Abstract
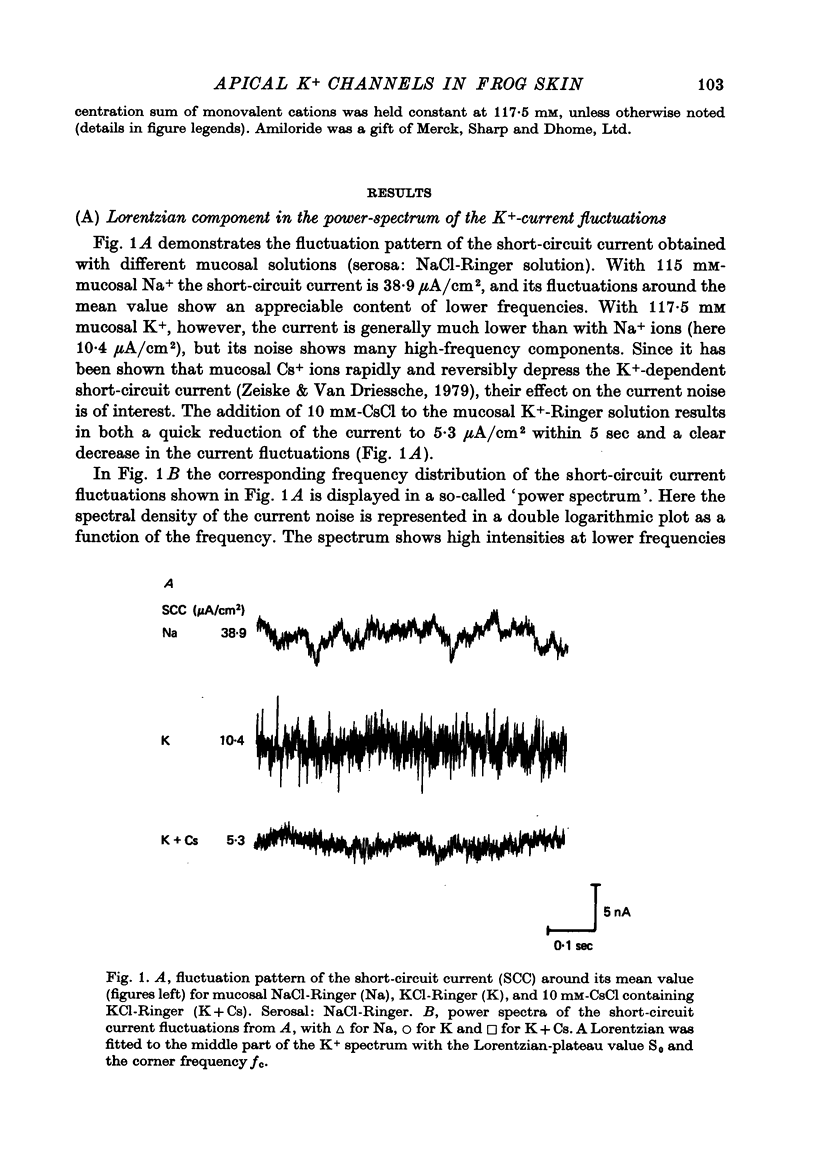
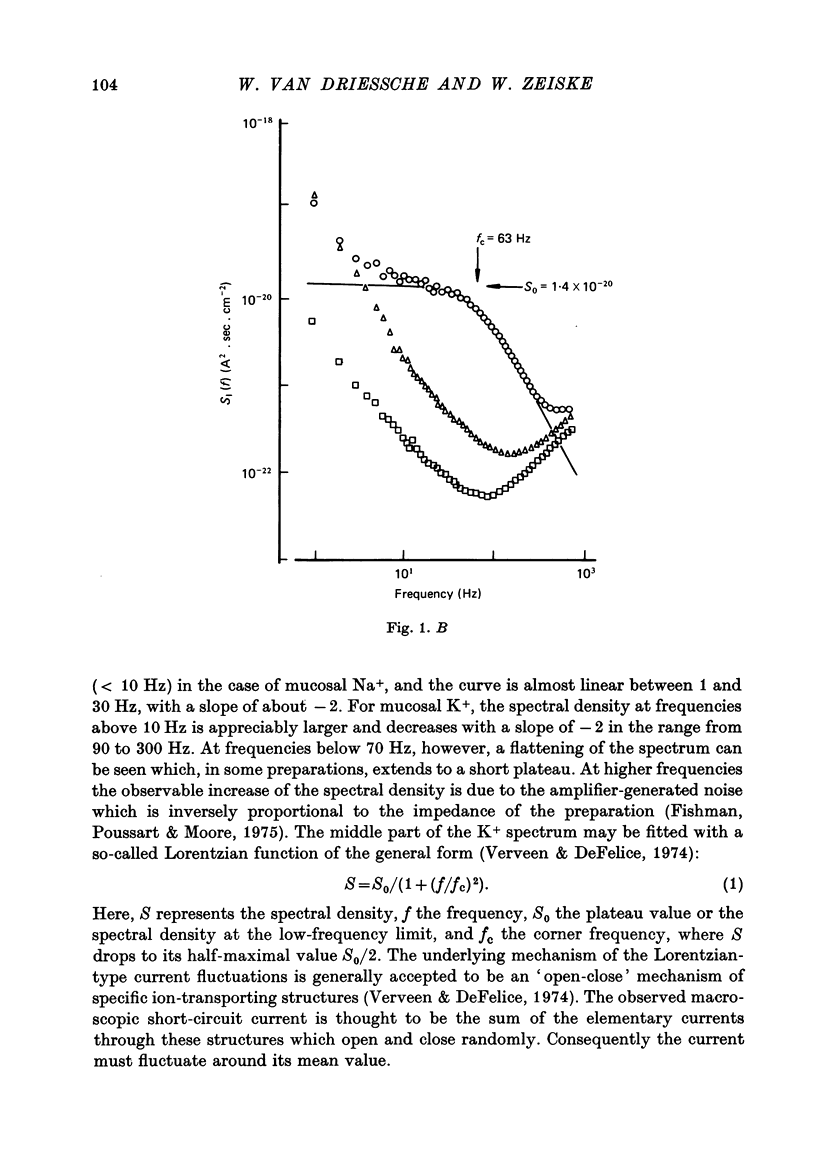
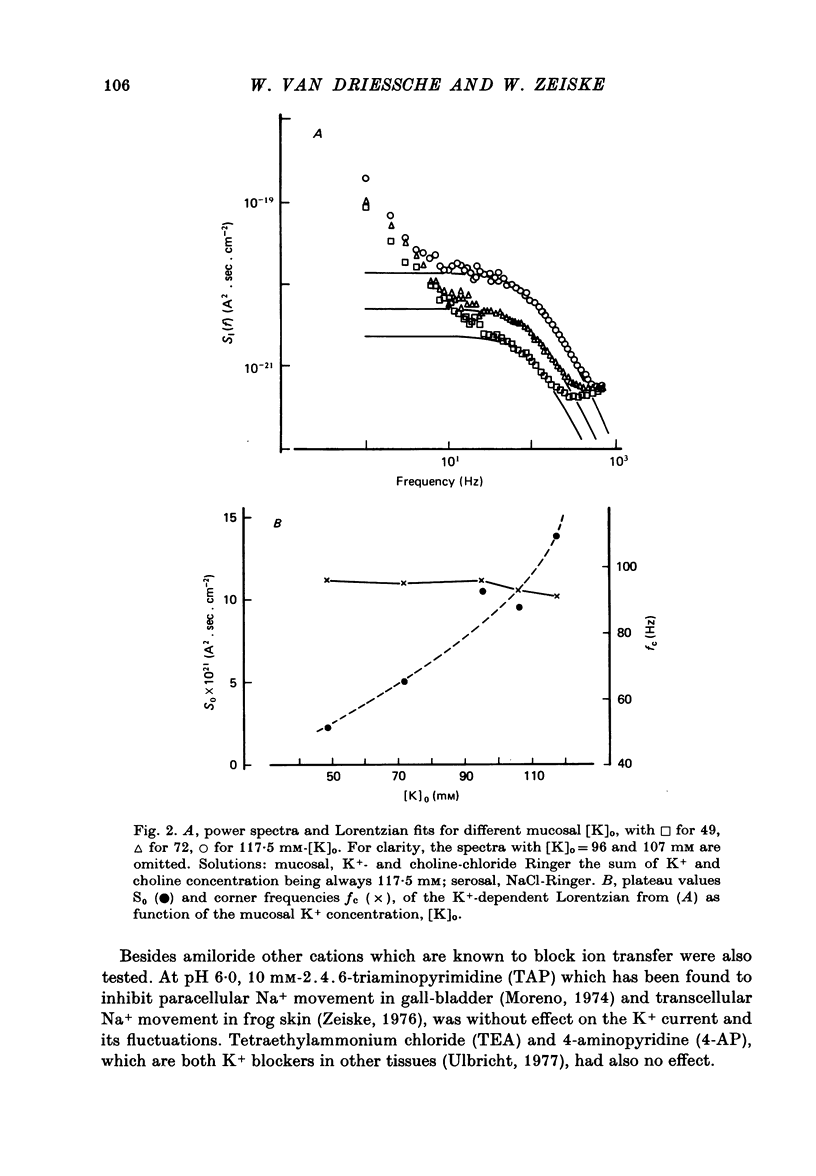
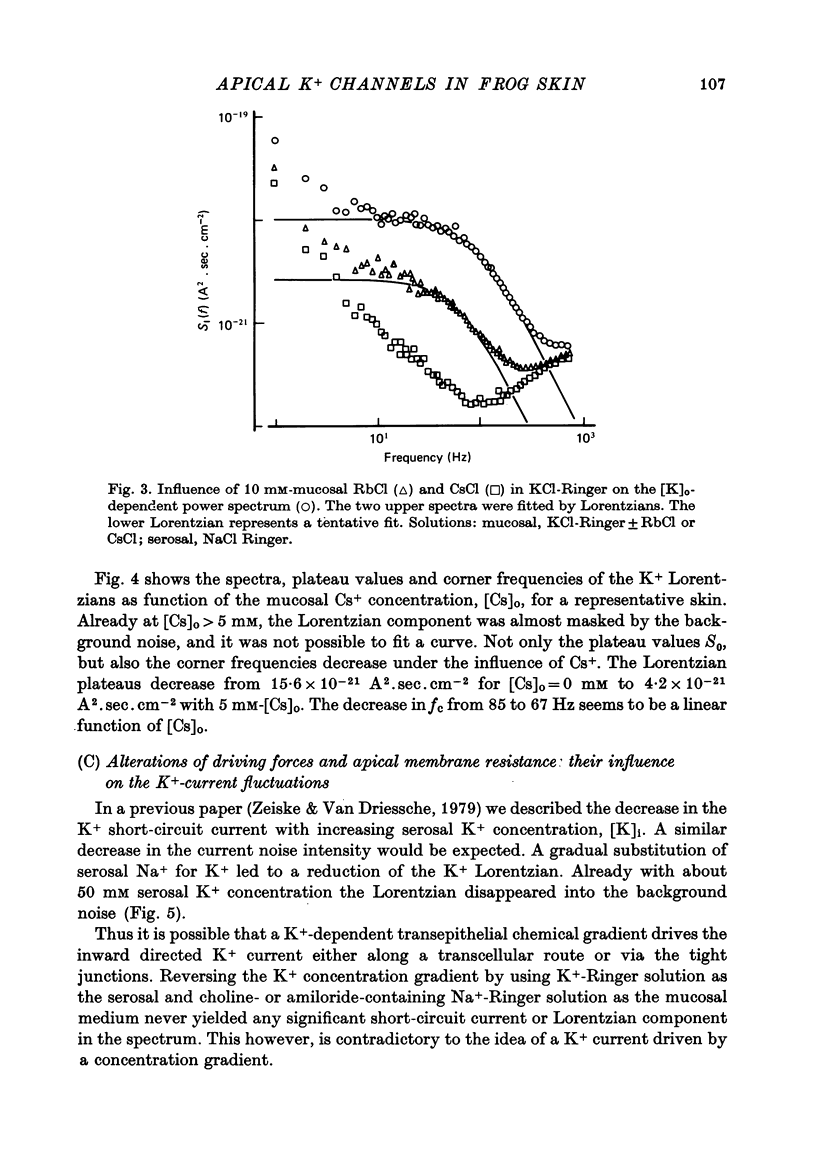
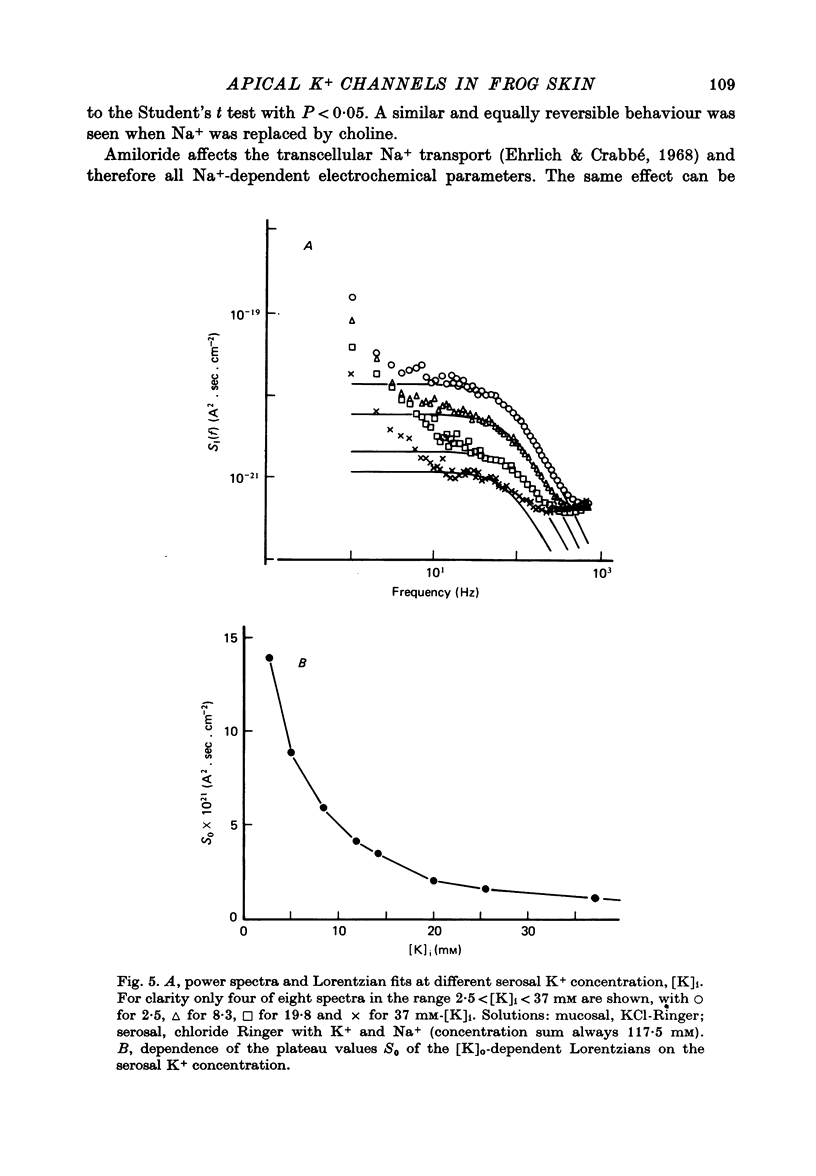
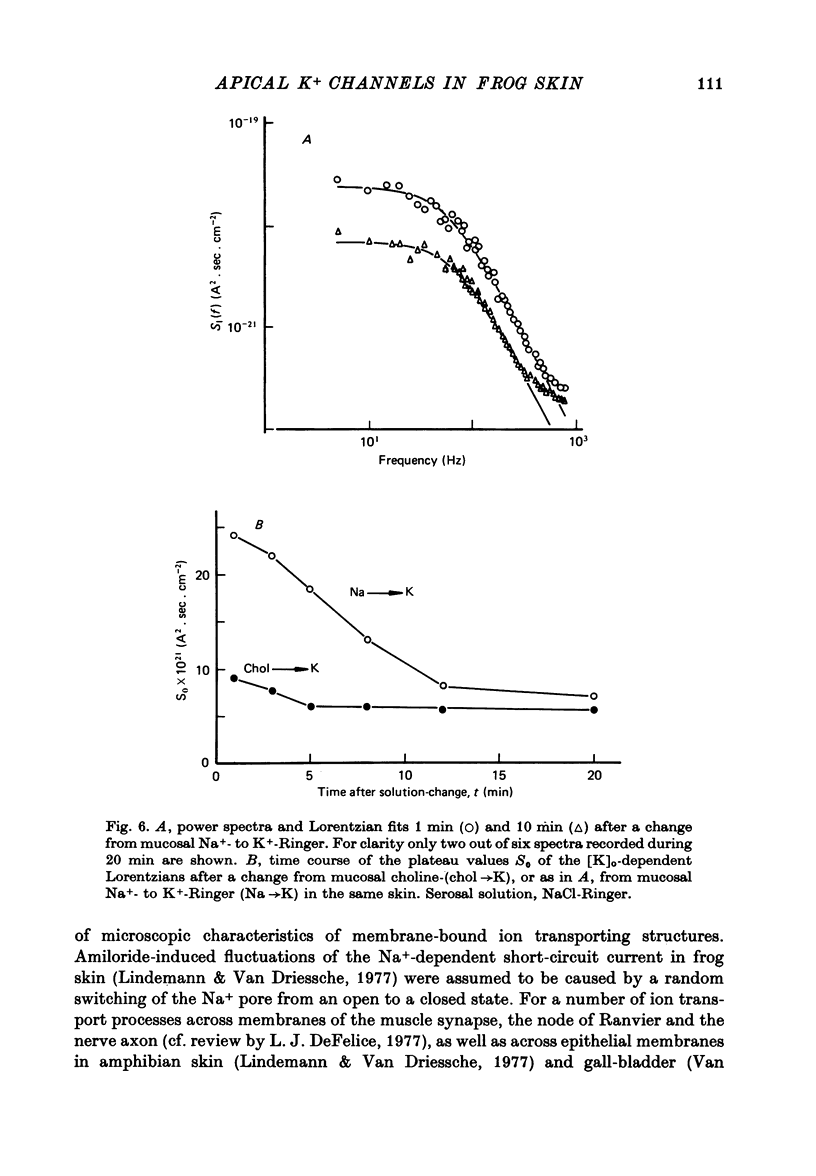
1. The previously demonstrated K+-dependent short-circuit current through the skin of frog species Rana temporaria (Zeiske & Van Driessche, 1979), bathed with mucosal K+- and serosal Na+-Ringer solution, was investigated with current-fluctuation analysis. 2. The current-noise spectra were recorded in the frequency range from 1 to 800 Hz and showed a Lorentzian component with a mean plateau value S0 = (1.50 +/- 0.05).10(-20) A2.s.cm-2 and a corner frequency of fc=(81.0 +/- 3.4)Hz(n=14). 3. S0 increased with mucosal K+ concentration, [K]o, while fc remained almost unchanged. A decrease in S0 was observed when serosal Na+ was replaced by K+. 4. Mucosal Cs+ (10 mM) depressed, reversibly, the K+-dependent current noise to the level of the background noise. Moreover, a linear decrease in fc with increasing Cs+ concentration was observed. 5. Among the other tested alkali cations, Rb+ was the only blocker though less potent than Cs+. Tetraethylammonium, 4-aminopyridine, 2.4.6-triaminopyrimidine and amiloride had no effect. 6. Alterations in the transcellular transport of Na+ contained in a mucosal solution with high [K]o resulted in significant changes in K+ current noise. 7. The current-fluctuation intensities decreased with increasing contact time to high [K]o; these changes were concomitant with the previously reported time dependence of the short-circuit current (Zeiske & Van Driessche, 1979). 8. The K+-dependent fluctuations are thought to originate from K+-selective pathways in the apical cell membranes. The description of the K+-current noise by a single Lorentzian suggests that the "K+ channels" switch randomly between an open and closed state. 9. Assuming a two state model for the channel-kinetics, the single channel current i and the channel density M were calculated as i=(0.37 +/- 0.05)pA and M=(0.53 +/- 0.08) mu-2 (n=13).
Full text
PDF


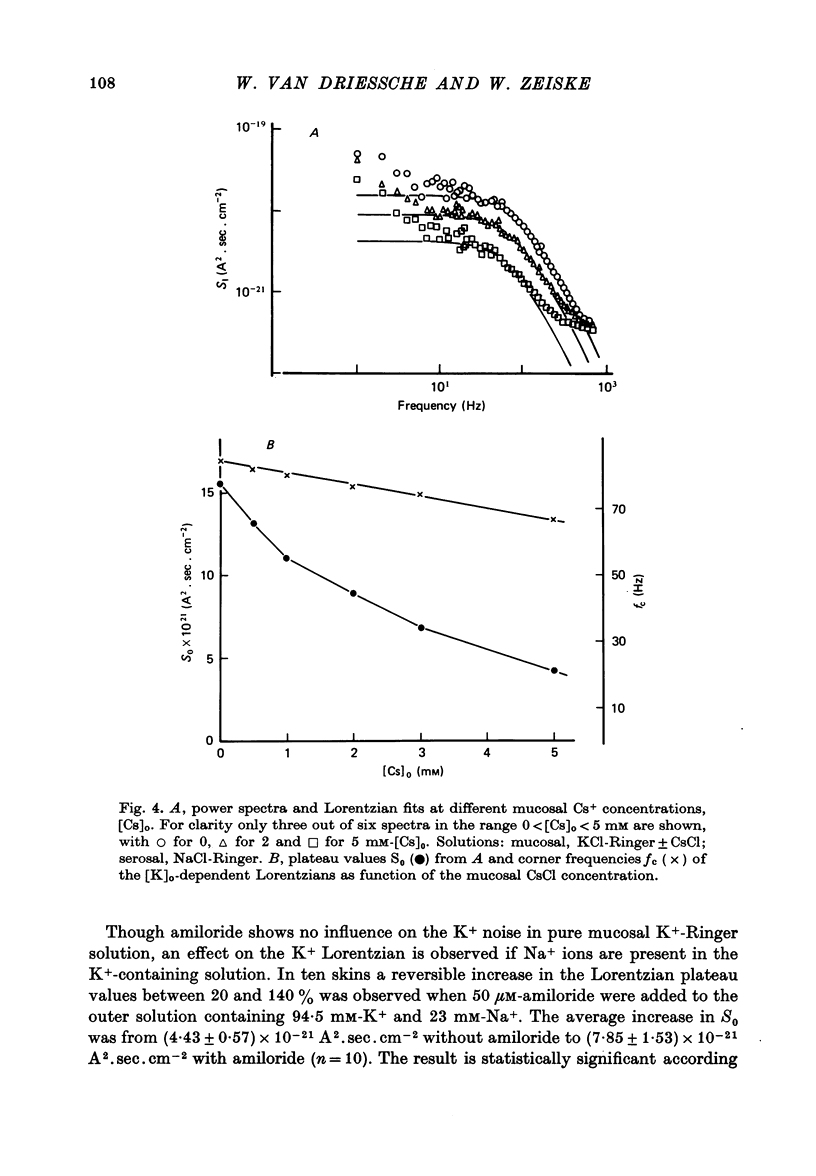






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeFelice L. J. Fluctuation analysis in neurobiology. Int Rev Neurobiol. 1977;20:169–208. doi: 10.1016/s0074-7742(08)60653-4. [DOI] [PubMed] [Google Scholar]
- Ehrlich E. N., Crabbé J. The mechanism of action of amipramizide. Pflugers Arch. 1968;302(1):79–96. doi: 10.1007/BF00586783. [DOI] [PubMed] [Google Scholar]
- Fishman H. M., Moore L. E., Poussart D. M. Potassium-ion conduction noise in squid axon membrane. J Membr Biol. 1975 Dec 4;24(3-4):305–328. doi: 10.1007/BF01868629. [DOI] [PubMed] [Google Scholar]
- Fishman H. M., Poussart D. M., Moore L. E. Noise measurements in squid axon membrane. J Membr Biol. 1975 Dec 4;24(3-4):281–304. doi: 10.1007/BF01868628. [DOI] [PubMed] [Google Scholar]
- Helman S. I., Fisher R. S. Microelectrode studies of the active Na transport pathway of frog skin. J Gen Physiol. 1977 May;69(5):571–604. doi: 10.1085/jgp.69.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G. Cardiac Purkinje fibers: cesium as a tool to block inward rectifying potassium currents. Pflugers Arch. 1976 Sep 30;365(2-3):99–106. doi: 10.1007/BF01067006. [DOI] [PubMed] [Google Scholar]
- KOEFOED-JOHNSEN V., USSING H. H. The nature of the frog skin potential. Acta Physiol Scand. 1958 Jun 2;42(3-4):298–308. doi: 10.1111/j.1748-1716.1958.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Katz U. Changes in ionic conductances and in sensitivity to amiloride during the natural moulting cycle of toad skin (Bufo viridis, L.). J Membr Biol. 1978 Jan 12;38(1-2):1–9. doi: 10.1007/BF01875159. [DOI] [PubMed] [Google Scholar]
- Leblanc G. The mechanism of lithium accumulation in the isolated frog skin epithelium. Pflugers Arch. 1972;337(1):1–18. doi: 10.1007/BF00587867. [DOI] [PubMed] [Google Scholar]
- Lindemann B., Van Driessche W. Sodium-specific membrane channels of frog skin are pores: current fluctuations reveal high turnover. Science. 1977 Jan 21;195(4275):292–294. doi: 10.1126/science.299785. [DOI] [PubMed] [Google Scholar]
- MUELLER P. POTASSIUM AND RUBIDIUM EXCHANGE ACROSS THE SURFACE MEMBRANE OF CARDIAC PURKINJE FIBRES. J Physiol. 1965 Apr;177:453–462. doi: 10.1113/jphysiol.1965.sp007604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J. H. Blockage of cation permeability across the tight junctions of gallbladder and other leaky epithelia. Nature. 1974 Sep 13;251(5471):150–151. doi: 10.1038/251150a0. [DOI] [PubMed] [Google Scholar]
- Nagel W. Evidence for electrogenic Na transport from the cytoplasmatic tissue pool of frog skin epithelium [proceedings]. J Physiol. 1978 Nov;284:146P–147P. [PubMed] [Google Scholar]
- Nielsen R. Effect of amphotericin B on the frog skin in vitro. Evidence for outward active potassium transport across the epithelium. Acta Physiol Scand. 1971 Sep;83(1):106–114. doi: 10.1111/j.1748-1716.1971.tb05056.x. [DOI] [PubMed] [Google Scholar]
- Shum W. K., Fanelli G. M., Jr Does intracellular sodium regulate sodium transport across the mucosal surface of frog skin? Biochim Biophys Acta. 1978 Oct 4;512(3):593–597. doi: 10.1016/0005-2736(78)90168-2. [DOI] [PubMed] [Google Scholar]
- Ulbricht W. Ionic channels and gating currents in excitable membranes. Annu Rev Biophys Bioeng. 1977;6:7–31. doi: 10.1146/annurev.bb.06.060177.000255. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Gögelein H. Potassium channels in the apical membrane of the toad gallbladder. Nature. 1978 Oct 19;275(5681):665–667. doi: 10.1038/275665a0. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Zeiske W. Fluctuations of the K+-current in the frog skin (Rana temporaria) [proceedings]. Arch Int Physiol Biochim. 1978 Aug;86(3):685–687. [PubMed] [Google Scholar]
- Verveen A. A., DeFelice L. J. Membrane noise. Prog Biophys Mol Biol. 1974;28:189–265. doi: 10.1016/0079-6107(74)90019-4. [DOI] [PubMed] [Google Scholar]
- Zeiske W., Van Driessche W. Saturable K+ pathway across the outer border of frog skin (rana temporaria): kinetics and inhibition by Cs+ and other cations. J Membr Biol. 1979 May 7;47(1):77–96. doi: 10.1007/BF01869048. [DOI] [PubMed] [Google Scholar]


