Abstract
The mature MALP-404 surface lipoprotein of Mycoplasma fermentans comprises a membrane-anchored N-terminal lipid-modified region responsible for macrophage activation (P. F. Mühlradt, M. Kieβ, H. Meyer, R. Süβmuth, and G. Jung, J. Exp. Med. 185:1951-1958, 1997) and an external hydrophilic region that contains the selective lipoprotein-associated (SLA) motif defining a family of lipoproteins from diverse but selective prokaryotes, including mycoplasmas (M. J. Calcutt, M. F. Kim, A. B. Karpas, P. F. Mühlradt, and K. S. Wise, Infect. Immun. 67:760-771, 1999). This family generally corresponds to a computationally defined group of orthologs containing the basic membrane protein (BMP) domain. Two discrete lipid-modified forms of the abundant MALP product which vary dramatically in ratio among isolates of M. fermentans occur on the mycoplasma surface: (i) MALP-404, the full-length mature product, and (ii) MALP-2, the Toll-like receptor 2-mediated macrophage-activating lipopeptide containing the N-terminal 14 residues of the mature lipoprotein. The role of posttranslational processing in the biogenesis of MALP-2 from the prototype MALP-404 SLA-containing lipoprotein was investigated. Detergent phase fractionation of cell-bound products and N-terminal sequencing of a newly discovered released fragment (RF) demonstrated that MALP-404 was subject to site-specific proteolysis between residues 14 and 15 of the mature lipoprotein, resulting in the cell-bound MALP-2 and soluble RF products. This previously unknown mechanism of posttranslational processing among mycoplasmas suggests that specific cleavage of some surface proteins may confer efficient “secretion” of extracellular products by these organisms, with concurrent changes in the surface phenotype. This newly identified form of variation may have significant implications for host adaptation by mycoplasmas, as well as other pathogens expressing lipoproteins of the SLA (BMP) family.
Mycoplasmas are small, wall-less bacteria of gram-positive lineage that rely on diverse interactions with their hosts to maintain a chronic and parasitic lifestyle. Their limited genomic capacity and the paucity of recognizable orthologs for regulatory proteins (4, 9, 12, 18) suggests that other means have evolved by which these organisms adapt to and survive in changing host environments. Perhaps as an alternative to classic gene regulation, mycoplasmas are now known to utilize a broad array of mechanisms to generate phenotypic and genetic variation in propagating populations. Variation of mycoplasma surface proteins in particular is thought to support adaptive survival strategies of these organisms within the host and to mediate various aspects of pathogenesis, as recently reviewed (40). Whereas several systems affecting variation of membrane-bound surface proteins have been studied, less is known about the elaboration and possibly variable production of other extracellular mycoplasmal components, analogous to the diverse, secreted virulence factors released by many pathogenic gram-positive bacteria (1, 10, 28).
Lacking an external cell wall, mycoplasmas rely on surface lipoproteins for many direct interactions with the host environment. Several of these are highly abundant and are subject to surface variation resulting from mutational mechanisms affecting the primary gene that encodes the product (6, 13, 14, 27, 47, 50, 56, 57). This class of protein is becoming increasingly important in the biology and pathogenesis of mycoplasmas, in regard to both specific functions and the broader “modulin” activities associated with the lipid moieties of these products (5, 16, 26, 32, 39). Structure-function relationships of the anchored lipid moieties (31, 48), as well as the diverse roles attributed to external hydrophilic regions of these proteins, are of considerable interest. Recently, we investigated a prominent surface lipoprotein of Mycoplasma fermentans that occurs in alternative forms, apparently as a result of posttranscriptional (and probably posttranslational) processing (3). That study showed that the single-copy malp gene of M. fermentans contains one open reading frame encoding the sequences of two lipid-modified, membrane-anchored protein products that are demonstrable on the organism's surface. The smaller product, MALP-2, is a 2-kDa, 14-amino-acid lipopeptide with potent immunomodulatory activity mediated via Toll-like receptor 2 (26, 31, 32, 48). MALP-2 corresponds to the N-terminal region of the mature, full-length MALP-404 lipoprotein that was earlier shown to be expressed on the surface as an abundant membrane protein of 41 kDa (53). Interestingly, different strains of M. fermentans express markedly different ratios of these two products, despite the consistent presence of full-length transcripts of the malp gene (3).
An additional feature of interest is the presence of a motif (SLA, for selective lipoprotein associated) within the MALP-404 product (3). The SLA motif occurs in putative or characterized lipoproteins of diverse microbial species, including multiple genera of gram-positive and, to a lesser extent, gram-negative eubacteria (3, 41). Recently, this group of proteins has been shown to coincide with a family of orthologous proteins containing the computationally derived basic membrane protein (BMP) domain (http://www.sanger.ac.uk/cgi-bin/Pfam/getacc?PF02608). The predominant association of the motif with surface lipoproteins and its irregular occurrence in diverse bacteria suggest a possible common function. In addition, studies (15, 29) attributing possible host modulatory functions to the external hydrophilic (versus lipid-modified) portion of MALP-404 raise further interest in this class of protein. Because the SLA motif and these purported functions are absent from the shorter MALP-2 product, the possibility also arises that a distinctive and variable functional phenotype may occur at the mycoplasma surface in association with this altered lipoprotein. An important, untested corollary is that a functional molecule might also be released from cells as a result of MALP-404 processing.
In this study, the biogenesis of MALP-2 was explored, first to help distinguish among possible processing pathways previously speculated to generate these products (3) and, second, to understand possibly novel and general aspects of surface protein processing in mycoplasmas and, more generally, of SLA-containing lipoproteins. In particular, the possibility that MALP-2 is derived from a posttranslational proteolytic processing step may have broader ramifications, if alternative forms of the SLA-containing product arise concurrently.
We provide evidence in this report supporting the extracellular, site-specific processing of MALP-404, yielding the alternative MALP-2 surface-bound lipopeptide as well as a stable, released fragment of MALP-404 containing the SLA motif. This defines (i) a new mechanism of “secretion” in mycoplasmas, possibly shared by other organisms expressing lipoproteins of the SLA family, and (ii) a novel means of altering the phenotype of mycoplasmas through posttranslational processing that governs both the presence of a full-length or truncated surface lipoprotein and the respective retention or release of a soluble gene product.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
M. fermentans PG18 (clone 39) was grown in modified Hayflick medium containing 20% heat-inactivated horse serum (Gibco BRL, Grand Island, N.Y.) as described previously (3, 53). M. fermentans strain II-29/1 (31) was obtained from Peter Mühlradt, and early-passage (passage 3 to 5) cultures, propagated as previously described (3, 31), were used.
Generation of antibodies.
The following specific monoclonal antibodies (MAbs), as well as specific mouse polyclonal antibodies (PAbs) to synthetic peptides, were used for analysis in this study: PAb to PEP-C, a peptide sequence near the C terminus of the MALP-404 open reading frame (3); PAb to SLA-1, generated to a synthetic peptide (CVLITDEGKIDDKSFNQSAFEALKAIN) containing the SLA-1 motif (underlined) and spanning amino acid residues 41 to 66 in the mature MALP-404 lipoprotein (3); MAb F208C2B1 to a 14-amino-acid peptide comprising the MALP-2 amino acid sequence (3); MAb 4444H7.A to an epitope mapped to a region of MALP-404 between residues 97 and 139 (3; K. Davis, M. Kim, and K. S. Wise, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr G-22, p. 326, 1999); and MAbs F202C19A and 44437E12 to epitopes in the N-terminal and C-terminal regions, respectively, of the abundant P29 surface lipoprotein of M. fermentans (49). PAbs to synthetic peptides were prepared as previously described (3). Briefly, BALB/c mice were injected intraperitoneally three times at weekly intervals with 20 to 50 μg of keyhole limpet hemocyanin-conjugated peptide emulsified in incomplete Freund adjuvant. Preimmune serum and serum samples taken at least 1 week after the last injection were used at a dilution of 1:200.
Detergent phase fractionation, gel electrophoresis, immunoblotting, and immunoprecipitation.
M. fermentans PG18 cells from a late-logarithmic-phase broth culture were washed with phosphate-buffered saline and subjected to Triton X-114 (TX-114) phase fractionation as previously described (54) using 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (Pefablock SC; Roche Diagnostics, Indianapolis, Ind.) as a proteinase inhibitor. TX-114 phase fractionation of the supernatant was performed using the same method, but without proteinase inhibitor.
Proteins were resolved by discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with either Tris-glycine buffer (25) as previously described (42) or a Tris-Tricine buffer system (46) for resolution of low-molecular-weight proteins, using a 10% resolving gel and a 4% stacking gel as previously described (3). Samples were suspended in SDS-PAGE buffer and heated at 100°C for 5 min under reducing conditions (5% [vol/vol] β-mercaptoethanol) prior to SDS-PAGE. Prestained low-molecular-mass protein markers (Gibco BRL) were run as standards. Western immunoblotting was performed by electrophoretically transferring proteins either to nitrocellulose membranes for proteins resolved by Tris-glycine SDS-PAGE (42) or to polyvinylidene difluoride (PVDF) membranes for proteins resolved by Tris-Tricine SDS-PAGE. The membranes were blocked and immunostained as described elsewhere (3).
Immunoprecipitation employed hybridoma supernatant-derived MAb 4444H7.A immobilized on Sepharose 4B immunosorbent beads conjugated with affinity-purified goat antibody to mouse immunoglobulin G (ICN-Cappel, Durham, N.C.) as described previously (3). Immunoprecipitation of TX-114 phase proteins of M. fermentans (adjusted to 0.05% TX-114) was performed as previously described (3, 52). Supernatant from late-logarithmic-phase culture of M. fermentans was obtained by centrifugation at 14,000 × g for 20 min at 4°C and passed through a 0.1-μm-pore-size Millex-VV PVDF membrane filter (Millipore, Bedford, Mass.) to remove any residual cells or debris prior to TX-114 phase fractionation and/or incubation with immobilized MAb.
Amino-terminal protein sequencing.
Immunoprecipitated protein from M. fermentans PG18 culture supernatant was resolved by SDS-PAGE using Tris-glycine and electrophoretically transferred to a PVDF membrane (Sequiblot; Bio-Rad, Hercules, Calif.). The membrane was stained with Coomassie brilliant blue R-250 (0.1% [wt/vol] in 1% acetic acid-40% methanol) for 20 s, destained in 50% methanol, and dried. The prominent 39-kDa stained band was excised, and sequence analysis was performed by automated Edman degradation on an ABI model 494 Procise sequencer (PE Applied Biosystems, Inc., Foster City, Calif.). Sequence analysis was performed by Young Moo Lee and the staff at the Molecular Structure Facility at the University of California, Davis.
RESULTS
Lipid-modified malp products are cell associated.
The alternative pathways that can be envisioned for MALP-2 biogenesis (3) all require the proteolytic cleavage of a longer product to generate this 14-amino-acid-residue lipopeptide. An implied corollary is that a hydrophilic product and/or degradation products corresponding to the C-terminal portion of the MALP-04 sequence (beyond Lys14) would be produced. Depending on the compartment in which proteolysis occurs, these putative products could be generated and reside within cells or, alternatively, might be released from the cell surface by extracellular processing events. Understanding the nature and fate of the hydrophilic protein segment C-terminal to Lys14 was therefore thought likely to be particularly informative in understanding the biogenesis of MALP-2 from MALP-404.
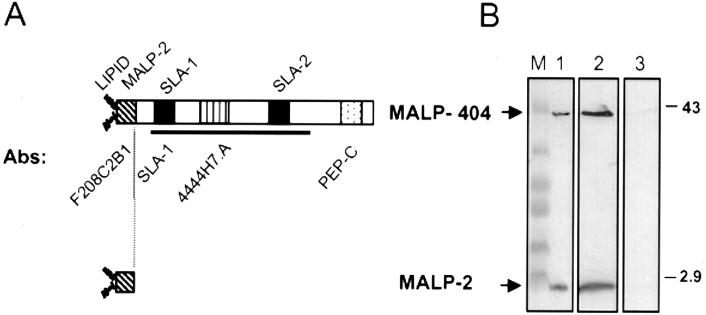
Both MALP-404 and MALP-2 (Fig. 1A) have been shown to be lipid modified (31, 53), indicating that they are membrane bound and should partition into the detergent phase during TX-114 detergent phase fractionation (54). To confirm the predicted properties of MALP-2, and to determine whether other cell-associated forms of malp-encoded gene products might occur, TX-114 phase fractionation of washed cells from a propagating culture of M. fermentans PG18 was performed. Proteins from the TX-114 and aqueous phases, as well as nonfractionated total washed cells, were resolved utilizing the Tris-Tricine gel system. The subsequent immunoblot (Fig. 1B) was developed first with MAb F208C2B1, generated to a peptide representing the MALP-2 sequence (3), and subsequently with MAb 4444H7.A to an epitope mapped to the central region of MALP-404 (Davis et al., Abstr. 99th Gen. Meet. Am. Soc. Microbiol.). These two reagents verified that MALP-404 and MALP-2 are both present in the total (Fig. 1B, lane 1) and TX-114 phase (lane 2) samples but are absent from the aqueous phase (lane 3). This result confirms the expectation that both surface products are cell associated and integrally bound in the membrane. Importantly, using the MAb 4444H7.A epitope as a marker, no other MALP-related products were found to be cell associated, either in the TX-114 phase or in the aqueous phase that contains the bulk of the proteins of this organism (53). The absence of cell-associated hydrophilic MALP products is noteworthy in that it fails to provide evidence (by this criterion) for the intracellular biogenesis of MALP-2.
FIG. 1.
Characteristics of MALP products from M. fermentans PG18. (A) Features of MALP-404 (top) and MALP-2 (bottom). The two products are shown by boxes. Above MALP-404 are shown different features of the protein, including diacylglyceryl lipid linkage of the mature protein at the +1 Cys (LIPID), the 14-amino-acid N-terminal sequence (31) of MALP-2 (diagonal hatching) and the SLA-1 and SLA-2 motifs (3, 41) (solid). Below MALP-404 are shown specific antibodies (described in Materials and Methods) recognizing the 14-amino-acid MALP-2 sequence (MAb F208C2B1), the SLA-1 region of MALP-404 (PAb SLA-1), an epitope located between residues 97 and 139 (MAb 4444H7.A; vertical hatching), and a 30-amino-acid C-terminal polypeptide sequence (PAb PEP-C; stippled). The solid line depicts the region comprising the BMP domain (http://www.sanger.ac.uk/cgi-bin/Pfam/getacc?PF02608). (B) Properties of MALP-404 and MALP-2 determined by detergent phase fractionation of whole PG18 cells. Total cellular protein (lane 1), TX-114 phase proteins (lane 2), or aqueous-phase proteins (lane 3) from a late-logarithmic-phase culture of M. fermentans PG18 were separated by Tris-Tricine SDS-PAGE, transferred to a PVDF membrane, and immunostained with MAbs F208C2B1 and 4444H7.A. Lane M, prestained protein standards. The masses of selected markers are indicated (in kilodaltons) on the right. The positions of MALP-2 and MALP-404 are indicated on the left.
A stable hydrophilic MALP-404 fragment containing the SLA motif is released from cells.
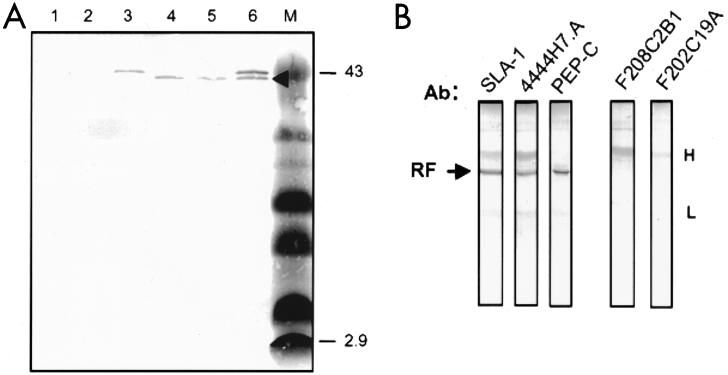
One model of MALP-2 biogenesis entails extracellular processing of MALP-404. This predicts a hydrophilic product(s) that could be released from cells and, depending on the extent of proteolytic activity, might comprise recognizable portions of the MALP-404 polypeptide. To identify such a putative product(s), the supernatant from a culture of M. fermentans PG18 was filtered to remove any remaining mycoplasma cells or cell fragments and subjected to TX-114 phase fractionation and immunoprecipitation. Filtered, unfractionated supernatant, as well as subsequently derived TX-114 and aqueous phases of the supernatant, was incubated with immobilized MAb 4444H7.A to immunoprecipitate epitope-bearing, MALP-related products that might have been released from the mycoplasma cells (Fig. 2A). A Western blot of immunoprecipitates from the whole filtered supernatant (or from the aqueous fraction of the supernatant) revealed a single band of approximately 39 kDa, apparently released by these cells (Fig. 2A, lanes 4 and 5). This product was distinctly smaller than MALP-404 immunoprecipitated from the TX-114 phase fraction of whole cells (lane 3). This size difference was formally confirmed by analysis of combined immunoprecipitates (lane 6). It is noteworthy that immunoprecipitation of aqueous-phase proteins from whole cells again revealed no epitope-bearing MALP fragments (lane 1). In control experiments, analogous immunoprecipitation of supernatant with immobilized MAb F208C2B1 (to the MALP-2 peptide) revealed no epitope-bearing products released from cells, nor did immunoprecipitation with two MAbs to the unrelated P29 surface lipoprotein (49, 53) reveal any hydrophilic fragments of that abundant product (data not shown).
FIG. 2.
Characterization of RF from M. fermentans PG18. (A) Immunoprecipitates using MAb 4444H7.A were separated by Tris-Tricine SDS-PAGE, transferred to a PVDF membrane, and stained with the same MAb. The samples include immunoprecipitates from the following: TX-114 phase of filtered mycoplasma culture supernatant (lane 1), aqueous-phase proteins of whole cells (lane 2), TX-114 phase proteins of whole cells (lane 3), filtered supernatant prior to phase fractionation (lane 4), and aqueous phase of filtered supernatant (lane 5). A mixture of immunoprecipitates from the TX-114 phase of washed cells and from the aqueous phase of filtered supernatant is shown in lane 6. RF is indicated by the arrowhead. The markers (lane M) are the same as those described in the legend to Fig. 1. (B) Distribution of epitopes on RF. Immunoprecipitated protein (arrow) was separated by Tris-glycine SDS-PAGE, transferred to nitrocellulose as for panel A, and immunostained with a series of Abs, including PAb to SLA-1, MAb 4444H7.A (used for immunoprecipitation), and PAb to PEP-C. As negative controls, the same immunoprecipitated protein was immunostained with MAb F208C2B1 to the MALP-2 peptide or an irrelevant MAb, F202C19A, to the abundant M. fermentans surface protein P29 (53). The positions of the immunoprecipitating immunoglobulin heavy (H) and light (L) chains (lightly stained by the secondary Ab reagent) are indicated on the right.
In order to further characterize the 39-kDa released fragment (termed RF), the distribution of epitopes on this product was determined (Fig. 2B). The immunoprecipitated RF protein was recognized by three of the four specific Abs to epitopes shown in Fig. 1A: SLA-1, MAb 4444H7.A, and PEP-C. MAb F208C2B1 to the MALP-2 sequence failed to bind RF, as did the irrelevant MAb F202C19A to P29. RF therefore appeared to be a full-length, stable hydrophilic protein lacking only the N-terminal portion of MALP-404.
RF is generated from MALP-404 by site-specific endoproteolytic cleavage.
The size of the RF, in combination with the presence of all but the N-terminal MALP-2 epitope, suggested that the fragment might be the result of a specific proteolytic cleavage. To examine this possibility, the immunoprecipitated RF protein from culture supernatant (example shown in Fig. 3, inset) was subjected to N-terminal sequencing. For the PG18 strain, this yielded an unambiguous sequence of 10 amino acids (DISKYTTTNA) which corresponded precisely with residues 15 through 24 of the MALP-404 sequence. This result provided strong evidence that the proteolytic event generating MALP-2 is site specific and occurs C-terminal to Lys14 in the mature lipoprotein. These data also support the hypothesis that the proteolytic event is extracellular, based on (i) the absence of RF or related hydrophilic products associated with whole cells and (ii) the lack of known precedents for mycoplasmal systems capable of secreting hydrophilic proteins.
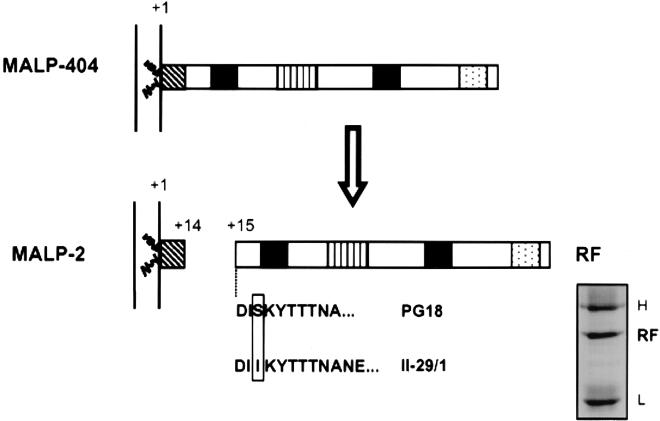
FIG. 3.
Proposed model for the generation of MALP-2 and RF. The mature MALP-404 is lipid modified and anchored on the external face of the plasma membrane. Site-specific proteolytic cleavage of the peptide bond between Lys14 and Asp15 results in two stable products, the MALP-2 lipopeptide, which remains lipid modified and membrane bound, and RF, which is released from the cell. Features of MALP-404 and MALP-2 are described in the legend to Fig. 1. Shown below RF are the experimentally determined N-terminal sequences of RF obtained from M. fermentans strain PG18 or from strain II-29/1, preferentially expressing MALP-2 (3). An allelic polymorphism at residue 17 is boxed. The inset shows immunoprecipitate of RF separated by Tris-glycine SDS-PAGE, blotted to a PVDF membrane, and stained with Coomassie blue prior to N-terminal sequencing. The gel shows RF obtained from 2 ml of late-logarithmic-phase culture from strain II-29/1. The locations of heavy (H) and light (L) chains are indicated on the right.
To confirm that the generation of RF was not confined to the PG18 strain of M. fermentans, filtered supernatant from strain II-29/1, previously shown to express predominantly the MALP-2 surface lipopeptide (3), was subjected to an identical immunoprecipitation protocol, and N-terminal sequencing of the purified protein was performed. The abundant RF derived from this isolate had the same N terminus as RF from the PG18 strain and revealed a predicted allelic polymorphism (3) at residue 17, two residues from the cleavage site (Fig. 3).
DISCUSSION
Our discovery and initial examination of an RF encoded by the single-copy malp gene of M. fermentans (i) provides an explanation for a previously noted variable surface phenotype in this species, (ii) reveals that the complete phenotype also includes the release or retention of a SLA-containing polypeptide sequence of a major lipoprotein, (iii) suggests a novel form of mycoplasmal surface variation governed by specific proteolysis of a surface lipoprotein, and (iv) raises the prospect that other members of the SLA-containing family of lipoproteins may be subject to surface processing and variation.
The diacylglyceryl-modified MALP-2 lipopeptide has been extensively characterized (31, 32, 48) and shown to be a potent activator of macrophages and monocytes, operating through Toll-like receptor 2 pattern recognition (23, 26, 30-33, 43, 45, 48). This compound also activates interleukin-6 expression in cultured murine calvaria (36). The origin of MALP-2, from a gene encoding the longer MALP-404 lipoprotein, was recently determined by our laboratory, as were striking differences in the ratio of these two surface components expressed among some strains of M. fermentans (3). Notably, other studies of this gene product have attributed additional functions to soluble (recombinant or mycoplasma-derived) forms of the protein, including Ab-independent binding of complement (29) and promotion of monocyte differentiation (15). We show here that a soluble derivative of the MALP-404 lipoprotein is naturally released in culture by M. fermentans through precise processing of the lipoprotein sequence to create an alternative phenotype that comprises the membrane-bound MALP-2 product and “secretion” of the RF exoprotein. Although the stoichiometry of components in the latter phenotype has yet to be quantified, the ready acquisition from culture medium of microgram quantities of RF per milliliter (estimated by protein-stained SDS-PAGE gels [data not shown]) suggests a highly efficient mechanism for conversion, particularly in populations known to display a predominance of MALP-2 on the surface (3; M. F. Kim, K. Davis, M. Calcutt, and K. S. Wise, Abstr. 12th Int. Congr. Int. Org. for Mycoplasmol., abstr. E19, p. 168, 1998). The stability of RF in mycoplasma broth is also noteworthy. Supernatants stored for several weeks showed no breakdown products of this component by immunoprecipitation (data not shown). Whether RF is produced, or further processed, in the human host is unknown, as is its precise function. Nevertheless, human serum Ab to MALP-404 has been noted previously (19). This product may now be more fully explored by retrieval of soluble, authentic RF product from in vitro culture.
The site-specific processing that generates RF is noteworthy from at least two aspects. First, it appears to occur through cleavage at a single peptide bond, most likely displayed on the surface of the organism. Indeed, the RF released into the supernatant could not originate by release from damaged cells, since cell-associated RF was not detected by sensitive immunoassays. Moreover, mycoplasmas are not known to contain any of the secretion apparatuses described in bacteria (2, 8, 21, 44) that are capable of transporting hydrophilic proteins such as RF, nor are orthologs of these secretory systems found in any of four mycoplasma genomes sequenced to date (4, 9, 12, 18). The most likely pathway for the biogenesis of MALP-2 and RF therefore appears to be (i) Sec-dependent translocation of the prolipoprotein of MALP-404, (ii) acylation and signal peptide cleavage characteristic of mycoplasmal lipoproteins (31, 55), and (iii) subsequent proteolytic processing of the mature MALP-404 external to the plasma membrane to create the MALP-2 and RF products.
A second critical, yet unknown, feature of MALP-404 proteolysis is the identity and specificity of the corresponding proteinase. Notable examples of surface proteinases of gram-positive bacteria include (i) signal peptidases (7, 11), (ii) the “sortase” family of transpetidases that act on diverse membrane-anchored proteins carrying a sortase-specific target sequence substrate motif (22, 35, 51), and (iii) the recently identified HtrA proteinase of Lactococcus lactis, which acts (in a less specific manner) as a housekeeping proteinase for general degradation and processing of surface proteins (38). These proteinases are predicted to be integral membrane proteins with active sites residing on the external face of the plasma membrane. Mycoplasmas may express some but not all of these activities. For example, Mycoplasma pulmonis has been predicted to have a gene encoding a signal peptidase I (4). In contrast, sortases (mediating transpeptidation to cell wall peptidoglycan) or their orthologous genes have not been found, nor are they anticipated, in the wall-less mycoplasmas. HtrA orthologs, while predicted to occur in small-genome organisms such as mycoplasmas (38), have not been reported in these organisms to date. Our finding that an apparently distinctive substrate specificity is involved in the processing of MALP-404 suggests a novel proteinase activity. Identification of a separately encoded, trans-acting surface proteinase possibly specific to the MALP-404 product will be an important subject for further investigations. Equally plausible, however, would be the autocatalytic processing of MALP-404 to yield the two proteolytic products. Interestingly, either model of surface proteolysis (trans acting or autocatalytic) would mimic one of the hallmark features of autotransporter secretion mechanisms characterized in gram-negative bacteria (2, 17). These rely on specific proteolytic cleavage, either by autocatalysis or distinct proteinase products, to release functional domains of diverse proteins from the outer membrane. Evidence of posttranslational processing in mycoplasmas has been reported in the assembly of a complex mycoplasma adhesin organelle (24, 37), the apparent processing of a ciliary adhesin (20), and the processing of a lipoprotein of unknown function (34). Our results extend the known roles of processing to the production of exoprotein and the concomitant alteration of surface antigenic phenotypes. It is likely that such processing is generally employed as an important factor in determining several aspects of the mycoplasma-host interactions.
An intriguing finding in this study is the release from MALP-404 of a fragment containing the SLA motif. Two sequence signatures, SLA-1 (3) and, subsequently, SLA-2 (41), were identified at corresponding positions within diverse lipoproteins of gram-positive and other prokaryotic genera. More recently, independent computational comparisons have shown that SLA motifs are among multiple sequence signatures defining a family of lipoproteins that contain the BMP domain (http://www.sanger.ac.uk/cgi-bin/Pfam/getacc?PF02608). Interestingly, in each of the four complete genome sequences of mycoplasmas, SLA orthologs occur as single genomic copies. These include Mycoplasma pneumoniae MPN052 (MG040 homologue) (18); Mycoplasma genitalium MG040 (9); M. pulmonis Mypu 3640 (4); and Ureaplasma urealyticum UU016 (12). Partial sequences of three additional mycoplasmas also contain orthologous genes encoding SLA lipoproteins. These include Mycoplasma capricolum and Mycoplasma hyorhinis (3) and Mycoplasma gallisepticum (P. Markham, A. Kanci, G. Czifra, B. Sundquist, and G. Browning, Abstr. 13th Int. Congr. Int. Org. Mycoplasmol., abstr. P-D08, p. 138, 2000). Comparison of the sequences flanking the cleavage site in MALP-404 with similar regions of other SLA lipoproteins from mycoplasmas and diverse prokaryotes reveals no canonical sequence signature for processing, such as that associated with the sortase substrate motif (22, 35, 51), nor has any SLA family member other than the prototype MALP protein of M. fermentans yet been shown to undergo proteolytic processing. Sequence divergence of these proteins outside the SLA and BMP signatures suggests a high level of selection for this family of products. Whether the SLA motifs or BMP domain per se represents a common processing function or some other common function associated with these surface proteins, or their released counterparts, remains to be determined.
Acknowledgments
We thank Michael Calcutt for helpful discussions regarding SLA and BMP protein families and Mary Kim for generation of critical experimental reagents.
This work was supported by DHHS grants AI32219 (K.S.W.) from the National Institute of Allergy and Infectious Diseases and T32 GM08396-10 (K.L.D.) from the National Institute of General Medical Sciences. K.L.D. was supported as a trainee of the University of Missouri—Columbia Molecular Biology Program.
REFERENCES
- 1.Alouf, J. E. 1980. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol. Ther. 11:661-717. [DOI] [PubMed] [Google Scholar]
- 2.Burns, D. L. 1999. Biochemistry of type IV secretion. Curr. Opin. Microbiol. 2:25-29. [DOI] [PubMed] [Google Scholar]
- 3.Calcutt, M. J., M. F. Kim, A. B. Karpas, P. F. Mühlradt, and K. S. Wise. 1999. Differential posttranslational processing confers intraspecies variation of a major surface lipoprotein and a macrophage-activating lipopeptide of Mycoplasma fermentans. Infect. Immun. 67:760-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambaud, I., R. Heilig, S. Ferris, V. Barbe, D. Samson, F. Galisson, I. Moszer, K. Dybvig, H. Wroblewsky, A. Viari, E. Rocha, and A. Blanchard. 2001. The complete genome sequence of the murine respiratory pathogen Mycoplasma pulmonis. Nucleic Acids Res. 29:2145-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambaud, I., H. Wroblewski, and A. Blanchard. 1999. Interactions between mycoplasma lipoproteins and the host immune system. Trends Microbiol. 7:493-499. [DOI] [PubMed] [Google Scholar]
- 6.Citti, C., and K. S. Wise. 1995. Mycoplasma hyorhinis vlp gene transcription: critical role in phase variation and expression of surface lipoproteins. Mol. Microbiol. 18:649-660. [DOI] [PubMed] [Google Scholar]
- 7.Dalbey, R., M. O. Lively, S. Bron, and J. M. van Dijl. 1997. The chemistry and enzymology of the type I signal peptidases. Protein Sci. 6:1129-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fath, M. J., and R. Kolter. 1993. ABC transporters: bacterial exporters. Microbiol. Rev. 57:995-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser, C. M., J. D. Gocayne, O. White, M. D. Adams, R. A. Clayton, R. D. Fleischmann, C. J. Bult, A. R. Kerlavage, G. Sutton, J. M. Kelley, J. L. Fritchman, J. F. Weidman, K. V. Small, M. Sandusky, J. Fuhrmann, D. Nguyen, T. R. Utterback, D. M. Saudek, C. A. Phillips, J. M. Merrick, J. F. Tomb, B. A. Dougherty, K. F. Bott, P. C. Hu, T. S. Lucier, S. N. Peterson, H. O. Smith, C. A. Hutchison III, and J. C. Venter. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270:397-403. [DOI] [PubMed] [Google Scholar]
- 10.Freudl, R. 1992. Protein secretion in Gram-positive bacteria. J. Biotechnol. 23:231-240. [DOI] [PubMed] [Google Scholar]
- 11.Geukens, N., E. Lammertyn, L. Van Mellaert, S. Schacht, K. Schaerlaekens, V. Parro, S. Bron, Y. Engelborghs, R. P. Mellado, and J. Anne. 2001. Membrane topology of the Streptomyces lividans type I signal peptidases. J. Bacteriol. 183:4752-4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass, J. I., E. J. Lefkowitz, J. S. Glass, C. R. Heiner, E. Y. Chen, and G. H. Cassell. 2000. The complete sequence of the mucosal pathogen Ureaplasma urealyticum. Nature 407:757-762. [DOI] [PubMed] [Google Scholar]
- 13.Glew, M. D., N. Baseggio, P. F. Markham, G. F. Browning, and I. D. Walker. 1998. Expression of the pMGA genes of Mycoplasma gallisepticum is controlled by variation in the GAA trinucleotide repeat lengths within the 5′ noncoding regions. Infect. Immun. 66:5833-5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groisman, E. A., and H. Ochman. 1996. Pathogenicity islands: bacterial evolution in quantum leaps. Cell 87:791-794. [DOI] [PubMed] [Google Scholar]
- 15.Hall, R. E., S. Agarwal, D. P. Kestler, J. A. Cobb, K. M. Goldstein, and N. S. Chang. 1996. cDNA and genomic cloning and expression of the P48 monocytic differentiation/activation factor, a Mycoplasma fermentans gene product. Biochem. J. 319:919-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson, B., S. Poole, and M. Wilson. 1996. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol. Rev. 60:316-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson, I. R., R. Cappello, and J. P. Nataro. 2000. Autotransporter proteins, evolution and redefining protein secretion. Trends Microbiol. 8:529-534. [DOI] [PubMed] [Google Scholar]
- 18.Himmelreich, R., H. Hilbert, H. Plagens, E. Pirkl, B.-C. Li, and R. Herrmann. 1996. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res. 24:4420-4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman, R. W., F. X. O'Sullivan, K. R. Schafermeyer, T. L. Moore, D. Roussell, R. Watson-McKown, M. F. Kim, and K. S. Wise. 1997. Mycoplasma infection and rheumatoid arthritis: analysis of their relationship using immunoblotting and an ultrasensitive polymerase chain reaction detection method. Arthritis Rheum. 40:1219-1228. [DOI] [PubMed] [Google Scholar]
- 20.Hsu, T., S. Artiushin, and F. C. Minion. 1997. Cloning and functional analysis of the P97 swine cilium adhesin gene of Mycoplasma hyopneumoniae. J. Bacteriol. 179:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janulczyk, R., and M. Rasmussen. 2001. Improved pattern for genome-based screening identifies novel cell wall-attached proteins in gram-positive bacteria. Infect. Immun. 69:4019-4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann, A., P. F. Mühlradt, D. Gemsa, and H. Sprenger. 1999. Induction of cytokines and chemokines in human monocytes by Mycoplasma fermentans-derived lipoprotein MALP-2. Infect. Immun. 67:6303-6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krause, D. C. 1998. Mycoplasma pneumoniae cytadherence: organization and assembly of the attachment organelle. Trends Microbiol. 6:15-18. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 26.Lien, E., T. J. Sellat, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 27.Lysnyansky, I., R. Rosengarten, and D. Yogev. 1996. Phenotypic switching of variable surface lipoproteins in Mycoplasma bovis involves high-frequency chromosomal rearrangements. J. Bacteriol. 178:5395-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madden, J. C., N. Ruiz, and M. Caparon. 2001. Cytolysin-mediated translocation (CMT): a functional equivalent of Type III secretion in Gram-positive bacteria. Cell 104:143-152. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto, M., M. Nishiguchi, S. Kikkawa, H. Nishimura, S. Nagasawa, and T. Seya. 1998. Structural and functional properties of complement-activating protein M161Ag, a Mycoplasma fermentans gene product that induces cytokine production by human monocytes. J. Biol. Chem. 273:12407-12414. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto, M., and T. Seya. 1999. M161Ag is a potent cytokine inducer with complement activating function. Int. J. Mol. Med. 3:291-295. [DOI] [PubMed] [Google Scholar]
- 31.Mühlradt, P. F., M. Kieβ, H. Meyer, R. Süβmuth, and G. Jung. 1997. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J. Exp. Med. 185:1951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mühlradt, P. F., H. Meyer, and R. Jansen. 1996. Identification of S-(2,3-dihydroxypropyl)cystein in a macrophage-activating lipopeptide from Mycoplasma fermentans. Biochemistry 35:7781-7786. [DOI] [PubMed] [Google Scholar]
- 33.Nishiguchi, M., M. Matsumoto, T. Takao, M. Hoshino, Y. Shimonishi, S. Tsuji, N. A. Begum, O. Takeuchi, S. Akira, K. Toyoshima, and T. Seya. 2001. Mycoplasma fermentans lipoprotein M161 Ag-induced cell activation is mediated by Toll-like receptor 2: role of N-terminal hydrophobic portion in its multiple functions. J. Immunol. 166:2610-2616. [DOI] [PubMed] [Google Scholar]
- 34.Noormohammadi, A. H., P. F. Markham, M. F. Duffy, K. G. Whithear, and G. F. Browning. 1998. Multigene families encoding the major hemagglutinins in phylogenetically distinct mycoplasmas. Infect. Immun. 66:3470-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pallen, M. J., A. C. Lam, M. Antonio, and K. Dunbar. 2001. An embarrassment of sortases—a richness of substrates? Trends Microbiol. 9:97-101. [DOI] [PubMed] [Google Scholar]
- 36.Piec, G., J. Mirkovitch, S. Palacio, P. F. Mühlradt, and R. Felix. 1999. Effect of MALP-2, a lipopeptide from Mycoplasma fermentans, on bone resorption in vitro. Infect. Immun. 67:6281-6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popham, P. L., T. W. Hahn, K. A. Krebes, and D. C. Krause. 1997. Loss of HMW1 and HMW3 in noncytadhering mutants of Mycoplasma pneumoniae occurs post-translationally. Proc. Natl. Acad. Sci. USA 94:13979-13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poquet, I., V. Saint, E. Seznec, N. Simoes, A. Bolotin, and A. Gruss. 2000. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol. Microbiol. 35:1042-1051. [DOI] [PubMed] [Google Scholar]
- 39.Rawadi, G. 2000. Mycoplasma fermentans interaction with monocytes/macrophages: molecular basis. Microbes Infect. 2:955-964. [DOI] [PubMed] [Google Scholar]
- 40.Razin, S., D. Yogev, and Y. Naot. 1998. Molecular biology and pathogenicity of Mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosati, S., S. Pozzi, P. Robino, B. Montinaro, A. Conti, M. Fadda, and M. Pittau. 1999. P48 major surface antigen of Mycoplasma agalactiae is homologous to a malp product of Mycoplasma fermentans and belongs to a selected family of bacterial lipoproteins. Infect. Immun. 67:6213-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosengarten, R., and K. S. Wise. 1991. The Vlp system of Mycoplasma hyorhinis: combinatorial expression of distinct size variant lipoproteins generating high-frequency surface antigenic variation. J. Bacteriol. 173:4782-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sacht, G., A. Märten, U. Deiters, R. Süβmuth, G. Jung, E. Wingender, and P. F. Mühlradt. 1998. Activation of nuclear factor-κB in macrophages by mycoplasmal lipopeptides. Eur. J. Immunol. 28:4207-4212. [DOI] [PubMed] [Google Scholar]
- 44.Sandkvist, M. 2001. Minireview. Type II secretion and pathogenesis. Infect. Immun. 69:3523-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato, S., F. Nomura, T. Kawai, O. Takeuchi, P. F. Mühlradt, K. Takeda, and S. Akira. 2000. Synergy and cross-tolerance between Toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J. Immunol. 165:7096-7101. [DOI] [PubMed] [Google Scholar]
- 46.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 47.Simmons, W. L., C. Zuhua, J. I. Glass, J. W. Simecka, G. H. Cassell, and H. L. Watson. 1996. Sequence analysis of the chromosomal region around and within the V-1-encoding gene of Mycoplasma pulmonis: evidence for DNA inversion as a mechanism for V-1 variation. Infect. Immun. 64:472-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeuchi, O., A. Kaufmann, K. Grote, T. Kawai, K. Hoshino, M. Morr, P. F. Mühlradt, and S. Akira. 2000. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a Toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164:554-557. [DOI] [PubMed] [Google Scholar]
- 49.Theiss, P., A. Karpas, and K. S. Wise. 1996. Antigenic topology of the P29 surface lipoprotein of Mycoplasma fermentans: differential display of epitopes results in high-frequency phase variation. Infect. Immun. 64:1800-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Theiss, P., and K. S. Wise. 1997. Localized frameshift mutation generates selective, high-frequency phase variation in a surface lipoprotein encoded by a mycoplasma ABC transporter operon. J. Bacteriol. 179:4013-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ton-That, H., and O. Schneewind. 1999. Anchor structure of staphylococcal surface proteins. J. Biol. Chem. 274:24316-24320. [DOI] [PubMed] [Google Scholar]
- 52.Wise, K. S., and M. F. Kim. 1987. Major membrane surface proteins of Mycoplasma hyopneumoniae selectively modified by covalently bound lipid. J. Bacteriol. 169:5546-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wise, K. S., M. F. Kim, P. M. Theiss, and S.-C. Lo. 1993. A family of strain-variant surface lipoproteins of Mycoplasma fermentans. Infect. Immun. 61:3327-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wise, K. S., M. F. Kim, and R. Watson-McKown. 1995. Variant membrane proteins, p. 227-241. In S. Razin and J. G. Tully (ed.), Molecular and diagnostic procedures in mycoplasmology. Academic Press, New York, N.Y.
- 55.Wu, H. C., and M. Tokunaga. 1986. Biogenesis of lipoproteins in bacteria. Curr. Top. Microbiol. Immunol. 125:127-157. [DOI] [PubMed] [Google Scholar]
- 56.Yogev, D., R. Rosengarten, R. Watson-McKown, and K. S. Wise. 1991. Molecular basis of Mycoplasma surface antigenic variation: a novel set of divergent genes undergo spontaneous mutation of periodic coding regions and 5′ regulatory sequences. EMBO J. 10:4069-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang, Q., and K. S. Wise. 1996. Molecular basis of size and antigenic variation of a Mycoplasma hominis adhesin encoded by divergent vaa genes. Infect. Immun. 64:2737-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]