Abstract
It is accepted that cell-mediated immune responses predominate in mycobacterial infections. Many studies have shown that CD4+ T cells produce Th1 cytokines, such as gamma interferon (IFN-γ), in response to mycobacterial antigens and that the cytolytic activity of CD8+ cells toward infected macrophages is important. However, the extent and manner in which γδ T cells participate in this response remain unclear. In ruminants, γδ T cells comprise a major proportion of the peripheral blood mononuclear cell population. We have previously shown that WC1+ γδ T cells are involved early in Mycobacterium bovis infection of cattle, but their specific functions are not well understood. Here we describe an in vivo model of bovine tuberculosis in which the WC1+ γδ T cells were depleted from the peripheral circulation and respiratory tract, by infusion of WC1+-specific monoclonal antibody, prior to infection. While no effects on disease pathology were observed in this experiment, results indicate that WC1+ γδ T cells, which become significantly activated (CD25+) in the circulation of control calves from 21 days postinfection, may play a role in modulating the developing immune response to M. bovis. WC1+-depleted animals exhibited decreased antigen-specific lymphocyte proliferative response, an increased antigen-specific production of interleukin-4, and a lack of specific immunoglobulin G2 antibody. This suggests that WC1+ γδ TCR+ cells contribute, either directly or indirectly, toward the Th1 bias of the immune response in bovine tuberculosis—a hypothesis supported by the decreased innate production of IFN-γ, which was observed in WC1+-depleted calves.
Infection with Mycobacterium bovis, the causative organism of bovine tuberculosis, occurs in many countries in both domestic and wild animals (64). Persistence of this zoonotic disease coupled with the spread of human immunodeficiency virus infection, particularly in sub-Saharan Africa, is also giving cause for concern (15).
Protective immunity against tuberculosis is considered to be essentially cell mediated and dependent upon the interaction of T cells with infected macrophages (4). The role of the CD4+ T-cell subpopulation during infection is well defined in humans and mice (28, 49). These cells respond to infection principally through the production of cytokines such as gamma interferon (IFN-γ) (3), which are considered to be involved in the activation of macrophages (14, 46). The CD8+ subpopulation has also been shown to produce IFN-γ, although recent work suggests that only a small percentage of CD8+ T cells actually produce this cytokine at any given time during infection (55). An additional important role for CD8+ cells in early infection may be their ability to act as cytotoxic T lymphocytes (CTLs), and this has already been described in both humans and mice (31, 40). The release of live M. bovis from infected bovine macrophages by antigen-stimulated CD8+ cells has recently been described and indicates that CTL responses also exist in cattle (33). Such CTLs may not only be involved in the lysis of specific target cells, but may also release molecules, such as granulysin, which have been shown to kill mycobacteria directly (61).
The role of the γδ T-cell subpopulation is less well understood. Protection studies in γδ T-cell receptor (TCR) knockout mice have shown that M. bovis bacillus Calmette-Guérin (BCG) infection can be controlled (29), but that inoculation with a high intravenous dose of virulent Mycobacterium tuberculosis is rapidly lethal (27). However, lower doses (including aerosol exposure) of M. tuberculosis have been found to grow identically in γδ knockout mice and wild-type control mice (17). In addition, it has been observed that γδ knockout mice develop larger and less well organized granulomas in response to intravenous M. tuberculosis than control mice, leading to the proposal that γδ T cells may not have a direct role in protection but are primarily involved in the regulation of granuloma formation (17). Recently, however, it has been shown that human γδ T cells can have a direct effect on the viability of M. tuberculosis (16).
In ruminants, γδ T cells can be divided into two main subpopulations, based on the expression of the workshop cluster 1 (WC1) molecule (11). These two distinct γδ T-cell subsets, WC1+ and WC1−, are also differentially distributed throughout the tissues (37). WC1, encoded by a large family of genes, exists as a number of isoforms and belongs to the scavenger receptor cysteine-rich domain family (65). However, although two human gene sequences exist which are more than 85% homologous with the bovine WC1 gene sequence, no evidence of WC1 expression on human γδ T cells has been reported (66). Neither has any evidence of WC1 expression on mouse γδ T cells been reported to date. In young cattle, however, circulating γδ T cells can represent up to 75% of peripheral blood lymphocytes (21), and the majority of these also express WC1 (68).
Studies on the WC1 molecule are limited. It has been proposed that it may control the tissue-specific homing of γδ T cells (66). Anti-WC1 antibody has also been shown to induce G0/G1 cell cycle growth arrest in interleukin-2 (IL-2)-dependent γδ T-cell lines, suggesting that WC1 may have a significant biological role in the control of γδ T-cell proliferation (62).
Previous work carried out in the Veterinary Sciences Division, the Department of Agriculture and Rural Development, Belfast, United Kingdom, has shown a decrease in circulating numbers of WC1+ γδ T cells during early M. bovis infection of cattle and increased numbers of WC1+ cells in early lung lesions (9, 10, 51), suggesting that WC1+ γδ T cells migrate from the peripheral circulation to a primary site of infection. WC1+ γδ T cells have also been found to respond in vitro to mycobacterial antigens (51, 57). However, the in vivo function of these cells remains unclear.
A novel, severe combined immunodeficient mouse, reconstituted with a bovine immune system (SCID-bo), has been used to investigate the role of WC1+ γδ T cells (56). The results obtained from M. bovis infection in this model suggest a role for WC1+ γδ T cells in cattle similar to that already postulated for γδ T cells in mice (17).
The relative contribution of WC1+ γδ T cells to the overall immune response following M. bovis infection has not, to date, been examined in cattle. The aim of the present study was to investigate the in vivo role of WC1+ γδ T cells by depleting them from the circulation of cattle prior to experimental M. bovis infection. Any difference in overall immune response observed between WC1+-depleted and -nondepleted animals would provide the first characterization of a role for WC1+ γδ T cells in bovine tuberculosis beyond that of regulating granuloma formation. Such an approach has the advantage of using the natural host for infection and may, therefore, prove relevant to the understanding of mycobacterial immunity in general.
MATERIALS AND METHODS
Experimental animals.
Fourteen calves were used in this study. All were Friesian-cross males of approximately 6 months of age, which had been obtained from farms with no history of M. bovis infection for at least 5 years.
Depletion of WC1+ γδ T cells and experimental infection.
Calves were depleted of WC1+ γδ T cells by intravenous infusion of monoclonal antibodies (MAb). Animals undergoing depletion received 5 mg of CC15 anti-WC1 immunoglobulin G2a (IgG2a) MAb (22) intravenously on each of 7 consecutive days. Control animals not depleted of WC1+ cells received a nonrelated, isotype-matched MAb, AV37 (a chicken leukocyte-specific MAb) (IAH, Compton, United Kingdom), following an identical protocol.
Two calves (one depleted and one not depleted of WC1+) were used initially to confirm the successful depletion of WC1+ γδ T cells using this protocol. Blood samples were taken from both animals immediately prior to the first administration of MAb, and on 4 separate days during the MAb administration process. On the 8th day, 24 h after the last administration of MAb and immediately prior to postmortem examination, a final blood sample was taken. Peripheral blood mononuclear cells (PBMC) were isolated from each sample on the day of bleeding, and the proportion of each major lymphocyte cell population (CD4+, CD8+, TCR1+, WC1+, and B cells) was determined by single-color flow cytometric analysis (FCA), with appropriate controls, as described below. Following postmortem examination, lung tissue sections from both calves were stained immunohistochemically using a WC1+ γδ T-lymphocyte antibody as described previously (10). In brief, following blocking of endogenous peroxidase activity by hydrogen peroxide, test sections were microwaved in an aqueous urea solution. Sections were then treated with normal horse serum before overnight incubation at room temperature with CC15 MAb, diluted 1:100. Sequential incubation with biotinylated anti-mouse IgG and with an avidin-biotin-peroxidase complex reagent (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, Calif.) followed, and peroxidase activity was developed with diaminobenzidine-tetrahydrochloride (DAB-peroxidase substrate kit; Vector Laboratories). Sections were then counterstained lightly with Harris's hematoxylin. As a negative control, the staining procedure was carried out, omitting the use of the primary antibody.
Single-color FCA of PBMC indicated an even spread of WC1+ γδ T-cell population sizes across the 12 remaining calves. These were allocated randomly into groups of equal numbers of WC1+-depleted and -nondepleted animals. Animals were housed in two separate high-security isolation houses (three WC1+-depleted and three-nondepleted per house), held under negative pressure, with expelled air filtered through absolute filters (43). The depleted animals received CC15 MAb on days −1 to 5 inclusive, while the non-depleted animals received AV37 MAb on the same days. On day 0 and day 1, in addition to MAb, all 12 animals each received a total of 7 × 106 CFU of M. bovis by intranasal instillation as described previously (43). The strain of M. bovis used (T/91/1378) was isolated originally in Northern Ireland from a field case of bovine tuberculosis. Blood samples were taken from each animal on the following days: −1, 0, 1, 4, 7, 11, 14, 19, 21, 25, 28, 32, 35, and 39. PBMC were isolated and used on each day both for assessment of cell-mediated immune functions (including IFN-γ production) and for FCA. In addition, PBMC were used each day (except day 39) to prepare supernatants for measurement of IL-2 production and on days 0, 19, 25, and 32 for measurement of IL-4 production. Serum samples were also collected weekly for measurement of specific anti-M. bovis IgM, IgG1, and IgG2 activity.
Postmortem examinations.
A detailed necropsy was performed on each calf (one WC1+-depleted and one nondepleted animal on each of days 34 and 36, respectively, and the remaining eight animals on day 40) as described previously (9). In brief, these examinations included close visual assessment of approximately 5-mm-thick, serial, transverse sections of each lung lobe and of upper respiratory tract surfaces and tonsils following longitudinal midline sectioning of the head. Lymph nodes were inspected, and the retropharyngeal, parotid, submandibular, cervical, bronchial, mediastinal, and mesenteric nodes were serially sliced at 2-mm intervals, to facilitate thorough examination. A wide range of tissues were taken for histopathological and bacteriological examination, including upper respiratory tract surfaces; palatine and pharyngeal tonsils; cranial, middle, and caudal lung lobes; and lymph nodes (9). Tissue samples were fixed in 10% neutral buffered formalin and processed by a standard paraffin wax technique. Sections were cut and stained with hematoxylin and eosin and also by the Ziehl-Neelsen method. They were examined for the presence of tuberculous lesions and acid-fast bacteria.
Lymphocyte proliferation assay (LPA) and preparation of antigen-stimulated culture supernatants.
PBMC were separated from heparinized blood by density centrifugation, resuspended at a concentration of 106/ml in tissue culture medium (TCM), and dispensed in 200-μl volumes into wells of 96-well tissue culture plates as described previously (50). Purified protein derivatives (PPD) from M. bovis and Mycobacterium avium (PPD-B and PPD-A) (Veterinary Laboratory Agency, Weybridge, United Kingdom) and pokweed mitogen (PWM) (Sigma Chemical Co., Poole, United Kingdom) were diluted in phosphate-buffered saline (PBS) and added to triplicate wells at a final optimal concentration of 4 μg/ml. For each animal, a further three wells remained unstimulated as controls (PBS only added). The cultures were incubated in 6% CO2 at 37°C for 5 days. For the final 16 h, each well was pulsed with 1 μCi of tritiated thymidine ([methyl-3H]thymidine) (Amersham International, Amersham, United Kingdom). The contents of each well were harvested onto a glass fiber filter mat (Wallac, Turku, Finland), and incorporated radioactivity was determined as counts per minute by liquid scintillation counting. Responses where the mean number of counts per minute was greater than that of the control by a factor of at least two were considered positive.
To provide supernatants for cytokine analyses, similar cultures were established at each time point using PPD-B and PBS only (six wells each). These were incubated in 6% CO2 at 37°C for 4 days, and cells were pelleted by centrifugation at 220 × g for 15 min at 10°C. Supernatants were aspirated from individual wells and stored at −20°C for subsequent analysis of secreted IL-2, IFN-γ, IL-4, and granulocyte-macrophage colony-stimulating factor (GM-CSF).
Flow cytometry.
Cell phenotype, together with activation status (CD25+ expression) and expression of major histocompatibility complex class II (MHC-II), were determined by two-color flow cytometry as described previously (57). The primary MAbs and secondary, isotype-specific, conjugates used are shown in Table 1. FCA was performed using a FACS Vantage (Becton Dickinson, Oxford, United Kingdom) equipped with an Innova Enterprise ion laser (Coherent Laser Group, San Jose, Calif.). The mononuclear cell population was identified on the basis of forward and orthogonal light scatter and was gated appropriately. Green (fluorescein isothiocyanate [FITC]) and orange (phycoerythrin [PE]) log integral signals were obtained from the gated population. Ten thousand cells were counted for each sample, and analyses were performed using LYSIS II software (Becton Dickinson). Isotype-matched control MAbs (AV20) (29, 37) gave negative staining with both FITC and PE conjugates and were used to set compensation.
TABLE 1.
Primary antibodies and conjugates used for FCA
| MAb or clone | Specificity | Isotype | MAb reference or source | Conjugatea |
|---|---|---|---|---|
| CC8 | CD4 | IgG2a | 22 | Anti-mouse IgG2a-FITC |
| CC63 | CD8 | IgG2a | 35 | Anti-mouse IgG2a-FITC |
| CC15 | WC1 | IgG2a | 22 | Anti-mouse IgG2a-FITC |
| GB21A | TCR1 | IgG2b | VMRD Inc., Pullman, Wash. | Anti-mouse IgG2b-FITC |
| CC51 | CD21 | IgG2b | IAH, Compton | Anti-mouse IgG2b-FITC |
| CACT116A | CD25 | IgG1 | VMRD Inc., Pullman, Wash. | Anti-mouse IgG1-PE |
| CC108 | MHC II | IgG1 | IAH, Compton | Anti-mouse IgG1-PE |
| CC158 | MHC II | IgG2a | IAH, Compton | Anti-mouse IgG2a-FITC |
| CCG33 | CD14 | IgG1 | 60 | Anti-mouse IgG1-PE |
| AV20 | Chick leukocyte antigen | IgG1 | IAH, Compton | Anti-mouse IgG1-PE |
| AV37 | Chick leukocyte antigen | IgG2a | IAH, Compton | Anti-mouse IgG2a-FITC |
| Av29 | Chick leukocyte antigen | IgG2b | IAH, Compton | Anti-mouse IgG2b-FITC |
Southern Biotech Associates Inc., Birmingham, Al.
IL-2 bioassay.
Detection of IL-2 was based on the measurement of concanavalin A (ConA) blast proliferation following stimulation by IL-2-containing culture supernatants (18).
PBMC, isolated from an animal free of M. bovis infection, were diluted in TCM to a final concentration of 2 × 106/ml. ConA (Sigma) was added at a concentration of 5 μg per ml of cell suspension, and cells were incubated in 6% CO2 at 37°C for 4 days to ensure up-regulation of the IL-2 receptor. The ConA blasts were washed twice in PBS and resuspended in TCM. Into each well of a 96-well tissue culture plate (Nunc, Roskilde, Denmark) was placed 150 μl of cell suspension (8 × 104 cells), and into appropriate wells were placed (in triplicate) 50-μl aliquots of test supernatants harvested from PBMC cultures as described earlier. Recombinant bovine IL-2 (IAH, Compton) was used as a positive control on each plate, and TCM was used as a negative control. Cells were incubated overnight in 6% CO2 at 37°C, and then each well was pulsed with 1 μCi of tritiated thymidine and incubated for a further 16 h prior to harvesting and counting as before. Results were expressed as net counts per minute (mean counts per minute for supernatant obtained from three PPD-B wells minus mean counts per minute for supernatant obtained from three PBS control wells). An IL-2 response to PPD-B was considered positive where the net number of counts per minute was greater than that of the mean control (PBS) by a factor of at least 2.
IL-4 bioassay.
IL-4 activity was measured using a B-cell bioassay (26) in which PBMC were isolated from an animal free of M. bovis infection and B cells were magnetically sorted using bovine immunoglobulin light chain-specific MAb ILA-58 (67). Following incubation with microbeads coated with rat anti-mouse IgG2a and -2b (Miltenyi Biotech, Bergisch Gladbach, Germany), B cells were isolated using the magnetically activated cell sorting column separation system (Miltenyi) and then resuspended in TCM. Into each well of a 96-well tissue culture plate (Nunc) was placed 75 μl of cell suspension (7.5 × 104 cells), and into appropriate wells were placed (in triplicate) 50-μl aliquots of test supernatants harvested from PBMC cultures as described earlier. Recombinant bovine IL-4 (IAH, Compton) was used as a positive control on each plate, and TCM was used as a negative control. Cells were incubated for 24 h in 6% CO2 at 37°C, and then each well was pulsed with 1 μCi of tritiated thymidine and incubated for a further 24 h prior to harvesting and counting as before. Results were expressed as net counts per minute (mean counts per minute for supernatant obtained from three PPD-B wells minus mean counts per minute for supernatant obtained from three PBS control wells), and an IL-4 response to PPD-B was considered positive when the net number of counts per minute was greater than that of the control by a factor of at least 2.
IFN-γ capture enzyme linked immunosorbent assay (ELISA).
Wells of microtitre plates (Maxisorp; Nunc) were coated with bovine IFN-γ-specific MAb 5D10 (IAH, Compton) overnight at room temperature. Wells were washed with PBS containing 0.05% (vol/vol) Tween 20 (Sigma) (PBS-T), and the remaining sites were blocked with 1% bovine serum albumin (wt/vol) (Sigma). After washing, test supernatants harvested from PBMC cultures were added. Recombinant bovine IFN-γ (IAH, Compton) was used as a positive control on each plate, and recombinant bovine IL-6 (IAH, Compton) was used as a negative control. The plates were again washed, and IFN-γ-specific biotinylated CC302 MAb (IAH, Compton) was added to all wells. Plates were again washed, and to each well was added streptavidin-horseradish peroxidase (HRP) conjugate (Amersham International). Following incubation, the plates were washed and bound HRP was detected by incubation with the substrate 3,3′,5,5′-tetramethylbenzidine (TMB) (Chemicon, Harrow, United Kingdom). Results were expressed as net optical density (OD) (OD of supernatant obtained from PPD-B wells minus OD of supernatant obtained from PBS wells). The IFN-γ response to PPD-B was considered positive when the net OD was greater than the IL-6 negative control's OD by a factor of at least 2.
GM-CSF capture ELISA.
Wells of microtiter plates (Becton Dickinson) were coated with bovine GM-CSF-specific MAb CC305 (IAH, Compton) overnight at room temperature. After washing with PBS-T, test supernatants harvested from PBMC cultures were added. Recombinant GM-CSF (IAH, Compton) was used as a positive control on each plate, and recombinant bovine IFN-γ was used as a negative control. The plates were again washed, and then anti-GM-CSF biotinylated 3C2 MAb (IAH, Compton) was added to all wells. Detection of bound biotinylated MAb was carried out with streptavidin-HRP conjugate and TMB substrate, as described earlier.
Antibody ELISA.
A measurement of serum anti-M. bovis IgM, IgG1, and IgG2 activity was made by ELISA based on M. bovis sonic extract, produced from the T/91/1378 strain of M. bovis (50). M. bovis sonic extract was used as the solid-phase antigen, and the ELISA protocol has been described previously (34). Sera from each animal were diluted 1:100 in PBS-T for testing. A high-titer serum sample from a previously experimentally infected animal (51) was used as a positive control on each plate. Preinfection serum from this same animal was used as a negative control, and PBS-T alone was used as a background control. In order to detect the presence of anti-M. bovis antibody, subclass-specific mouse MAbs were each diluted in PBS-T as follows: anti-IgG1 clone K372G6 (Serotec, Oxford, United Kingdom) was diluted 1:2,500, anti-IgG2 clone K1924F10 (Serotec) was diluted 1:2,500, and IgM clone BM-23 (Sigma) was diluted 1:4,000. Affinity-purified goat anti-mouse Ig-HRP conjugate (Sigma) was diluted 1:5,000 in PBS-T and used to label bound subclass-specific MAb, and bound HRP was detected with TMB as described earlier. Although there was no evidence of non-specific binding of either MAbs or conjugate in these ELISAs, several animals had very low, but detectable, IgM or IgG1 binding at the outset of the experiment. For this reason, results for each animal were expressed as net OD (OD of postinfection sample at each time point minus OD of preinfection sample). Antibody responses were considered positive when the net OD was greater than the PBS-T background control's OD by a factor of at least 2.
Statistical analyses.
The effects of WC1+ γδ T-cell depletion upon T-cell phenotypes and activation status were determined by analysis of variance for repeated measurements, which compared the means for the groups depleted and not depleted of WC1+ cells over the various time periods and tested for interaction between groups and time. For the effect of depletion upon discrete immunological measurements, where it was inappropriate to assume a normal distribution for the observations, the nonparametric Mann-Whitney U test based on comparing the ranks for the two groups at each time point was used instead.
RESULTS
Initial assessment of WC1+ γδ T-cell depletion.
The percentage of WC1+ γδ T cells in the total PBMC population was monitored initially in one calf by flow cytometry over a 7-day period of CC15 MAb administration and compared with the percentage in a second calf, which had received equal doses of AV37 isotype control MAb. Depletion of WC1+ PBMC from the peripheral circulation was almost complete following the first CC15 MAb injection (Table 2). The relative percentage of CD4+, CD8+, and B cells remained largely unchanged, while the relative percentage of total γδ (TCR1+) T cells fell. The removal of WC1+ γδ T cells from the peripheral circulation in this animal accounts for these observed changes. In contrast, the percentage of CD4+, CD8+, WC1+, γδ (TCR1+), and B cells in total PBMC remained relatively unchanged in the animal receiving AV37 MAb.
TABLE 2.
Phenotype analysis of PBMC following administration of CC15 or AV37 MAba
| Day | % Gated PBMC in:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal receiving AV37 (nondepleted)
|
Animal receiving CC15 (depleted)
|
||||||||||
| WC1 | TCR1 | CD4 | CD8 | B cells | WC1 | TCR1 | CD4 | CD8 | B cells | ||
| 1 | 18.5 | 24.0 | 33.5 | 13.5 | 17.5 | 8.0 | 18.0 | 26.0 | 14.5 | 23.5 | |
| 2 | 10.5 | 24.0 | 31.5 | 12.0 | 17.5 | 0.5 | 9.5 | 28.0 | 15.5 | 25.0 | |
| 5 | 17.0 | 28.5 | 20.0 | 12.0 | 13.0 | 0.1 | 9.0 | 25.0 | 13.5 | 19.0 | |
| 7 | 19.0 | 28.0 | 23.5 | 15.0 | 12.0 | 0.1 | 6.0 | 28.0 | 16.0 | 19.5 | |
| 8 (postmortem) | 18.0 | 26.0 | 26.0 | 13.5 | 13.5 | 0.4 | 5.5 | 30.0 | 17.5 | 21.5 | |
Results expressed as percentage of gated PBMC, as assessed by FCA.
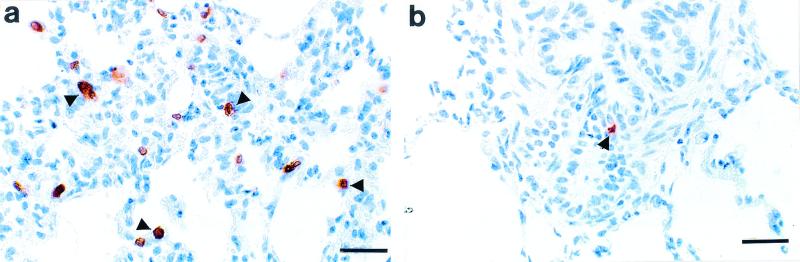
On the 8th day (24 h after the last administration of either CC15 or AV37 MAb), both animals were examined postmortem, including immunohistochemical labeling of WC1+ γδ T cells in lung tissue. Histologically, in both calves depleted and those not depleted of WC1+ cells with a round cytoplasmic outline with large centrally placed, round-to-ovoid nuclei and a thin peripheral rim of cytoplasm consistent with lymphocyte morphology exhibited both nuclear and cytoplasmic staining with the CC15 anti-WC1+ MAb (Fig. 1). Although the location of these cells within pulmonary parenchyma was similar in both calves, there was a significant difference in cell numbers in each case. In both animals positive-staining cells were randomly distributed within interalveolar septae, bronchial and bronchiolar epithelia, and submucosae and within bronchus- and bronchiolus-associated lymphoid tissue. Upon quantitative assessment of cell numbers, an average of 33 cells expressing WC1 were observed per high-power microscopic field (40× objective) in the lungs of the calf not depleted of WC1+ cells (Fig. 1a). This contrasted with a cell count averaging one positively staining cell per three high-power microscopic lung fields (40× objective) in the animal depleted of WC1+ (Fig. 1b).
FIG. 1.
Immunohistochemical labeling with avidin-biotin-peroxidase of WC1+ γδ T cells in lung tissue. (a) Numerous WC1+ γδ T lymphocytes (arrowheads) within interlobular septae in the lung of a calf not depleted of WC1+ cells. Bar, 25 μm. (b) One WC1+ γδ T lymphocyte (arrowhead) beneath bronchiolar epithelium in the lung of calf depleted of WC1+ cells. Bar, 25 μm.
The results from these first calves indicated that successful depletion of WC1+ γδ T cells from the peripheral circulation and lower respiratory tract could be achieved by intravenous administration of CC15 MAb. This method was therefore used to deplete six calves of WC1+ γδ T cells prior to experimental M. bovis infection. The immune responses observed in these WC1+-depleted animals were then compared directly with those of six control animals that were given AV37 MAb.
WC1+ γδ T-cell depletion and response to M. bovis infection. (i) Flow cytometry.
Statistical analysis showed a highly significant effect of MAb depletion upon the percentage of circulating WC1+ γδ T cells from day 0 until the close of the experiment (P < 0.001) (Fig. 2a). In calves not depleted of WC1+ cells an initial decrease in circulating WC1 numbers within 1 week of infection, which has been reported previously (9, 10, 51), was again observed. This initial decrease was followed closely by a steady rise, to a final level which was higher than that observed before infection. Over the course of the experiment, changes in the circulating numbers of γδ T cells (TCR1+) in both groups of animals mirrored those described for the WC1+ γδ T-cell population. After day 7 until the close of the experiment, relatively greater numbers of CD4+ cells were circulating in the animals depleted of WC1+ cells (P < 0.01), which may reflect the decrease in the WC1+ cell population. No evidence of an effect due to depletion of WC1+ γδ T cells on the circulating percentage of the CD8+ cells was observed (P > 0.05). Pre- and postinfection, the proportions of CD8+ cells in the group not depleted of WC1+ cells were consistently lower than those observed for the group depleted of these cells (P = 0.04). This was due to the presence of high numbers of circulating CD8+ cells in one animal from the group depleted of WC1+ cells throughout the course of the experiment. Such variation is an inherent aspect of any study that uses outbred cattle.
FIG. 2.
FCA of T cells in the peripheral circulation of WC1+ γδ T-cell-depleted and -nondepleted calves during experimental M. bovis infection. (a) Percentage phenotype of WC1+, TCR1+, CD4+, and CD8+ T cells in both WC1+-depleted and -nondepleted calves. (b) Percentage activation of each phenotype (as measured by CD25+ expression). Error bars, standard errors of the means.
Activation was determined by the expression of the IL-2 receptor (IL-2R [CD25+]) and by upregulation of MHC-II on each subpopulation of cells. Importantly, in calves not depleted of WC1+ cells a pronounced in vivo activation of WC1+ γδ T cells was seen to occur early in infection (P < 0.001) (Fig. 2b). In these animals, levels of WC1+ CD25+ cells were significantly raised by day 21. From Fig. 2 it appears that, following M. bovis infection, it is the CD8+ T cells which show the first peak of activation (at day 14), followed several days later by activation of the γδ T cells (WC1+ and TCR1+). Such activation appears to be occurring slightly in advance of CD4+ T-cell activation. Increased levels of the MHC-II molecule were not detected by flow cytometry on either WC1+ or TCR1+ γδ T cells from any animal during the course of the experiment (data not shown).
(ii) Lymphocyte proliferation assay.
Figure 3 shows the lymphocyte proliferative responses of calves (both depleted and not depleted of WC1+ cells) in response to stimulation with PPD-B and PWM throughout the course of infection. No animal showed a proliferative response to either PPD-A or PPD-B prior to infection. Responses to PPD-B remained negative until approximately 19 to 21 days following infection, after which there were two phases of increased proliferation (Fig. 3a). Initially, responses to PPD-B rose rapidly, but they showed a marked decline at around day 28. Following the commencement of proliferative responses in both sets of animals, at all subsequent times the mean level of response to PPD-B in the group depleted of WC1+ cells was lower than that of the group not depleted of these cells. From day 32, a second rise in lymphocyte proliferation commenced, and differences between the calves in the WC1+-depleted and nondepleted groups were observed on days 32 and 35 (P = 0.05). Four of the five remaining calves not depleted of WC1+ cells showed a rapid rise in response to PPD-B which peaked at day 39 with a similar magnitude of response to that seen in the initial peak. In contrast, only a small degree of proliferation was observed at day 35 in only one of the five remaining animals from the depleted group. Importantly, this failure to mount a pronounced second peak of proliferation to PPD-B did not appear to be due to a general nonresponsiveness of PBMC in the WC1+-depleted calves. At each time point during the experiment, PBMC from both WC1+-depleted and nondepleted calves responded equally well and, indeed, almost identically to nonspecific stimulation with PWM (P > 0.1) (Fig. 3b).
FIG. 3.
Lymphocyte proliferation responses of WC1+ γδ T-cell-depleted and -nondepleted calves during experimental M. bovis infection. All results are expressed as mean counts per minute ± standard errors of the means (error bars). (a) PBMC cultured with either PBS or PPD-B. (b) PBMC cultured with PWM.
Cytokine measurements.
In order to investigate the differences in proliferative responses observed, cytokine production was examined in antigen-stimulated cultures from both WC1+-depleted and -nondepleted groups of calves.
(i) IL-2.
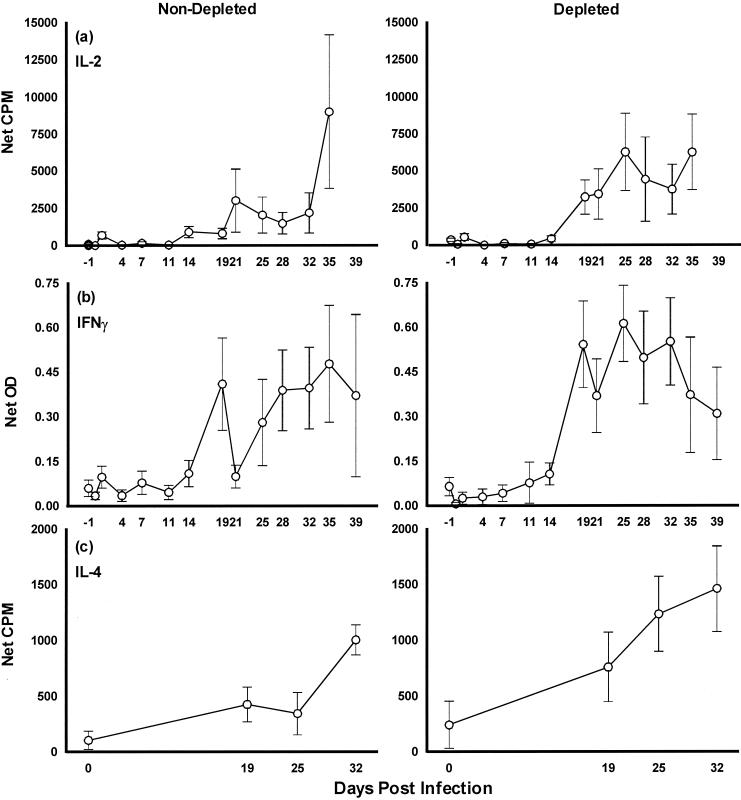
Levels of IL-2 production, in response to mycobacterial antigens, were similar in both groups of calves (P > 0.1) (Fig. 4a). In parallel with the development of a lymphocyte proliferative response, IL-2 production began at around day 19 to 21. Interestingly, in the group depleted of WC1+ cells, despite the lack of a second peak of proliferative response, IL-2 production continued throughout the course of the experiment.
FIG. 4.
Cytokine production by antigen-stimulated PBMC from WC1+ γδ T-cell-depleted and -nondepleted calves during experimental M. bovis infection. Results for IL-2 and IL-4 are expressed as mean net counts per minute ± standard errors of the means (error bars). Results for IFN-γ are expressed as net OD ± standard errors of the means (error bars). (a) IL-2; (b) IFN-γ; (c) IL-4.
(ii) IFN-γ.
Antigen-driven production of IFN-γ was similar in both groups of animals (P > 0.1), with specific release of this cytokine commencing between 14 and 19 days postinfection (Fig. 4b). However, careful examination of the early time points reveals that an initial fall in IFN-γ production occurred in the WC1+-depleted animals. This was first seen on day 0, was immediately following the administration of CC15 MAb on day −1, and was statistically significant only for cultures stimulated with PPD-B (P = 0.03) (Table 3). The trend for similar reduction of IFN-γ production in unstimulated cultures at day 0 may indicate that this response is nonspecific or innate.
TABLE 3.
IFN-γ release, in response to PBS or PPD-B, by cells from calves treated with anti-WC1 MAb (depleted) or control MAb (nondepleted)a
| Day | Mean OD ± SEM in response to PPD-B
|
P | Mean OD ± SEM in response to PBS
|
P | ||
|---|---|---|---|---|---|---|
| Nondepleted | Depleted | Nondepleted | Depleted | |||
| −1 | 0.45 ± 0.12 | 0.47 ± 0.09 | 0.47 | 0.47 ± 0.10 | 0.41 ± 0.08 | 0.41 |
| 0 | 0.48 ± 0.13 | 0.21 ± 0.06 | 0.03b | 0.49 ± 0.15 | 0.23 ± 0.08 | 0.12 |
| 1 | 0.53 ± 0.09 | 0.38 ± 0.09 | 0.24 | 0.47 ± 0.10 | 0.38 ± 0.10 | 0.15 |
| 4 | 0.46 ± 0.15 | 0.38 ± 0.16 | 0.35 | 0.45 ± 0.15 | 0.40 ± 0.15 | 0.24 |
IFN-γ production expressed as mean OD ± standard error of the mean.
No significant differences between animals depleted of WC1+ cells and those not depleted of these cells, except on day 0 with PPD-B (P = 0.03).
(iii) IL-4.
Differences were observed between the two groups of calves with respect to the amount of IL-4 produced (Fig. 4c). By day 25, the animals depleted of WC1+ cells were producing significantly greater quantities of this cytokine (P = 0.04). By day 32, a single animal from the group not depleted of WC1+ cells was also producing IL-4 at a level comparable with that observed in animals depleted of these cells (P = 0.2).
(iv) GM-CSF.
GM-CSF production was not detectable at any time in any animal used in this experiment (data not shown).
Antibody ELISA.
In light of the difference found in IL-4 production between groups of animals depleted and not depleted of these cells it was relevant to examine any possible differences in antibody isotype responses.
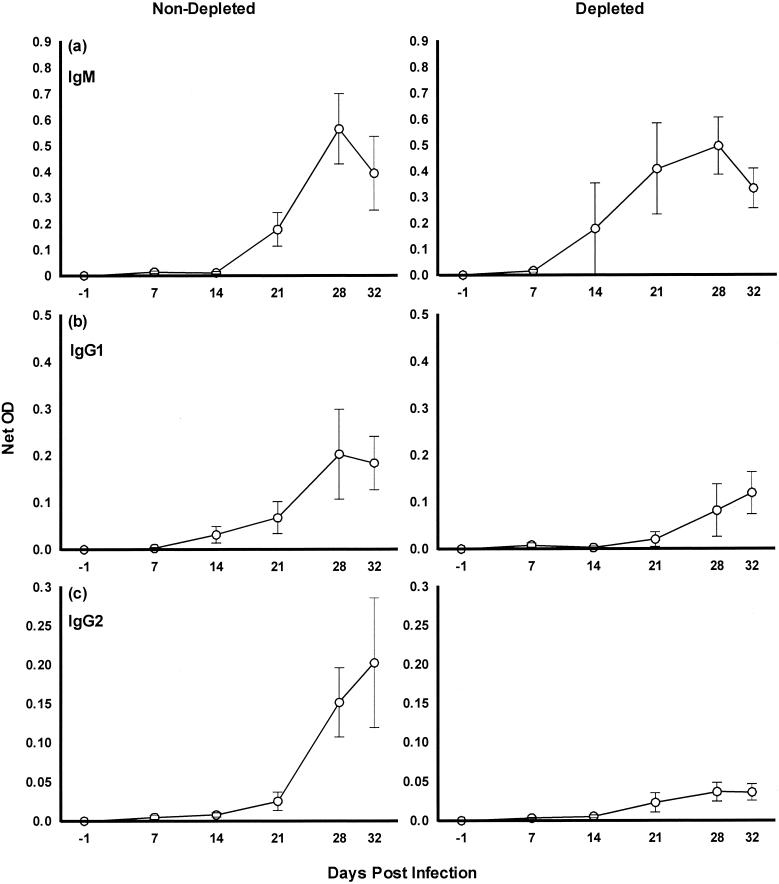
Results for detection of anti-M. bovis serum antibody are shown in Fig. 5. Although there was no significant difference in the amount of IgM produced in both groups of calves (P > 0.1), production of this antibody commenced 1 week earlier in calves depleted of WC1+ cells (Fig. 5a). Similar results were seen in both sets of animals for IgG1 antibody production (P > 0.1) (Fig. 5b). However, calves depleted of WC1+ cells exhibited negligible production of IgG2, which was clearly lower than that produced by calves not depleted of WC1+ cells, on both day 28 (P = 0.06) and day 32 (P = 0.05) (Fig. 5c).
FIG. 5.
M. bovis-specific antibody isotype responses in WC1+ γδ T-cell-depleted and -nondepleted calves during experimental infection. All results are expressed as net OD ± standard errors of the means (error bars). (a) IgM; (b) IgG1; (c) IgG2.
Summary of immunological responses.
Table 4 shows a summary of the immunological responses observed in each calf during the course of this experiment. All animals exhibited antigen-specific proliferation responses, and all produced both IFN-γ and IL-2. However, the production of IL-4 between groups was not the same, which became even more apparent when the numbers of calves with a positive IL-4 response were considered.
TABLE 4.
Summary of positive immunological responses which developed following M. bovis challenge in calves depleted and not depleted of WC1+ γδ T cells
| Animal | Maximum response index (fold increase)c
|
||||||
|---|---|---|---|---|---|---|---|
| LPA | IL-2 | IFN-γ | IL-4 | IgM | IgG1 | IgG2 | |
| Da | 11 | 8 | 11 | 6 | 9 | ||
| D | 8 | 5 | 9 | 4 | 16 | 10 | |
| D | 28 | 9 | 10 | 2 | |||
| D | 69 | 5 | 12 | 7 | 18 | 6 | |
| D | 15 | 3 | 10 | 4 | 15 | ||
| D | 100 | 2 | 11 | 7 | 4 | ||
| NDb | 6 | 2 | 3 | 11 | |||
| ND | 13 | 6 | 8 | 18 | |||
| ND | 29 | 2 | 6 | 8 | 9 | 10 | |
| ND | 67 | 2 | 11 | 25 | 10 | ||
| ND | 27 | 6 | 10 | 3 | 9 | 10 | 25 |
| ND | 28 | 13 | 11 | 3 | 13 | ||
Depleted animal.
Nondepleted animal.
For each animal, when positive immune responses were detected postinfection, the maximum response index (fold increase) is given. Cutoffs and calculation of indices are defined in Materials and Methods.
Four of the six animals from the group depleted of WC1+ cells had levels of IL-4 above the cutoff for positivity, compared with only one of six from the group not depleted of these cells. A similar pattern emerged when antibody responses were examined. All animals (with the exception of one calf not depleted of WC1+ cells) had detectable M. bovis-specific IgM responses. Three out of six animals from the WC1+-depleted group and four out of six from the WC1+-nondepleted group produced M. bovis-specific IgG1 antibody. The production of IgG2 was, however, very different between the two groups of animals. No M. bovis-specific IgG2 was observed in any animal prior to day 21 of the experiment. After day 21, IgG2 production was seen in three of the six animals not depleted of WC1+ cells. In contrast, no M. bovis-specific IgG2 responses, which were classified as positive, were observed in the group of animals depleted of WC1+ cells.
Postmortem examinations.
Tuberculous lesions were observed in the respiratory tract and associated lymph nodes of all 12 calves. Sites affected included the nasal mucosa; nasopharynx; pharyngeal tonsils; trachea; lungs and retropharyngeal; and parotid, submandibular, cervical, and bronchomediastinal lymph nodes. There were no obvious differences detected in the nature, extent or distribution of the lesions between calves depleted and those not depleted of WC1+ cells. Histologically, lesions consisted of a necrotic core, a surrounding mantle of macrophages and multinucleate macrophage giant cells, and a further circumscribing zone of lymphocytes. The microscopic appearance of the lesions did not differ significantly between the two groups. A proportion of lesions in both groups exhibited aggregations of degenerate and intact neutrophils in necrotic areas, mineralization of necrotic cores, and vascular congestion and erythrocyte extravasation peripheral to the macrophage mantle. While variations in the numbers of acid-fast organisms within lesions were noted there was no clear difference in bacterial numbers in the lesions of animals depleted and those not depleted of WC1+ cells.
DISCUSSION
The involvement of γδ T cells in immune responses to mycobacterial infections has been suggested from a large number of studies, but a precise definition of their function remains elusive (5, 54). Detailed studies of the bovine immune response to M. bovis infection are limited by the availability of secure facilities in which to house large animals. For this reason, most of the data which have accumulated on the role of γδ T cells has been restricted to human infection with M. tuberculosis and murine infections with M. tuberculosis or M. bovis. If investigation of the WC1+ subset of γδ T cells is required, the problem is complicated further by the absence of WC1+ expression on human and murine cells (66). The development of a SCID-bo mouse model (56) has gone some way to addressing these problems. However, WC1+ γδ T-cell depletion studies and infection in this model have, to date, focused on the detection of Th1 cytokine production and definition of effects on lesion architecture.
Within the context of bovine tuberculosis, experimental infections have indicated a role for γδ T cells, particularly those expressing the WC1 molecule (9, 10, 51, 57). Since bovine tuberculosis can be reproduced experimentally in the natural host, this model provides an important opportunity to derive new data on the function of γδ T cells in antimycobacterial immunity.
Previous studies in cattle reported that depletion of WC1+ T cells following administration of CC15 MAb resulted in an enhanced IgM response to a nonreplicating antigen, human red blood cells, and an enhanced local IgM and IgA antibody response following an acute respiratory infection with respiratory syncytial virus (22, 63). These observations indicated that in vivo depletion of WC1+ T cells is feasible and that biological effects are evident as a consequence. In the present experiment, the ability of MAb CC15 to deplete WC1+ γδ T cells from the peripheral circulation and lower respiratory tract in calves was confirmed. As expected from previous studies, WC1+ γδ T-cell depletion by MAb cannot be permanent. However, the return of the WC1+ cells to the peripheral circulation did not commence for approximately 3 weeks. The expected return of the WC1+ cells influenced the protocol used. An intranasal inoculum containing greater numbers of M. bovis than had been used in recent experiments (32, 51) was chosen to ensure development of immune responses and pathological changes within a 5-week period. In order to mimic a more natural, slowly progressing, infection, it would be necessary to decrease the inoculation dose significantly and possibly to choose another route for M. bovis delivery. However, under such experimental conditions, a number of animals fail to become infected and those which are successfully infected take longer to mount immune responses (8, 44). Therefore, depletion of the WC1+ population cannot be maintained long enough to make experiments with a low challenge feasible, as continued exposure to mouse MAb produces a bovine anti-mouse antibody response, leading to anaphylaxis (22).
The present study confirmed that, following infection with M. bovis, a response was evident within the γδ T-cell population in vivo. The numbers of circulating γδ T cells decreased within 5 days of the time of inoculation, as has been reported previously (9, 10, 51). This may be a result of localization to the initial site of infection. Additionally, it was observed that circulating γδ T cells became activated, as evidenced from a fivefold upregulation of IL-2R expression in vivo following infection with M. bovis. Upregulation of IL-2R expression was most pronounced for the γδ T cells, although expression of IL-2R on CD4+ and CD8+ T cells was also upregulated postinfection, indicating in vivo activation of these populations as well as the γδ T cells. Peak activation of the CD8+ T cells and activation of the γδ T-cell population occurred in advance of CD4+ T-cell activation. This indicates that the concept that CD8+ T cells are predominantly involved in later-stage antimycobacterial immune responses (1, 48, 51) may require reevaluation. The presence of large numbers of activated CD8+ T cells in the lungs of mice during the early stages of M. tuberculosis infection has been described (55), and these cells may have an important early role in the developing immune response. The observation that the WC1+ cells become activated in vivo before the CD4+ cells indicates that this is not simply a consequence of secondary responses to cytokine production by the MHC-II-restricted population.
All the animals in the present experiment developed an antigen-specific proliferative response 19 to 21 days postinfection. However, a second peak of lymphocyte proliferative activity was seen in the calves not depleted of WC1+ cells. This was not so apparent in the WC1+-depleted calves, possibly indicating a diminished cell-mediated immune response in these animals.
The results of the present experiment provide evidence for a reduction in the levels of IFN-γ produced in vitro, immediately following depletion of WC1+ γδ T cells. The immunological pathways leading to this observation are currently unknown but may involve innate or nonspecific mechanisms. Later, the two groups of calves produced similar levels of the proinflammatory cytokines IFN-γ and IL-2. Furthermore, IFN-γ production was evident slightly in advance of the proliferative response, a finding that is consistent with previous studies using this model (9). There have, to date, been several reports investigating the production of IFN-γ by WC1+ γδ T cells. There appears to be general agreement that mitogen stimulation of WC1+ cells from PBMC induces little, if any, synthesis of IFN-γ (2, 12), However, it has been reported that WC1+ cells can synthesize IFN-γ after stimulation by autologous irradiated monocytes (2). The synthesis of IFN-γ by WC1+ cells from lymph nodes has also been observed (59), which suggests that different functional subsets of the WC1+ cells population may exist. If so, the distribution of these cells might change post infection. Furthermore, synthesis of IFN-γ occurs in WC1+ cell lines (13). Thus, it is apparent that, given the appropriate stimulus, WC1+ γδ T cells are able to synthesize IFN-γ. Synthesis of proinflammatory cytokines by the WC1+ cells early in infection as part of an innate response would have important functional consequences, by affecting the subsequent immune response and promoting a Th1 bias (30).
There has been increasing evidence from mouse models that γδ T cells have a role in host protection from bacterial infections. For example, mice depleted of γδ T cells by MAb infusions have been found to be more susceptible to Listeria monocytogenes, although the disparate roles of γδ T-cell subpopulations remain to be clarified (42, 47). Consistent with the findings of the present study, it has been postulated that γδ T cells provide a link between innate and acquired immune responses to pathogens (38), and there is evidence that murine responses to Salmonella are modulated through γδ T-cell cytokine release (41).
The molecular basis for activation of the WC1+ T cells is not well defined. The WC1 molecule itself may affect signal transduction (25, 62). Although these cells will proliferate in response to IL-2 alone, stimulation is enhanced by a second signal (11, 12). There is evidence that the WC1 molecule is the product of a large family of genes (36, 65), and the different gene products may provide an element of differential functionality in the response of this population. An obvious need therefore exists for the characterization of WC1+-specific target antigens in bovine tuberculosis.
Of particular note within the present experiment is the observation that, later in the infection, the number of calves producing detectable IL-4 and the level of that production were markedly higher in the group depleted of WC1+ cells than in the group not depleted of these cells. This increased production of IL-4 in the WC1+-depleted group may account for the lack of a second-phase lymphocyte proliferative response observed in these calves. As infection with M. bovis progresses there is an association between increased pathology and an increasing antibody response (45). Such an association has also been reported for tuberculosis in humans (23). Interestingly, clear differences were noted in the humoral responses between calves depleted and those not depleted of WC1+ cells. This was particularly evident with respect to IgG2 antibody. Half of the animals not depleted of WC1+ cells produced M. bovis-specific IgG2 antibody, whereas none of the calves depleted of WC1+ cells did so. Production of the IgG2 isotype in cattle has been associated with Th1 cytokines (19, 20), and the increased levels of IL-4 evident in WC1+-depleted animals compared to WC1+-nondepleted calves may have inhibited class switching and synthesis of IgG2 antibody.
It has been widely accepted that a clear division exists between murine Th1 and Th2 responses, leading to domination by either cell-mediated immunity or humoral responses following exposure to a given antigen or pathogen (39). The same Th1/Th2 paradigm was, until recently, also generally accepted to exist in humans (53). However, such a concept may be an oversimplification of more complex immunoregulatory networks (24). It has been demonstrated recently that, in humans and cattle, there is a gradual increase in both Th1 (IFN-γ and IL-2) and Th2 (IL-4 and IL-5) cytokine expression with the progression of tuberculosis (52, 58). Others have also reported this lack of a clear-cut Th1 versus Th2 dichotomy in M. tuberculosis infection of humans (5). Recent evidence has indicated that, in cattle, the T-cell response to a given antigen or pathogen is heterogeneous although a predominant Th1 or Th2 response can, and does, occur (7). For example, it has been shown that stimulation of (PPD-B specific) bovine Th clones from cattle with no known exposure to M. bovis or M. tuberculosis can induce a Th1 cytokine profile in 75% of all clones (6).
In conclusion, although gross postmortem and histopathological examinations indicated no major differences between calves depleted of WC1+ cells and calves not depleted of these cells, depletion of WC1+ γδ T cells resulted in differences evident in the immune response during the course of the infection. The study provides evidence that WC1+ γδ T cells play a role in directing the initial cellular response to M. bovis following infection of the natural host. The essential observations were that the WC1+ cells are activated after infection in advance of the CD4+ T cells, and depletion immediately results in less IFN-γ production, a diminished proliferative response later, increased IL-4, and reduced levels of IgG2 antibody. Taken together, these suggest that production of IFN-γ early by WC1+ cells, possibly as part of the innate immune response, promotes lymphocyte activation and influences the cytokine bias by contributing to proinflammatory cytokine production. This could comprise part of a process that results in the Th1 bias associated with an IgG2 antibody response. Another possibility is that WC1+ γδ T cells may influence the innate production of IFN-γ by a second population, such as NK cells. By implication, these results therefore also suggest that the role of γδ T cells in mycobacterial infections is not limited to the containment of disease through granuloma formation but may have a more general effect on immune responses, particularly through their contribution to the proinflammatory cytokine pool.
Acknowledgments
This work was supported by a grant from the Biotechnology and Biological Sciences Research Council (grant 81/509909; BBSRC, Swindon, United Kingdom) and by the Department of Agriculture and Rural Development for Northern Ireland (DARDNI).
We thank David Kilpatrick for statistical analyses, Dermot Mackie for assistance, Martyn Girvin for the preparation of figures, and Sara Duggan for help with the cytokine assays.
REFERENCES
- 1.Ainslie, G. M., J. A. Solomon, and E. D. Bateman. 1992. Lymphocyte and lymphocyte subset numbers in blood and bronchoalveolar lavage and pleural fluid in various forms of human pulmonary tuberculosis at presentation and during recovery. Thorax 47:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin, C. L., T. Sathiyaseelan, M. Rocchi, and D. McKeever. 2000. Rapid changes occur in the percentage of circulating bovine WC1+ γδ Th1 cells. Res. Vet. Sci. 69:175-180. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, P. F., S. Z. Lu, J. S. Abrams, E. Wang, M. Yamamura, and R. L. Modlin. 1993. Cytokine production at the site of disease in human tuberculosis. Infect. Immun. 61:3482-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boom, W. H. 1996. The role of T-cell subsets in Mycobacterium tuberculosis infection. Infect. Agents Dis. 5:73-81. [PubMed] [Google Scholar]
- 5.Boom, W. H. 1999. Gamma delta T cells and Mycobacterium tuberculosis. Microbes Infect. 1:187-195. [DOI] [PubMed] [Google Scholar]
- 6.Brown, W. C., and D. M. Estes. 1997. Type 1 and type 2 responses in cattle and their regulation, p. 15-33. In M. Horzinek and V. E. C. J. Schijns (ed.), Cytokines in veterinary medicine. CAB International, Wallingford, United Kingdom.
- 7.Brown, W. C., A. C. RiceFicht, and D. M. Estes. 1998. Bovine type 1 and type 2 responses. Vet. Immunol. Immunopathol. 63:45-55. [DOI] [PubMed] [Google Scholar]
- 8.Buddle, B. M., D. Keen, A. Thomson, G. Jowett, A. R. McCarthy, J. Heslop, G. W. Delisle, J. L. Stanford, and F. E. Aldwell. 1995. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res. Vet. Sci. 59:10-16. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy, J. P., D. G. Bryson, J. M. Pollock, R. T. Evans, F. Forster, and S. D. Neill. 1998. Early lesion formation in cattle experimentally infected with Mycobacterium bovis. J. Comp. Pathol. 119:27-44. [DOI] [PubMed] [Google Scholar]
- 10.Cassidy, J. P., D. G. Bryson, M. M. Gutiérrez Cancela, F. Forster, J. M. Pollock, and S. D. Neill. 2001. Lymphocyte subtypes in experimentally induced early-stage bovine tuberculosis lesions. J. Comp. Pathol. 124:. 46-51. [DOI] [PubMed]
- 11.Clevers, H., N. D. MacHugh, A. Bensaid, S. Dunlap, C. L. Baldwin, A. Kaushal, K. Iams, C. J. Howard, and W. I. Morrison. 1990. Identification of a bovine surface antigen uniquely expressed on CD4− CD8− T-cell receptor γδ T lymphocytes. Eur. J. Immunol. 20:809-817. [DOI] [PubMed] [Google Scholar]
- 12.Collins, R. A., P. Sopp, K. I. Gelder, K. Griffith, W. I. Morrison, and C. J. Howard. 1996. Bovine γδ TcR+ T lymphocytes are stimulated to proliferate by autologous Theileria annulata-infected cells in the presence of interleukin-2. Scand. J. Immunol. 44:444-452. [DOI] [PubMed] [Google Scholar]
- 13.Collins, R. A., D. Werling, S. E. Duggan, A. P. Bland, K. R. Parsons, and C. J. Howard. 1998. γδ T cells present antigen to CD4+ αβ T cells. J. Leukoc. Biol. 63:707-714. [DOI] [PubMed] [Google Scholar]
- 14.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon-gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosivi, O., J. M. Grange, C. J. Daborn, M. C. Raviglione, T. Fujikura, D. Cousins, R. A. Robinson, H. F. A. K. Huchzermeyer, I. DeKantor, and F. X. Meslin. 1998. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg. Infect. Dis. 4:59-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dieli, F., M. Troye-Blomberg, J. Ivanyi, J. J. Fournie, M. Bonneville, M. A. Peyrat, G. Sireci, and A. Salerno. 2000. Vγ9/Vδ2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Eur. J. Immunol. 30:1512-1519. [DOI] [PubMed] [Google Scholar]
- 17.D'Souza, C. D., A. M. Cooper, A. A. Frank, R. J. Mazzaccaro, B. R. Bloom, and I. M. Orme. 1997. An anti-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J. Immunol. 158:1217-1221. [PubMed] [Google Scholar]
- 18.Emery, D. L., J. H. Dufty, and P. R. Wood. 1988. An analysis of cellular proliferation, and synthesis of lymphokines and specific antibody in vitro by leukocytes from immunised cattle. Vet. Immunol. Immunopathol. 18:67-80. [DOI] [PubMed] [Google Scholar]
- 19.Estes, D. M., N. M. Closser, and G. K. Allen. 1994. IFN-gamma stimulates IgG2 production from bovine B-cells costimulated with anti-mu and mitogen. Cell. Immunol. 154:287-295. [DOI] [PubMed] [Google Scholar]
- 20.Estes, D. M., W. Tuo, W. C. Brown, and J. Goin. 1998. Effects of type I and type II interferons and transforming growth factor-beta on B-cell differentiation and proliferation. Definition of costimulation and cytokine requirements for immunoglobulin synthesis and expression. Immunology 95:604-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hein, W. R., and C. R. Mackay. 1991. Prominence of gamma delta T cells in the ruminant immune-system. Immunol. Today 12:30-34. [DOI] [PubMed] [Google Scholar]
- 22.Howard, C. J., P. Sopp, K. R. Parsons, and J. Finch. 1989. In vivo depletion of BoT4 (CD4) and of non-T4/T8 lymphocyte subsets in cattle with monoclonal antibodies. Eur. J. Immunol. 19:757-764. [DOI] [PubMed] [Google Scholar]
- 23.Hussain, R., H. Shiratsuchi, J. J. Ellner, and R. S. Wallis. 2000. PPD-specific IgG1 antibody subclass upregulates tumour necrosis factor expression in PPD-stimulated monocytes: possible link with disease pathogenesis in tuberculosis. Clin. Exp. Immunol. 119:449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelso, A. 1995. Th1 and Th2 subsets: paradigms lost. Immunol. Today 16:374-379. [DOI] [PubMed] [Google Scholar]
- 25.Kirkham, P. A., H. H. Takamatsu, and R. M. E. Parkhouse. 1997. Growth arrest of γδ T cells induced by monoclonal antibody against WC1 correlates with activation of multiple tyrosine phosphatases and dephosphorylation of MAP kinase erk2. Eur. J. Immunol. 27:717-725. [DOI] [PubMed] [Google Scholar]
- 26.Kuhnle, G., R. A. Collins, J. E. Scott, and G. M. Keil. 1996. Bovine interleukins 2 and 4 expressed in recombinant bovine herpesvirus are biologically active secreted glycoproteins. J. Gen. Virol. 77:2231-2240. [DOI] [PubMed] [Google Scholar]
- 27.Ladel, C. H., C. Blum, A. Dreher, K. Reifenberg, and S. H. E. Kaufmann. 1995. Protective role of gamma/delta T cells and alpha/beta T cells in tuberculosis. Eur. J. Immunol. 25:2877-2881. [DOI] [PubMed] [Google Scholar]
- 28.Ladel, C. H., S. Daugelat, and S. H. E. Kaufmann. 1995. Immune response to Mycobacterium bovis Bacille Calmette-Guerin infection in major histocompatibility complex class I-deficient and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur. J. Immunol. 25:377-384. [DOI] [PubMed] [Google Scholar]
- 29.Ladel, C. H., J. Hess, S. Daugelat, P. Mombaerts, S. Tonegawa, and S. H. E. Kaufmann. 1995. Contribution of alpha/beta and gamma/delta T lymphocytes to immunity against Mycobacterium bovis Bacillus Calmette Guérin: studies with T cell receptor-deficient mutant mice. Eur. J. Immunol. 25:838-846. [DOI] [PubMed] [Google Scholar]
- 30.Ladel, C. H., C. Blum, and S. H. E. Kaufmann. 1996. Control of natural killer cell-mediated innate resistance against the intracellular pathogen Listeria monocytogenes by gamma/delta T lymphocytes. Infect. Immun. 64:1744-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalvani, T., R. Brookes, R. J. Wilkinson, A. S. Malin, A. A. Pathan, P. Andersen, H. Dockrell, G. Pasvol, and A. V. S. Hill. 1998. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 95:270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liébana, E., R. M. Girvin, M. Welsh, S. D. Neill, and J. M. Pollock. 1999. Generation of CD8+ T-cell responses to Mycobacterium bovis and mycobacterial antigen in experimental bovine tuberculosis. Infect. Immun. 67:1034-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liébana, E., A. Aranaz, F. E. Aldwell, J. McNair, S. D. Neill, A. J. Smyth, and J. M. Pollock. 2000. Cellular interactions in bovine tuberculosis: release of active mycobacteria from infected macrophages by antigen-stimulated T cells. Immunology 99:23-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lightbody, K. A., R. A. Skuce, S. D. Neill, and J. M. Pollock. 1998. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet. Rec. 142:295-300. [DOI] [PubMed] [Google Scholar]
- 35.MacHugh, N. D., A. Bensaid, C. J. Howard, W. C. Davis, and W. I. Morrison. 1991. Analysis of the reactivity of anti-bovine CD8 monoclonal antibodies with cloned T-cell lines and mouse L-cells transfected with bovine CD8. Vet. Immunol. Immunopathol. 27:169-172. [DOI] [PubMed] [Google Scholar]
- 36.MacHugh, N. D., P. L. J. Wijngaard, H. C. Clevers, and W. C. Davis. 1993. Clustering of monoclonal antibodies recognising different members of the WC1 gene family. Vet. Immunol. Immunopathol. 39:155-160. [DOI] [PubMed] [Google Scholar]
- 37.MacHugh, N. D., J. K. Mburu, M. J. Carrol, C. R. Wyatt, J. A. Orden, and W. C. Davis. 1997. Identification of two distinct subsets of bovine gamma delta T cells with unique cell surface phenotype and tissue distribution. Immunology 92:340-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mak, T. W., and D. A. Ferrick. 1991. The γδ T-cell bridge: linking innate and acquired immunity. Nat. Med. 4:764-765. [DOI] [PubMed] [Google Scholar]
- 39.Mossman, T. R., and R. L. Coffman. 1989. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:45-173. [DOI] [PubMed] [Google Scholar]
- 40.Muller, I., S. P. Cobbold, H. Waldmann, and S. H. E. Kaufmann. 1987. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T-cells. Infect. Immun. 55:2037-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naiki, Y., H. Nishimura, S. Itohara, and Y. Yoshikai. 2000. γδ T cells may dichotomously modulate infection with avirulent Salmonella choleraesuis via IFNγ and IL-13 in mice. Cell. Immunol. 202:61-69. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura, T., G. Matsuzaki, and K. Nomoto. 1999. The protective role of T cell receptor Vγ1+ T cells in primary infection with Listeria monocytogenes. Immunology 96:29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neill, S. D., J. Hanna, J. J. O'Brien, and R. M. McCracken. 1988. Excretion of Mycobacterium bovis by experimentally infected cattle. Vet. Rec. 123:340-343. [DOI] [PubMed] [Google Scholar]
- 44.Neill, S. D., J. J. O'Brien, and J. Hanna. 1991. A mathematical model for Mycobacterium bovis excretion from tuberculous cattle. Vet. Microbiol. 28:103-109. [DOI] [PubMed] [Google Scholar]
- 45.Neill, S. D., J. M. Pollock, D. G. Bryson, and J. Hanna. 1994. Pathogenesis of Mycobacterium bovis infection in cattle. Vet. Microbiol. 40:41-52. [DOI] [PubMed] [Google Scholar]
- 46.Newport, M. J., C. M. Huxley, S. Huston, C. M. Hawrylowicz, B. A. Oostra, R. Williamson, and M. Levin. 1996. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941-1949. [DOI] [PubMed] [Google Scholar]
- 47.O'Brien, R. L., L. Yin, S. A. Huber, K. Ikuta, and W. K. Born. 2000. Depletion of a γδ T cell subset can increase host resistance to a bacterial infection. J. Immunol. 165:6472-6479. [DOI] [PubMed] [Google Scholar]
- 48.Orme, I. M. 1991. The molecular targets of T cells in acquired immunity to tuberculosis. Immunol. Lett. 30:207-212. [DOI] [PubMed] [Google Scholar]
- 49.Orme, I. M., A. D. Roberts, J. P. Griffin, and J. S. Abrams. 1993. Cytokine secretion by CD4 T-lymphocytes acquired in response to Mycobacterium tuberculosis infection. J. Immunol. 151:518-525. [PubMed] [Google Scholar]
- 50.Pollock, J. M., A. J. Douglas, D. P. Mackie, and S. D. Neill. 1994. Identification of bovine T-cell epitopes for 3 Mycobacterium bovis antigens: MPB70, 19,000MW and MPB57. Immunology 82:9-15. [PMC free article] [PubMed] [Google Scholar]
- 51.Pollock, J. M., D. A. Pollock, D. G. Campbell, R. M. Girvin, A. D. Crockard, S. D. Neill, and D. P. Mackie. 1996. Dynamic changes in circulating and antigen-responsive T-cell subpopulations post-Mycobacterium bovis infection in cattle. Immunology 87:236-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rhodes, S. G., N. Palmer, S. P. Graham, A. E. Bianco, R. G. Hewinson, and H. M. Vordermeier. 2000. Distinct response kinetics of gamma interferon and interleukin-4 in bovine tuberculosis. Infect. Immun. 68:5393-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romagnani, S. 1994. Lymphokine production by human T-cells in disease states. Annu. Rev. Immunol. 12:227-257. [DOI] [PubMed] [Google Scholar]
- 54.Salerno, A., and F. Dieli. 1998. Role of gamma delta T lymphocytes in immune response in humans and mice. Crit. Rev. Immunol. 18:327-357. [DOI] [PubMed] [Google Scholar]
- 55.Serbina, N. V., and J. L. Flynn. 1999. Early emergence of CD8+ T cells primed for production of type 1 cytokines in the lungs of Mycobacterium tuberculosis-infected mice. Infect. Immun. 67:3980-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith, R. A., J. M. Kreeger, A. J. Alvarez, J. C. Goin, W. C. Davis, D. L. Whipple, and D. M. Estes. 1999. Role of CD8+ and WC-1+ gamma/delta T cells in resistance to Mycobacterium bovis infection in the SCID-bo mouse. J. Leukoc. Biol. 65:28-34. [DOI] [PubMed] [Google Scholar]
- 57.Smyth, A. J., M. D. Welsh, R. M. Girvin, and J. M. Pollock. 2001. In vitro responsiveness of γδ T-cells from Mycobacterium bovis-infected cattle to mycobacterial antigens: predominant involvement of WC1+ cells. Infect. Immun. 69:89-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Somoskovi, A., G. Zissel, P. F. Zipfel, M. W. Ziegenhagen, J. Klaucke, H. Haas, M. Schlaak, and J. Muller-Quernheim. 1999. Different cytokine patterns correlate with the extension of disease in pulmonary tuberculosis. Eur. Cytokine Netw. 10:135-141. [PubMed] [Google Scholar]
- 59.Sopp, P., and C. J. Howard. 2001. IFN gamma and IL-4 production by CD4, CD8, and WC1 gamma delta TCR+ T cells from cattle lymph nodes and blood. Vet. Immunol. Immunopathol. 81:85-96. [DOI] [PubMed] [Google Scholar]
- 60.Sopp, P., L. S. Kwong, and C. J. Howard. 1996. Identification of bovine CD14. Vet. Immunol. Immunopathol. 52:323-328. [DOI] [PubMed] [Google Scholar]
- 61.Stenger, S., D. A Hanson, R. Teitelbaum, P. Dewan, K. R. Niazi, C. J. Froelich, T. Ganz, S. ThomaUszynski, A. Melian, C. Bogdan, S. A. Porcelli, B. R. Bloom, A. M. Krensky, and R. L. Modlin. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282:121-125. [DOI] [PubMed] [Google Scholar]
- 62.Takamatsu, H. H., P. A. Kirkham, R. Michael, and E. Parkhouse. 1997. A gamma delta T cell specific surface receptor (WC1) signalling G0/G1 cell cycle arrest. Eur. J. Immunol. 27:105-110. [DOI] [PubMed] [Google Scholar]
- 63.Taylor, G., L. H. Thomas, S. G. Wyld, J. Furze, P. Sopp, and C. J. Howard. 1995. Role of T-lymphocyte subsets in recovery from respiratory syncytial virus-infection in calves. J. Virol. 69:6658-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thoen, C. O., and R. Chodini. 1993. Mycobacterium, p. 44-56. In C. L. Gyles, and C. O. Thoen (ed.), Pathogenesis of bacterial infections in animals. Iowa State University Press, Ames.
- 65.Wijngaard, P. L. J., M. J. Metzelaar, N. D. MacHugh, W. I. Morrison, and H. C. Clevers. 1992. Molecular characterisation of the WC1 antigen expressed specifically on bovine CD4− CD8− gamma-delta T-lymphocytes. J. Immunol. 149:3273-3277. [PubMed] [Google Scholar]
- 66.Wijngaard, P. L. J., N. D. MacHugh, M. J. Metzelaar, S. Romberg, A. Bensaid, L. Pepin, W. C. Davis, and H. C. Clevers. 1994. Members of the novel WC1 gene family are differentially expressed on subsets of bovine CD4− CD8− gamma delta T lymphocytes. J. Immunol. 152:3476-3482. [PubMed] [Google Scholar]
- 67.Williams, D. J. L., J. Newson, and J. Naessens. 1990. Quantitation of bovine immunoglobulin isotypes and allotypes using monoclonal antibodies. Vet. Immunol. Immunopathol. 24:267-283. [DOI] [PubMed] [Google Scholar]
- 68.Wyatt, C. R., C. Madruga, C. Cluff, S. Parish, M. J. Hamilton, W. Goff, and W. C. Davis. 1994. Differential distribution of gamma-delta-T-cell receptor lymphocyte subpopulations in blood and spleen of young and adult cattle. Vet. Immunol. Immunopathol. 40:187-199. [DOI] [PubMed] [Google Scholar]