Abstract
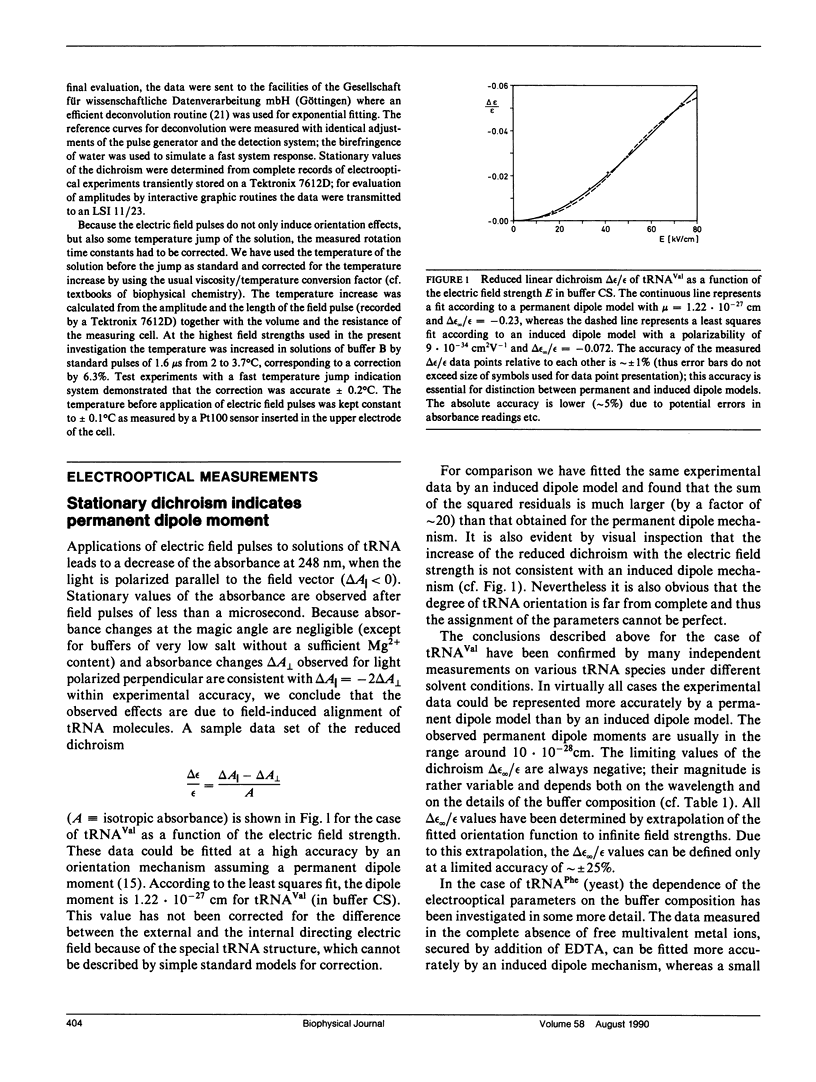
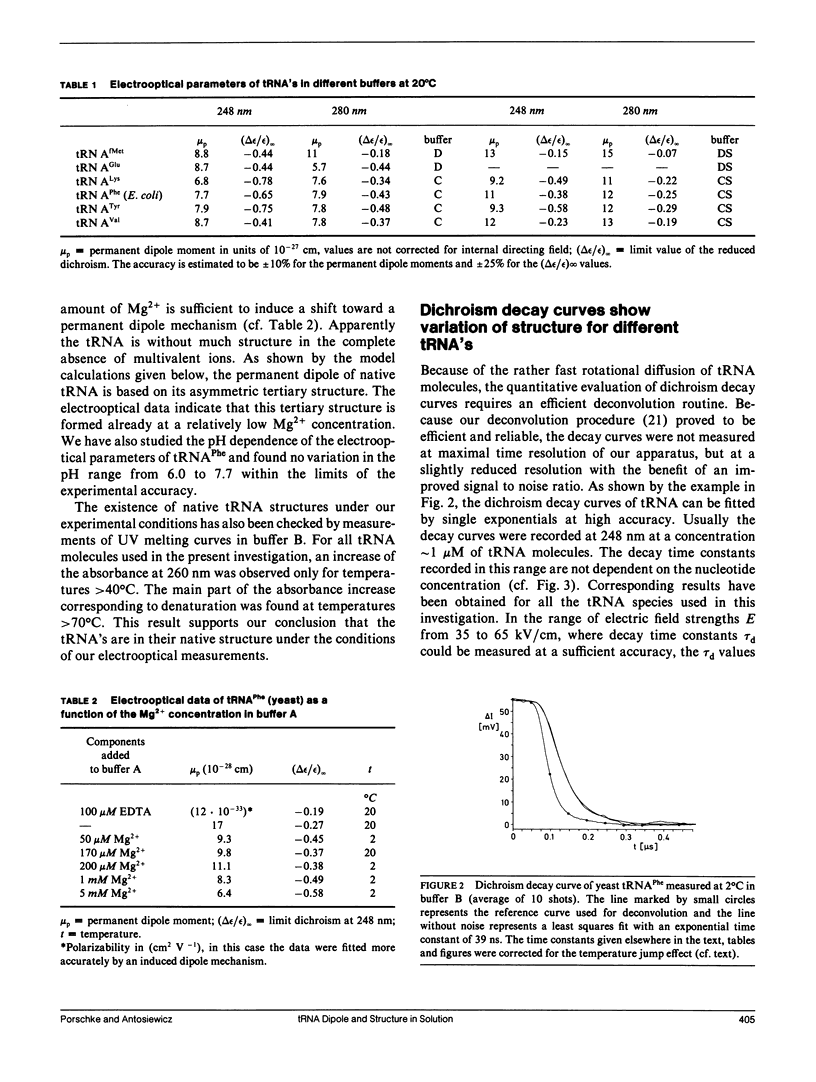
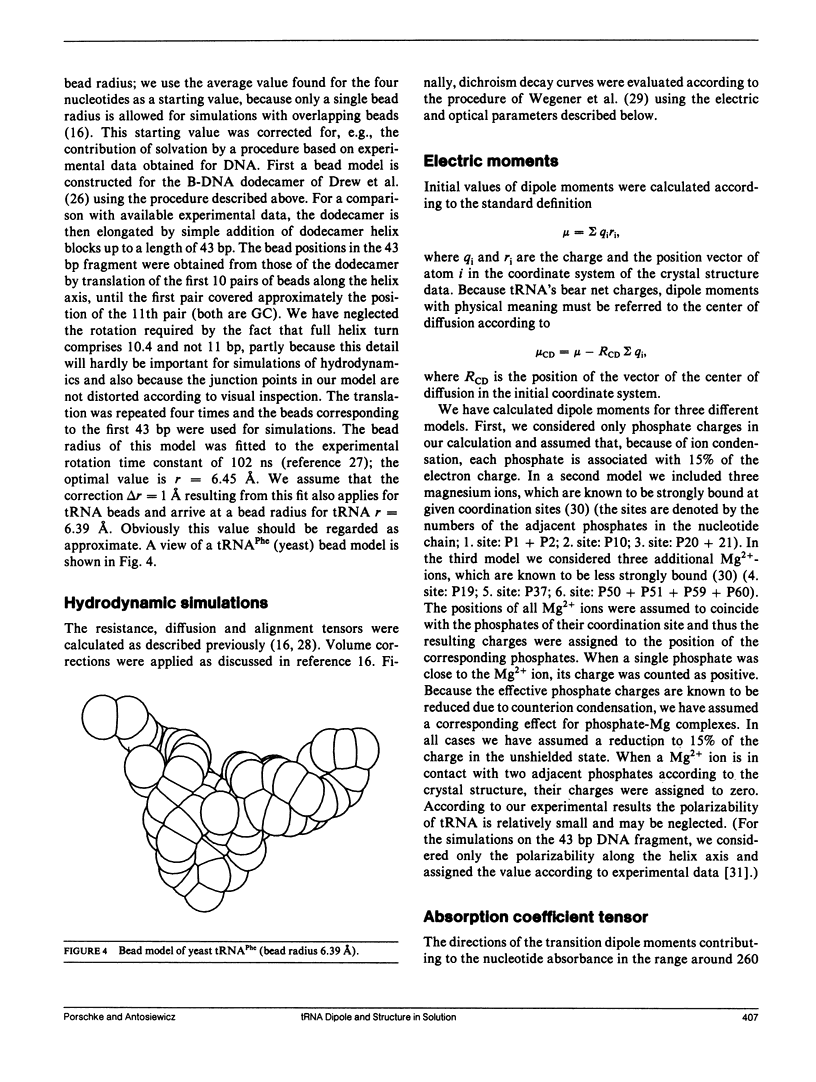
The structure of six different tRNA molecules has been analyzed in solution by electrooptical measurements and by bead model simulations. The electric dichroism measured as a function of the field strength shows that tRNA's are associated with substantial permanent dipole moments, which are in the range of 1 x 10(-27) cm(identical to 300 D; before correction for the internal directing field). Rotational diffusion time constants of tRNA molecules in their native state at 2 degrees C show a considerable variation. A particularly large value found for tRNA(Tyr) (50 ns) can be explained by its nine additional nucleotide residues. However, remarkable variations remain for tRNA molecules with the standard number of 76 nucleotide residues (tRNA(Phe) [yeast] 41.6 ns, tRNA(Val) [Escherichia coli] 44.9 ns, tRNA(Glu) [E. coli] 46.8 ns; tRNA(Phe) [E. coli] 48.3 ns). These variations indicate modulations of the tertiary structure, which may be due to a change of the L-hinge angle. Bead models are used to simulate both electric and hydrodynamic parameters of tRNA molecules according to the crystal structure of tRNA(Phe) (yeast). The asymmetric distribution of phosphate charges with respect to the center of diffusion leads, under the assumption of a constant charge reduction to 15% by ion condensation, to a theoretical dipole moment of 7.2 x 10(-28) cm, which is in reasonable agreement with the measurements. The dichroism decay curve calculated for tRNA(Phe) (yeast) is also consistent with the measurements and thus the structure in solution and in the crystal must be very similar in this case. However, our measurements also indicate that the structure of some other tRNA's in solution is different, even in cases with the same number of nucleotide residues.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antosiewicz J., Porschke D. An unusual electrooptical effect observed for DNA fragments and its apparent relation to a permanent electric moment associated with bent DNA. Biophys Chem. 1989 Mar;33(1):19–30. doi: 10.1016/0301-4622(89)80003-1. [DOI] [PubMed] [Google Scholar]
- Antosiewicz J., Porschke D. Turn of promotor DNA by cAMP receptor protein characterized by bead model simulation of rotational diffusion. J Biomol Struct Dyn. 1988 Feb;5(4):819–837. doi: 10.1080/07391102.1988.10506429. [DOI] [PubMed] [Google Scholar]
- Chen M. C., Giegé R., Lord R. C., Rich A. Raman spectra and structure of yeast phenylalanine transfer RNA in the crystalline state and in solution. Biochemistry. 1975 Oct 7;14(20):4385–4391. doi: 10.1021/bi00691a007. [DOI] [PubMed] [Google Scholar]
- Claesens F., Rigler R. Conformational dynamics of the anticodon loop in yeast tRNAPhe as sensed by the fluorescence of wybutine. Eur Biophys J. 1986;13(6):331–342. doi: 10.1007/BF00265669. [DOI] [PubMed] [Google Scholar]
- Diekmann S., Hillen W., Jung M., Wells R. D., Pörschke D. Electric properties and structure of DNA-restriction fragments from measurements of the electric dichroism. Biophys Chem. 1982 May;15(2):157–167. doi: 10.1016/0301-4622(82)80028-8. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Wing R. M., Takano T., Broka C., Tanaka S., Itakura K., Dickerson R. E. Structure of a B-DNA dodecamer: conformation and dynamics. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2179–2183. doi: 10.1073/pnas.78.4.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de la Torre J. G., Bloomfield V. A. Hydrodynamic properties of complex, rigid, biological macromolecules: theory and applications. Q Rev Biophys. 1981 Feb;14(1):81–139. doi: 10.1017/s0033583500002080. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Söll D. G., Crothers D. M. Studies of the complex between transfer RNAs with complementary anticodons. I. Origins of enhanced affinity between complementary triplets. J Mol Biol. 1976 May 25;103(3):499–519. doi: 10.1016/0022-2836(76)90214-x. [DOI] [PubMed] [Google Scholar]
- Heerschap A., Mellema J. R., Janssen H. G., Walters J. A., Haasnoot C. A., Hilbers C. W. Imino-proton resonances of yeast tRNAPhe studied by two-dimensional nuclear Overhauser enhancement spectroscopy. Eur J Biochem. 1985 Jun 18;149(3):649–655. doi: 10.1111/j.1432-1033.1985.tb08973.x. [DOI] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Klug A. Crystallographic refinement of yeast phenylalanine transfer RNA at 2-5A resolution. J Mol Biol. 1976 Dec 25;108(4):619–649. doi: 10.1016/s0022-2836(76)80109-x. [DOI] [PubMed] [Google Scholar]
- Labuda D., Striker G., Porschke D. Mechanism of codon recognition by transfer RNA and codon-induced tRNA association. J Mol Biol. 1984 Apr 25;174(4):587–604. doi: 10.1016/0022-2836(84)90085-8. [DOI] [PubMed] [Google Scholar]
- Ladner J. E., Jack A., Robertus J. D., Brown R. S., Rhodes D., Clark B. F., Klug A. Atomic co-ordinates for yeast phenylalanine tRNA. Nucleic Acids Res. 1975 Sep;2(9):1629–1637. doi: 10.1093/nar/2.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois R., Kim S. H., Cantor C. R. A comparison of the fluorescence of the Y base of yeast tRNA-Phe in solution and in crystals. Biochemistry. 1975 Jun 3;14(11):2554–2558. doi: 10.1021/bi00682a040. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Moras D., Dock A. C., Dumas P., Westhof E., Romby P., Ebel J. P., Giegé R. Anticodon-anticodon interaction induces conformational changes in tRNA: yeast tRNAAsp, a model for tRNA-mRNA recognition. Proc Natl Acad Sci U S A. 1986 Feb;83(4):932–936. doi: 10.1073/pnas.83.4.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porschke D., Jung M. The conformation of single stranded oligonucleotides and of oligonucleotide-oligopeptide complexes from their rotation relaxation in the nanosecond time range. J Biomol Struct Dyn. 1985 Jun;2(6):1173–1184. doi: 10.1080/07391102.1985.10507631. [DOI] [PubMed] [Google Scholar]
- Porschke D. Structure and dynamics of double helices in solution: modes of DNA bending. J Biomol Struct Dyn. 1986 Dec;4(3):373–389. doi: 10.1080/07391102.1986.10506356. [DOI] [PubMed] [Google Scholar]
- Porschke D. The mechanism of ion polarisation along DNA double helices. Biophys Chem. 1985 Aug;22(3):237–247. doi: 10.1016/0301-4622(85)80046-6. [DOI] [PubMed] [Google Scholar]
- Pörschke D. Structure and dynamics of a tryptophanepeptide-polynucleotide complex. Nucleic Acids Res. 1980 Apr 11;8(7):1591–1612. doi: 10.1093/nar/8.7.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pörschke D. Threshold effects observed in conformation changes induced by electric fields. Biopolymers. 1976 Oct;15(10):1917–1928. doi: 10.1002/bip.1976.360151004. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Seeman N. C., Wang A. H., Suddath F. L., Rich A. Yeast phenylalanine transfer RNA: atomic coordinates and torsion angles. Nucleic Acids Res. 1975 Dec;2(12):2329–2341. doi: 10.1093/nar/2.12.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigler R., Wintermeyer W. Dynamics of tRNA. Annu Rev Biophys Bioeng. 1983;12:475–505. doi: 10.1146/annurev.bb.12.060183.002355. [DOI] [PubMed] [Google Scholar]
- Rizzo V., Schellman J. A. Matrix-method calculation of linear and circular dichroism spectra of nucleic acids and polynucleotides. Biopolymers. 1984 Mar;23(3):435–470. doi: 10.1002/bip.360230305. [DOI] [PubMed] [Google Scholar]
- Romby P., Moras D., Dumas P., Ebel J. P., Giegé R. Comparison of the tertiary structure of yeast tRNA(Asp) and tRNA(Phe) in solution. Chemical modification study of the bases. J Mol Biol. 1987 May 5;195(1):193–204. doi: 10.1016/0022-2836(87)90336-6. [DOI] [PubMed] [Google Scholar]
- Schevitz R. W., Podjarny A. D., Krishnamachari N., Hughes J. J., Sigler P. B., Sussman J. L. Crystal structure of a eukaryotic initiator tRNA. Nature. 1979 Mar 8;278(5700):188–190. doi: 10.1038/278188a0. [DOI] [PubMed] [Google Scholar]
- Schimmel P. R., Redfield A. G. Transfer RNA in solution: selected topics. Annu Rev Biophys Bioeng. 1980;9:181–221. doi: 10.1146/annurev.bb.09.060180.001145. [DOI] [PubMed] [Google Scholar]
- Striker G., Labuda D., Vega-Martin M. C. The three conformations of the anticodon loop of yeast tRNA(Phe). J Biomol Struct Dyn. 1989 Oct;7(2):235–255. doi: 10.1080/07391102.1989.10507768. [DOI] [PubMed] [Google Scholar]
- Tao T., Nelson J. H., Cantor C. R. Conformational studies on transfer ribonucleic acid. Fluorescence lifetime and nanosecond depolarization measurements on bound ethidium bromidee. Biochemistry. 1970 Sep 1;9(18):3514–3524. doi: 10.1021/bi00820a004. [DOI] [PubMed] [Google Scholar]
- Thompson M. R., Williams R. C., O'Neal C. H. Studies on proteins and tRNA with transient electric birefringence. Biophys J. 1978 Oct;24(1):264–266. doi: 10.1016/S0006-3495(78)85373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells B. D., Lakowicz J. R. Intensity and anisotropy decays of the Wye base of yeast tRNA(Phe) as measured by frequency-domain fluorometry. Biophys Chem. 1987 Apr;26(1):39–43. doi: 10.1016/0301-4622(87)80005-4. [DOI] [PubMed] [Google Scholar]
- Woo N. H., Roe B. A., Rich A. Three-dimensional structure of Escherichia coli initiator tRNAfMet. Nature. 1980 Jul 24;286(5771):346–351. doi: 10.1038/286346a0. [DOI] [PubMed] [Google Scholar]


