Abstract
1. Two types of effects of tetraethylammonium chloride (TEA) have been found in the smooth muscle cells of the rabbit main pulmonary artery. (a) With rapid onset of action TEA depolarizes the cell membrane, increases the membrane resistance, causes anomalous rectification and occasionally spike potentials in response to externally applied depolarizing current pulses and produces tonic contractions. (b) During prolonged (greater than 30 min) incubation in TEA phasic contractions develop progressively and the vascular strips respond to electrical stimulation with synchronized and powerful contractions. 2. There is a linear relationship between log concentration TEA and depolarization over the range of 10-100 mM-TEA. TEA (10 and 30 mM) raises the membrane resistance and decreases the core resistance. The latter effect appeared to develop more slowly than the former. 3. During short exposure to TEA part of the smooth muscle cells respond to depolarizing current pulses with spike potentials of variable amplitude and duration. These spikes are very sensitive to inhibition by verapamil or nickel chloride but are not affected by tetrodotoxin. The amplitude of electrotonic potentials, increased by TEA, is slightly further elevated by verapamil or nickle chloride. 4. TEA (10 mM) increases the mechanical response to low and intermediate potassium concentrations but has no effect on maximal contractions to high potassium. The slope of the line relating log potassium concentration to membrane potential is decreased by TEA. 5. TEA (10 mM) shifts the concentration response curve for the contractile effect of noradrenaline to the left and increases the maximum of noradrenaline-induced contractions. In the presence of TEA, noradrenaline reduces the membrane potential to markedly lower values than under control conditions. 6. It is concluded that the rapidly occurring effects of TEA on the vascular smooth muscle cells of the rabbit main pulmonary artery are a decrease in potassium and an increase in calcium conductance. The latter effect may be related to a blockade of potassium channels; however, we cannot rule out the possibility that TEA affects calcium conductance independent of its presumed action on potassium channels. The slowly developing effects of TEA may be ascribed to the formation of gap junctions and/or (less likely) to an intracellular accumulation of TEA.
Full text
PDF


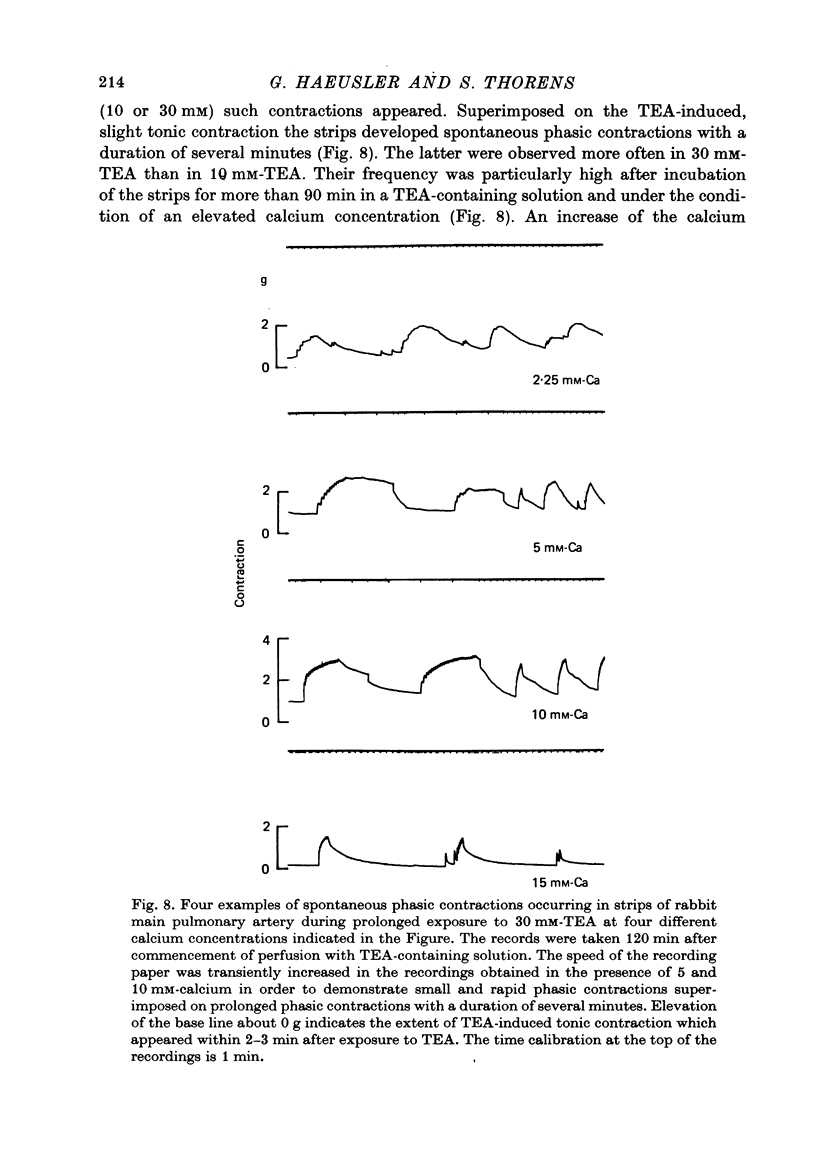


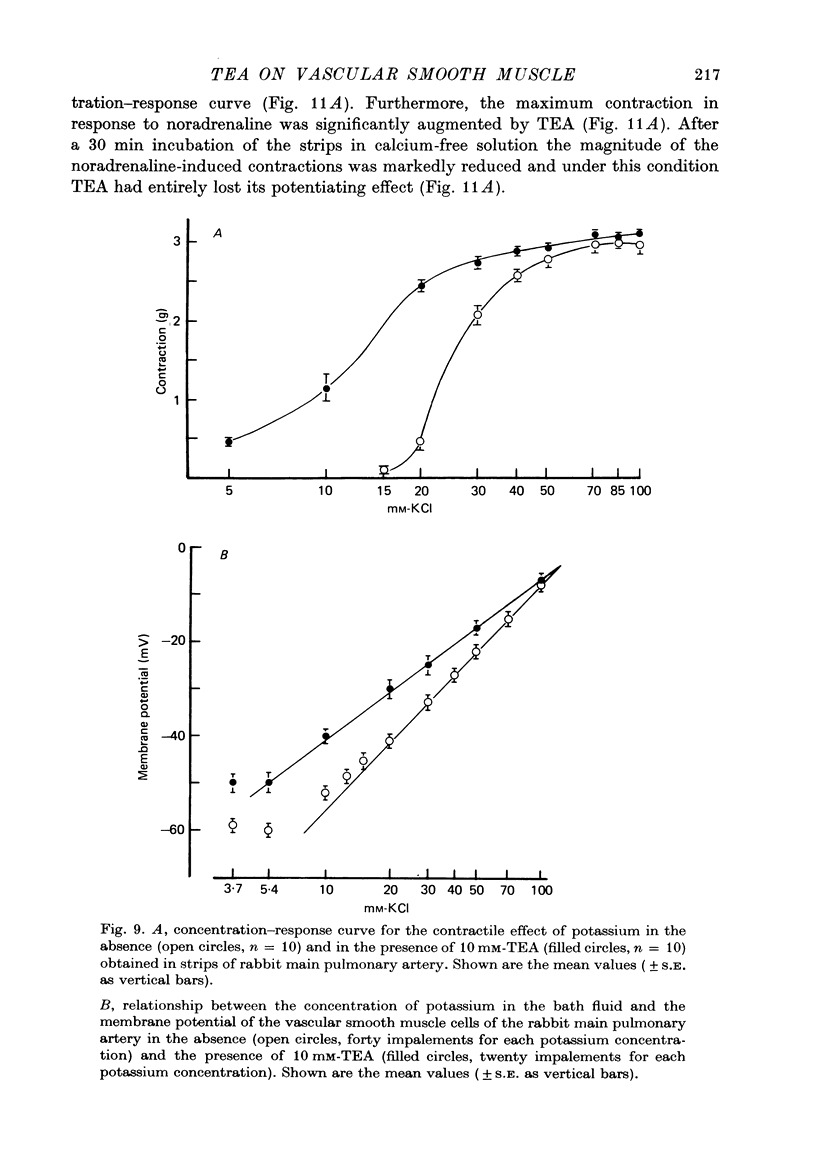
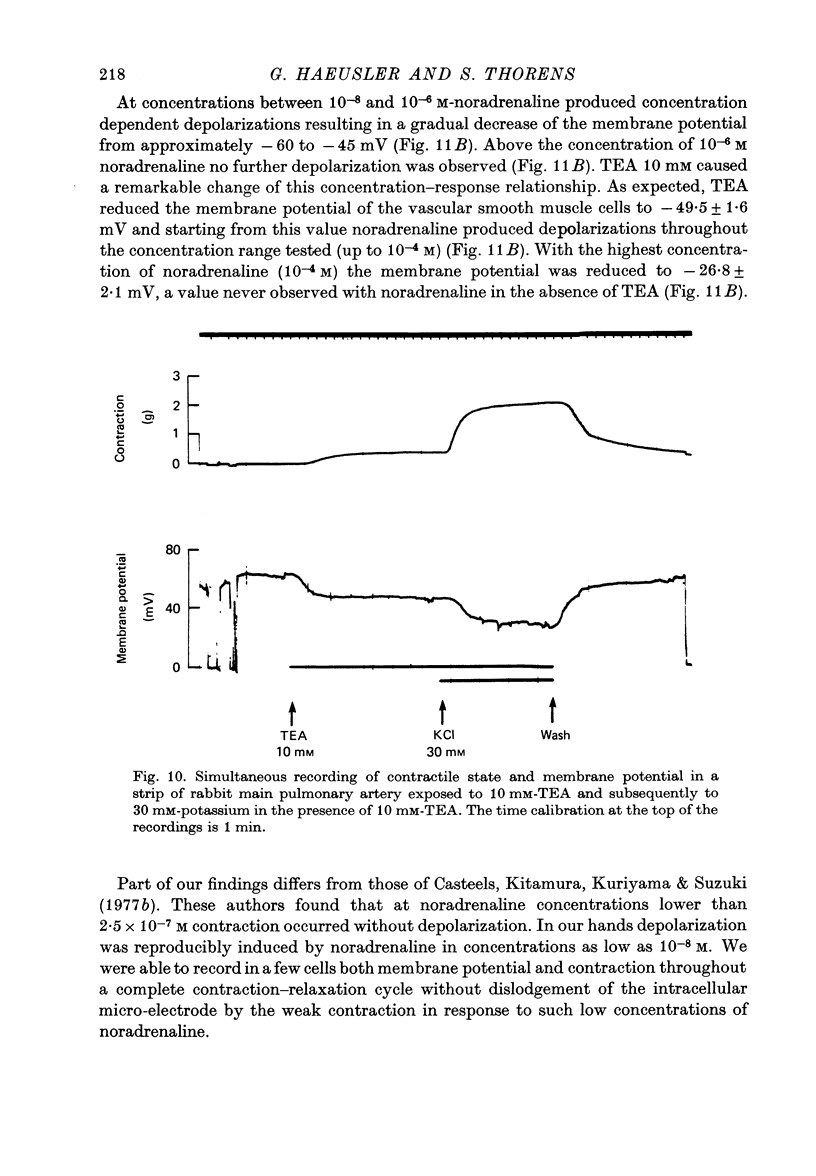
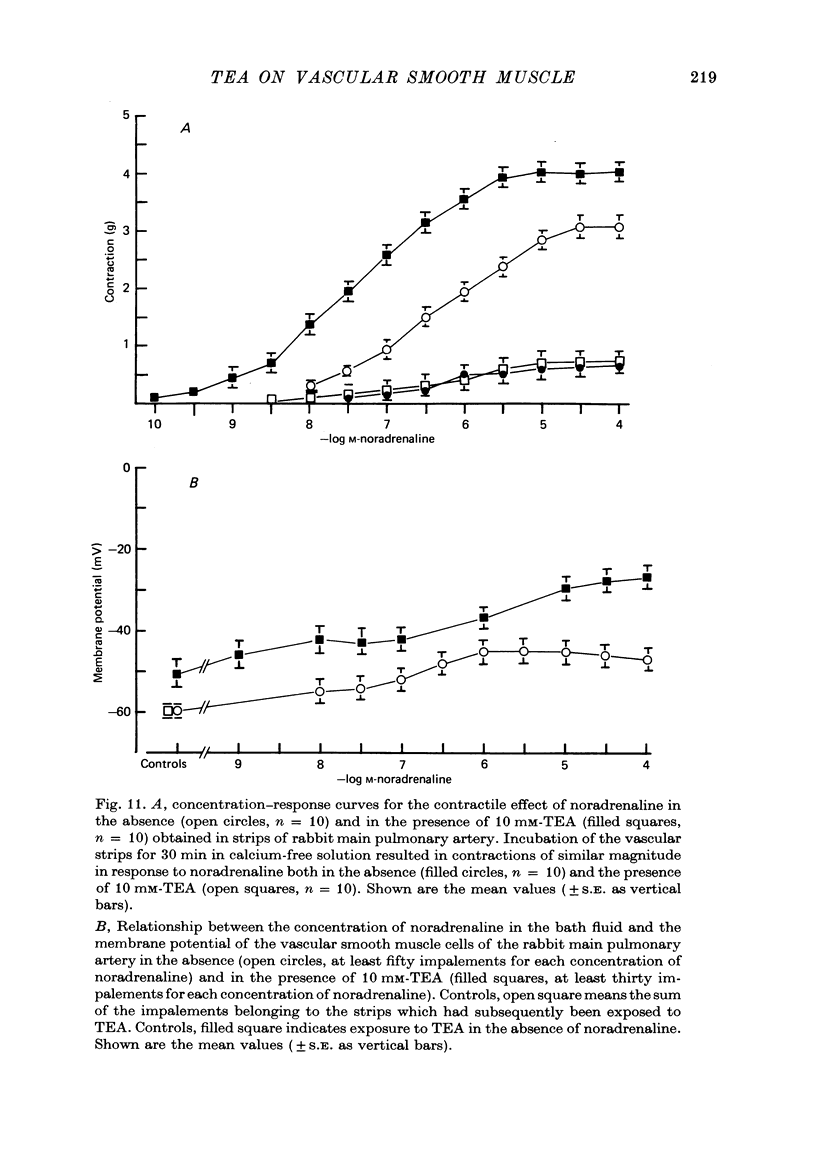





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Inactivation of the potassium conductance and related phenomena caused by quaternary ammonium ion injection in squid axons. J Gen Physiol. 1969 Nov;54(5):553–575. doi: 10.1085/jgp.54.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Dawson C. M., Ribalet B., Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J Physiol. 1979 Mar;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- BEVAN J. A. Action of tetraethylammonium chloride on the sympathetic ganglia-pulmonary artery preparation. J Pharmacol Exp Ther. 1963 May;140:193–198. [PubMed] [Google Scholar]
- Bassingthwaighte J. B., Fry C. H., McGuigan J. A. Relationship between internal calcium and outward current in mammalian ventricular muscle; a mechanism for the control of the action potential duration? J Physiol. 1976 Oct;262(1):15–37. doi: 10.1113/jphysiol.1976.sp011583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. Excitation-contraction coupling in the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):63–79. doi: 10.1113/jphysiol.1977.sp011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. The membrane properties of the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):41–61. doi: 10.1113/jphysiol.1977.sp011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. S., Di Palma J. R. Studies on the interaction of norepinephrine with some other drugs on the aortic smooth muscle of the rabbit. Arch Int Pharmacodyn Ther. 1973 Sep;205(1):11–22. [PubMed] [Google Scholar]
- Creed K. E., Gillespie J. S., Muir T. C. The electrical basis of excitation and inhibition in the rat anoccygeus muscle. J Physiol. 1975 Feb;245(1):33–47. doi: 10.1113/jphysiol.1975.sp010833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Capacitance of the surface and transverse tubular membrane of frog sartorius muscle fibers. J Gen Physiol. 1969 Mar;53(3):265–278. doi: 10.1085/jgp.53.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler G. Differential effect of verapamil on excitation-contraction coupling in smooth muscle and on excitation-secretion coupling in adrenergic nerve terminals. J Pharmacol Exp Ther. 1972 Mar;180(3):672–682. [PubMed] [Google Scholar]
- Haeusler G., Kuhn H., Thorens S. The effect of tetraethylammonium chloride on calcium fluxes in smooth muscle from rabbit main pulmonary artery. J Physiol. 1980 Jun;303:225–241. doi: 10.1113/jphysiol.1980.sp013282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler G. Relationship between noradrenaline-induced depolarization and contraction in vascular smooth muscle. Blood Vessels. 1978;15(1-3):46–54. doi: 10.1159/000158152. [DOI] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H., Sakamoto Y. Effects of tetraethylammonium chloride on the membrane activity of guinea-pig stomach smooth muscle. J Physiol. 1970 Dec;211(2):445–460. doi: 10.1113/jphysiol.1970.sp009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELKAR V. V., GULATI O. D., GOKHALE S. D. EFFECT OF GANGLION BLOCKING DRUGS ON THE RESPONSES OF THE RABBIT AORTIC STRIP TO ADRENALINE, NORADRENALINE AND OTHER VASOACTIVE SUBSTANCES. Arch Int Pharmacodyn Ther. 1964 May 1;149:209–222. [PubMed] [Google Scholar]
- Kannan M. S., Daniel E. E. Formation of gap junctions by treatment in vitro with potassium conductance blockers. J Cell Biol. 1978 Aug;78(2):338–348. doi: 10.1083/jcb.78.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y., Stanfield P. R. Actions of some cations on the electrical properties and mechanical threshold of frog sartorius muscle fibers. J Gen Physiol. 1970 May;55(5):620–639. doi: 10.1085/jgp.55.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. T. Excitation and contraction in bovine tracheal smooth muscle. J Physiol. 1975 Jan;244(2):263–281. doi: 10.1113/jphysiol.1975.sp010796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhaus A. L., Prichard J. W. Calcium dependent action potentials produced in leech Retzius cells by tetraethylammonium chloride. J Physiol. 1975 Mar;246(2):351–369. doi: 10.1113/jphysiol.1975.sp010894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger E. A., Stephens N. L. Effect of tetraethylammonium on tonic airway smooth muscle: initiation of phasic electrical activity. Am J Physiol. 1975 Feb;228(2):633–636. doi: 10.1152/ajplegacy.1975.228.2.633. [DOI] [PubMed] [Google Scholar]
- LUM B. K., RASHLEIGH P. L. Potentiation of vasoactive drugs by ganglionic blocking agents. J Pharmacol Exp Ther. 1961 Apr;132:13–18. [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Electrophysiological studies of the smooth muscle cell membrane of the rabbit common carotid artery. J Gen Physiol. 1971 Jun;57(6):738–751. doi: 10.1085/jgp.57.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Lux H. D. Differential action of TEA + on two K + -current componentss of a molluscan neurone. Pflugers Arch. 1972;336(2):87–100. doi: 10.1007/BF00592924. [DOI] [PubMed] [Google Scholar]
- Ochi R., Nishiye H. Effect of intracellular tetraethylammonium ion on action potential in the guinea-pig's myocardium. Pflugers Arch. 1974 May 6;348(4):305–316. doi: 10.1007/BF00589220. [DOI] [PubMed] [Google Scholar]
- Osa T., Kuriyama H. The membrane properties and decremental conduction of excitation in the fundus of the guinea-pig stomach. Jpn J Physiol. 1970 Dec 15;20(6):626–639. doi: 10.2170/jjphysiol.20.626. [DOI] [PubMed] [Google Scholar]
- Porzig H. Comparative study of the effects of propranolol and tetracaine on cation movements in resealed human red cell ghosts. J Physiol. 1975 Jul;249(1):27–49. doi: 10.1113/jphysiol.1975.sp011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Blaustein M. P., Haeusler G. Na-Ca exchange and tension development in arterial smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):87–94. doi: 10.1098/rstb.1973.0011. [DOI] [PubMed] [Google Scholar]
- Romero P. J., Whittam R. The control by internal calcium of membrane permeability to sodium and potassium. J Physiol. 1971 May;214(3):481–507. doi: 10.1113/jphysiol.1971.sp009445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI T., NISHIYAMA A., INOMATA H. Effect of tetraethyl ammonium ion on the electrical activity of smooth muscle cell. Nature. 1963 Mar 2;197:908–909. doi: 10.1038/197908a0. [DOI] [PubMed] [Google Scholar]
- Sandow A. Skeletal muscle. Annu Rev Physiol. 1970;32:87–138. doi: 10.1146/annurev.ph.32.030170.000511. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968 Jan;159(1):129–145. [PubMed] [Google Scholar]
- Somlyo A. V., Vinall P., Somlyo A. P. Excitation-contraction coupling and electrical events in two types of vascular smooth muscle. Microvasc Res. 1969 Oct;1(4):354–373. doi: 10.1016/0026-2862(69)90014-4. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. The onset of the effects of zinc and tetraethylammonium ions on action potential duration and twitch amplitude of single muscle fibres. J Physiol. 1973 Dec;235(3):639–654. doi: 10.1113/jphysiol.1973.sp010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurszewski J. H. A study of the canine gastric action potential in the presence of tetraethylammonium chloride. J Physiol. 1978 Apr;277:91–102. doi: 10.1113/jphysiol.1978.sp012262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Haefely W., Staehelin H. Potentiation by tetraethylammonium of the response of the cat spleen to postganglionic sympathetic nerve stimulation. J Pharmacol Exp Ther. 1967 Sep;157(3):532–540. [PubMed] [Google Scholar]


