Abstract
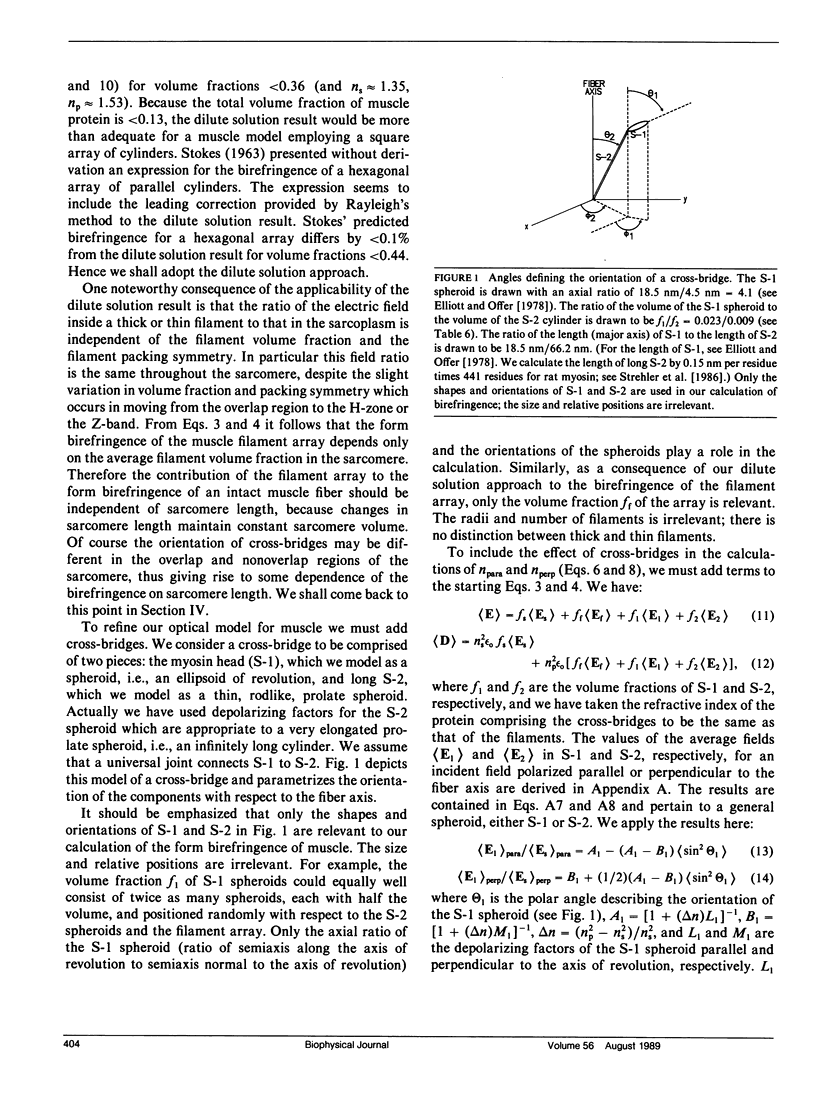
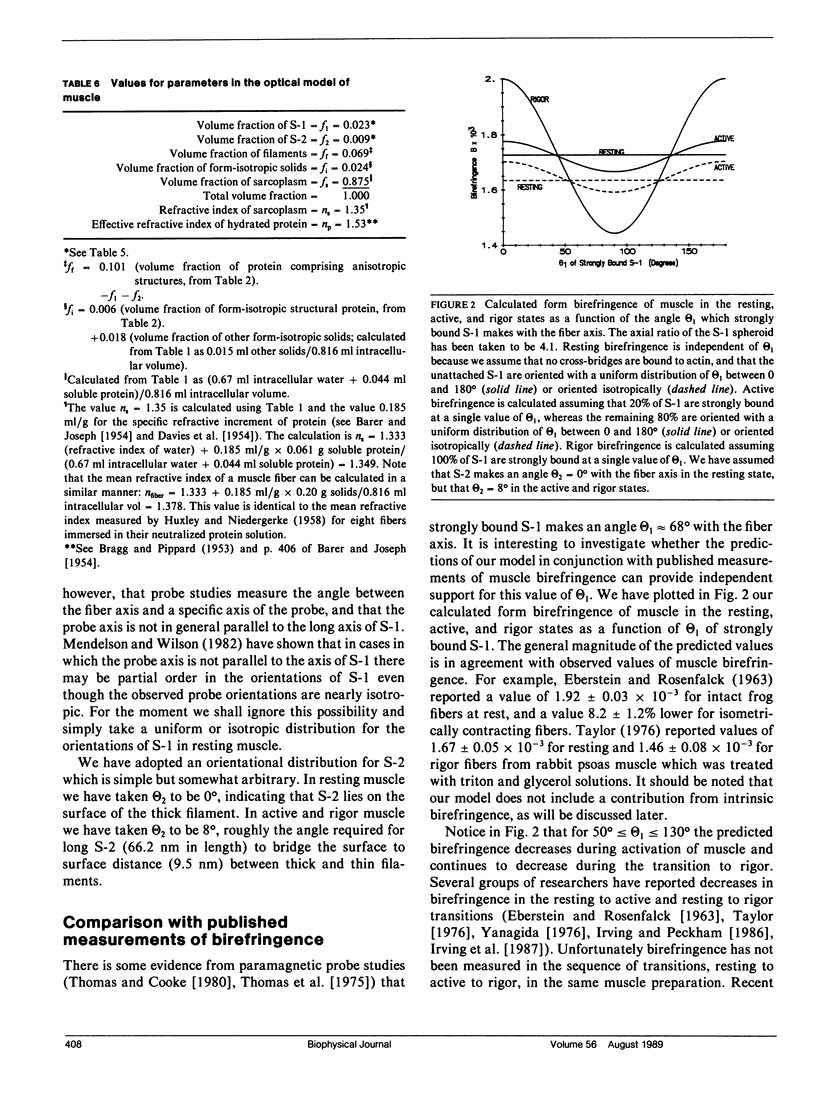
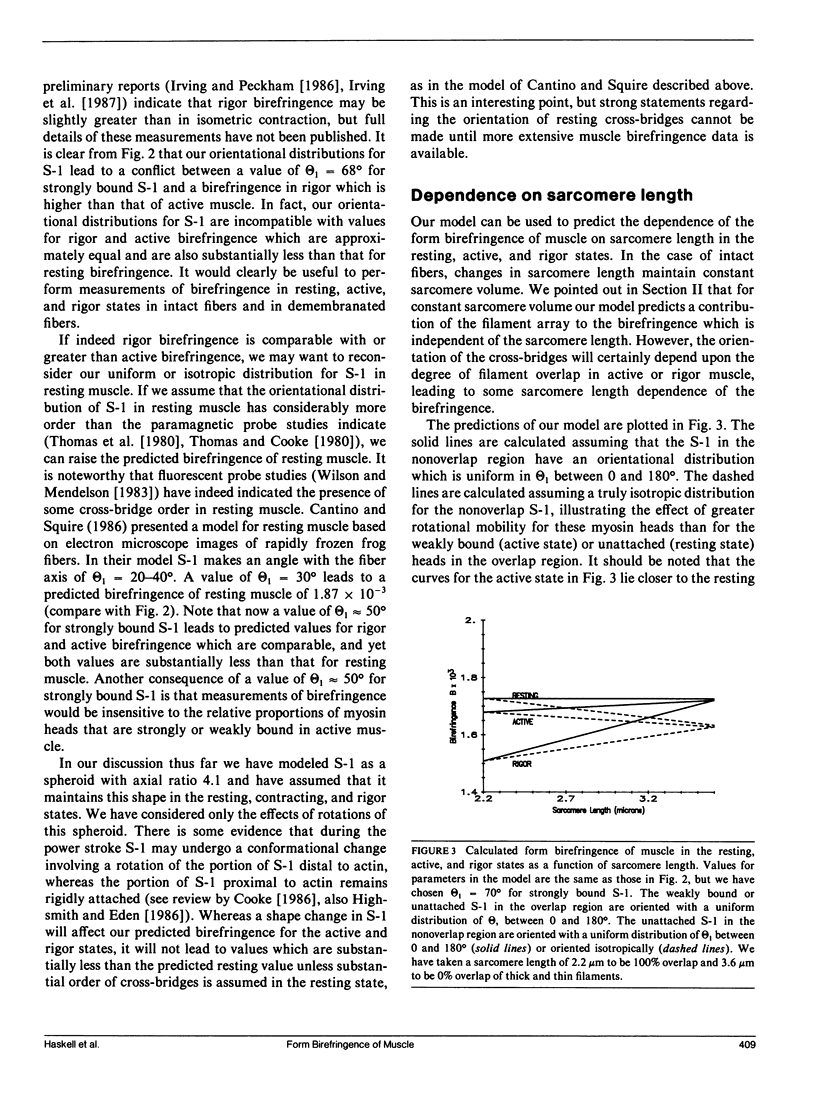
We investigate the sensitivity of measurements of muscle birefringence to cross-bridge dynamics in the resting, active, and rigor states. The theory of form birefringence is reviewed, and an optical model is constructed for the form birefringence of muscle. Values for the parameters in the model are selected or deduced from the literature. As an illustration of the use of the model, plausible distributions for the orientations of cross-bridges in the resting, active, and rigor states are constructed using a model for cross-bridge dynamics suggested by Huxley and Kress (1985). The general magnitude of the predictions of our model is comparable with that of published measurements of muscle birefringence. However, the precise values of the predicted birefringence for the resting, active, and rigor states are sensitive to the assumed orientations of cross-bridges. We also investigate the dependence of muscle birefringence on sarcomere length and on disorder in the orientation of the myofilament array. We conclude that measurements of muscle birefringence can play a useful role in distinguishing between proposed models of cross-bridge dynamics.
Full text
PDF




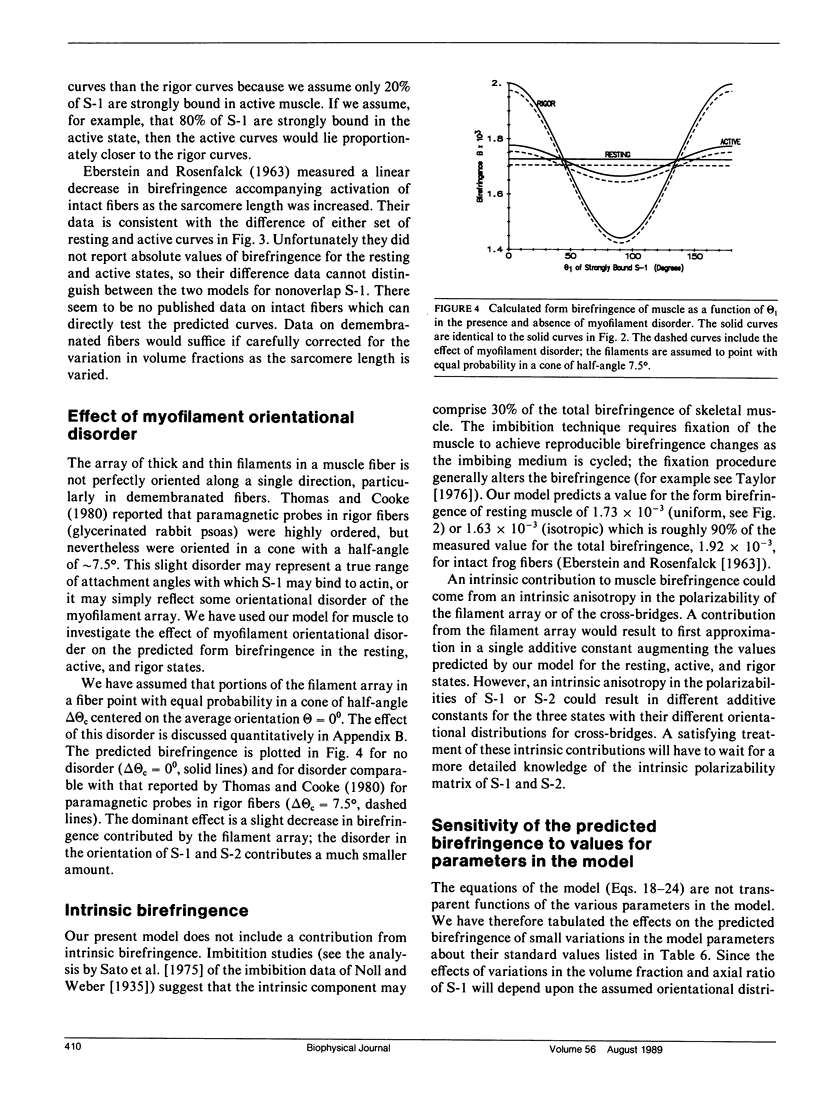



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle P. J., Conway E. J., Kane F., O'reilly H. L. Volume of interfibre spaces in frog muscle and the calculation of concentrations in the fibre water. J Physiol. 1941 Jun 30;99(4):401–414. doi: 10.1113/jphysiol.1941.sp003911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt T. P., Ando T., Borejdo J. Evidence for cross-bridge order in contraction of glycerinated skeletal muscle. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7515–7519. doi: 10.1073/pnas.80.24.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantino M., Squire J. Resting myosin cross-bridge configuration in frog muscle thick filaments. J Cell Biol. 1986 Feb;102(2):610–618. doi: 10.1083/jcb.102.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Crowder M. S., Thomas D. D. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982 Dec 23;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Cooke R. The mechanism of muscle contraction. CRC Crit Rev Biochem. 1986;21(1):53–118. doi: 10.3109/10409238609113609. [DOI] [PubMed] [Google Scholar]
- Crowder M. S., Cooke R. Orientation of spin-labeled nucleotides bound to myosin in glycerinated muscle fibers. Biophys J. 1987 Feb;51(2):323–333. doi: 10.1016/S0006-3495(87)83338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott A., Offer G. Shape and flexibility of the myosin molecule. J Mol Biol. 1978 Aug 25;123(4):505–519. doi: 10.1016/0022-2836(78)90204-8. [DOI] [PubMed] [Google Scholar]
- Godfrey J. E., Harrington W. F. Self-association in the myosin system at high ionic strength. I. Sensitivity of the interaction to pH and ionic environment. Biochemistry. 1970 Feb 17;9(4):886–893. doi: 10.1021/bi00806a025. [DOI] [PubMed] [Google Scholar]
- HANSON J., HUXLEY H. E. Quantitative studies on the structure of cross-striated myofibrils. II. Investigations by biochemical techniques. Biochim Biophys Acta. 1957 Feb;23(2):250–260. doi: 10.1016/0006-3002(57)90326-8. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F., NIEDERGERKE R. Measurement of the striations of isolated muscle fibres with the interference microscope. J Physiol. 1958 Dec 30;144(3):403–425. doi: 10.1113/jphysiol.1958.sp006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Highsmith S., Eden D. Myosin subfragment 1 has tertiary structural domains. Biochemistry. 1986 Apr 22;25(8):2237–2242. doi: 10.1021/bi00356a058. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Faruqi A. R., Kress M., Bordas J., Koch M. H. Time-resolved X-ray diffraction studies of the myosin layer-line reflections during muscle contraction. J Mol Biol. 1982 Jul 15;158(4):637–684. doi: 10.1016/0022-2836(82)90253-4. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Kress M. Crossbridge behaviour during muscle contraction. J Muscle Res Cell Motil. 1985 Apr;6(2):153–161. doi: 10.1007/BF00713057. [DOI] [PubMed] [Google Scholar]
- Mendelson R. A., Wilson M. G. Three-dimensional disorder of dipolar probes in a helical array. Application to muscle cross-bridges. Biophys J. 1982 Aug;39(2):221–227. doi: 10.1016/S0006-3495(82)84511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Ellis G. W., Inoué S. Microtubular origin of mitotic spindle form birefringence. Demonstration of the applicability of Wiener's equation. J Cell Biol. 1975 Dec;67(3):501–517. doi: 10.1083/jcb.67.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler E. E., Strehler-Page M. A., Perriard J. C., Periasamy M., Nadal-Ginard B. Complete nucleotide and encoded amino acid sequence of a mammalian myosin heavy chain gene. Evidence against intron-dependent evolution of the rod. J Mol Biol. 1986 Aug 5;190(3):291–317. doi: 10.1016/0022-2836(86)90003-3. [DOI] [PubMed] [Google Scholar]
- Svensson E. C., Thomas D. D. ATP induces microsecond rotational motions of myosin heads crosslinked to actin. Biophys J. 1986 Nov;50(5):999–1002. doi: 10.1016/S0006-3495(86)83541-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Ishiwata S., Seidel J. C., Gergely J. Submillisecond rotational dynamics of spin-labeled myosin heads in myofibrils. Biophys J. 1980 Dec;32(3):873–889. doi: 10.1016/S0006-3495(80)85023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Seidel J. C., Hyde J. S., Gergely J. Motion of subfragment-1 in myosin and its supramolecular complexes: saturation transfer electron paramagnetic resonance. Proc Natl Acad Sci U S A. 1975 May;72(5):1729–1733. doi: 10.1073/pnas.72.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D. Spectroscopic probes of muscle cross-bridge rotation. Annu Rev Physiol. 1987;49:691–709. doi: 10.1146/annurev.ph.49.030187.003355. [DOI] [PubMed] [Google Scholar]
- Toylor D. L. Quantitative studies on the polarization optical properties of striated muscle. I. Birefringence changes of rabbit psoas muscle in the transition from rigor to relaxed state. J Cell Biol. 1976 Mar;68(3):497–511. doi: 10.1083/jcb.68.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner P. D. Preparation and fractionation of myosin light chains and exchange of the essential light chains. Methods Enzymol. 1982;85(Pt B):72–81. doi: 10.1016/0076-6879(82)85010-6. [DOI] [PubMed] [Google Scholar]
- Wilson M. G., Mendelson R. A. A comparison of order and orientation of crossbridges in rigor and relaxed muscle fibres using fluorescence polarization. J Muscle Res Cell Motil. 1983 Dec;4(6):671–693. doi: 10.1007/BF00712160. [DOI] [PubMed] [Google Scholar]
- Yanagida T. Angles of nucleotides bound to cross-bridges in glycerinated muscle fiber at various concentrations of epsilon-ATP, epsilon-ADP and epsilon-AMPPNP detected by polarized fluorescence. J Mol Biol. 1981 Mar 15;146(4):539–560. doi: 10.1016/0022-2836(81)90046-2. [DOI] [PubMed] [Google Scholar]
- Yanagida T. Birefringence of glycerinated crab muscle fiber under various conditions. Biochim Biophys Acta. 1976 Feb 20;420(2):225–235. doi: 10.1016/0005-2795(76)90314-7. [DOI] [PubMed] [Google Scholar]
- Yates L. D., Greaser M. L. Quantitative determination of myosin and actin in rabbit skeletal muscle. J Mol Biol. 1983 Jul 25;168(1):123–141. doi: 10.1016/s0022-2836(83)80326-x. [DOI] [PubMed] [Google Scholar]


