Abstract
The gating kinetics of single-ion channels are generally modeled in terms of Markov processes with relatively small numbers of channel states. More recently, fractal (Liebovitch et al. 1987. Math. Biosci. 84:37-68) and diffusion (Millhauser et al. 1988. Proc. Natl. Acad. Sci. USA. 85:1502-1507) models of channel gating have been proposed. These models propose the existence of many similar conformational substrates of the channel protein, all of which contribute to the observed gating kinetics. It is important to determine whether or not Markov models provide the most accurate description of channel kinetics if progress is to be made in understanding the molecular events of channel gating. In this study six alternative classes of gating model are tested against experimental single-channel data. The single-channel data employed are from (a) delayed rectifier K+ channels of NG 108-15 cells and (b) locust muscle glutamate receptor channels. The models tested are (a) Markov, (b) fractal, (c) one-dimensional diffusion, (d) three-dimensional diffusion, (e) stretched exponential, and (f) expo-exponential. The models are compared by fitting the predicted distributions of channel open and closed times to those observed experimentally. The models are ranked in order of goodness-of-fit using a boot-strap resampling procedure. The results suggest that Markov models provide a markedly better description of the observed open and closed time distributions for both types of channel. This provides justification for the continued use of Markov models to explore channel gating mechanisms.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball F. G., Kerry C. J., Ramsey R. L., Sansom M. S., Usherwood P. N. The use of dwell time cross-correlation functions to study single-ion channel gating kinetics. Biophys J. 1988 Aug;54(2):309–320. doi: 10.1016/S0006-3495(88)82961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball F. G., Sansom M. S. Ion-channel gating mechanisms: model identification and parameter estimation from single channel recordings. Proc R Soc Lond B Biol Sci. 1989 May 22;236(1285):385–416. doi: 10.1098/rspb.1989.0029. [DOI] [PubMed] [Google Scholar]
- Ball F. G., Sansom M. S. Single-channel autocorrelation functions: the effects of time interval omission. Biophys J. 1988 May;53(5):819–832. doi: 10.1016/S0006-3495(88)83161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Adjacent interval analysis distinguishes among gating mechanisms for the fast chloride channel from rat skeletal muscle. J Physiol. 1989 Mar;410:561–585. doi: 10.1113/jphysiol.1989.sp017549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. A note on correlations in single ion channel records. Proc R Soc Lond B Biol Sci. 1987 Feb 23;230(1258):15–52. doi: 10.1098/rspb.1987.0008. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Easton D. M. Exponentiated exponential model (Gompertz kinetics) of Na+ and K+ conductance changes in squid giant axon. Biophys J. 1978 Apr;22(1):15–28. doi: 10.1016/S0006-3495(78)85467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- French A. S., Stockbridge L. L. Fractal and Markov behavior in ion channel kinetics. Can J Physiol Pharmacol. 1988 Jul;66(7):967–970. doi: 10.1139/y88-159. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
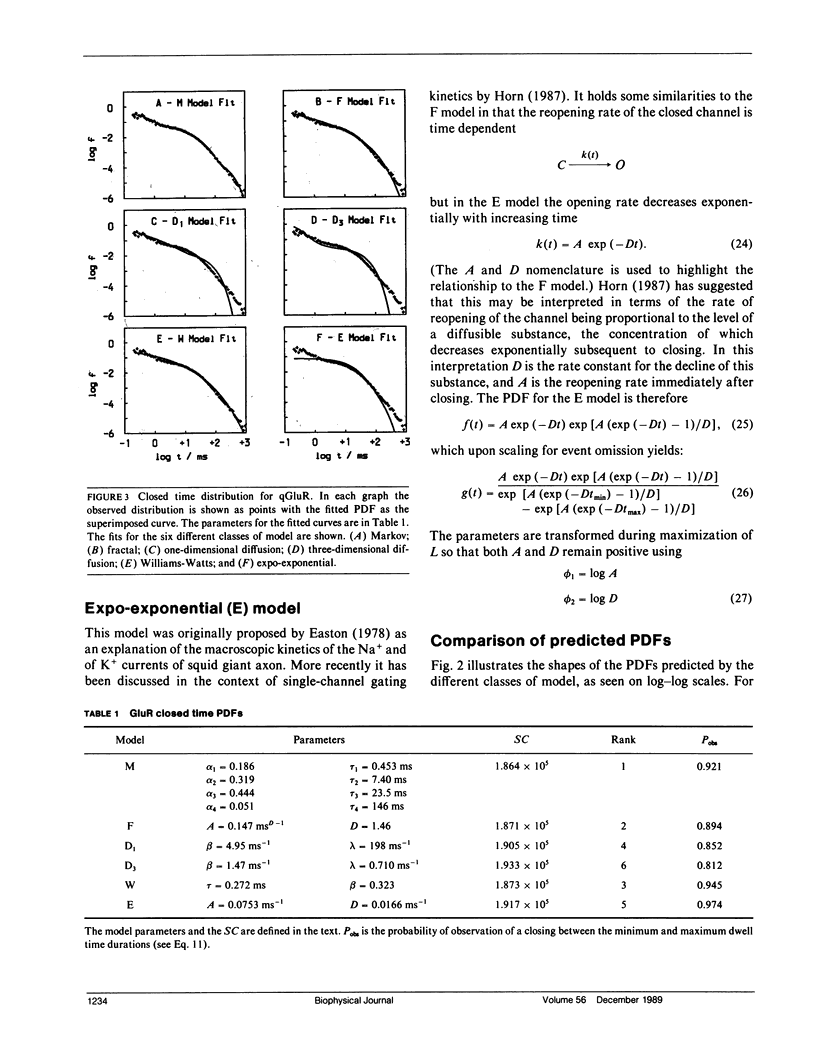
- Horn R., Korn S. J. Model selection: reliability and bias. Biophys J. 1989 Feb;55(2):379–381. doi: 10.1016/S0006-3495(89)82816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Lange K. Estimating kinetic constants from single channel data. Biophys J. 1983 Aug;43(2):207–223. doi: 10.1016/S0006-3495(83)84341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R. Statistical methods for model discrimination. Applications to gating kinetics and permeation of the acetylcholine receptor channel. Biophys J. 1987 Feb;51(2):255–263. doi: 10.1016/S0006-3495(87)83331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
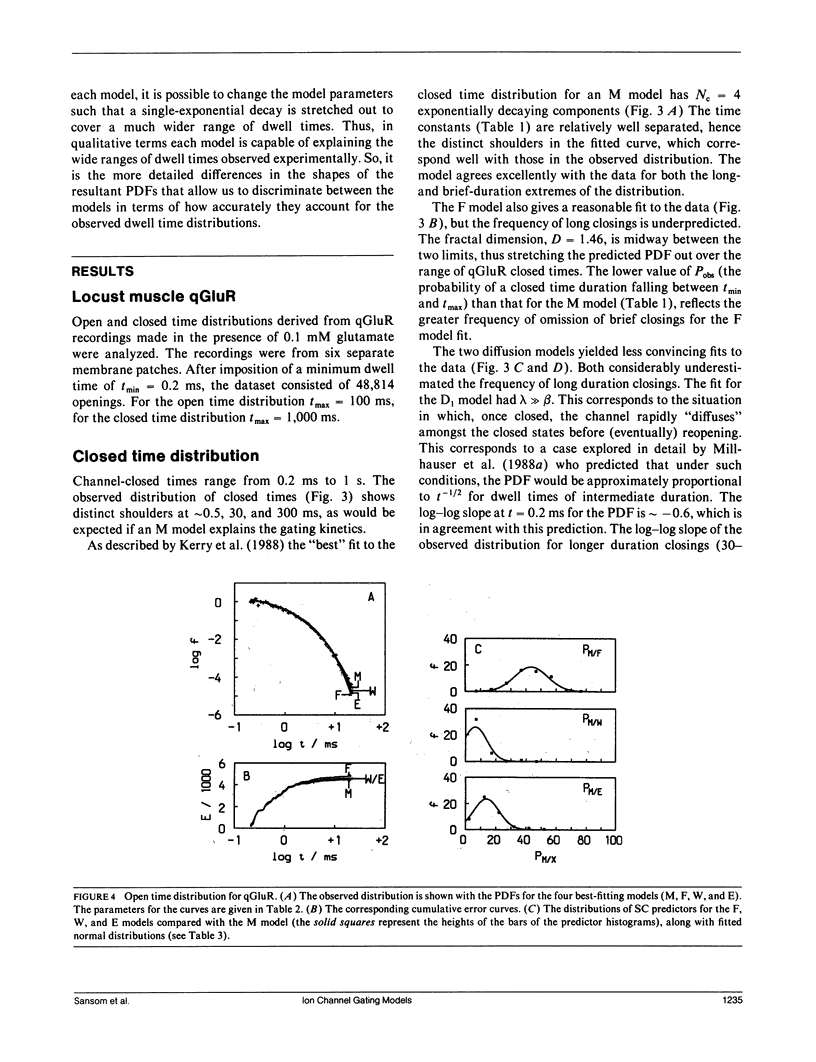
- Kerry C. J., Kits K. S., Ramsey R. L., Sansom M. S., Usherwood P. N. Single channel kinetics of a glutamate receptor. Biophys J. 1987 Jan;51(1):137–144. doi: 10.1016/S0006-3495(87)83318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerry C. J., Ramsey R. L., Sansom M. S., Usherwood P. N. Glutamate receptor channel kinetics: the effect of glutamate concentration. Biophys J. 1988 Jan;53(1):39–52. doi: 10.1016/S0006-3495(88)83064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klafter J., Shlesinger M. F. On the relationship among three theories of relaxation in disordered systems. Proc Natl Acad Sci U S A. 1986 Feb;83(4):848–851. doi: 10.1073/pnas.83.4.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S. J., Horn R. Statistical discrimination of fractal and Markov models of single-channel gating. Biophys J. 1988 Nov;54(5):871–877. doi: 10.1016/S0006-3495(88)83023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca P., Rice J. A., Fredkin D. R., Montal M. Kinetic analysis of channel gating. Application to the cholinergic receptor channel and the chloride channel from Torpedo californica. Biophys J. 1985 Apr;47(4):469–478. doi: 10.1016/S0006-3495(85)83939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landaw E. M., DiStefano J. J., 3rd Multiexponential, multicompartmental, and noncompartmental modeling. II. Data analysis and statistical considerations. Am J Physiol. 1984 May;246(5 Pt 2):R665–R677. doi: 10.1152/ajpregu.1984.246.5.R665. [DOI] [PubMed] [Google Scholar]
- Liebovitch L. S. Analysis of fractal ion channel gating kinetics: kinetic rates, energy levels, and activation energies. Math Biosci. 1989 Mar;93(1):97–115. doi: 10.1016/0025-5564(89)90015-1. [DOI] [PubMed] [Google Scholar]
- Liebovitch L. S., Fischbarg J., Koniarek J. P., Todorova I., Wang M. Fractal model of ion-channel kinetics. Biochim Biophys Acta. 1987 Jan 26;896(2):173–180. doi: 10.1016/0005-2736(87)90177-5. [DOI] [PubMed] [Google Scholar]
- Liebovitch L. S., Sullivan J. M. Fractal analysis of a voltage-dependent potassium channel from cultured mouse hippocampal neurons. Biophys J. 1987 Dec;52(6):979–988. doi: 10.1016/S0006-3495(87)83290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebovitch L. S. Testing fractal and Markov models of ion channel kinetics. Biophys J. 1989 Feb;55(2):373–377. doi: 10.1016/S0006-3495(89)82815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
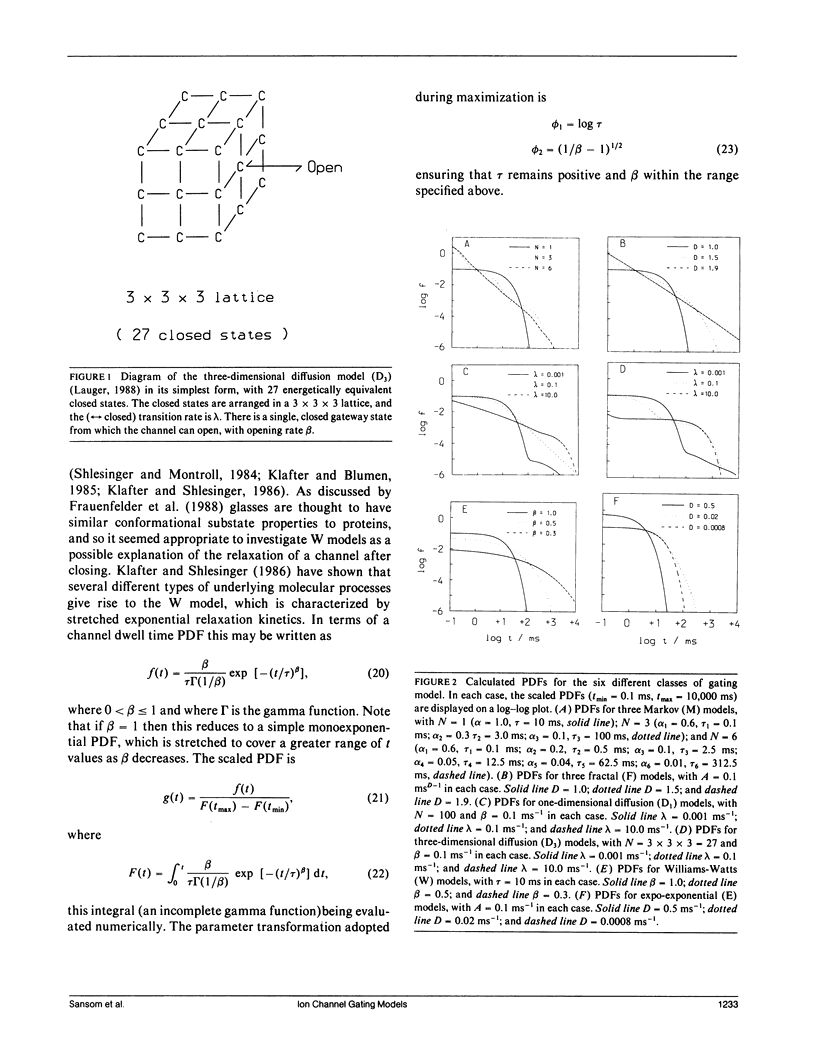
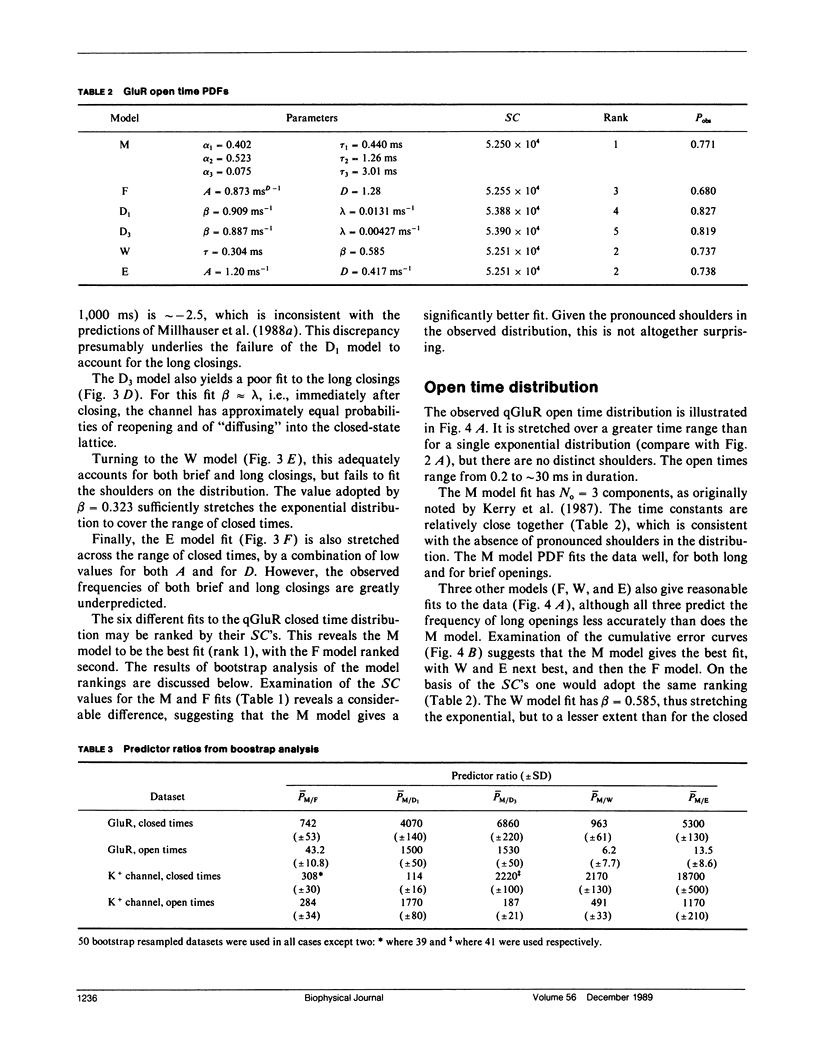
- Läuger P. Internal motions in proteins and gating kinetics of ionic channels. Biophys J. 1988 Jun;53(6):877–884. doi: 10.1016/S0006-3495(88)83168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
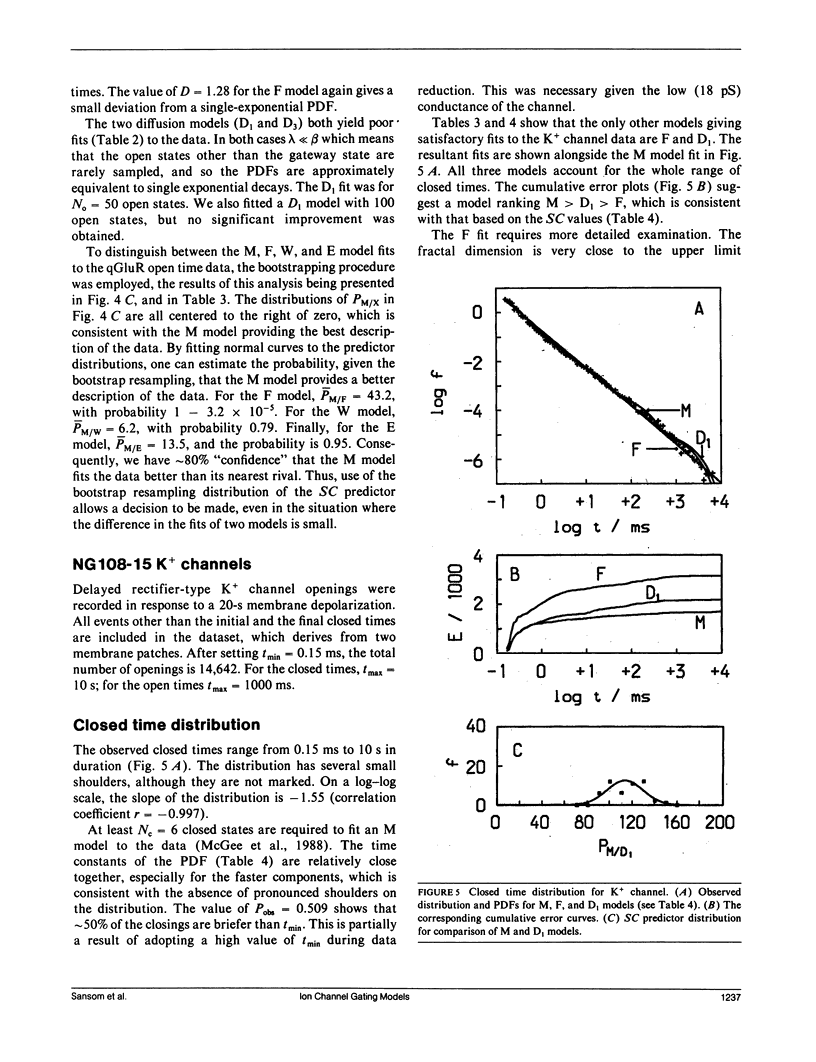
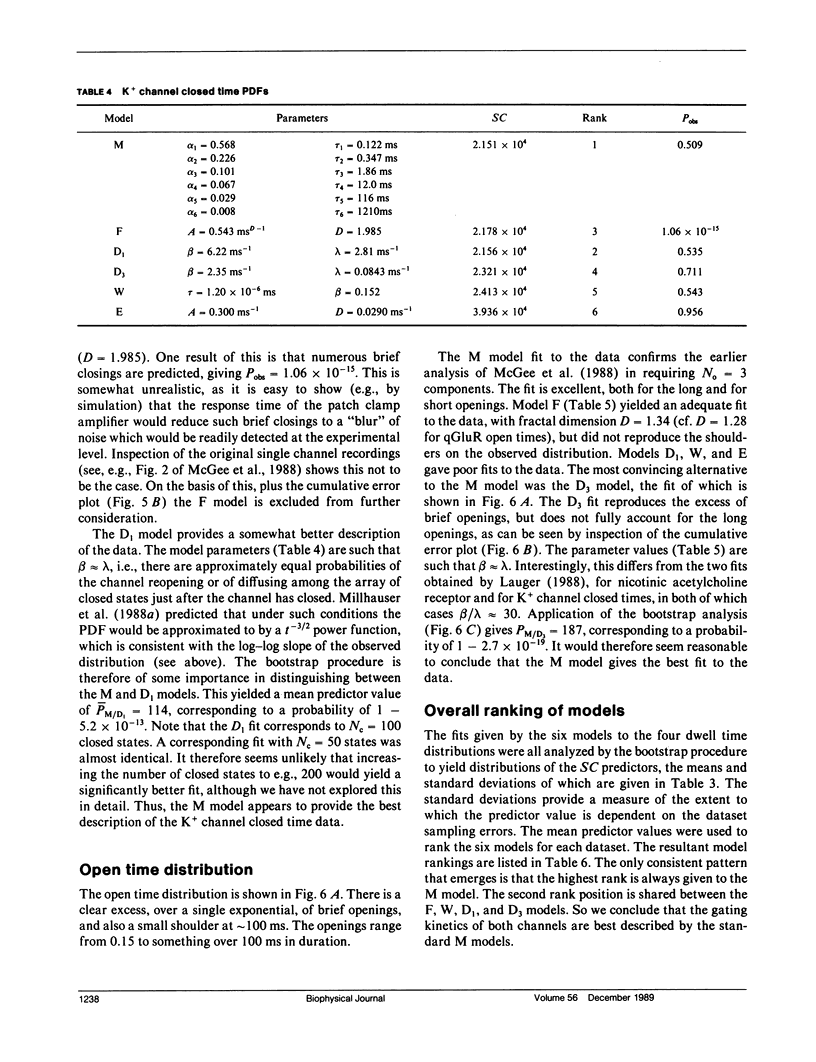
- McGee R., Jr, Sansom M. S., Usherwood P. N. Characterization of a delayed rectifier K+ channel in NG108-15 neuroblastoma X glioma cells: gating kinetics and the effects of enrichment of membrane phospholipids with arachidonic acid. J Membr Biol. 1988 Apr;102(1):21–34. doi: 10.1007/BF01875350. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Inverse relationship of the durations of adjacent open and shut intervals for C1 and K channels. Nature. 1985 Oct 17;317(6038):625–627. doi: 10.1038/317625a0. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Kinetic states and modes of single large-conductance calcium-activated potassium channels in cultured rat skeletal muscle. J Physiol. 1988 Aug;402:79–120. doi: 10.1113/jphysiol.1988.sp017195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Spivak C. E., Blatz A. L., Weiss D. S., Magleby K. L. Fractal models, Markov models, and channel kinetics. Biophys J. 1989 Feb;55(2):383–385. doi: 10.1016/S0006-3495(89)82817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Weiss D. S., Spivak C. E., Blatz A. L., Magleby K. L. Fractal models are inadequate for the kinetics of four different ion channels. Biophys J. 1988 Nov;54(5):859–870. doi: 10.1016/S0006-3495(88)83022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhauser G. L., Salpeter E. E., Oswald R. E. Diffusion models of ion-channel gating and the origin of power-law distributions from single-channel recording. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1503–1507. doi: 10.1073/pnas.85.5.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhauser G. L., Salpeter E. E., Oswald R. E. Rate-amplitude correlation from single-channel records. A hidden structure in ion channel gating kinetics? Biophys J. 1988 Dec;54(6):1165–1168. doi: 10.1016/S0006-3495(88)83051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne R. K., Yeo G. F., Edeson R. O., Madsen B. W. Stochastic modelling of a single ion channel: an alternating renewal approach with application to limited time resolution. Proc R Soc Lond B Biol Sci. 1988 Apr 22;233(1272):247–292. doi: 10.1098/rspb.1988.0022. [DOI] [PubMed] [Google Scholar]
- Papke R. L., Millhauser G., Lieberman Z., Oswald R. E. Relationships of agonist properties to the single channel kinetics of nicotinic acetylcholine receptors. Biophys J. 1988 Jan;53(1):1–10. doi: 10.1016/S0006-3495(88)83059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
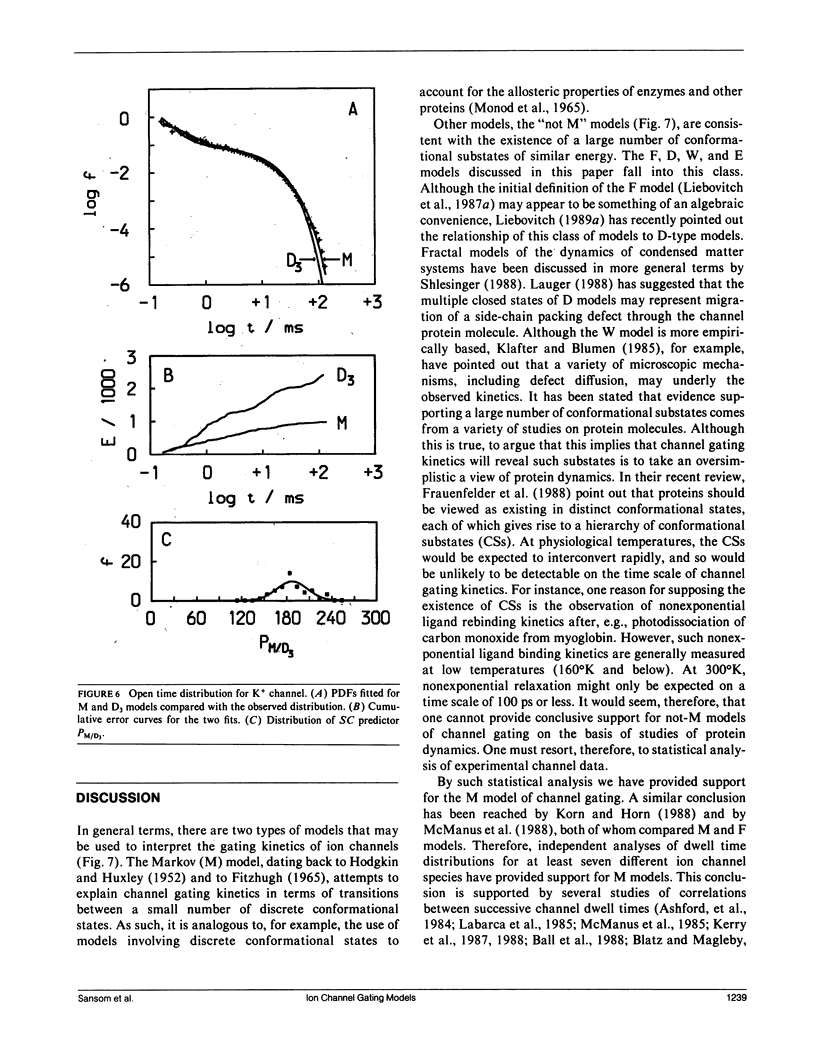
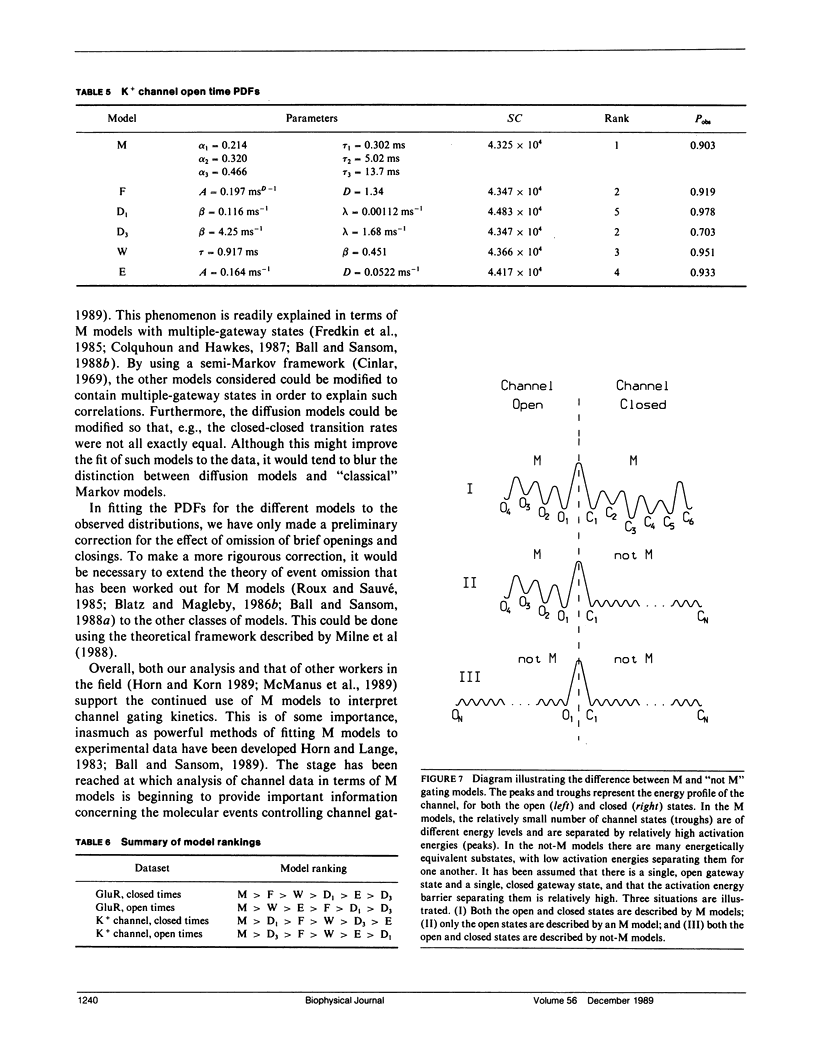
- Roux B., Sauvé R. A general solution to the time interval omission problem applied to single channel analysis. Biophys J. 1985 Jul;48(1):149–158. doi: 10.1016/S0006-3495(85)83768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlesinger M. F., Montroll E. W. On the Williams-Watts function of dielectric relaxation. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1280–1283. doi: 10.1073/pnas.81.4.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]


