Abstract
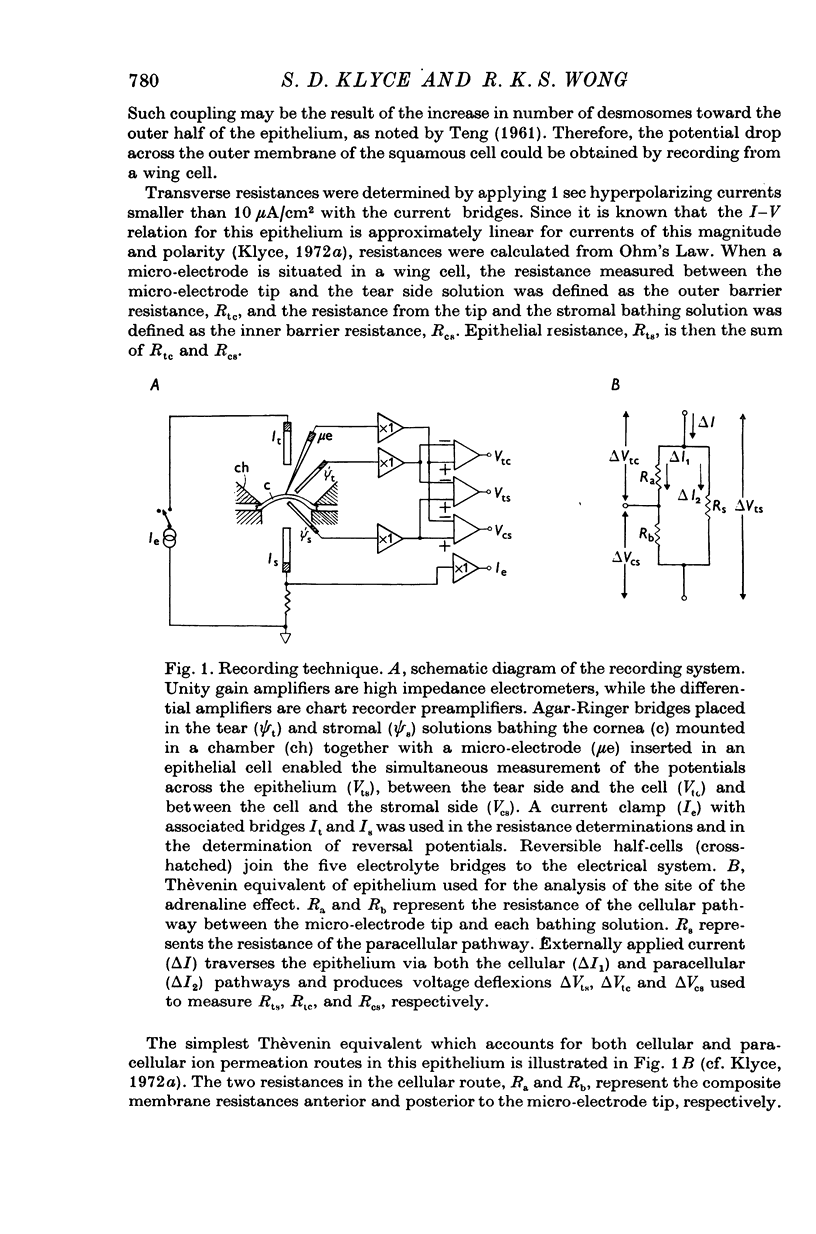
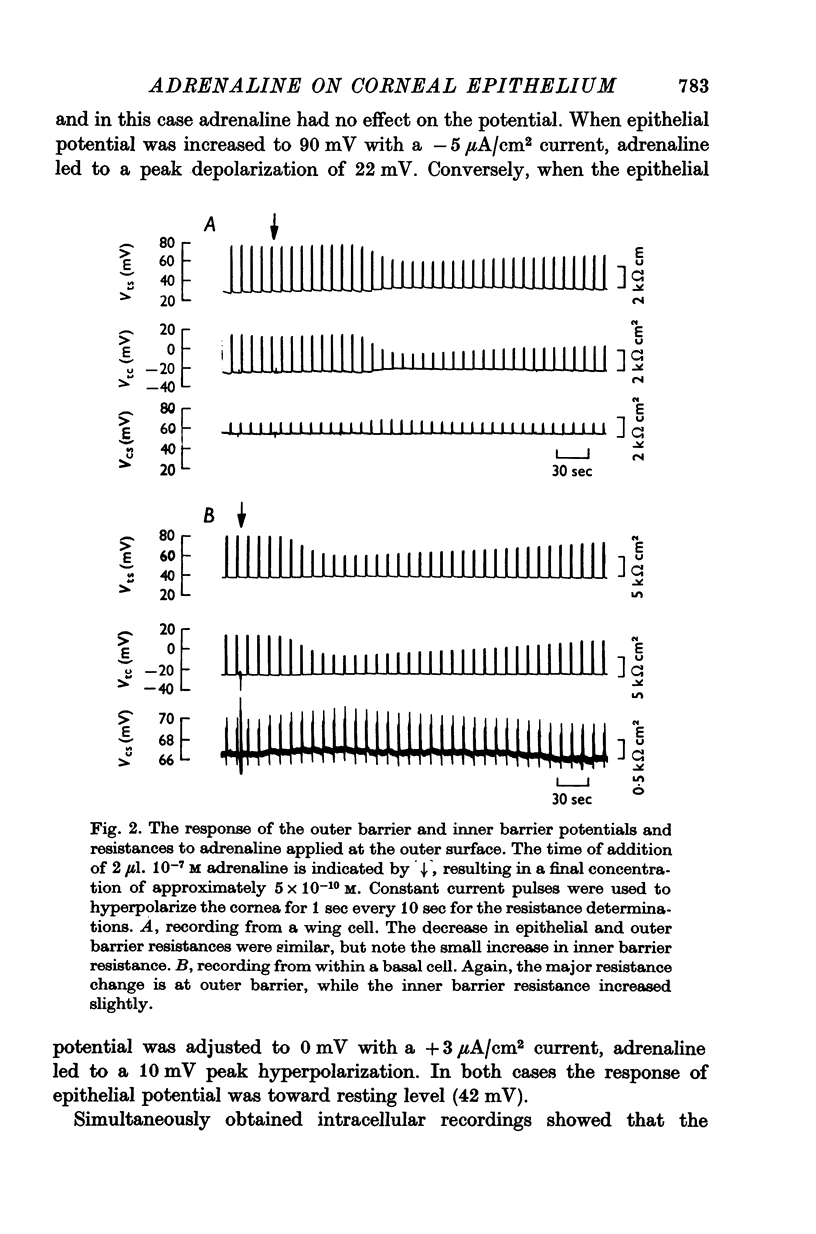
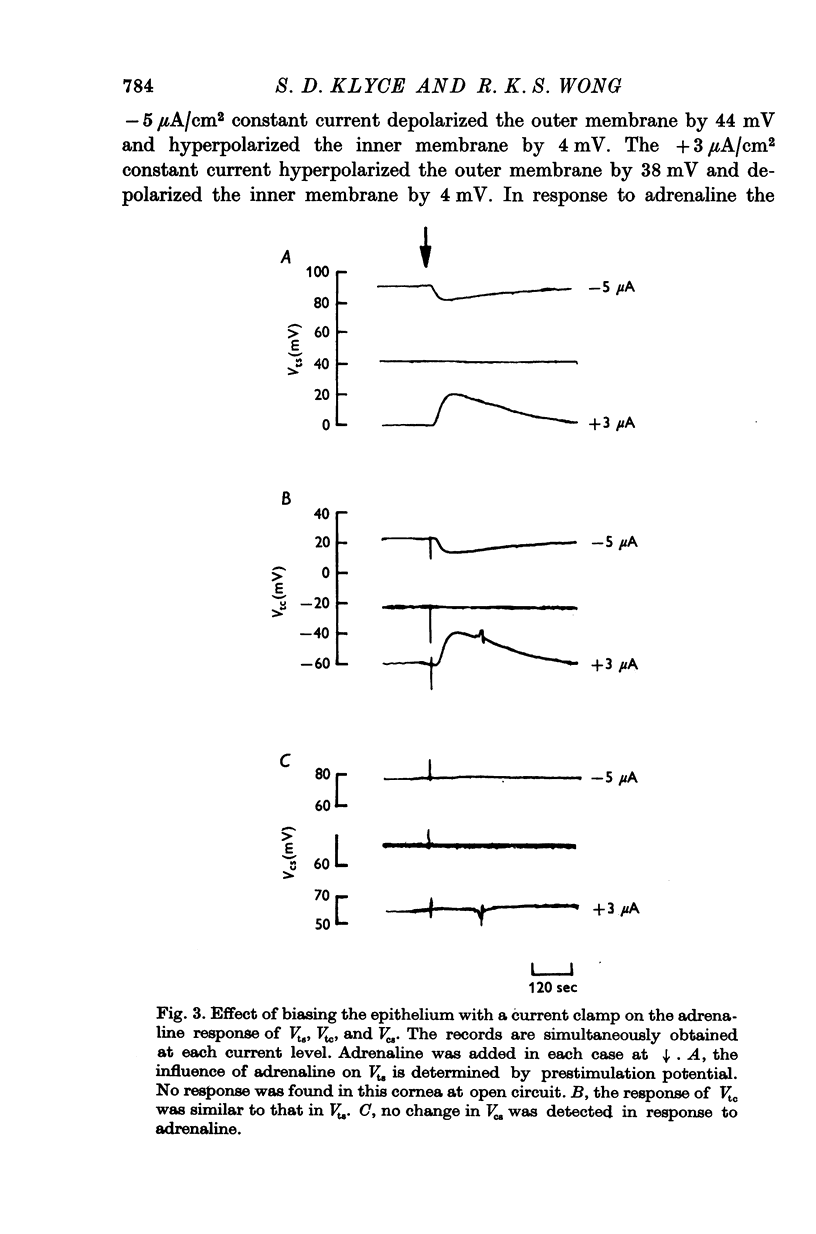
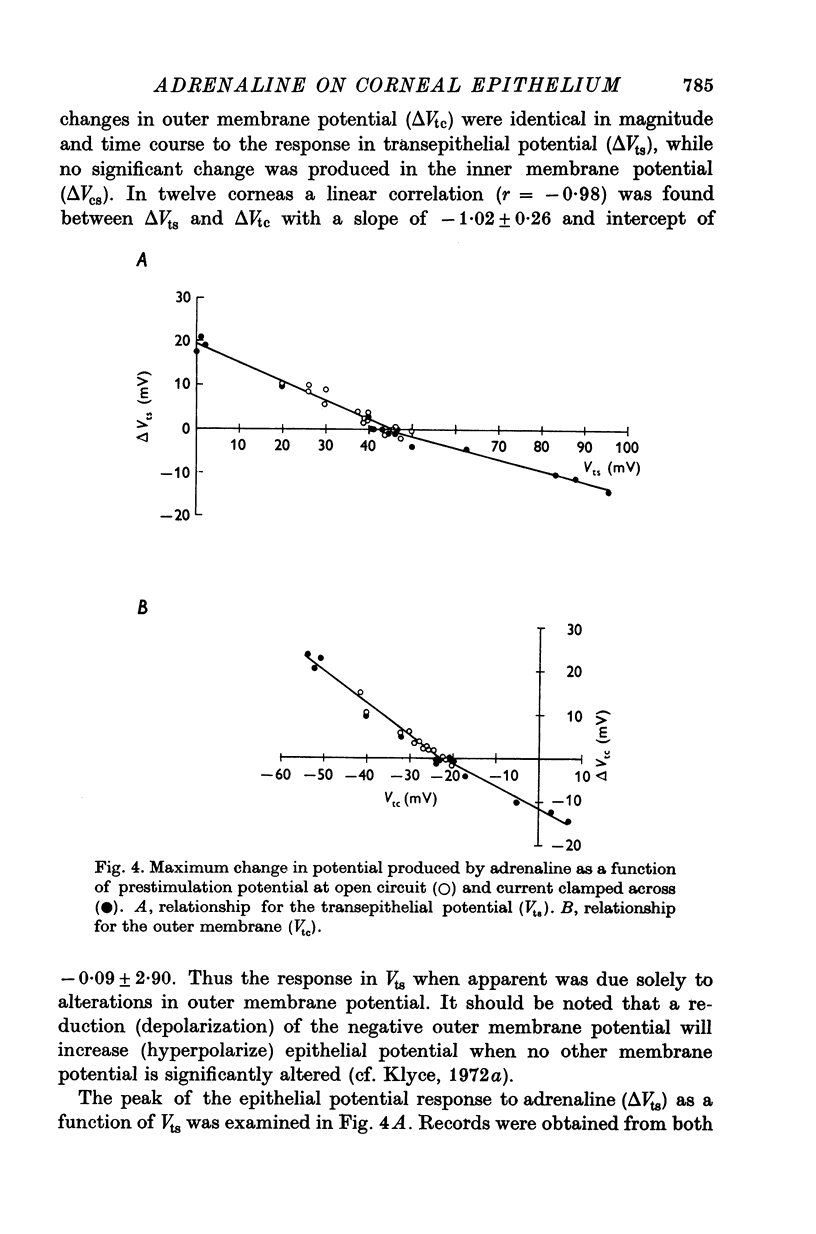
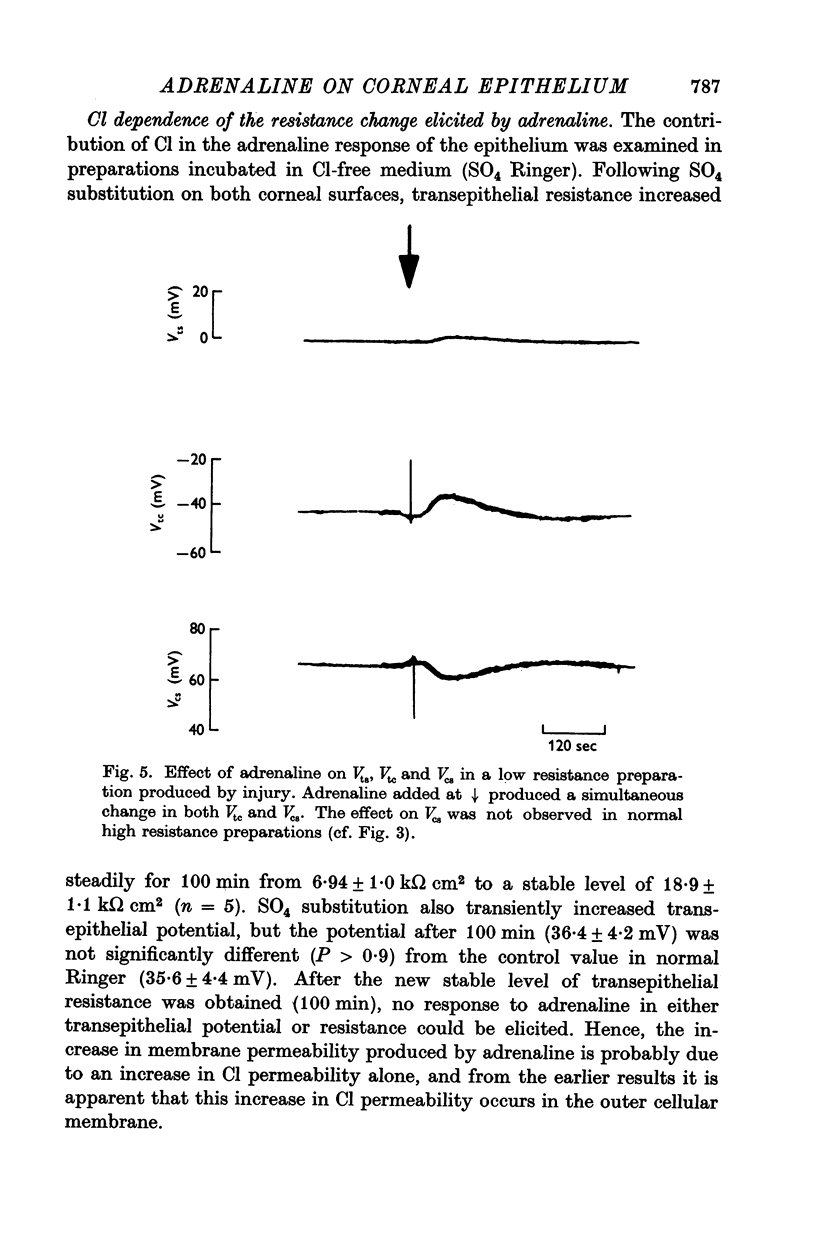
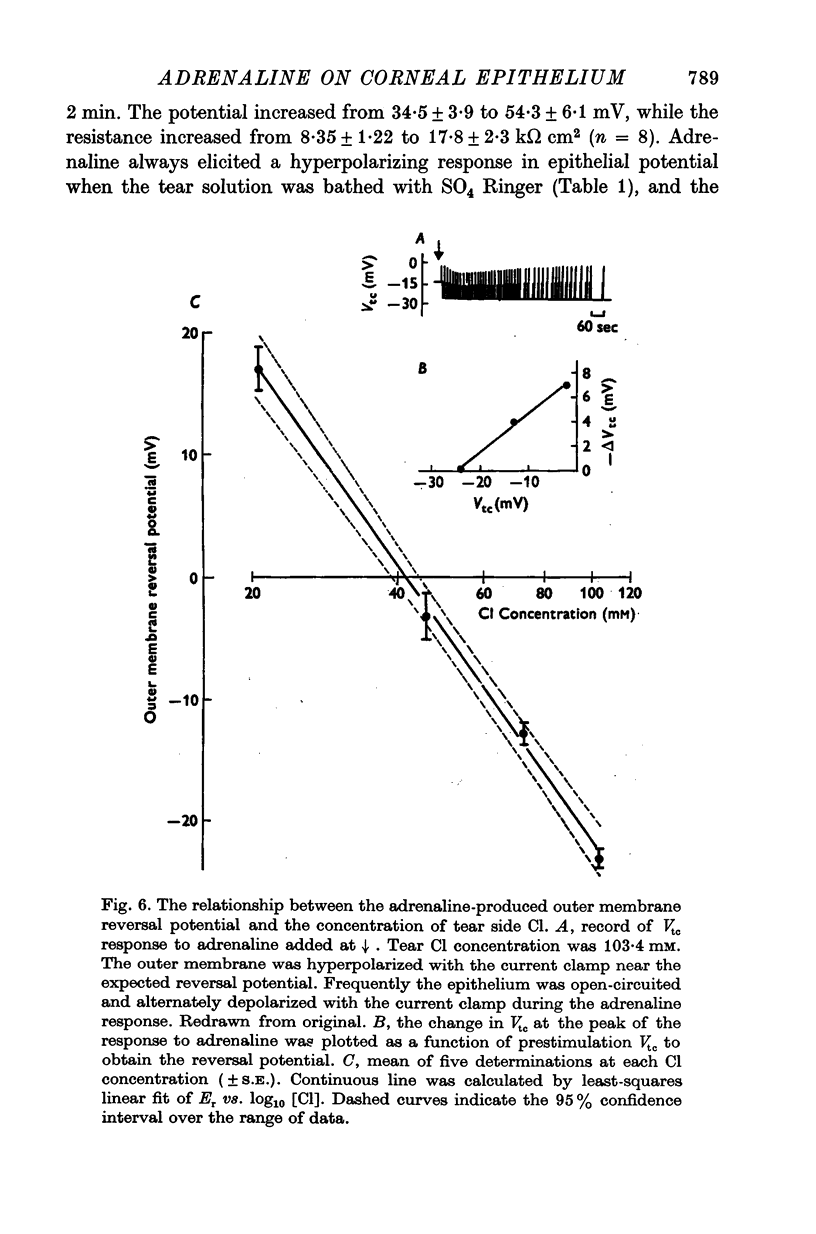
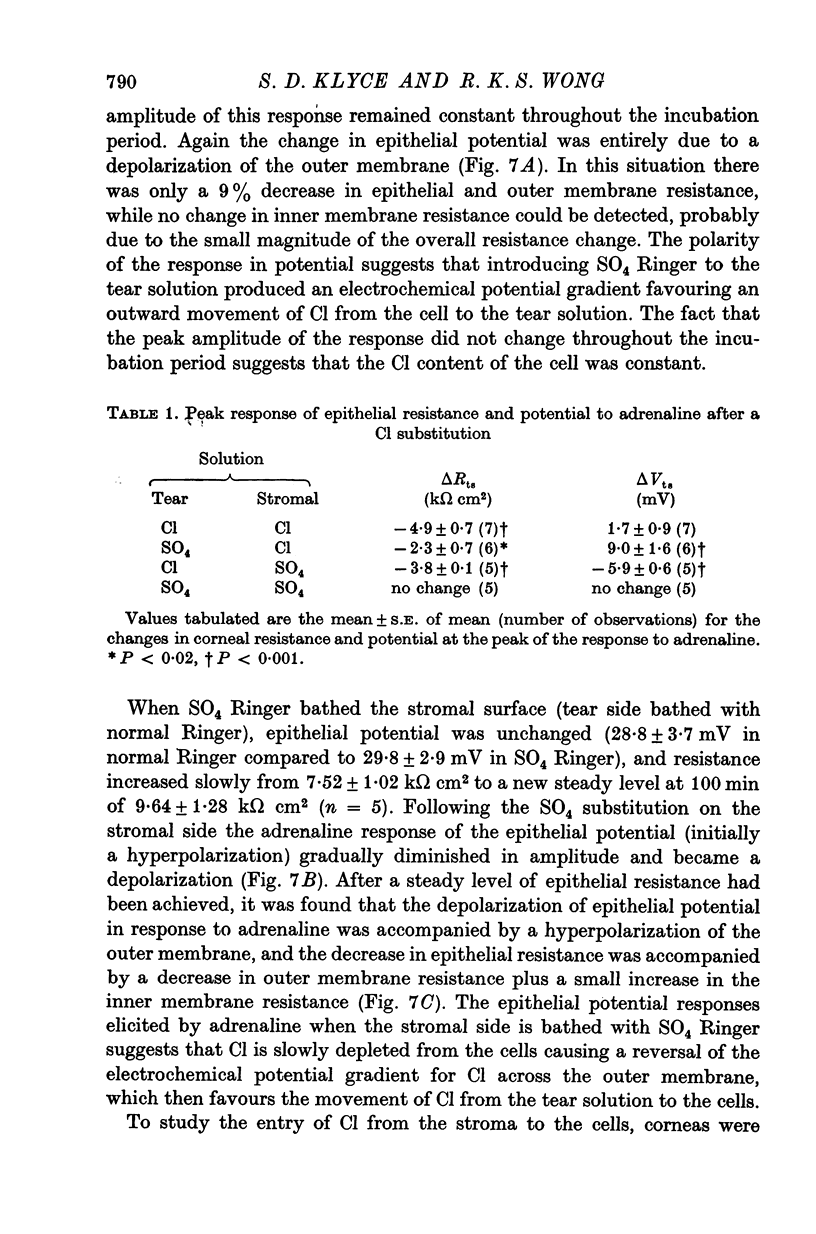
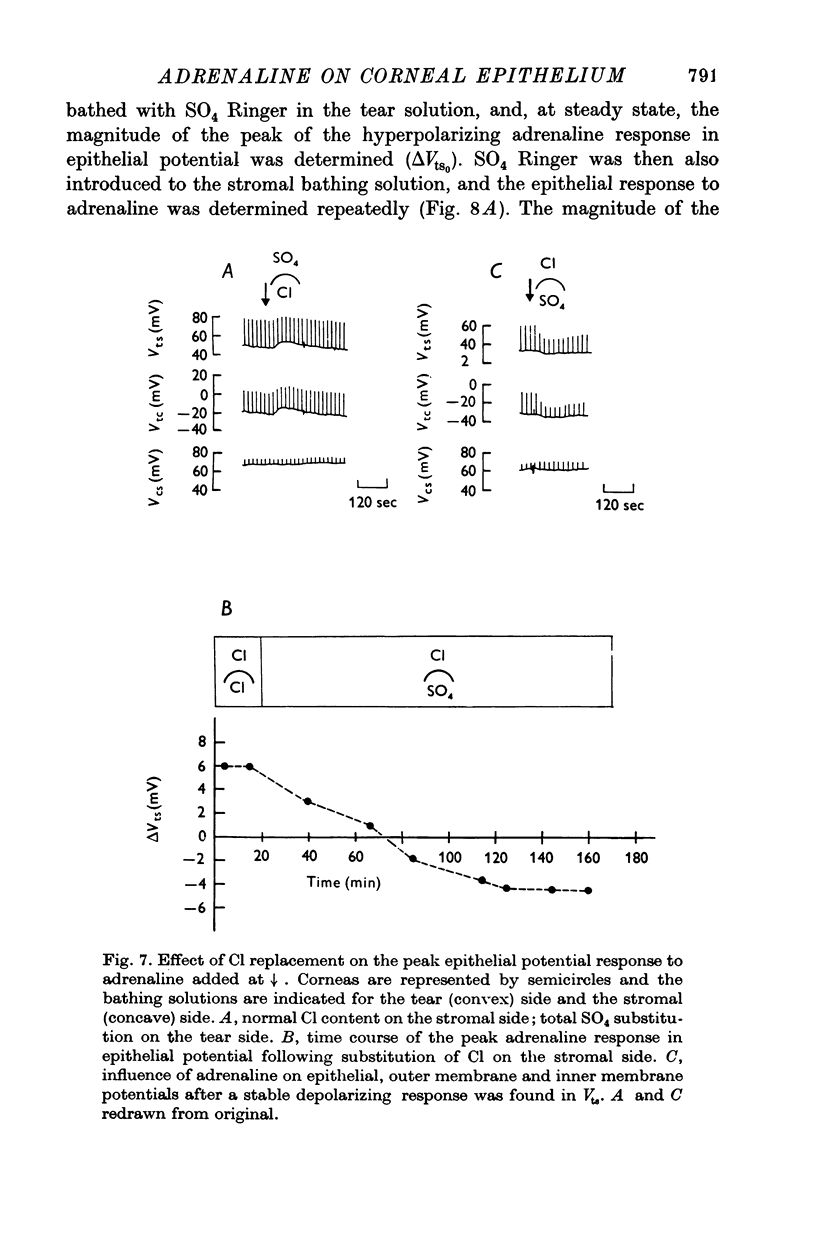
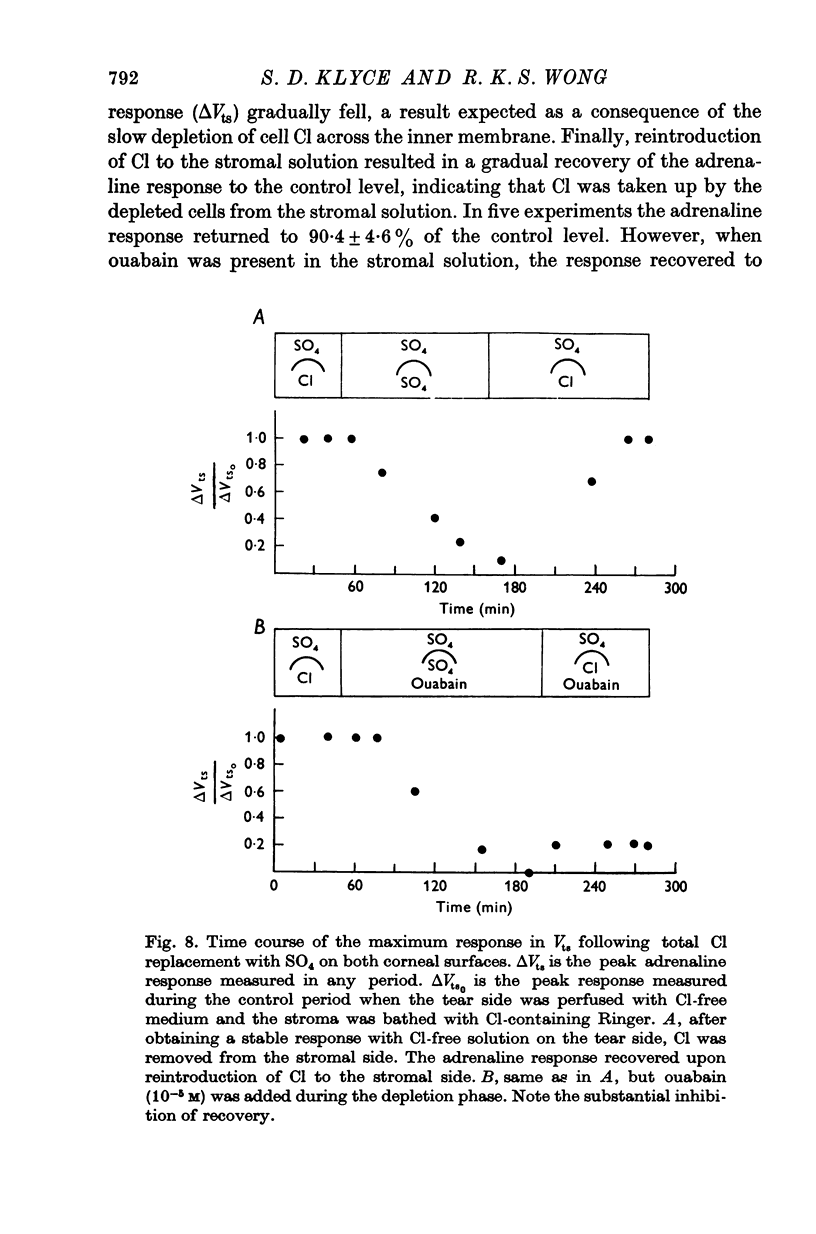
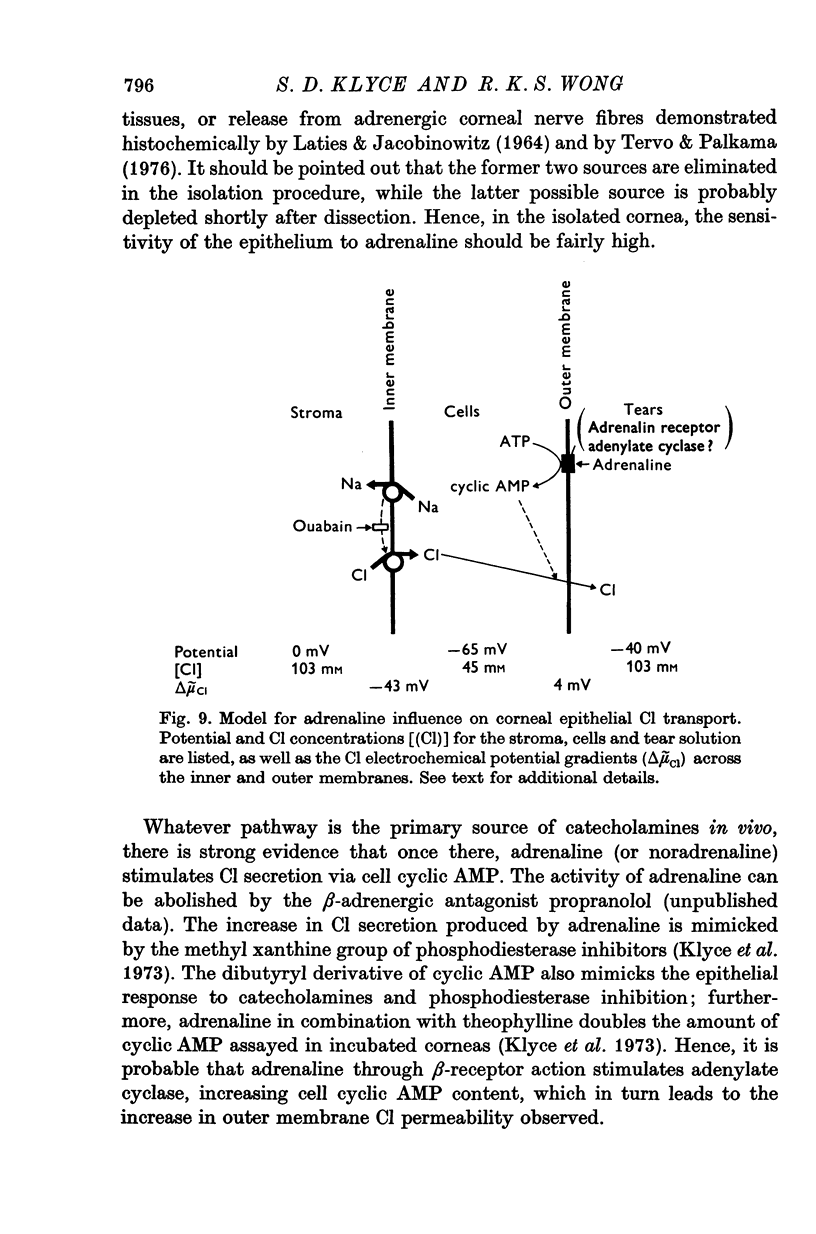
1. Membrane events accompanying adrenaline-stimulated Cl secretion by the isolated rabbit corneal epithelium were investigated with micro-electrodes. 2. Pulses of adrenaline (5 X 10(-10) M final concentration) delivered to either side of the epithelium produced a transient decrease in epithelial resistance occuring at the outer membrane of the squamous cell. This response was reversible and could be blocked completely by total Cl substitution with SO4. 3. Adrenaline generally produced a small transient increase in epithelial potential occuring also at the squamous cell outer membrane. Reversal potentials obtained for the adrenaline response were 45-1 mV for corneal potential and 22-8 mV for outer membrane. 4. Adrenaline always hyperpolarized epithelial potential when the tear side was bathed in Cl-free solution. Reversing the gradient (Cl-free on the stromal side) slowly and consistently changed the response to a depolarization which reached a steady level after 2 hr. 5. The reversal potential of the outer membrane for the adrenaline response was found to be a semilogarithmic function of the tear side Cl concentration over a broad range with a slope of 56 mV/decade. The reversal potential was zero at a tear side Cl concentration of 41-5 mM, which value may be taken to be representative of cell Cl concentration. 6. After abolishing the adrenaline response by perfusing both sides of the tissue with Cl-free solution, reintroduction of Cl to the stromal side led to a recovery of the epithelial potential response in the hyperpolarizing direction. The recovery of the response was inhibited by ouabain (10(-5) M). 7. The results supported the following model for the influence of adrenaline on anion transport in the epithelium: Cl is transported against an electrochemical potential gradient into the cells from the stromal side by an active process linked to Na-k activated ATPase. Normally a slight gradient exists from cells to tears favouring the passive outward diffusion of Cl. This latter process is enhanged by adrenaline, which increases cell cyclic AMP, in turn increasing the passive Cl permeability of the outer cellular membrane.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bülbring E., Tomita T. Suppression of spontaneous spike generation by catecholamines in the smooth muscle of the guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):103–119. doi: 10.1098/rspb.1969.0014. [DOI] [PubMed] [Google Scholar]
- CEREIJIDO M., CURRAN P. F. INTRACELLULAR ELECTRICAL POTENTIALS IN FROG SKIN. J Gen Physiol. 1965 Mar;48:543–557. doi: 10.1085/jgp.48.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The electrical properties of the motoneurone membrane. J Physiol. 1955 Nov 28;130(2):291–325. doi: 10.1113/jphysiol.1955.sp005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONN A., MAURICE D. M., MILLS N. L. Studies on the living cornea in vitro. II. The active transport of sodium across the epithelium. Arch Ophthalmol. 1959 Nov;62:748–757. doi: 10.1001/archopht.1959.04220050010002. [DOI] [PubMed] [Google Scholar]
- Ehlers N., Ehlers D. Effects of hydrostatic and colloid-osmotic pressure on electrical potential and short-circuit current across the explanted rabbit cornea. Acta Ophthalmol (Copenh) 1968;46(4):767–778. doi: 10.1111/j.1755-3768.1968.tb02875.x. [DOI] [PubMed] [Google Scholar]
- Green K. Ion transport in isolated cornea of the rabbit. Am J Physiol. 1965 Dec;209(6):1311–1316. doi: 10.1152/ajplegacy.1965.209.6.1311. [DOI] [PubMed] [Google Scholar]
- Hax W. M., van Venrooij G. E., Vossenberg J. B. Cell communication: a cyclic AMP mediated phenomenon. J Membr Biol. 1974;19(3):253–266. doi: 10.1007/BF01869981. [DOI] [PubMed] [Google Scholar]
- House C. R. The effect of different ionic levels on the electrical response of toad skin to noradrenaline. J Physiol. 1971 Oct;218(2):305–324. doi: 10.1113/jphysiol.1971.sp009619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C. R. The effect of noradrenaline on the toad skin potential. J Physiol. 1970 Aug;209(3):513–537. doi: 10.1113/jphysiol.1970.sp009177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C. R. The role of glandular activity in the electrical response of amphibian skin to noradrenaline. J Physiol. 1969 Jun;202(3):631–644. doi: 10.1113/jphysiol.1969.sp008831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOEFOED-JOHNSEN V., USSING H. H. The nature of the frog skin potential. Acta Physiol Scand. 1958 Jun 2;42(3-4):298–308. doi: 10.1111/j.1748-1716.1958.tb01563.x. [DOI] [PubMed] [Google Scholar]
- KOEFOED-JOHNSEN V., USSING H. H., ZERAHN K. The origin of the short-circuit current in the adrenaline stimulated frog skin. Acta Physiol Scand. 1952;27(1):38–48. doi: 10.1111/j.1748-1716.1953.tb00922.x. [DOI] [PubMed] [Google Scholar]
- Klyce S. D., Neufeld A. H., Zadunaisky J. A. The activation of chloride transport by epinephrine and Db cyclic-AMP in the cornea of the rabbit. Invest Ophthalmol. 1973 Feb;12(2):127–139. [PubMed] [Google Scholar]
- Klyce S. D. Relationship of epithelial membrane potentials to corneal potential. Exp Eye Res. 1973 May 10;15(5):567–575. doi: 10.1016/0014-4835(73)90068-7. [DOI] [PubMed] [Google Scholar]
- Klyce S. D. Transport of Na, Cl, and water by the rabbit corneal epithelium at resting potential. Am J Physiol. 1975 May;228(5):1446–1452. doi: 10.1152/ajplegacy.1975.228.5.1446. [DOI] [PubMed] [Google Scholar]
- LATIES A., JACOBOWITZ D. A HISTOCHEMICAL STUDY OF THE ADRENERGIC AND CHOLINERGIC INNERVATION OF THE ANTERIOR SEGMENT OF THE RABBIT EYE. Invest Ophthalmol. 1964 Dec;3:592–600. [PubMed] [Google Scholar]
- Leuenberger P. M. Lanthanium hydroxide tracer studies on rat corneal endothelium. Exp Eye Res. 1973 Jan 1;15(1):85–91. doi: 10.1016/0014-4835(73)90193-0. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Diamond J. M. Active sodium transport by mammalian urinary bladder. Nature. 1975 Feb 27;253(5494):747–748. doi: 10.1038/253747a0. [DOI] [PubMed] [Google Scholar]
- Lindley B. D. Nerve stimulation and electrical properties of frog skin. J Gen Physiol. 1969 Apr;53(4):427–449. doi: 10.1085/jgp.53.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice D. M. Epithelial potential of the cornea. Exp Eye Res. 1967 Apr;6(2):138–140. doi: 10.1016/s0014-4835(67)80065-4. [DOI] [PubMed] [Google Scholar]
- Maurice D. M. The location of the fluid pump in the cornea. J Physiol. 1972 Feb;221(1):43–54. doi: 10.1113/jphysiol.1972.sp009737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otori T. Electrolyte content of the rabbit corneal stroma. Exp Eye Res. 1967 Oct;6(4):356–367. doi: 10.1016/s0014-4835(67)80010-1. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Dependence of serosal membrane potential on mucosal membrane potential in toad urinary bladder. Biophys J. 1975 Jan;15(1):71–75. doi: 10.1016/S0006-3495(75)85792-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOFFENIELS E., SALEE M. L. THE EFFECTS OF THE ELECTRICAL STIMULATION OF THE BRACHIAL PLEXUS ON THE POTENTIAL DIFFERENCE OF FROG SKIN. Comp Biochem Physiol. 1965 Apr;14:587–602. doi: 10.1016/0010-406x(65)90248-3. [DOI] [PubMed] [Google Scholar]
- Salée M. L., Vidrequin-Deliège M. Nervous control of the permeability characteristics of the isolated skin of the toad Bufo bufo L. Comp Biochem Physiol. 1967 Nov;23(2):583–597. doi: 10.1016/0010-406x(67)90410-0. [DOI] [PubMed] [Google Scholar]
- Schultz S. G. Electrical potential differences and electromotive forces in epithelial tissues. J Gen Physiol. 1972 Jun;59(6):794–798. doi: 10.1085/jgp.59.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TENG C. C. The fine structure of the corneal epithelium and basement membrane of the rabbit. Am J Ophthalmol. 1961 Feb;51:278–297. [PubMed] [Google Scholar]
- Tomita T., Sakamoto Y., Oba M. Conductance increase by adrenaline in guinea pig taenia coli studied with voltage clamp method. Nature. 1974 Aug 2;250(465):432–433. doi: 10.1038/250432a0. [DOI] [PubMed] [Google Scholar]
- Tomlinson R. W., Wood A. W. Catecholamine-induced changes in ion transport in short-circuited frog skin and the effect of beta-blockade. J Physiol. 1976 May;257(2):515–530. doi: 10.1113/jphysiol.1976.sp011382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Heyden C., Weekers J. F., Schoffeniels E. Sodium and chloride transport across the isolated rabbit cornea. Exp Eye Res. 1975 Jan;20(1):89–96. doi: 10.1016/0014-4835(75)90111-6. [DOI] [PubMed] [Google Scholar]


