to F.B. We thank P. Dupree (University of Cambridge, UK) for providing GONST1-YFP DNA.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Federica Brandizzi (federica.brandizzi@usask.ca).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.105.034900.
References
- Andreeva, A.V., Zheng, H., Saint-Jore, C.M., Kutuzov, M.A., Evans, D.E., and Hawes, C.R. (2000). Organization of transport from endoplasmic reticulum to Golgi in higher plants. Biochem. Soc. Trans. 28, 505–512. [PubMed] [Google Scholar]
- Aridor, M., Bannykh, S.I., Rowe, T., and Balch, W.E. (1999). Cargo can modulate COPII vesicle formation from the endoplasmic reticulum. J. Biol. Chem. 274, 4389–4399. [DOI] [PubMed] [Google Scholar]
- Aridor, M., Fish, K.N., Bannykh, S., Weissman, J., Roberts, T.H., Lippincott-Schwartz, J., and Balch, W.E. (2001). The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J. Cell Biol. 152, 213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor, M., Weissman, J., Bannykh, S., Nuoffer, C., and Balch, W.E. (1998). Cargo selection by the COPII budding machinery during export from the ER. J. Cell Biol. 141, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin, T.C., Handford, M.G., Yuseff, M.I., Orellana, A., and Dupree, P. (2001). Identification and characterization of GONST1, a Golgi-localized GDP-mannose transporter in Arabidopsis. Plant Cell 13, 2283–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe, C. (2003). Signals for COPII-dependent export from the ER: What's the ticket out? Trends Cell Biol. 13, 295–300. [DOI] [PubMed] [Google Scholar]
- Batoko, H., Zheng, H.Q., Hawes, C., and Moore, I. (2000). A rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12, 2201–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benghezal, M., Wasteneys, G.O., and Jones, D.A. (2000). The C-terminal dilysine motif confers endoplasmic reticulum localization to type I membrane proteins in plants. Plant Cell 12, 1179–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi, F., Frangne, N., Marc-Martin, S., Hawes, C., Neuhaus, J.M., and Paris, N. (2002. a). The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain. Plant Cell 14, 1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi, F., Hanton, S., DaSilva, L.L., Boevink, P., Evans, D., Oparka, K., Denecke, J., and Hawes, C. (2003). ER quality control can lead to retrograde transport from the ER lumen to the cytosol and the nucleoplasm in plants. Plant J. 34, 269–281. [DOI] [PubMed] [Google Scholar]
- Brandizzi, F., Snapp, E.L., Roberts, A.G., Lippincott-Schwartz, J., and Hawes, C. (2002. b). Membrane protein transport between the endoplasmic reticulum and the Golgi in tobacco leaves is energy dependent but cytoskeleton independent: Evidence from selective photobleaching. Plant Cell 14, 1293–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, J.L., and Schekman, R. (1997). Selective packaging of cargo molecules into endoplasmic reticulum-derived COPII vesicles. Proc. Natl. Acad. Sci. USA 94, 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras, I., Ortiz-Zapater, E., and Aniento, F. (2004. a). Sorting signals in the cytosolic tail of membrane proteins involved in the interaction with plant ARF1 and coatomer. Plant J. 38, 685–698. [DOI] [PubMed] [Google Scholar]
- Contreras, I., Yang, Y., Robinson, D.G., and Aniento, F. (2004. b). Sorting signals in the cytosolic tail of plant p24 proteins involved in the interaction with the COPII coat. Plant Cell Physiol. 45, 1779–1786. [DOI] [PubMed] [Google Scholar]
- Crofts, A.J., Leborgne-Castel, N., Hillmer, S., Robinson, D.G., Phillipson, B., Carlsson, L.E., Ashford, D.A., and Denecke, J. (1999). Saturation of the endoplasmic reticulum retention machinery reveals anterograde bulk flow. Plant Cell 11, 2233–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva, L.L., Snapp, E.L., Denecke, J., Lippincott-Schwartz, J., Hawes, C., and Brandizzi, F. (2004). Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell 16, 1753–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva, L.L., Taylor, J.P., Hadlington, J.L., Hanton, S.L., Snowden, C.J., Fox, S.J., Foresti, O., Brandizzi, F., and Denecke, J. (2005). Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17, 132–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., Botterman, J., and Deblaere, R. (1990). Protein secretion in plant cells can occur via a default pathway. Plant Cell 2, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., De Rycke, R., and Botterman, J. (1992). Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J. 11, 2345–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., Goldman, M.H., Demolder, J., Seurinck, J., and Botterman, J. (1991). The tobacco luminal binding protein is encoded by a multigene family. Plant Cell 3, 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez, M., Dejgaard, K., Fullekrug, J., Dahan, S., Fazel, A., Paccaud, J.P., Thomas, D.Y., Bergeron, J.J., and Nilsson, T. (1998). gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J. Cell Biol. 140, 751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driouich, A., Zhang, G.F., and Staehelin, L.A. (1993). Effect of brefeldin A on the structure of the Golgi apparatus and on the synthesis and secretion of proteins and polysaccharides in sycamore maple (Acer pseudoplatanus) suspension-cultured cells. Plant Physiol. 101, 1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping, E.A., and Moye-Rowley, W.S. (2002). Identification of interdependent signals required for anterograde traffic of the ATP-binding cassette transporter protein Yor1p. J. Biol. Chem. 277, 34860–34869. [DOI] [PubMed] [Google Scholar]
- Fiedler, K., and Rothman, J.E. (1997). Sorting determinants in the transmembrane domain of p24 proteins. J. Biol. Chem. 272, 24739–24742. [DOI] [PubMed] [Google Scholar]
- Gillingham, A.K., and Munro, S. (2003). Long coiled-coil proteins and membrane traffic. Biochim. Biophys. Acta 1641, 71–85. [DOI] [PubMed] [Google Scholar]
- Gillingham, A.K., Pfeifer, A.C., and Munro, S. (2002). CASP, the alternatively spliced product of the gene encoding the CCAAT-displacement protein transcription factor, is a Golgi membrane protein related to giantin. Mol. Biol. Cell 13, 3761–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo, C.G., and Maccioni, H.J. (2003). Endoplasmic reticulum export of glycosyltransferases depends on interaction of a cytoplasmic dibasic motif with Sar1. Mol. Biol. Cell 14, 3753–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, L., and Chrispeels, M.J. (1993). Tonoplast and soluble vacuolar proteins are targeted by different mechanisms. Plant Cell 5, 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handford, M.G., Sicilia, F., Brandizzi, F., Chung, J.H., and Dupree, P. (2004). Arabidopsis thaliana expresses multiple Golgi-localised nucleotide-sugar transporters related to GONST1. Mol. Genet. Genomics 272, 397–410. [DOI] [PubMed] [Google Scholar]
- Hanton, S.L., Bortolotti, L.E., Renna, L., Stefano, G., and Brandizzi, F. (2005). Crossing the divide—transport between the endoplasmic reticulum and Golgi apparatus in plants. Traffic 6, 267–277. [DOI] [PubMed] [Google Scholar]
- Haseloff, J., Siemering, K.R., Prasher, D.C., and Hodge, S. (1997). Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc. Natl. Acad. Sci. USA 94, 2122–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofte, H., and Chrispeels, M.J. (1992). Protein sorting to the vacuolar membrane. Plant Cell 4, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, L.W., and Rogers, J.C. (1998). Integral membrane protein sorting to vacuoles in plant cells: Evidence for two pathways. J. Cell Biol. 143, 1183–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappeler, F., Klopfenstein, D.R., Foguet, M., Paccaud, J.P., and Hauri, H.P. (1997). The recycling of ERGIC-53 in the early secretory pathway. ERGIC-53 carries a cytosolic endoplasmic reticulum-exit determinant interacting with COPII. J. Biol. Chem. 272, 31801–31808. [DOI] [PubMed] [Google Scholar]
- Kuehn, M.J., Herrmann, J.M., and Schekman, R. (1998). COPII-cargo interactions direct protein sorting into ER-derived transport vesicles. Nature 391, 187–190. [DOI] [PubMed] [Google Scholar]
- Lee, H.I., Gal, S., Newman, T.C., and Raikhel, N.V. (1993). The Arabidopsis endoplasmic reticulum retention receptor functions in yeast. Proc. Natl. Acad. Sci. USA 90, 11433–11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M.H., Min, M.K., Lee, Y.J., Jin, J.B., Shin, D.H., Kim, D.H., Lee, K.H., and Hwang, I. (2002). ADP-ribosylation factor 1 of Arabidopsis plays a critical role in intracellular trafficking and maintenance of endoplasmic reticulum morphology in Arabidopsis. Plant Physiol. 129, 1507–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, D., Zerangue, N., Lin, Y.F., Collins, A., Yu, M., Jan, Y.N., and Jan, L.Y. (2001). Role of ER export signals in controlling surface potassium channel numbers. Science 291, 316–319. [DOI] [PubMed] [Google Scholar]
- Malkus, P., Jiang, F., and Schekman, R. (2002). Concentrative sorting of secretory cargo proteins into COPII-coated vesicles. J. Cell Biol. 159, 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, E.A., Beilharz, T.H., Malkus, P.N., Lee, M.C., Hamamoto, S., Orci, L., and Schekman, R. (2003). Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell 114, 497–509. [DOI] [PubMed] [Google Scholar]
- Miranda, M., Sorkina, T., Grammatopoulos, T.N., Zawada, W.M., and Sorkin, A. (2004). Multiple molecular determinants in the carboxyl terminus regulate dopamine transporter export from endoplasmic reticulum. J. Biol. Chem. 279, 30760–30770. [DOI] [PubMed] [Google Scholar]
- Misumi, Y., Sohda, M., Tashiro, A., Sato, H., and Ikehara, Y. (2001). An essential cytoplasmic domain for the Golgi localization of coiled-coil proteins with a COOH-terminal membrane anchor. J. Biol. Chem. 276, 6867–6873. [DOI] [PubMed] [Google Scholar]
- Mossessova, E., Bickford, L.C., and Goldberg, J. (2003). SNARE selectivity of the COPII coat. Cell 114, 483–495. [DOI] [PubMed] [Google Scholar]
- Nakamura, N., Yamazaki, S., Sato, K., Nakano, A., Sakaguchi, M., and Mihara, K. (1998). Identification of potential regulatory elements for the transport of Emp24p. Mol. Biol. Cell 9, 3493–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, N., and Balch, W.E. (1997). A di-acidic signal required for selective export from the endoplasmic reticulum. Science 277, 556–558. [DOI] [PubMed] [Google Scholar]
- Nishimura, N., Bannykh, S., Slabough, S., Matteson, J., Altschuler, Y., Hahn, K., and Balch, W.E. (1999). A di-acidic (DXE) code directs concentration of cargo during export from the endoplasmic reticulum. J. Biol. Chem. 274, 15937–15946. [DOI] [PubMed] [Google Scholar]
- Otte, S., and Barlowe, C. (2002). The Erv41p-Erv46p complex: Multiple export signals are required in trans for COPII-dependent transport from the ER. EMBO J. 21, 6095–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulhe, F., Imhof, B.A., and Wehrle-Haller, B. (2004). A specific endoplasmic reticulum export signal drives transport of stem cell factor (Kitl) to the cell surface. J. Biol. Chem. 279, 55545–55555. [DOI] [PubMed] [Google Scholar]
- Phillipson, B.A., Pimpl, P., daSilva, L.L., Crofts, A.J., Taylor, J.P., Movafeghi, A., Robinson, D.G., and Denecke, J. (2001). Secretory bulk flow of soluble proteins is efficient and COPII dependent. Plant Cell 13, 2005–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna, L., Hanton, S.L., Stefano, G., Bortolotti, L., Misra, V., and Brandizzi, F. (2005). Identification and characterization of AtCASP, a plant transmembrane Golgi matrix protein. Plant Mol. Biol. 58, 109–122. [DOI] [PubMed] [Google Scholar]
- Ritzenthaler, C., Nebenführ, A., Movafeghi, A., Stussi-Garaud, C., Behnia, L., Pimpl, P., Staehelin, L.A., and Robinson, D.G. (2002). Reevaluation of the effects of brefeldin A on plant cells using tobacco Bright Yellow 2 cells expressing Golgi-targeted green fluorescent protein and COPI antisera. Plant Cell 14, 237–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Jore, C.M., Evins, J., Batoko, H., Brandizzi, F., Moore, I., and Hawes, C. (2002). Redistribution of membrane proteins between the Golgi apparatus and endoplasmic reticulum in plants is reversible and not dependent on cytoskeletal networks. Plant J. 29, 661–678. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Press).
- Sato, K., and Nakano, A. (2002). Emp47p and its close homolog Emp46p have a tyrosine-containing endoplasmic reticulum exit signal and function in glycoprotein secretion in Saccharomyces cerevisiae. Mol. Biol. Cell 13, 2518–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevier, C.S., Weisz, O.A., Davis, M., and Machamer, C.E. (2000). Efficient export of the vesicular stomatitis virus G protein from the endoplasmic reticulum requires a signal in the cytoplasmic tail that includes both tyrosine-based and di-acidic motifs. Mol. Biol. Cell 11, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockklausner, C., Ludwig, J., Ruppersberg, J.P., and Klocker, N. (2001). A sequence motif responsible for ER export and surface expression of Kir2.0 inward rectifier K(+) channels. FEBS Lett. 493, 129–133. [DOI] [PubMed] [Google Scholar]
- Takeuchi, M., Ueda, T., Sato, K., Abe, H., Nagata, T., and Nakano, A. (2000). A dominant negative mutant of sar1 GTPase inhibits protein transport from the endoplasmic reticulum to the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J. 23, 517–525. [DOI] [PubMed] [Google Scholar]
- Votsmeier, C., and Gallwitz, D. (2001). An acidic sequence of a putative yeast Golgi membrane protein binds COPII and facilitates ER export. EMBO J. 20, 6742–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Matteson, J., An, Y., Moyer, B., Yoo, J.S., Bannykh, S., Wilson, I.A., Riordan, J.R., and Balch, W.E. (2004). COPII-dependent export of cystic fibrosis transmembrane conductance regulator from the ER uses a di-acidic exit code. J. Cell Biol. 167, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa, K., Toyooka, K., Fukuda, H., and Matsuoka, K. (2005). Membrane-anchored prolyl hydroxylase with an export signal from the endoplasmic reticulum. Plant J. 41, 81–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.