Abstract
Increased gastric production of interleukin 8 and tumor necrosis factor alpha (TNF-α) has been implicated in the pathogenesis of Helicobacter pylori-associated gastroduodenal disease. In the present study we used a mouse model to demonstrate whether loss of the tumor necrosis factor receptor 1 (TNF-R1) function leads to differences in gastric inflammation or the systemic immune response in H. pylori infection. Six different clinical isolates of H. pylori (three cytotoxin-positive and three cytotoxin-negative strains) were adapted to C57BL/6 mice. TNF-R1-deficient (TNF-R1−/−) mice (n = 19) and isogenetic controls (n = 24) were infected and sacrificed after 4 weeks of infection. Inflammation of the stomach and the humoral immune response to H. pylori were evaluated by histological, immunohistochemical, and serological methods. There was no detectable difference in the grade or activity of gastritis in TNF-R1−/− mice when they were compared with wild-type mice, but the number of lymphoid aggregates was slightly reduced in the gastric mucosa of TNF-R1−/− mice. Interestingly, total immunoglobulin G (IgG), as well as IgG1, IgG2b, and IgG3, H. pylori-specific antibody titers were significantly higher in wild-type mice. As revealed by immunoblot analysis, the difference in reactivity against H. pylori antigens was not based on a failure to recognize single H. pylori antigens in TNF-R1−/− mice. We therefore suggest that TNF-R1-mediated TNF-α signals might support a systemic humoral immune response against H. pylori and that the gastric inflammatory response to H. pylori infection seems to be independent of TNF-R1-mediated signals.
Helicobacter pylori colonization of the human gastric mucosa has been shown to be the main causative agent of chronic active type B gastritis and is closely associated with peptic and duodenal ulcer disease (6, 23, 42, 58), as well as gastric carcinoma (19, 45) and low-grade gastric B-cell lymphoma of the MALT type (19, 30, 59). There are two proposed explanations for how chronic H. pylori infection may lead to such diverse clinical outcomes. First, genetic diversity of virulence factors and antigenic profiles of various H. pylori strains may account for different disease entities. Second, genetically based differences in the individual immune responses to the pathogen may result in failure to eradicate the infection and lead to chronic mucosal damage (25); for example, interleukin-1 (IL-1) gene cluster polymorphisms followed by enhanced production of IL-1β seem to be associated with an increased risk of H. pylori-induced hypochlorhydria and gastric cancer (20).
Gastric mucosal damage in H. pylori infection depends on special virulence factors of H. pylori, including enhanced motility (28); production of adaptive enzymes, such as urease (41), catalase (27), and phospholipase C (12); specific adherence to gastric epithelial cells (17); production of a vacuolating cytotoxin (VacA) by some H. pylori strains (37); and the presence of a bacterial gene cluster (a pathogenicity island) (22) with cagA (cytotoxin-associated gene A) as a marker for the presence of the cag pathogenicity island (7). CagA- and VacA-expressing H. pylori strains enhance gastric inflammation in H. pylori infections and are strongly associated with gastroduodenal ulceration (13, 24), in which allelic variants of the vacA gene appear to regulate cytotoxic activity; vacA s1m1 strains produce higher levels of cytotoxin than s1m2 strains, which are essentially nontoxic in the HeLa cell assay but may be able to induce vacuolization in primary gastric cells or other cell lines. vacA s2m2 strains do not show detectable cytotoxin activity (2, 3, 47). Also important is the inflammatory reaction of the host, which is modulated by secretion of various cytokines, like IL-8 (11), gamma interferon (IFN-γ) (33), and tumor necrosis factor alpha (TNF-α) (12).
TNF-α is a key mediator in a host's response against gram-negative bacteria and in the septic shock syndrome induced by either lipopolysaccharide (LPS) or bacterial superantigens (5). Secretion of TNF-α from LPS-activated mononuclear phagocytes or antigen-stimulated T cells can be enhanced by IFN-γ. In H. pylori gastritis (13) the cytokine response is of the Th1 type since IFN-γ but not IL-4 is predominant (38). In mice lacking interferon regulatory factor 1 the defective Th1 response was associated with the total lack of gastritis and atrophy despite severe colonization with H. pylori (55). The multiple biological activities of TNF-α, like stimulation of expression of adhesion molecules such as intercellular adhesion molecule 1 on endothelial cells, which facilitates the extravasation of neutrophils into the lamina propria of mucosal tissue, activation of leukocytes and T-lymphocytes, stimulation of the production of cytokines by macrophages and monocytes (26, 56), and induction of apoptosis (34), are mediated by two distinct cell surface receptors. Tumor necrosis factor receptor 1 (TNF-R1), binding TNF-α and lymphotoxin alpha (LT-α) (= TNF-β), is generally known to mediate most of the TNF-α effects, especially apoptosis (57), whereas TNF-R2 is mainly implicated in lymphocyte proliferation (21).
Mice deficient for TNF-R1 are resistant to lethal doses of either LPS or Staphylococcus aureus endotoxin B but are severely impaired with respect to clearing Listeria monocytogenes and readily succumb to infection (51). Moreover, mice lacking TNF-R1 show a complete lack of Peyer's patches (46), and LT-α-deficient mice have defects in forming germinal centers (43), whereas the development of lymph nodes is not inhibited.
Marchetti et al. (40) developed in 1995 a mouse model of H. pylori infection that mimics human disease. The pathogenesis of H. pylori infection in vivo was studied by adapting fresh clinical isolates of bacteria to colonize the stomachs of mice, and a gastric pathology resembling human disease was observed, especially in infections with cytotoxin-producing strains.
In this study we used TNF-R1-deficient mice and isogenetic controls that were infected orally with different H. pylori strains and sacrificed after 4 weeks to show whether the loss of TNF-R1 function leads to differences in the systemic immune response or gastric inflammation. Our findings demonstrate that the systemic humoral immune response to H. pylori antigens might be enhanced by TNF-α mediated by the TNF-R1 pathway, whereas gastric inflammation in H. pylori infections seems to be independent of this pathway.
MATERIALS AND METHODS
Animals.
Twenty-four female TNF-R1-deficient C57BL/6 mice (GSF, Munich, Germany) (51) and 19 female isogenetic controls (Charles River, Sulzfeld, Germany), all of which were 10 weeks old, were housed in Mikrolon type stainless steel isolators; five mice of each group were used as uninfected controls. All materials were sterilized with pressurized steam, and the animals were fed a sterile, totally resorbable liquid nutrition diet (10 ml per mouse per day; Biosorbin MCT; Pfrimmer Nutricia, Erlangen, Germany), as well as water ad libitum. During the last 24 h before they were sacrificed, they were given only sterile water. The use of animals in research complied with all relevant federal guidelines and institutional policies.
Bacteria and growth conditions.
We used six different fresh clinical isolates of H. pylori. Three of them were cytotoxin positive and three were cytotoxin negative, as evaluated by a HeLa and Vero cell assay. These isolates were first passaged in wild-type C57BL/6 mice, which were sacrificed 2 weeks after H. pylori infection. Bacteria isolated from the stomachs and cultivated as described below were then used in experiments to infect TNF-R1-deficient mice and isogenetic controls. Cultures used for infecting mice were grown under microaerophilic conditions at 37°C for 48 h in brucella broth supplemented with 8% fetal calf serum and H. pylori-selective supplement (Dent) containing vancomycin (10 mg/liter), trimethoprim lactate (5 mg/liter), cefsulodin (5 mg/liter), and amphotericin B (5 mg/liter) (BBFCS-8% Dent), purchased from Oxoid (Basingstoke, Great Britain). Broth cultures were examined by phase-contrast microscopy for motility, purity, and coccoid forms. Bacteria were pelleted by centrifugation at 3,000 × g for 15 min, and the pellet was resuspended in brucella broth. Bacterial density was estimated by using a dilution series (about 109 CFU/ml).
Evaluation of cytotoxicity.
Detection of vacuolating cytotoxic activity was evaluated with HeLa and Vero cells (American Type Culture Collection, Rockville, Md.) as published previously (29, 37). H. pylori cells were grown for 48 h in BBFCS-8% Dent (Oxoid). Culture supernatants were centrifuged, sterilely filtered with a 0.22-μm-pore-size Millex-GV filter (Millipore, Eschborn, Germany), and tested for vacuolating cytotoxin activity with Vero cells (ATCC CCL 81) and HeLa cells (ATCC CCL 2) under standard conditions. After inoculation of 96-well microtiter plates with 2 × 104 cells per well, serial dilutions (1:2 to 1:8) of H. pylori culture supernatants were inoculated onto the coated plates and incubated in a humid atmosphere containing 5% CO2 at 37°C. After 24 h, the level of vacuolization was determined by inverse microscopy (magnification, ×100 to ×200). H. pylori cell lines were considered cytotoxin positive if vacuolization was observed in more than 50% of Vero and HeLa cells. H. pylori ATCC 49503 and ATCC 51932 were used as positive and negative controls. The oligonucleotide primer and PCR amplification conditions for vacA alleles (s1/s2, m1, m2) used have been described previously (2).
For cagA we used our own primers, cagAF (5′-AAAGGATTGTCCCCACAAGAA-3′) and cagAR (5′-TCCGTTACCTTTTGATTGATGA-3′).
Determination of IL-8 protein.
AGS cells (= ATCC CRL 1739) were cultured in RPMI 1640 medium (PAN Biotech, Aidenbach, Germany) supplemented with 10% heat-inactivated fetal calf serum, 1% penicillin G, and 1% streptomycin in 12-well plates with 5 × 105 cells per well at 37°C in a 5% CO2 incubator for 24 h. Then bacteria (cultured as described above) were harvested by centrifugation at 1,000 × g for 15 min, resuspended in antibiotic-free RPMI 1640 medium, and added to the cells at a final concentration of 2.5 × 107 CFU per well. After 8 h of incubation at 37°C in the presence of 5% CO2, cell supernatants were obtained after centrifugation at 10,000 × g for 15 min at 4°C to remove bacteria and then were stored at −80°C until they were used for analysis. The concentrations of IL-8 in the culture supernatants were determined by an enzyme-linked immunosorbent assay (ELISA) (Pharmingen, San Diego, Calif.) and expressed in picograms per milliliter.
Characterization of H. pylori strains.
The characteristics of the six different fresh clinical isolates evaluated by cell culture assay for vacuolating activity, by PCR for vacA and cagA, and by an IL-8 assay are summarized in Table 1.
TABLE 1.
Characterization of the six fresh clinical H. pylori isolates used for infection of mice
| Fresh clinical isolate of H. pylori | Cell assay for vacuolating activity | PCR for vacA | PCR for cagA | IL-8 assay (pg/ml) |
|---|---|---|---|---|
| I (Ly27a) | Negative | s1m1 | Positive | 827.0 |
| II (SE088) | Negative | s1m2 | Positive | 895.4 |
| III (OM1605) | Negative | NDa | ND | ND |
| IV (OM1626) | Positive | s1m1 | Positive | 1,162.0 |
| V (Ca077) | Positive | s1m1 | Negative | 33.7 |
| VI (Ca117) | Positive | s1mb | Positive | 1,174.1 |
ND, not determined. H. pylori strain OM1605 could not be recultivated for molecular analysis.
The m allele was not determinable.
Antibiotics and dosing scheme.
As the growth of H. pylori may be suppressed by lactobacilli previously inhabiting the stomachs of mice (32), every mouse was treated with ciprofloxacin (0.5 mg per day orally), amikacin (375 μg per day orally and intraperitoneally [i.p.]), imipenem (1.25 mg per day orally and i.p.), vancomycin (1 mg per day orally and i.p.), and fluconazole (150 μg per day orally) to decontaminate the gastrointestinal tract on three consecutive days. The doses were in accordance with the highest permissible human doses per day expressed in milligrams per kilogram of body weight.
H. pylori infection.
Beginning 2 days after the treatment with antibiotics, the mice were inoculated orally three times at 2-day intervals with the six different H. pylori strains described above. The mice were each given 0.25 ml of a solution of 0.2 M NaHCO3 orally to neutralize acidity, and 109 CFU of each strain in 100 to 200 μl of an H. pylori suspension was administered immediately after the bicarbonate treatment by using a blunt stainless steel tube.
Collection of gastric tissue and serum samples.
Mice infected with adapted H. pylori strains were sacrificed 4 weeks after infection. At the time of killing, a blood sample was drawn from each mouse from the vena cava to assess the postinfection immune status. The stomach was removed, washed in phosphate-buffered saline (PBS), and dissected longitudinally into four tissue fragments (so that each fragment contained parts of cardia, body, and antrum). Uninfected controls were treated in the same manner.
Assessment of H. pylori infection in mice.
For each stomach, one fragment was immediately placed in urea-containing medium (Helicobacter urease test; Astra, Wedel, Germany), and another was prepared for frozen tissue sectioning for use in immunohistochemistry. A third was placed on a special agar plate containing Wilkins-Chalgren agar (Oxoid), 10% horse blood, 125 mg of vancomycin per 0.5 liter, 5 mg of polymyxin B per 0.5 liter, 62.5 mg of trimethoprim per 0.5 liter, and 12.5 mg of amphotericin B (Sigma, Deisenhofen, Germany) per 0.5 liter and then incubated under microaerophilic conditions at 37°C for 48 to 72 h for bacterial isolation. The remaining fragment was placed in neutral buffered formalin for histological analysis. The presence of urease activity in tissue fragments was determined by monitoring biopsy urease agar for ≤24 h at room temperature.
Detection of antibodies by ELISA.
H. pylori antigen-specific immunoglobulin G (IgG) and IgG subclass antibodies in serum were detected by an ELISA technique by using the Enzygnost anti-Helicobacter ELISA (Behring, Marburg, Germany) with 96-well plates coated with extracts from cytotoxin-positive H. pylori strain NCTC 11637. Diluted (1:231) serum samples were added in 220-μl aliquots to antigen-coated microtiter wells. Bound H. pylori-specific immunoglobulins were detected with peroxidase-conjugated sheep anti-mouse antibodies (Dianova, Hamburg, Germany). Immune complexes were detected by reaction with a solution containing tetramethylbenzidine and hydrogen peroxide. Optical densities at 450 nm were determined with an Easy Reader EAR 400 AT (SLT-Labinstruments, Crailsheim, Germany). As the Behring Enzygnost ELISA is made for human serum samples, we determined the quantities (in mouse units) of IgG antibodies in sera by interpolation of a standard curve derived from a mouse serum sample with a high optical density value and an H. pylori infection confirmed by a biopsy urease reaction and H. pylori isolation from a gastric tissue sample. An optical density of 1.300 was determined to be 100 U (mouse units), and the cutoff was evaluated by using mean optical density values plus 2 standard deviations for native uninfected mice (optical density of 0.300 or 20 U). An IgG antibody concentration of ≥35 U (plus 3 standard deviations) was determined to confirm H. pylori infection serologically. For the IgG subclass antibodies detected as described above by using specific peroxidase-conjugated sheep anti-mouse IgG1, IgG2a, IgG2b, and IgG3 antibodies (Dianova), the optical densities were compared directly with a cutoff value (optical density, 0.100) evaluated as described above.
Immunoblotting.
Fresh clinical H. pylori isolate Ca117 was used as a standard antigen preparation for a native immunoblot. Bacteria were grown under microaerophilic conditions on Wilkins-Chalgren agar (Oxoid) with 10% horse blood and H. pylori selective supplement Dent (Oxoid) at 37°C for 48 h. Bacteria were harvested from agar plates, washed in 1 ml of PBS, and pelleted by centrifugation at 10,500 × g for 1 min. The pellet was resuspended in H2O (200 μl), 250 μl of sodium dodecyl sulfate (N-lauryl-sarcosine, sodium salt) sample buffer (Mikrogen, Munich, Germany) was added, and the suspension was then incubated at 100°C for 5 to 10 min. After a second centrifugation at 10,500 × g for 1 min, the pellet was used as an H. pylori protein sample for sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The samples were separated by using a 5% polyacrylamide stacking gel and a 15% polyacrylamide separation gel. Separated proteins were transferred to nitrocellulose paper (Schleicher & Schuell, Dassel, Germany) by semidry blotting for 2 h at 25 V, 102 mA, and 100 W in a Trans-Blot SD semidry transfer cell blotter (Bio-Rad, Munich, Germany). After blocking of nonspecific binding by incubation in Tris-buffered saline containing 0.2% Tween 20 (Serva, Heidelberg, Germany) for 2 h, the nitrocellulose sheets were incubated overnight with serum samples (1 in 200 dilution) in incubation buffer (Mikrogen). Bound IgG was detected by sequential incubation with peroxidase-conjugated sheep anti-mouse antibody (1 in 200 dilution) and 3,3′-diaminobenzidine-30% hydrogen peroxide. For interpretation of the results we used a scoring system that differentiated between specific and cross-reacting bands. The scoring system was evaluated for a commercial IgG blot in a study performed by one of us (N. Lehn, Institute of Medical Microbiology and Hygiene, Technical University of Munich, Munich, Germany) and Mikrogen in 1995 with 99 human sera from 59 H. pylori-positive and 40 H. pylori-negative individuals who were well characterized by histopathology and microbiology. Reactions to individual antigens were classified by points depending on immunodominant potency, and then all points were added to obtain a score. The following antigens in accordance with the commercial Mikrogen immunoblot were assessed and identified by size and monoclonal antibodies (Mikrogen): CagA/VacA (120 and 87 kDa), OMP (67 kDa), UreB (62 kDa), HspA/B (58 kDa), FlaA (54 kDa), 47 kDa, 33 kDa, UreA (29 kDa), 28 kDa, 25 kDa, and 19 kDa.
Immunohistochemistry.
Longitudinal sections of gastric tissue including cardia, body, and antrum were mounted in Jung tissue-freezing medium (Leica, Nussloch, Germany) and frozen in liquid-nitrogen-cooled 2-methylbutane (Aldrich, Steinheim, Germany). Tissue sections (thickness, 8 μm) were fixed with acetone and incubated with biotinylated rat anti-mouse monoclonal antibodies (MAb) and then with mouse anti-rat antibody conjugated to biotinylated horseradish peroxidase (Dianova). The controls included incubation with an MAb having unrelated specificity. Reagents were applied to tissue sections for 30 min, and the sections were washed three times with PBS. Cell-bound peroxidase was visualized with 5% 3-amino-9-ethylcarbazole (Sigma), 30% H2O2, and 50 mM acetate buffer (pH 5.2), and sections were counterstained with hematoxylin. The degree of gastric infiltration defined by the MAb was scored by determining the number of cells per field of vision (magnification, ×400).
MAb.
The following MAb were used in this study: anti-CD4 (clone H129.19; dilution, 1:100), anti-CD8 (clone 53-6.7; dilution, 1:100), anti-CD11b (clone M1/70; dilution, 1:2), anti-CD45 (B220, clone RA3-6B2; dilution, 1:100), and anti-Gr1 (clone RB6-8C5; dilution, 1:50). All MAb were obtained from Pharmingen, San Diego, Calif., and all dilutions were with PBS.
Histological analyses of gastric tissue samples.
Gastric sections were fixed in neutral buffered formalin, and formalin-fixed tissues were embedded in paraffin, sectioned (thickness, 4 μm), and stained with hematoxylin and eosin for assessment of histopathology (presence of inflammatory cell infiltrates, erosive lesions, edema, hyperplasia). The graded morphological variables (none to severe) examined were grade of gastritis (density of lymphocytes and plasma cells), activity of gastritis (density of polymorphonuclear leukocytes), glandular atrophy, intestinal metaplasia, lymphoid follicles, and epithelial regeneration in accordance with the updated Sydney system (16), and the data were summarized to obtain a total gastritis score. Histological analyses of tissues from knockout and wild-type mice were performed in a blinded fashion.
Statistical analysis.
The SPSS program package (version 7.5; SPSS Inc., Chicago, Ill.) was used for statistical analysis. Differences in the antibody response (ELISA), the presence of inflammatory cells in immunohistochemical analyses, or histopathological lesions between TNF-R1-deficient mice and isogenetic controls were analyzed by the nonparametric Mann-Whitney U test. Antibody responses in an immunoblot were compared by using a χ2 test. Differences were considered significant at a P value of ≤0.05.
RESULTS
Detection of H. pylori infection in mice.
H. pylori was isolated by culturing mouse gastric tissue for 4 weeks postinoculation from 100% of TNF-R1-deficient mice and isogenetic controls inoculated orally with different strains of H. pylori. Likewise, we obtained positive results with the biopsy urease agar assay, as well as with ELISA and immunoblot analyses, for 100% of both groups of mice (data not shown).
ELISA.
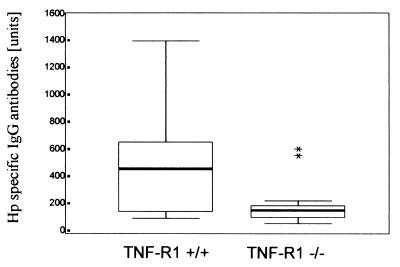
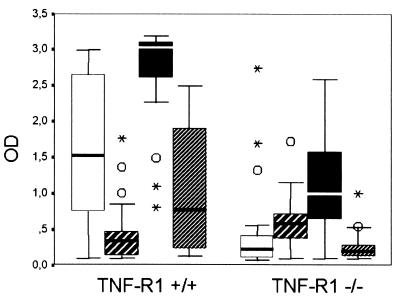
IgG antibody titers to H. pylori were significantly higher in infected mice than in uninfected controls (P < 0.0001). As detailed in Fig. 1, IgG antibody titers were also higher in infected isogenetic control mice than in TNF-R1-deficient mice (P = 0.0049). When the IgG subclass response was examined, significantly higher titers were found for IgG1 (P = 0.0002), IgG2b (P < 0.0001), and IgG3 (P = 0.0008), but not for IgG2a (P = 0.596), in infected isogenetic controls than in TNF-R1-deficient mice, as shown in Fig. 2. The use of cytotoxin-positive or cytotoxin-negative H. pylori strains did not influence IgG antibody production in infected TNF-R1-deficient (TNF-R1−/−) mice and isogenetic controls.
FIG. 1.
Box plots with medians, minimum and maximum values, upper and lower quartiles, and extremes (asterisks) of IgG antibody titers (ELISA units) to H. pylori (Hp) in infected TNF-R1-deficient mice (TNF-R1−/−, n = 19) and controls (TNF-R1+/+, n = 24). H. pylori-specific IgG antibody titers were significantly higher in infected isogenetic control mice than in TNF-R1-deficient mice (P = 0.0049).
FIG. 2.
Box plots with medians, minimum and maximum values, upper and lower quartiles, outliers (○), and extremes (asterisks) of IgG subclass antibody responses (optical density [OD] in ELISA analysis) to H. pylori in infected TNF-R1-deficient mice (TNF-R1−/−, n = 19) and controls (TNF-R1+/+, n = 24). Significantly higher titers were found for IgG1 (open bars) (P = 0.0002), IgG2b (solid bars) (P < 0.0001), and IgG3 (bars with narrow cross-hatching) (P = 0.0008), but not for IgG2a (bars with broad cross-hatching) (P = 0.596), in infected isogenetic controls than in TNF-R1-deficient mice.
Immunoblotting.
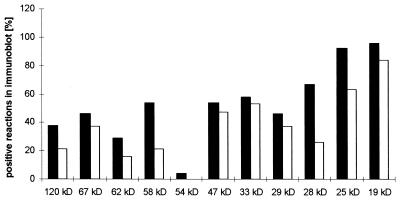
All H. pylori-infected mice reacted with specific antigens of H. pylori, whereas the uninfected controls did not react or reacted only with known nonspecific and cross-reacting antigens, like heat shock proteins or flagellin (data not shown). TNF-R1-deficient mice and wild-type mice did not differ in reactivity to specific H. pylori antigens, but the percentage of positive reactions to single antigens was lower in the TNF-R1−/− group (Fig. 3). For individual antigens, statistically significant differences between TNF-R1-deficient mice and wild-type controls were found for the 58-kDa antigen (= Hsp) (P = 0.02742), the 28-kDa antigen (P = 0.00857), and the 25-kDa antigen (P = 0.02248); the values were higher in wild-type mice. Antibodies against the 54-kDa antigen were found only in one wild-type mouse.
FIG. 3.
Positive reactions in immunoblots to individual antigens by infected TNF-R1-deficient mice (open bars) (n = 19) and isogenetic controls (solid bars) (n = 24). TNF-R1-deficient mice and wild-type mice did not differ significantly in reactivity against specific H. pylori antigens.
No association between the level of the IgG antibody responses in ELISA and immunoblots and the use of cytotoxin-positive or cytotoxin-negative H. pylori strains for infection of mice was found.
Immunohistochemistry.
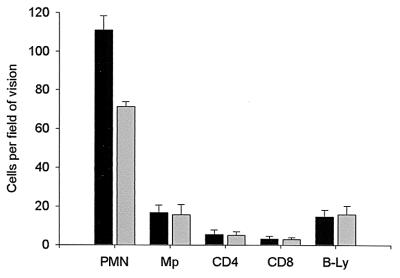
Uninfected controls showed significantly smaller numbers of polymorphonuclear leukocytes than infected mice and no infiltration with macrophages and CD4, CD8, and B cells in the gastric mucosa (data not shown). In addition, the gastric mucosa in mice infected with cytotoxin-positive H. pylori strains contained more polymorphonuclear leukocytes than the gastric mucosa in mice infected with cytotoxin-negative H. pylori strains (P < 0.0001) (Fig. 4).
FIG. 4.
Mean values and standard deviations for number of cells per field of vision (magnification, ×400) for mice infected with cytotoxin-positive (solid bars) (n = 22) and cytotoxin-negative (shaded bars) (n = 21) H. pylori strains. Gastric mucosa in mice infected with cytotoxin-positive H. pylori strains showed significantly more polymorphonuclear leukocytes than gastric mucosa in mice infected with cytotoxin-negative H. pylori-strains (P < 0.0001). No differences was detected for any other cell type analyzed.
Interestingly, all H. pylori-infected mice showed similar signs of gastritis, and there was no significant difference between isogenetic controls and TNF-R1-deficient mice in any of the cell populations analyzed. After H. pylori infection we observed marked infiltration with polymorphonuclear granulocytes (Fig. 5) and found about 90 granulocytes, about 20 macrophages, about 20 B-lymphocytes, about 10 CD4+ cells, and about five CD8+ cells in each field of vision (magnification, ×400).
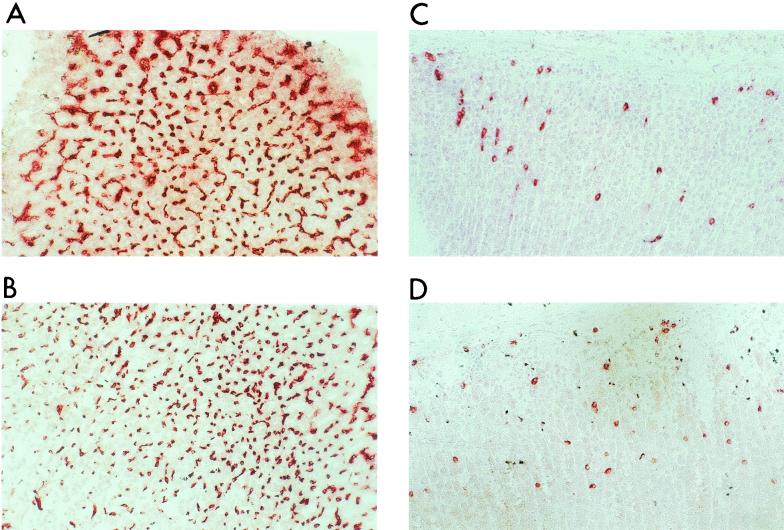
FIG. 5.
Infiltration of gastric mucosa with polymorphonuclear granulocytes (stained with MAb Gr1) showed no significant difference between TNF-R1-deficient mice (A and B) and wild-type mice (C and D) (magnification, ×200). Uninfected controls consisting of wild-type mice (C) and TNF-R1-deficient mice (D) showed significantly less (P < 0.0001) infiltration of the gastric mucosa with polymorphonuclear granulocytes.
Histology.
In the gastric mucosa of uninfected controls no inflammatory reactions could be detected, whereas all infected controls showed morphological changes due to gastritis (P < 0.0001). The accumulation of polymorphonuclear neutrophils (PMN) in the gastric mucosa was greater in mice infected with cytotoxin-positive H. pylori strains than in mice infected with cytotoxin-negative H. pylori strains, as evaluated with a scoring system in which 0 corresponded to no accumulation of PMN, 1 corresponded to low accumulation of PMN, 2 corresponded to intermediate accumulation of PMN, and 3 corresponded to high accumulation of PMN; the means ± standard deviations were 1.4 ± 0.5 and 1.2 ± 0.4, respectively, based on the immunohistochemical results, but the difference was not statistically significant. No significant difference between TNF-R1-deficient mice and isogenetic controls was detected when grade and activity of gastritis were examined. In contrast, TNF-R1-deficient mice had significantly fewer lymphoid aggregates in their gastric mucosa than isogenetic controls (means ± standard deviations, 0.0 ± 1.0 and 2.0 ± 0.99, respectively [scoring from 0 to 3 as described above]; P = 0.002).
DISCUSSION
In our study we used an H. pylori mouse model in which TNF-R1-deficient C57BL/6 mice and isogenetic wild-type controls were compared to evaluate the contribution of TNF-R1 in the systemic immune response and gastric inflammation in H. pylori-induced gastritis.
Activation of NF-κB, mediated by proteins of the H. pylori pathogenicity island, and the resulting increase in TNF-α secretion (22) seem to be critical in H. pylori-associated gastritis (45). Increased TNF-α secretion is associated with an increased level of apoptosis (34), independent of expression of the vacuolating cytotoxin (55) or the cagA status (50) of H. pylori. We therefore hypothesized that there was a reduced mucosal inflammatory response due to the loss of TNF-R1 function. The results of our study, however, demonstrate that TNF-α mediated by the TNF-R1 pathway might support primarily a systemic humoral immune response to H. pylori, whereas gastric inflammation in H. pylori infection seems to be independent of the function of the TNF-R1 receptor.
Surprisingly, TNF-R1-deficient mice exhibited significantly increased levels of serum IgG antibodies (as determined by ELISA) compared to the levels in wild-type mice at 4 weeks postinoculation (Fig. 1 and 2). In addition, we showed by immunoblotting that the weaker IgG production in TNF-R1-deficient mice cannot be explained by the failure to recognize specific H. pylori antigens overall (Fig. 3). Thus, the results of the immunoblot analysis may prove the generally weakened systemic humoral immune response of TNF-R1-deficient mice. All of these findings for the humoral immune response were completely independent of infection with cytotoxin-positive or cytotoxin-negative H. pylori strains (data not shown). Beyond that, isogenetic controls had significantly higher IgG antibody titers for IgG1, IgG2b, and IgG3, but not for IgG2a, than TNF-R1-deficient mice. Pasparakis et al. (48, 49) also demonstrated a strongly impaired secondary humoral immune response for all IgG subclass antibodies and IgE to thymus-dependent antigens in TNF-α-deficient mice and in addition defective formation of B-lymphocyte follicles in peripheral lymphoid organs of TNF-R1-deficient mice. Interestingly, we likewise found that the number of lymphoid aggregates was significantly lower in the mucosa of H. pylori-infected TNF-R1-deficient mice, but there was no effect on the grade and activity of gastritis. Similar findings were described by Neumann et al. (46) and Matsumoto et al. (43); there was a complete lack of Peyer's patches in TNF-R1-deficient mice and defective formation of germinal centers in LT-α-deficient mice. Thus, the reduced systemic immune response, together with fewer lymphoid follicles in gastric mucosa of TNF-R1-deficient mice, might lead to the conclusion that signals of TNF-α mediated by the TNF-R1 pathway support a systemic humoral immune response in a more meaningful manner than supposed until now. TNF-R1 may be necessary for activation and stimulation of macrophages for antigen presentation to CD4+ T-cells with consecutive T-cell-B-cell interaction. The different results for the IgG2a subclass response in TNF-α- and TNF-R1-deficient mice could be due to a decisive role of TNF-R2.
In the immunohistochemistry analysis, uninfected controls also showed some neutrophil infiltration in their gastric mucosa, possibly because of nonspecific reactions to various environmental antigens, but there was significantly less infiltration (P < 0.0001) than in H. pylori-infected mice (Fig. 5). However, mucosal infiltration with mononuclear cells and markers of chronic inflammation, particularly CD4+ T-helper cells (15), dominated by the Th1 type of inflammation (4, 14), and morphological changes for gastritis in histopathological analyses were found only in H. pylori-infected mice. Likewise, no difference were found between isogenetic controls and TNF-R1-deficient mice in any of the immunohistochemically analyzed different cell populations (Fig. 5) and in the histological grade of gastritis (data not shown). A possible explanation for this might be that TNF-α effects overlap with the effects of various other cytokines, such as IL-1 (35), so that the loss of one mediator does not result in a detectable reduction in acute inflammatory infiltration in the gastric mucosa. H. pylori infection in mice does not reach its peak before 8 weeks postinoculation (36). However, gastric mucosa in mice infected with cytotoxin-positive H. pylori strains in the cell culture assay for vacuolating activity showed higher accumulation of polymorphonuclear leukocytes than gastric mucosa in mice infected with cytotoxin-negative strains (Fig. 4). Interestingly, as shown in Table 1, we found that H. pylori isolates that were cytotoxin negative in the cell culture assay revealed a genotype for vacA that is indicative of cytotoxin activity (s1m1 and s1m2). In addition, although these strains tested positive for IL-8 induction in AGS cells, they were not associated with severe infiltration of polymorphonuclear cells, whereas one strain that was cytotoxin positive did not induce IL-8 but showed significant cellular infiltration. These findings suggest that expression of cytotoxic activity is related to severe gastric inflammation independent of a strain's capacity to induce IL-8 via the pathogenicity island. Reduced activity of gastritis due to infection with cytotoxin-negative H. pylori strains was reported also by Marchetti et al. (40), as well as Crabtree and Farmery (10). On the other hand, Eaton et al. demonstrated that there is no relationship among the presence of the cag pathogenicity island IL-8 induction, and neutrophilic gastritis (18).
In other animal models using cytokine knockout mice for analysis of the immunopathogenesis of H. pylori infection, it was shown that in contrast to TNF-α signals mediated by the TNF-R1 pathway, IFN-γ plays an important role in the induction of gastric inflammation caused by H. pylori infection. IFN-γ knockout mice get colonized but do not show any inflammatory signs after H. pylori infection (31, 52, 54). IL-4, in contrast, seems not to be critical in H. pylori infection (1, 8, 39), whereas it plays an important role in Helicobacter felis infection of mice (44), and Smythies et al. (53) found stronger gastric inflammation in IL-4-deficient mice. Chen et al. observed significantly reduced colonization of the gastric mucosa in IL-10-deficient mice so that IL-10 seemed to be an inhibitor of the protective immune response to H. pylori infection (9).
Thus, further investigations will be necessary to clarify the importance of TNF-α in pathogenesis and modulation of inflammatory processes following H. pylori infection leading to H. pylori-associated gastroduodenal disease. The effects mediated by TNF-R2 could be also of particular interest because they might be involved in the activation of IgG2a. In accordance with other results, our findings lead to the conclusion that TNF-α mediated by the TNF-R1 pathway is critical in maturation of the systemic humoral immune response, with formation of primary B-cell follicles, B-cell activation, and production of IgG antibodies (at least IgG1, IgG2b, and IgG3).
Acknowledgments
Our research was not supported by grants, special funds, or contracts, nor was it part of the official duties of any of the authors.
Editor: J. D. Clements
REFERENCES
- 1.Aebischer, T., S. Laforsch, R. Hurwitz, F. Brombacher, and T. F. Meyer. 2001. Immunity against Helicobacter pylori: significance of interleukin-4 receptor alpha chain status and gender of infected mice. Infect. Immun. 69:556-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atherton, J. C., P. Cao, R. M. Peek, Jr., M. K. Tummuru, M. J. Blaser, and T. L. Cover. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 270:17771-17777. [DOI] [PubMed] [Google Scholar]
- 3.Atherton, J. C., R. M. Peek, K. T. Tham, T. L. Cover, and M. J. Blaser. 1997. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology 112:92-99. [DOI] [PubMed] [Google Scholar]
- 4.Beales, I. L., and J. Calam. 1997. Stimulation of IL-8 production in human gastric epithelial cells by Helicobacter pylori, IL-1beta and TNF-alpha requires tyrosine kinase activity, but not protein kinase C. Cytokine 9:514-520. [DOI] [PubMed] [Google Scholar]
- 5.Beutler, B., and A. Cerami. 1988. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu. Rev. Biochem. 57:505-518. [DOI] [PubMed] [Google Scholar]
- 6.Blaser, M. J. 1987. Gastric Campylobacter-like organisms, gastritis, and peptic ulcer disease. Gastroenterology 93:371-383. [DOI] [PubMed] [Google Scholar]
- 7.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, W., D. Shu, and V. S. Chadwick. 1999. Helicobacter pylori infection in interleukin-4-deficient and transgenic mice. Scand. J. Gastroenterol. 34:987-992. [DOI] [PubMed] [Google Scholar]
- 9.Chen, W., D. Shu, and V. S. Chadwick. 2001. Helicobacter pylori infection: mechanism of colonization and functional dyspepsia reduced colonization of gastric mucosa by Helicobacter pylori in mice deficient in interleukin-10. J. Gastroenterol. Hepatol. 16:377-383. [DOI] [PubMed] [Google Scholar]
- 10.Crabtree, J. E., and S. M. Farmery. 1995. Helicobacter pylori and gastric mucosal cytokines: evidence that CagA-positive strains are more virulent. Lab. Investig. 73:742-745. [PubMed] [Google Scholar]
- 11.Crabtree, J. E., and I. J. Lindley. 1994. Mucosal interleukin-8 and Helicobacter pylori-associated gastroduodenal disease. Eur. J. Gastroenterol. Hepatol. 6:33-38. [PubMed] [Google Scholar]
- 12.Crabtree, J. E., T. M. Shallcross, R. V. Heatley, and J. I. Wyatt. 1991. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut 32:1473-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crabtree, J. E., J. D. Taylor, J. I. Wyatt, R. V. Heatley, T. M. Shallcross, D. S. Tompkins, and B. J. Rathbone. 1991. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet 338:332-334. [DOI] [PubMed] [Google Scholar]
- 14.D'Elios, M. M., M. Manghetti, M. De Carli, F. Costa, C. T. Baldari, D. Burroni, J. L. Telford, S. Romagnani, and G. Del Prete. 1997. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 158:962-967. [PubMed] [Google Scholar]
- 15.Deusch, K., C. Seifarth, A. Funk, I. Dahie, K. Reut, and M. Classen. 1992. Selective increase of CD4+ and CD25+ T cells but not of gamma-delta T cells in Helicobacter pylori associated gastritis. Isr. J. Med. Sci. 161:21.. [PubMed] [Google Scholar]
- 16.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed]
- 17.Dunn, B. E. 1993. Pathogenic mechanisms of Helicobacter pylori. Gastroenterol. Clin. N. Am. 22:43-57. [PubMed] [Google Scholar]
- 18.Eaton, K. A., D. Kersulyte, M. Mefford, S. J. Danon, S. Krakowka, and D. E. Berg. 2001. Role of Helicobacter pylori cag region genes in colonization and gastritis in two animal models. Infect. Immun. 69:2902-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eidt, S., M. Stolte, and R. Fischer. 1994. Helicobacter pylori gastritis and primary gastric non-Hodgkin's lymphomas. J. Clin. Pathol. 47:436-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Omar, E. M., M. Carrington, W. H. Chow, K. E. McColl, J. H. Bream, H. A. Young, J. Herrera, J. Lissowska, C. C. Yuan, N. Rothman, G. Lanyon, M. Martin, J. F. Fraumeni, Jr., and C. S. Rabkin. 2000. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404:398-402. [DOI] [PubMed] [Google Scholar]
- 21.Erikstein, B. K., E. B. Smeland, H. K. Blomhoff, S. Funderud, K. Prydz, W. Lesslauer, and T. Espevik. 1991. Independent regulation of 55-kDa and 75-kDa tumor necrosis factor receptors during activation of human peripheral blood B lymphocytes. Eur. J. Immunol. 21:1033-1037. [DOI] [PubMed] [Google Scholar]
- 22.Glocker, E., C. Lange, A. Covacci, S. Bereswill, M. Kist, and H. L. Pahl. 1998. Proteins encoded by the cag pathogenicity island of Helicobacter pylori are required for NF-κB activation. Infect. Immun. 66:2346-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham, D. Y., P. D. Klein, A. R. Opekun, and T. W. Boutton. 1988. Effect of age on the frequency of active Campylobacter pylori infection diagnosed by the [13C] urea breath test in normal subjects and patients with peptic ulcer disease. J. Infect. Dis 157:777-780. [DOI] [PubMed] [Google Scholar]
- 24.Gunn, M. C., J. C. Stephens, J. A. Stewart, B. J. Rathbone, and K. P. West. 1998. The significance of cagA and vacA subtypes of Helicobacter pylori in the pathogenesis of inflammation and peptic ulceration. J. Clin. Pathol. 51:761-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatz, R., E. Bayerdörffer, N. Lehn, and G. Enders. 1994. Immune response in Helicobacter pylori infection: implications for treatment of gastroduodenal disease. Clin. Immunother. 2:295-306. [Google Scholar]
- 26.Hatz, R. A., G. Rieder, M. Stolte, E. Bayerdorffer, G. Meimarakis, F. W. Schildberg, and G. Enders. 1997. Pattern of adhesion molecule expression on vascular endothelium in Helicobacter pylori-associated antral gastritis. Gastroenterology 112:1908-1919. [DOI] [PubMed] [Google Scholar]
- 27.Hazell, S. L., D. J. Evans, Jr., and D. Y. Graham. 1991. Helicobacter pylori catalase. J. Gen. Microbiol. 137:58-61. [DOI] [PubMed] [Google Scholar]
- 28.Hazell, S. L., A. Lee, L. Brady, and W. Hennessy. 1986. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J. Infect. Dis. 153:658-663. [DOI] [PubMed] [Google Scholar]
- 29.Hupertz, V., and S. Czinn. 1988. Demonstration of a cytotoxin from Campylobacter pylori. Eur. J. Clin. Micorbiol. Infect. Dis. 7:576-578. [DOI] [PubMed] [Google Scholar]
- 30.Hussell, T., P. G. Isaacson, J. E. Crabtree, and J. Spencer. 1993. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet 342:571-574. [DOI] [PubMed] [Google Scholar]
- 31.Kamradt, A. E., M. Greiner, P. Ghiara, and S. H. Kaufmann. 2000. Helicobacter pylori infection in wild-type and cytokine-deficient C57BL/6 and BALB/c mouse mutants. Microbes Infect. 2:593-597. [DOI] [PubMed] [Google Scholar]
- 32.Karita, M., Q. Li, D. Cantero, and K. Okita. 1994. Establishment of a small animal model for human Helicobacter pylori infection using germ-free mouse. Am. J. Gastroenterol. 89:208-213. [PubMed] [Google Scholar]
- 33.Karttunen, R., T. Karttunen, H. P. Ekre, and T. T. MacDonald. 1995. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut 36:341-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim, J. M., J. S. Kim, H. C. Jung, I. S. Song, and C. Y. Kim. 2000. Apoptosis of human gastric epithelial cells via caspase-3 activation in response to Helicobacter pylori infection: possible involvement of neutrophils through tumor necrosis factor alpha and soluble Fas ligands. Scand. J. Gastroenterol. 35:40-48. [DOI] [PubMed] [Google Scholar]
- 35.Le, J., and J. Vilcek. 1987. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab. Investig. 56:234-248. [PubMed] [Google Scholar]
- 36.Lee, A., J. G. Fox, G. Otto, and J. Murphy. 1990. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology 99:1315-1323. [DOI] [PubMed] [Google Scholar]
- 37.Leunk, R. D., P. T. Johnson, B. C. David, W. G. Kraft, and D. R. Morgan. 1988. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J. Med. Microbiol. 26:93-99. [DOI] [PubMed] [Google Scholar]
- 38.Lindholm, C., M. Quiding-Järbrink, H. Lönroth, A. Hamlet, and A. Svennerholm. 1998. Local cytokine response in Helicobacter pylori-infected subjects. Infect. Immun. 66:5964-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucas, B., D. Bumann, A. Walduck, J. Koesling, L. Develioglu, T. F. Meyer, and T. Aebischer. 2001. Adoptive transfer of CD4+ T cells specific for subunit A of Helicobacter pylori urease reduces H. pylori stomach colonization in mice in the absence of interleukin-4 (IL-4)/IL-13 receptor signaling. Infect. Immun. 69:1714-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marchetti, M., B. Aricò, D. Burroni, N. Figura, R. Rappuoli, and P. Ghiara. 1995. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science 267:1655-1658. [DOI] [PubMed] [Google Scholar]
- 41.Marshall, B. J., L. J. Barrett, and C. Prakash. 1990. Urea protects Helicobacter pylori but not Campylobacter jejuni from the bactericidal effect of acid. Gastroenterology 99:697-702. [DOI] [PubMed] [Google Scholar]
- 42.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed]
- 43.Matsumoto, M., S. Mariathasan, M. H. Nahm, F. Baranyay, J. J. Peschon, and D. D. Chaplin. 1996. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science 271:1289-1291. [DOI] [PubMed] [Google Scholar]
- 44.Mohammadi, M., J. Nedrud, R. Redline, N. Lycke, and S. J. Czinn. 1997. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology 113:1848-1857. [DOI] [PubMed] [Google Scholar]
- 45.Moss, S. F., J. Calam, B. Agarwal, S. Wang, and P. R. Holt. 1996. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut 38:498-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neumann, B., A. Luz, K. Pfeffer, and B. Holzmann. 1996. Defective Peyer's patch organogenesis in mice lacking the 55-kD receptor for tumor necrosis factor. J. Exp. Med. 184:259-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pagliaccia, C., M. de Bernard, P. Lupetti, X. Ji, D. Burroni, T. L. Cover, E. Papini, R. Rappuoli, J. L. Telford, and J. M. Reyrat. 1998. The m2 form of the Helicobacter pylori cytotoxin has cell type-specific vacuolating activity. Proc. Natl. Acad. Sci. USA 95:10212-10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pasparakis, M., L. Alexopoulou, V. Episkopou, and G. Kollias. 1996. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184:1397-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasparakis, M., L. Alexopoulou, M. Grell, K. Pfizenmaier, H. Bluethmann, and G. Kollias. 1997. Peyer's patch organogenesis is intact yet formation of B lymphocyte follicles is defective in peripheral lymphoid organs of mice deficient for tumor necrosis factor and its 55-kDa receptor. Proc. Natl. Acad. Sci. USA 94:6319-6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peek, R. M., Jr., S. F. Moss, K. T. Tham, G. I. Perez-Perez, S. Wang, G. G. Miller, J. C. Atherton, P. R. Holt, and M. J. Blaser. 1997. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J. Natl. Cancer Inst. 89:863-868. [DOI] [PubMed] [Google Scholar]
- 51.Pfeffer, K., T. Matsuyama, T. M. Kündig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P. S. Ohashi, M. Krönke, and T. W. Mak. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to Listeria monocytogenes infection. Cell 73:457-467. [DOI] [PubMed] [Google Scholar]
- 52.Sawai, N., M. Kita, T. Kodama, T. Tanahashi, Y. Yamaoka, Y. Tagawa, Y. Iwakura, and J. Imanishi. 1999. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect. Immun. 67:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smythies, L. E., K. B. Waites, J. R. Lindsey, P. R. Harris, P. Ghiara, and P. D. Smith. 2000. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J. Immunol. 165:1022-1029. [DOI] [PubMed] [Google Scholar]
- 54.Sommer, F., G. Faller, M. Rollinghoff, T. Kirchner, T. W. Mak, and M. Lohoff. 2001. Lack of gastritis and of an adaptive immune response in interferon regulatory factor-1-deficient mice infected with Helicobacter pylori. Eur. J. Immunol. 31:396-402. [DOI] [PubMed] [Google Scholar]
- 55.Takagi, A., S. Watanabe, M. Igarashi, J. Koike, K. Hasumi, R. Deguchi, Y. Koga, and T. Miwa. 2000. The effect of Helicobacter pylori on cell proliferation and apoptosis in gastric epithelial cell lines. Aliment. Pharmacol. Ther. 14:188-192. [DOI] [PubMed] [Google Scholar]
- 56.Talmadge, J. E., H. Phillips, M. Schneider, T. Rowe, R. Pennington, O. Bowersox, and B. Lenz. 1988. Immunomodulatory properties of recombinant murine and human tumor necrosis factor. Cancer Res. 48:544-550. [PubMed] [Google Scholar]
- 57.Tartaglia, L. A., T. M. Ayres, G. H. Wong, and D. V. Goeddel. 1993. A novel domain within the 55 kd TNF receptor signals cell death. Cell 74:845-853. [DOI] [PubMed] [Google Scholar]
- 58.Von Wulffen, H., J. Heesemann, G. H. Bützow, T. Löning, R. Laufs, G. H. Butzow, and T. Loning. 1986. Detection of Campylobacter pyloridis in patients with antrum gastritis and peptic ulcers by culture, complement fixation test, and immunoblot. J. Clin. Microbiol. 24:716-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wotherspoon, A. C., C. Doglioni, T. C. Diss, L. Pan, A. Moschini, M. Boni, and P. G. Isaacson. 1993. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 342:575-577. [DOI] [PubMed] [Google Scholar]