Abstract
Human gammaherpesvirus infections are associated with development of lymphoproliferative disease. Understanding of the mechanisms of gammaherpesvirus lymphomagenesis during chronic infection in a natural host has been limited by the exquisite species specificity of human gammaherpesviruses and the expense of primates. Murine gammaherpesvirus γHV68 is genetically and biologically related to human gammaherpesviruses and herpesvirus saimiri and has been reported to be associated with lymphoproliferative disease in mice (N. P. Sunil-Chandra, J. Arno, J. Fazakerley, and A. A. Nash, Am. J. Pathol. 145:818-826, 1994). We report the development of an animal model of γHV68 lymphomagenesis in BALB/c β2 microglobulin-deficient mice (BALB β2m−/−). γHV68 infection induced two lymphoproliferative lesions: B-cell lymphoma and atypical lymphoid hyperplasia (ALH). ALH lesion histology resembled lesions of Epstein-Barr virus-associated posttransplant lymphoproliferative disease and was characterized by the abnormal infiltration of the white pulp with cells expressing the plasma cell marker CD138. Lymphomas observed in γHV68-infected animals were B220+/CD3− large-cell lymphomas. γHV68-infected cells were common in ALH lesions as measured by in situ hybridization with a probe specific for viral tRNAs (vtRNAs), but they were scarce in γHV68-infected spleens with normal histology. Unlike ALH lesions, γHV68 vtRNA-positive cells were rare in lymphomas. γHV68 infection of BALB β2m−/− mice results in lymphoproliferation and lymphoma, providing a valuable tool for identifying viral and host genes involved in gammaherpesvirus-associated malignancies. Our findings suggest that γHV68 induces lymphomas via hit-and-run oncogenesis, paracrine effects, or stimulation of chronic inflammation.
Gammaherpesviruses establish lifelong latency in lymphoid and myeloid cells of the host. Importantly, many gammaherpesviruses, including human Epstein-Barr virus (EBV), Kaposi's sarcoma-associated herpesvirus (KSHV), and simian herpesvirus saimiri (HVS) are associated with a variety of lymphoproliferative diseases (LPDs) (13, 31, 71). In addition to Kaposi's sarcoma, a tumor of endothelial cell origin, KSHV has also been associated with at least two types of lymphoproliferative B-cell diseases, multicentric Castleman's disease and primary effusion lymphoma (PEL) (6). EBV induces hyperproliferation of infected B cells that can evolve into lymphoma in an immunocompromised host (7, 14). Other lymphomas with a strong link to EBV infection include Burkitt's lymphoma, Hodgkin's lymphoma, and AIDS-associated lymphomas (40, 71). HVS is associated with T-cell lymphomas in New World primates and in certain rabbits (31). Immunocompromised hosts have an increased incidence of gammaherpesvirus-associated lymphoproliferative disease, suggesting a critical role of the immune system in controlling lymphoproliferation and lymphomagenesis (71).
The mechanism of gammaherpesvirus-induced lymphomagenesis in animal models is not clear. The four major models of virus-associated oncogenesis include (i) direct transformation, (ii) “hit and run,” (iii) paracrine, and (iv) chronic inflammation. Direct transformation is the only model that requires viral presence in a majority of malignant cells (38). Such is the case in early onset posttransplant lymphomas that are uniformly associated with EBV (71). EBV genes expressed in infected B cells of these lymphomas represent the latency III program of EBV gene expression that provides the necessary signals for uncontrolled B-cell proliferation. In other cases, such as EBV-associated Hodgkin's disease, virus is present in a minority of cells found in the tumor (71). HVS-induced T-cell lymphomas uniformly retain viral genome (10). According to the “hit-and-run” model, the presence of the virus is only required to initiate events leading to the transformation of infected cells (1). Once a fully transformed state is achieved, virus is lost (44). In support of the “hit-and-run” mechanism, cell lines derived from the primary Kaposi's sarcoma lesions lose viral episome upon passage in vitro (24, 47). In vivo, EBV-associated Hodgkin's lymphoma may no longer harbor virus upon relapse (15). The existence of the paracrine mechanism of lymphomagenesis is supported by studies showing that cell-type-specific expression of the KSHV G protein-coupled receptor (vGPCR) in lymphocytes of transgenic mice induces angioproliferative lesions characteristic of Kaposi's sarcoma (69). KSHV vGPCR expression upregulates expression of vascular endothelial growth factor and thus contributes to the transformation of human endothelial cells in vitro (2). Although the role of chronic inflammation in the development of gammaherpesvirus-associated malignancies has not been examined, other chronic inflammatory stimuli, such as Helicobacter pylori infection, are major contributors to the development of cancer (12). EBV-specific CD8+ T cells isolated from latently infected individuals have immediate effector functions (direct ex vivo cytotoxic activity and epitope-specific cytokine production) (30, 59), suggesting continuous stimulation by viral antigens. Therefore, chronic inflammation associated with persistent EBV and KSHV infections might contribute to gammaherpesvirus lymphomagenesis. While the direct transformation model requires the continuous presence of virus in most malignant cells, the other three potential mechanisms of gammaherpesvirus-associated lymphomagenesis require short-term or no viral infection of lymphoma cells.
Analysis of the mechanisms responsible for gammaherpesvirus-associated lymphomagenesis in vivo has been hampered by the exquisite species specificity of human gammaherpesviruses and the expense and availability of primates. Murine γHV68 readily infects rodents, including laboratory mice (3). Genetically, γHV68 is related to the human viruses EBV and KSHV and the primate virus HVS (21, 63) and shares important features of its biology with its human and primate relatives. During acute infection, γHV68 lytically replicates in lymphoid and nonlymphoid compartments, including lung epithelial cells (52). Upon resolution of the acute infection, γHV68 latency is primarily maintained in B cells, macrophages, and dendritic cells (25, 53, 67, 68). CD8+ and CD4+ T cells are important for controlling acute γHV68 replication and γHV68 reactivation during latency (5, 9, 22, 46, 48, 58, 60, 61, 64).
Similar to human gammaherpesviruses, γHV68 infection is reported to be associated with lymphoproliferative disease in wild-type mice; however, γHV68-associated lymphomas develop at a low incidence (9%) and after a prolonged incubation period (up to 3 years) (51). Only a few cells in tumors were reported to harbor virus as determined by in situ hybridization (ISH) with probes specific for γHV68 sequences (51). γHV68 can infect and establish persistent infection in cultured B-cell lines (54), raising the possibility that γHV68-positive lymphoma cells isolated from a tumor in previous studies could have arisen from infection of virus negative tumor cells during the isolation process. Cyclosporine treatment was reported to increase the incidence of lymphoproliferative disease in γHV68-infected mice, suggesting the critical role for immune control of gammaherpesvirus-associated lymphomagenesis (51). However, these initial studies have not been extended and a robust model for γHV68-induced lymphomas is still needed. Interestingly, while infection with γHV68 activates and stimulates proliferation of primary murine B cells both in vitro and in vivo, attempts to use γHV68 to transform primary B cells in vitro have to date been unsuccessful (20, 37a, 49).
We considered the hypothesis that development of lymphomas after γHV68 infection may be dependent on both immune status and mouse genetic background. Therefore, we asked whether the absence of β2 microglobulin (β2m) expression in mice could potentiate γHV68-associated lymphoproliferative disease. β2 microglobulin is required for control of both latent and persistent γHV68 infection (11, 64; unpublished observation). In this report, we demonstrate that γHV68 infection of BALB β2 microglobulin-deficient mice (BALB β2m−/−), but not 129 β2m−/− mice, led to a high incidence and accelerated development of B-cell lymphoma and a second lymphoproliferative lesion we termed atypical lymphoid hyperplasia (ALH). ALH lesions were characterized by the abnormal expansion of the white pulp infiltrated with cells bearing the plasma cell CD138 marker and significant numbers of γHV68-positive cells. Lymphomas observed in γHV68-infected animals were of B-cell origin with a propensity to disseminate. Unlike cells in ALH lesions, few lymphoma cells expressed viral tRNAs (vtRNAs), as measured by in situ hybridization. These findings argue that lymphomagenesis in γHV68-infected immunocompromised hosts does not require the continuous presence of virus in malignant cells.
MATERIALS AND METHODS
Animals.
BALB/cJ (BALB) and β2m knockout mice (B2mtm1Unc) on the BALB/cJ (BALB β2m−/−) genetic background were obtained from Jackson Laboratories (Bar Harbor, Maine) and bred at Washington University, St. Louis, MO. 129Pas mice (129-β2m−/−) were derived from 129/BL6-β2m−/− mice (Jackson Laboratories, Bar Harbor, Maine) and were subsequently bred to 129/Pas (19) for 10 generations. All mice were housed in a specific-pathogen-free barrier facility at Washington University in accordance with federal and institutional guidelines. Mice were infected between 1.9 and 2.4 months of age.
Virus infections.
γHV68 WUMS (ATCC VR1465) was passaged and the titer was determined on NIH 3T12 cells (64). All injections were done intraperitoneally with 107 PFU γHV68 diluted in 500 μl of Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. Mock infections were performed with 500 μl of Dulbecco's modified Eagle's medium containing 10% fetal calf serum.
Organ harvest and tissue processing.
At various times postinfection, mice were sacrificed, and the spleen, liver, lungs, and thymus as well as any enlarged lymph nodes or macroscopically abnormal organs were removed. Portions of the organ samples were fixed in 10% buffered formalin (Fisher Scientific). Additionally, small fragments of splenic tissue and lymph nodes, when available, were snap-frozen in liquid nitrogen and stored at −70°C.
Histology and immunohistochemistry.
Organs were embedded in paraffin, and 5-μm sections were cut and stained with hematoxylin and eosin (H&E). For immunohistochemistry, sections were deparaffinized in Citri-Solv buffer (Fisher Scientific) and antigen retrieval was performed by boiling in 10 mM citrate buffer (pH 6.0) or 1 mM EDTA (pH 8.0) in a microwave oven. Endogenous peroxidase was quenched with 3% H2O2 in methanol for 30 min, and the sections were blocked 30 min in a blocking buffer (1% bovine serum albumin, 0.2% powdered skim milk, 0.3% Triton-X in phosphate-buffered saline [PBS]). Primary antibodies were applied to the sections overnight at 4°C in a humid chamber. Horseradish peroxidase (HRP)-conjugated secondary antibody diluted in the blocking buffer (goat anti-rabbit; BD Pharmingen, San Jose, CA) or donkey anti-rat (Jackson ImmunoResearch, West Grove, PA) was applied for 1 h at room temperature. Signal was enhanced with one round of tyramide signal amplification using the Trypticase soy agar-biotin system (Perkin-Elmer Life Sciences, Inc., Boston, MA), and color development was performed with diamidinobenzidine (DAB) substrate kit (Vector laboratories, Burlingame, CA). Immunostained slides were counterstained with hematoxylin (Sigma, St. Louis, MO). The following antibodies were used: B-cell marker (purified rat anti-B220, BD Pharmingen), plasma cell marker (purified rat anti-CD138/Syndecan-1, BD Pharmingen), T-cell marker (purified rat anti-CD3; Serotec, Oxford, United Kingdom), and rabbit polyclonal anti-γHV68 (65).
In situ hybridization.
Sections were deparaffinized and rehydrated using xylene washes followed by ethanol gradients. Postfixation was performed for 20 min in fresh 4% paraformaldehyde (pH 7.4), followed by 0.3% H2O2 in PBS for 30 min, acetylation in two 10-min incubations in 0.1 M triethanolamine and 0.25% acetic anhydride, and permeabilization with proteinase K (40 μg/ml; Sigma, St. Louis, MO) in PBS at room temperature for 20 min. Hybridization was conducted for 40 h at 37°C using a hybridization mix containing 4× SSC (1× SSC is 0.15 M NaCl plus 0.015M sodium citrate); 20% dextran sulfate; 50% formamide; 0.25 mg/ml each of poly(A), salmon sperm DNA, and tRNA; 5× Denhardt's solution (all from Sigma); and 100 mM dithiothreitol (Fisher). GreenStar digoxigenin-labeled oligonucleotide probes (Genedetect, Auckland, New Zealand) specific for vtRNAs were used at a final concentration of 200 ng/ml in hybridization mix. A cocktail of probes antisense to vtRNAs 1 to 4 was utilized (vtRNA1, nucleotides [nt] 12 to 41; vtRNA2, nt 13 to 42; vtRNA3, nt 3 to 32; and vtRNA4, nt 33 to 62). Stringent posthybridization washes were performed with 1× and 0.5× SSC at 55°C, and the hybridized probes were detected using a goat HRP-conjugated anti-digoxigenin Fab fragment (Dako) followed by a round of tyramide signal amplification and DAB substrate color development.
Molecular analysis of clonality.
DNA was extracted from frozen spleen fragments using standard procedures. Analysis of the rearranged status of the immunoglobulin heavy chain (IgH) locus was performed by PCR amplification using the family-specific degenerate forward primers Vh558, Vh7183, VhQ52, and DhL with the reverse primer Jh3 (35, 43). Amplified products were separated by agarose gel electrophoresis. When a predominant fragment was observed, it was cut, purifed, and reamplified in a second round using the appropriate primers. The resulting product was cloned in pGEM-T and sequenced. Sequence analysis was performed by aligning the sequenced product using V-QUEST search on the Immunogenetics database (http://imgt.cines.fr:8104/textes/vquest/).
Statistical analysis.
The data were analyzed using GraphPad Instat software (San Diego, CA). All results were compared using unpaired Student's t test or chi-square tests.
RESULTS
γHV68 infection is associated with an increased incidence of lymphoproliferative diseases in BALB-β2m-deficient mice.
Gammaherpesvirus-associated lymphoproliferative disease is increased in immunocompromised hosts. Given the importance of CD8 T cells and β2 microglobulin in the control of γHV68 replication and latency (11, 22, 58, 61, 64), we assessed the development of lymphoproliferative disease in mock-infected and γHV68-infected sex- and age-matched BALB/c mice lacking β2 microglobulin expression. Several defects are attributed to the β2 microglobulin deficiency, including lack of classical CD8 T cells, hepatic iron overload, and abnormal IgG homeostasis due to the inefficient expression of neonatal Fc receptor (28, 42, 70, 73). Mice were infected between 1.9 and 2.4 months of age and sacrificed at the indicated times (Table 1). We then assessed lymphoid histology in spleens, lymph nodes, livers, lungs, and kidneys.
TABLE 1.
Demographics of animals used in this study
| Infection | Strain | No. of animals
|
Mean age (mo) at:
|
||
|---|---|---|---|---|---|
| Total | With LPD | Infection | Termination | ||
| Mock | BALB | 17 | 0 | 1.9 | 13.4 |
| BALB β2m | 50 | 11 | 2.0 | 11.1 | |
| 129 β2m | 7 | 0 | 1.9 | 17.7 | |
| γHV68 | BALB | 23 | 0 | 2.2 | 11.5 |
| BALB β2m | 55 | 37 | 2.0 | 10.6 | |
| 129 β2m | 20 | 0 | 2.4 | 19.6 | |
Two lymphoproliferative lesions were observed disproportionately in infected compared to uninfected BALB β2m−/− mice: atypical lymphoid hyperplasia with plasmacytosis (ALH), and large-cell lymphomas. The pathology of these lesions is described below. Follicular hyperplasia, as defined by the presence of a prominent germinal center reaction with otherwise normal histology, was occasionally observed and was not scored as lymphoproliferative disease. Overall 37/55 (67%) of the infected BALB β2m−/− mice developed LPD (ALH or lymphoma) compared to 11/50 (22%) of the mock-infected controls (P < 0.0001) (Fig. 1A). There were significantly more lymphomas (29% versus 6%, P = 0.0022) and atypical lymphoid hyperplasias (38% versus 16%, P = 0.0157) in the γHV68-infected group as compared to the mock-infected animals (Fig. 1A).
FIG. 1.
γHV68 infection increases incidence of lymphoproliferative disease in BALB β2 microglobulin-deficient mice. Age- and sex-matched BALB β2m animals were mock infected or infected with γHV68 and monitored for the development of LPD. Total incidence of LPD (A) was subdivided into incidence of each of the two primary lesions (ALH and lymphoma) in male and female animals. (B and C) Incidence of LPD at indicated times postinfection in γHV68 (B)- and mock (C)-infected groups. n, number of mice.
The development of γHV68-associated lymphoproliferative lesions was not detected in BALB (0% LPD, n = 23) or 129/Pas β2m−/− animals (0% LPD, n = 20) (Table 1), demonstrating that both the β2m deficiency and the genetic background of the mice are key factors in determining susceptibility to γHV68-associated LPD.
Kinetics of lymphoproliferative disease development in γHV68-infected and control BALB-β2m−/− mice.
We analyzed the kinetics of lymphoproliferative lesions in γHV68-infected or mock-infected BALB β2m−/− mice (Fig. 1B and C). Overall, atypical lymphoid hyperplasia and lymphomas were detected at a higher incidence and at an earlier time in the γHV68-infected group as compared to the mock-infected group. The mean age of mice with a diagnosis of lymphoma was 11.6 months in the γHV68-infected group compared to 15.8 months in the mock-infected controls (P = 0.011). Atypical lymphoid hyperplasia was observed earlier and at an increased incidence in the γHV68-infected group. At 6 to 7 months postinfection, over 50% of the animals had atypical lymphoid hyperplasia lesions in the spleen, whereas none of the mock-infected animals had detectable lymphoproliferative disease (compare Fig. 1B and C). In both mock- and γHV68-infected groups, atypical lymphoid hyperplasia was detected at an earlier time postinfection than lymphomas. Thus γHV68 infection was associated with both an increased penetrance and an earlier onset of lymphoproliferative lesions.
Role of gender in development of lymphoproliferative disease.
An increased incidence of spontaneous lymphomas has been previously observed in BALB female but not male animals (23). Interestingly, we also observed a gender-dependent difference in the incidence of lymphoproliferative disease in mock-infected BALB β2m−/− animals (Fig. 1A). Mock-infected male BALB β2m−/− animals had a low level of atypical lymphoid hyperplasia and no spontaneous lymphomas (Fig. 1A, middle panel). The incidence of lymphoproliferative lesions was significantly increased in γHV68-infected males. In contrast, while there was a trend towards an increase in lymphoproliferative disease in γHV68-infected versus mock-infected females, the difference did not reach statistical significance. Mock-infected females had a higher incidence and severity of lymphoproliferative lesions (6 of 27 females with atypical lymphoid hyperplasia, 3 of 27 with lymphomas). The mean time of development of LPD in γHV68-infected females with lymphomas was shorter (13.2 months) than that observed in mock-infected females (15.8 months), suggesting that γHV68 accelerates the development of lymphomas in female BALB β2m−/− animals. A larger study will be required to determine whether γHV68 induces lymphoproliferative disease in female BALB β2m−/− mice.
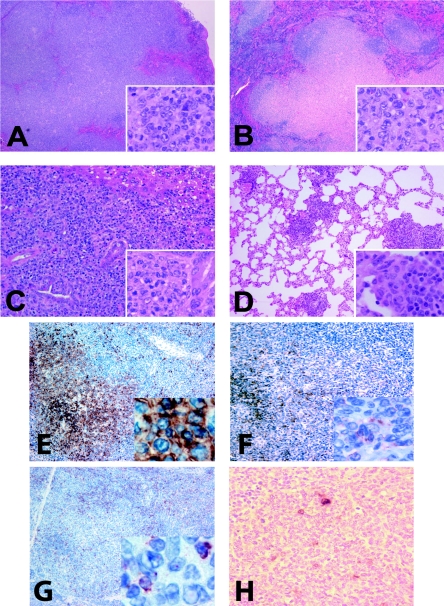
Histopathology of atypical lymphoid hyperplasia.
Four conditions were represented in spleens of the γHV68-infected mice in this study. Normal spleen sections displayed well-delineated areas of white pulp containing tightly packed lymphocytes with dark-staining nuclei (Fig. 2A and B). Follicular hyperplasia was scored based on the presence of reactive B-cell follicles (Fig. 2C and D, arrows) with otherwise normal splenic histology. Two examples of the atypical lymphoid hyperplasia lesions described below are shown in Fig. 2E and F. Of note, atypical lymphoid hyperplasia lesions displayed a spectrum of severity ranging from only a mild effacement of a few white pulp areas on a section (Fig. 2E, outlined) to a dramatic expansion of most white pulp areas (Fig. 2F). Spleens with lymphomas displayed a loss of normal splenic architecture and expansion of large cells with moderate amounts of cytoplasm and vesicular nuclei (Fig. 2G and H).
FIG. 2.
Splenic histology of γHV68-infected BALB β2m−/− mice. H&E staining of formalin-fixed paraffin-embedded tissues. (A and B) Normal (×20). (C and D) Follicular hyperplasia (×20). Arrows point to germinal centers abundant throughout the section. (E and F) Atypical lymphoid hyperplasia (×10). (E) A hyperplastic lesion is outlined. (G and H) Lymphoma (×4).
Atypical lymphoid hyperplasia lesions presented with a partial disruption of the normal splenic architecture with an irregular expansion of the white pulp. In contrast to the lymphomas, the distinction between red and white pulp remained apparent. While disorganized in more severe lesions, the expansion involved primarily the periarteriolar lymphoid sheath and sometimes the marginal zone (Fig. 3A and B). Atypical lymphoid hyperplasia lesions were also present in nonlymphoid organs, mainly the lungs, where they appeared as nodular infiltrates in parenchyma (Fig. 3C). The expansion reflected the presence of large lymphoid cells with moderate amounts of amphophilic cytoplasm and vesicular nuclei with clumped chromatin, features characteristic of plasmacytic differentiation (Fig. 3A to C, insets). Supporting the morphological findings, immunohistochemistry demonstrated an increased number of cells bearing the plasma cell CD138 marker in the areas of ALH lesions (Fig. 3D). B220 staining was confined to the otherwise normal B-cell areas of the white pulp (Fig. 3E).
FIG. 3.
Histopathology of atypical lymphoid hyperplasia. (A and B) ALH lesions in spleens (×4). The splenic architecture is partially disrupted with expanded white pulp areas. (C) Lung lymphocytic infiltrates seen in γHV68-infected animals with ALH lesions (×4). (Insets in panels A to C) Prominent plasmacytic features of cells in ALH lesions (×100). (D) The majority of the ALH-associated cells stain with the plasma cell marker CD138 (×10). (E) B220 is detected in residual B-cell follicles (×10). Adjacent spleen sections obtained from γHV68-infected BALB β2m−/− mice were stained with hematoxylin and eosin (F and H) or hybridized with antisense digoxigenin-labeled vtRNA probe (G, I, and J) and counterstained with hematoxylin. Sections originated from spleens exhibiting follicular hyperplasia (F and G) or atypical lymphoid hyperplasia (H, I, and J). Magnification: F to I, ×4; J, ×40.
The presence of γHV68 in lymphoproliferative lesions.
To determine whether γHV68 was present in lymphoproliferative lesions, we performed ISH for expression of viral tRNAs on spleen sections harvested from γHV68-infected BALB β2m−/− animals. Sections were hybridized to a digoxigenin-labeled probe antisense to the viral tRNA transcripts. γHV68 tRNA genes are expressed during viral latency and are a robust target for detection by ISH (4, 45, 50). Spleen sections from γHV68-infected BALB mice (16 days postinfection) and naïve animals were utilized as positive and negative controls. No staining was observed with vtRNA-specific probes on mock-infected spleen sections or with the control sense probe on γHV68-infected spleen sections (data not shown).
In order to test the sensitivity of detection of γHV68-infected cells by in situ hybridization, we calculated the frequency of vtRNA-positive cells on spleen sections obtained from γHV68-infected BL6 mice at 16 days postinfection. The nature of γHV68 latency in these mice is well established in the literature. At 16 days postinfection, approximately 1 in 1,000 splenocytes are γHV68 genome positive as determined by limited dilution nested PCR (56). Out of over 12,500 cells counted in random fields of spleen sections stained with vtRNA-specific probe, on average, 1 in 407 nucleated cells was vtRNA positive (range 1 in 186 cells to 1 in 1,256 cells). Therefore, the sensitivity of detection of the γHV68-infected cell by in situ hybridization using a vtRNA probe is comparable to the sensitivity of established methods, such as limited dilution PCR. These data show that in situ hybridization is not likely to significantly underestimate the number of vtRNA-expressing cells in tumors.
When spleens from BALB β2m−/− mice were examined by in situ hybridization, no or a few small vtRNA-positive foci were consistently detected in spleens from γHV68-infected animals in the absence of pathological lesions (defined as normal or follicular hyperplasia). Consistent with a previous report (50), the positive cells, when present, colocalized with B-cell follicles on the adjacent section stained with H&E (Fig. 3F and G, arrow).
Interestingly, spleen sections derived from γHV68-infected animals diagnosed with atypical lymphoid hyperplasia showed a greater abundance of vtRNA-positive foci. Based on the H&E-stained adjacent spleen section, the positive cells were localized to the B-cell follicles with some positive cells present in the abnormally expanded T-cell zone (Fig. 3H and I, arrow). The intensity of vtRNA staining of cells in a positive focus was variable, ranging from intense to low (Fig. 3J). Based on the nuclear morphology, the positive staining was detected in lymphocytes as well as cells with monocytoid-like nuclei.
Histopathology of splenic lymphomas found in γHV68-infected BALB β2m−/− mice.
Lymphomas were characterized by nodular and/or diffuse proliferation of pleiomorphic large cells, showing a mixture of immunoblastic and centroblastic features (Fig. 4A and B). The involved spleens had a largely disrupted architecture with a massively expanded white pulp and a general loss of the distinction between B- and T-cell areas. The malignant proliferation involved mainly the white pulp. The malignant cells had large nuclei with open chromatin and single prominent nucleoli (immunoblasts) or several small but conspicuous nucleoli (centroblasts). Aside from the large cells, some of the lymphomas showed various degrees of plasmacytosis. These lesions were histologically aggressive as judged by the high mitotic index and the number of apoptotic bodies present.
FIG. 4.
Histopathology of splenic lymphomas. (A and B) Lymphoma-bearing spleens (×4). The general architecture of the spleen is disrupted by the massive expansion of the white pulp. (Insets in panels A and B) Large lymphoma cells with irregular nuclei with conspicuous nucleoli (×40). Frequent mitotic figures are present. (C) Metastasis to the liver (×10). Lymphoma cells are preferentially localized in the portal areas of the liver surrounding the bile ducts. (Inset) Tumor cells adjacent to a bile duct (×40). (D) Metastasis to the lung (×10). (E to G) HRP immunostaining with hematoxylin counterstain. Magnification at ×10 (insets at ×40). (E) Expression of B-cell marker B220. (F) Expression of the plasma cell marker CD138. (G) Expression of T-cell marker CD3. Peritumoral and tumor-infiltrating small lymphocytes but not lymphoma cells stain positive. (H) Lymphoma-bearing spleen section was hybridized with antisense digoxigenin-labeled vtRNA probe and counterstained with hematoxylin (×40).
Although lymphomas were primarily detected in the spleens, metastatic dissemination was also observed. Two of the nine lymphomas found in γHV68-infected males displayed metastases to the liver (periportal distribution, Fig. 4C); six out of nine lymphomas metastasized to the lung (peribronchiovascular distribution, Fig. 4D).
By immunohistochemistry, lymphoma cells expressed the B220 isoform of CD45, although the staining was often weaker than that on the remaining normal B cells (Fig. 4E). Staining with a monoclonal antibody to CD138 specific for plasma cells indicated that some of the atypical cells had detectable levels of CD138 expression (Fig. 4F). CD3 immunostaining showed only interspersed T cells but was consistently negative on the tumor cells (Fig. 4G).
In contrast to atypical lymphoid hyperplasia lesions, spleens derived from lymphoma-bearing γHV68-infected animals displayed a very limited vtRNA staining. Overt vtRNA-positive foci were not detected in the tumors analyzed. Instead, a small number of individual cells in the tumors stained vtRNA positive (Fig. 4H). The γHV68-harboring cells were scattered throughout the lesion, with most of the positive cells showing low levels of vtRNA expression. Occasionally, small vtRNA-positive lymphocytes were found outside the lesions in the tumor-bearing spleens (data not shown). It was not possible to determine with certainty whether these virus-positive tumor cells were malignant.
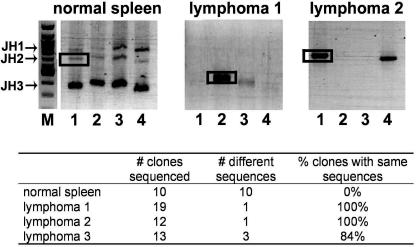
Clonality of splenic lymphomas.
Because of the apparent B-cell nature of the γHV68-associated lymphomas in infected animals, clonality of lymphomas was assessed by PCR analysis of the IgH locus (43). Although this clonality assay is quite robust in detection of specific rearrangements, it is not comprehensive in detection of all possible heavy chain rearrangements. In this assay, rearranged, but not germ line, heavy chain sequences are amplified using forward primers homologous to conserved framework region 3 of three murine Vh gene families (Fig. 5, lanes 1, 2, and 3 throughout) or primers homologous to a majority of known murine D minigenes (lanes 4). A reverse primer specific for the Jh3 sequence is used in all cases. Based on the utilization of the specific J region during heavy chain rearrangement (Jh1, Jh2, or Jh3), up to three major PCR products are obtained in each reaction from a heterogeneous population of splenic B cells (Fig. 5, normal spleen). A variety of sequences are expected to be present in any of the major PCR products obtained from a nonclonal population of B cells (Fig. 5, normal spleen and summary in the table below). The primers selected for the PCR analysis of the IgH locus are capable of amplifying only a select number of Vh families and are unable to detect heavy chain rearrangements utilizing Jh4.
FIG. 5.
Clonality assessment of γHV68-associated lymphomas. VH-to-DJH gene rearrangement was analyzed by PCR using forward primers specific for members of the Vh558 (lane 1), Vh7183 (lane 2), and VhQ52 (lane 3) families. DH-to-JH gene rearrangements were analyzed with the DhL primer (lane 4). A Jh3 reverse primer was used in all cases. PCR products were analyzed on a 1% agarose gel. M, 100-bp molecular size marker. Bands indicated by boxes were excised from the gel, reamplified using the same primers, cloned, and sequenced. The table shows the sequencing results.
Importantly, out of seven lymphomas analyzed, three tumors were found to be clonal based on the limited number of sequences present in the amplified major PCR product (Fig. 5). Specifically, two lymphomas were monoclonal; three sequences were detected upon analysis of the third lymphoma with majority of the sequences (11/13) belonging to one clone. Detection of more than one sequence could be due to amplification of the heavy chain of normal B cells present in the lymphoma-bearing spleen or due to the presence of several clonal transformed populations. The clonality of the remaining four lymphomas was indeterminate.
DISCUSSION
Gammaherpesvirus infection is associated with development of malignancies in immunocompromised hosts. To facilitate analysis of mechanisms and pathogenesis of this process, we have sought a murine model for gammaherpesvirus induction of lymphoproliferative disease. In this report, we demonstrate that γHV68 infection induces two lymphoproliferative diseases, each with histologic similarities to human diseases associated with gammaherpesvirus infection. We found that lack of the immunologically important molecule β2 microglobulin, genetic background, and gender all contributed to the risk of lymphoproliferative disease. The role of genetic background is of particular interest since, while immunocompromise is a clear risk factor for gammaherpesvirus-associated tumors, the reason that these tumors arise in only a small proportion of infected hosts is obscure. Interestingly, γHV68 infection was undetectable in most tumor cells, suggesting that direct transformation is not responsible for induction of malignancies in this model.
β2 microglobulin deficiency of γHV68-infected BALB mice contributes to development of lymphoproliferative disease.
BALB β2m−/− animals developed lymphoproliferative lesions in response to γHV68 infection, while their wild-type counterparts did not. One explanation for this is the paucity of CD8+ T cells capable of recognizing classical major histocompatibility complex class I-antigen complex in β2m−/− mice (73). CD8+ T cells play an important role in the control of γHV68 replication, latency, and persistent replication and can contribute to vaccination against latency in addition to control of lymphoproliferative disease associated with human gammaherpesviruses (5, 9, 22, 46, 48, 58, 60, 61, 64). CD4+ T cells also play a role in control of latent and persistent γHV68 infection (5, 9, 22, 46, 48, 58, 60, 61, 64). CD4+ T cells infiltrated and were critical for control of the γHV68-infected S11 tumor cell line in nude mice (41). A profound deficiency of CD8+ T cells and disruptions of immunoglobulin homeostasis due to the absence of neonatal Fc receptor are also observed in β2 microglobulin-deficient mice (28, 70, 73). As B cells and antibody play a role in regulating γHV68 latency (27, 32, 57, 65, 66), we cannot rule out a contribution of abnormal immunoglobulin homeostasis to tumorigenesis in BALB β2m−/− mice. Interestingly, β2 microglobulin deficiency on a C57BL/6 background resulted in a dramatic increase of polyoma virus-induced tumors in spite of equivalent levels of persistent viral gene expression and serum virus neutralizing antibodies in β2m−/− and wild-type mice (18).
It is also possible that the inability of BALB β2m−/− animals to control γHV68-induced lymphoproliferative disease is due instead to a lack of surface expression of nonclassical β2m-dependent class I molecules. These molecules are recognized by nonclassical CD8+ T cells, as well as NK cells, and have been shown to be important in tumor rejection (8, 34). Other abnormalities associated with the loss of β2 microglobulin expression, including iron overload, could have also played a role in the development of lymphoproliferative lesions in this strain (28, 42, 70, 73).
Role of mouse genetic background in susceptibility to lymphoproliferative disease.
The differences in oncogenic properties of gammaherpesviruses within species have been extensively documented for the HVS infection of primates. While HVS is ubiquitously present in T cells of healthy squirrel monkeys (36), it rapidly induces T-cell lymphomas in New World monkeys and marmosets (37). We also examined the role of background genes in development of lymphoproliferative disease. In contrast to the robust tumorigenesis observed in γHV68-infected BALB-β2m−/− mice, no tumors were detected in 129-β2m−/− mice, demonstrating that background genes contribute to susceptibility to or control of γHV68-associated lymphomagenesis.
The fact that mock-infected BALB β2m−/− mice develop similar lymphoproliferative disease as γHV68-infected mice (Fig. 1), albeit at a lower incidence and after a longer incubation time, suggests that the role of γHV68 infection may be to simply exacerbate an existing propensity to develop lymphoproliferative lesions which clearly seems to depend on the background and gender of the animal. It is interesting to speculate that the clear effect of background genes in our system may reflect a more general role for allelic variation in the susceptibility to gammaherpesvirus-associated disease. In this scenario, the reason that some hosts develop lymphoproliferative disease when immunosuppressed while others do not is that polymorphic susceptibility genes play a role. For example, polymorphisms in the gamma interferon (IFN-γ) gene have been associated with development of human posttransplant lymphoproliferative disease (PTLD), a lesion that is histologically similar to the atypical lymphoid hyperplasia described here. In a small study, an IFN-γ gene polymorphism associated with low production of IFN-γ positively segregated with development of PTLD in renal transplant patients (62). Importantly, IFN-γ is a key regulator of γHV68 reactivation from latency (47a). Recently this same gene polymorphism was associated with the development of EBV+ lymphoproliferative disease in the human peripheral blood lymphocyte-SCID mouse model (16).
The BALB/c background is associated with a functional polymorphism of the coding region of DNA dependent protein kinase catalytic subunit involved in DNA repair following ionizing radiation exposure (72). The BALB variant of this gene produces a kinase with decreased activity and, therefore, increases susceptibility of BALB mice to ionizing radiation-induced genomic instability (72). Another functional coding polymorphism unique to BALB/c results in decreased activity of p16INK4a, a cyclin-dependent kinase inhibitor (17). Crossing of BALB/c, but not other strains, to p53-null mice was important to establish the mouse model reflecting the critical role of p53 heterozygosity in breast cancer development (33). In our study, the presence of atypical lymphoid hyperplasia in uninfected BALB β2m−/− mice revealed that β2m deficiency combined with the BALB background predisposes to lymphoproliferative disease. Although γHV68-dependent lymphoproliferative disease has been reported in BALB/c mice (51), the shorter duration of this study, differences in mouse strains/suppliers, and animal housing conditions could have contributed to the absence of tumors in BALB/c γHV68-infected animals in our study.
Relationship between γHV68-associated atypical lymphoid hyperplasia and lymphoma.
γHV68 infection accelerated development and increased incidence of two lymphoproliferative lesions in BALB β2m−/− mice: lymphoma and atypical lymphoid hyperplasia. Compared to lymphomas, atypical lymphoid hyperplasia lesions appeared at a higher frequency and at an earlier time postinfection in animals infected with γHV68. Expression of CD138, which we observed in atypical lymphoid hyperplasia lesions, has also been reported to be present at high frequency in KSHV-associated primary effusion lymphoma (26).
Atypical lymphoid hyperplasia lesions have significant similarities to lesions of human PTLD. We have selected the term ALH rather than PTLD to emphasize that there is no current proof that the mechanisms underlying γHV68-associated ALH are shared with human PTLD. Importantly, atypical lymphoid hyperplasia lacked features typical for malignant lesions. ALH lesions were spatially restricted within the disrupted white pulp area and lacked mitotic figures and apoptotic bodies. Because of the earlier appearance of ALH as compared to lymphomas in γHV68-infected animals and because of the retention of some CD138+ cells in the γHV68-associated lymphomas, it is tempting to speculate that ALH is a precursor to malignancy. However, a causal relationship between the two lymphoproliferative diseases cannot be established by these morphological studies.
Role of γHV68 infection in the development of atypical lymphoid hyperplasia.
Clusters of vtRNA-positive cells were observed in atypical lymphoid hyperplasia lesions, but not in spleens with normal histology taken from γHV68-infected animals. It is not clear at this point whether these cells represent expansion of latent or lytically infected cells. Interestingly, EBV-triggered PTLD in humans is characterized by increased numbers of EBV-positive cells and increased viral replication and leads to malignancies if not controlled (55). Perhaps lymphoma cells arise from the vtRNA-positive cells associated with atypical lymphoid hyperplasia. In the future, it will be important to determine the type of cell expressing vtRNAs in ALH lesions.
Role of γHV68 infection in the development of lymphoma.
The increased incidence and accelerated development of lymphoproliferative lesions in γHV68-infected BALB β2m−/− mice (Fig. 1) strongly argues for the role of γHV68 infection in the genesis of lymphomas. However, unlike certain EBV-associated tumors, only a few tumor cells in γHV68-infected animals were positive for vtRNA expression. Our results are in agreement with previously published studies of γHV68-associated lymphomagenesis in wild-type mice, where only a small proportion of malignant cells were reported to harbor γHV68 in infected animals (39, 51). Thus, the direct transformation model that operates in certain EBV-associated tumors is clearly not applicable to γHV68-associated lymphomagenesis in BALB β2m−/− mice.
The role of γHV68 vtRNA-positive cells in lymphomas is not clear. They may be incidental to the malignancy process. However, it is also possible that these cells are driving the malignancy process. For example, although only a small portion of cells in Hodgkin's lymphoma are EBV positive, it is thought that these cells are the malignant cells surrounded by nonmalignant inflammatory cell infiltrate. Interestingly, it was reported that EBV was lost from a malignant Hodgkin's tumor upon relapse of the same malignancy, raising an intriguing possibility that EBV presence may not be required to maintain a malignant phenotype of tumor in vivo (15). Unfortunately, a true case of hit-and-run oncogenesis may be difficult to prove for human gammaherpesviruses, especially, in the case of a “clean exit” where the viral genome is completely lost (1).
Although the direct transformation model with continuous presence of viral genome in malignant cells is not applicable to our tumor system, several other mechanisms of virus-associated lymphomagenesis may contribute to the γHV68-induced lymphoproliferative disease. γHV68-infected cells might stimulate proliferation and transformation of uninfected malignant B cells through release of growth factors (paracrine model). A similar situation may occur in Kaposi's sarcomas where release of vascular endothelial growth factor by KSHV-infected cells expressing viral G protein-coupled receptor can stimulate angiogenesis and proliferation of uninfected cells in the lesion (6).
Alternatively, chronic inflammation associated with γHV68 infection may drive proliferation of antigen-specific and bystander B cells that could subsequently acquire deleterious mutations leading to transformation. The monoclonal nature of some γHV68-associated tumors suggests that a single transformed B cell gave rise to the observed malignancies. Interestingly, in humans, B-cell receptors of chronic lymphocytic lymphoma cells are strikingly similar among several independent cases and resemble monoclonal antibodies reactive with carbohydrates found on pathogens (29), suggesting that chronic B-cell stimulation may play a role in human lymphomagenesis. If chronic inflammation is indeed the primary driving force behind γHV68-associated lymphomagenesis in our tumor model, then other mouse pathogens that cause chronic infections (i.e., mouse cytomegalovirus) may also trigger lymphoproliferative disease in BALB β2m−/− mice. Therefore, monitoring of lymphoproliferative disease in BALB β2m−/− mice chronically infected with other viruses is an important priority for future studies.
In summary, β2m deficiency in BALB/c mice greatly increases susceptibility to γHV68-associated lymphomagenesis. γHV68 infection of these animals leads to increased incidence and accelerated development of atypical lymphoid hyperplasia and B-cell lymphoma and represents a valuable tool to study host and viral factors involved in gammaherpesvirus-associated pathogenesis in a natural host. Because γHV68 is genetically and biologically related to the human gammaherpesviruses, EBV and KSHV, identification of mechanisms of lymphomagenesis associated with γHV68 infection in vivo might contribute to the understanding of lymphomagenesis associated with human gammaherpesviruses. The availability of a model in which one can study potential mechanisms of tumorigenesis such as hit and run, paracrine, and chronic inflammation should enhance chances of understanding fundamental mechanisms responsible for virus-associated tumors.
Acknowledgments
H.W.V. and S.H.S. were supported by National Institutes of Health (NIH) grant CA74730. V.L.T. was supported by an NIH training grant T32 CA 09547 and NIH/NRSA fellowship F32 AI065079-01. F.S. was supported by and American Cancer Society postdoctoral fellowship. S.A.T. is a Leukemia and Lymphoma Society special fellow. We acknowledge the assistance of core histopathology facilities funded by DRTC and NIH grant P30 DK52514.
We thank Darren Kreamalmayer for his outstanding expertise in the breeding of knockout animals and all members of the Virgin lab for helpful discussions. A special acknowledgment goes to Stacy Efstathiou, who selflessly provided reagents and helpful suggestions for analysis of tumors by in situ hybridization.
REFERENCES
- 1.Ambinder, R. F. 2000. Gammaherpesviruses and “Hit-and-Run” oncogenesis. Am. J. Pathol. 156:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, E. A. Mesri, and M. C. Gerhengorn. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator Nature 391:86-89. (Erratum, 392: 6672.) [DOI] [PubMed]
- 3.Blaskovic, D., M. Stancekova, J. Svobodova, and J. Mistrikova. 1980. Isolation of five strains of herpesviruses from two species of free living small rodents. Acta Virol. 24:468. [PubMed] [Google Scholar]
- 4.Bowden, R. J., J. P. Simas, A. J. Davis, and S. Efstathiou. 1997. Murine gammaherpesvirus 68 encodes tRNA-like sequences which are expressed during latency. J. Gen. Virol. 78:1675-1687. [DOI] [PubMed] [Google Scholar]
- 5.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cathomas, G. 2003. Kaposi's sarcoma-associated herpesvirus (KSHV)/human herpesvirus 8 (HHV-8) as a tumor virus. Herpes 10:72-77. [PubMed] [Google Scholar]
- 7.Chadburn, A., J. M. Chen, D. T. Hsu, G. Frizzera, E. Cesarman, T. J. Garrett, J. G. Mears, S. D. Zangwill, L. J. Addonizio, R. E. Michler, and D. M. Knowles. 1998. The morphologic and molecular genetic categories of posttransplantation lymphoproliferative disorders are clinically relevant. Cancer 82:1978-1987. [PubMed] [Google Scholar]
- 8.Chiang, E. Y., and I. Stroynowski. 2004. A nonclassical MHC class I molecule restricts CTL-mediated rejection of a syngeneic melanoma tumor. J. Immunol. 173:4394-4401. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, J. P., R. D. Cardin, K. C. Branum, and P. C. Doherty. 1999. CD4(+) T cell-mediated control of a gamma-herpesvirus in B cell-deficient mice is mediated by IFN-gamma. Proc. Natl. Acad. Sci. USA 96:5135-5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins, C. M., and P. G. Medveczky. 2001. Oncogenic transformation of T cells by Herpesvirus saimiri, p. 140-158. In L. J. Rosenthal (ed.), Mechanisms of DNA tumor virus transformation. Karger, Basel, Switzerland.
- 11.Coppola, M. A., E. Flano, P. Nguyen, C. L. Hardy, R. D. Cardin, N. Shastri, D. L. Woodland, and M. A. Blackman. 1999. Apparent MHC-independent stimulation of CD8+ T cells in vivo during latent murine gammaherpesvirus infection. J. Immunol. 163:1481-1489. [PubMed] [Google Scholar]
- 12.Crowe, S. E. 2005. Helicobacter infection, chronic inflammation, and the development of malignancy. Curr. Opin. Gastroenterol. 21:32-38. [PubMed] [Google Scholar]
- 13.Damania, B. 2004. Oncogenic gammaherpesviruses: comparison of viral proteins involved in tumorigenesis. Nat. Rev. Microbiol. 2:656-668. [DOI] [PubMed] [Google Scholar]
- 14.Darenkov, I. A., M. A. Marcarelli, G. P. Basadonna, A. L. Friedman, K. M. Lorber, J. G. Howe, J. Crouch, M. J. Bia, A. S. Kliger, and M. I. Lorber. 1997. Reduced incidence of Epstein-Barr virus-associated posttransplant lymphoproliferative disorder using preemptive antiviral therapy. Transplantation 64:848-852. [DOI] [PubMed] [Google Scholar]
- 15.Delecluse, H. J., T. Marafioti, M. Hummel, F. Dallenbach, I. Anagnostopoulos, and H. Stein. 1997. Disappearance of the Epstein-Barr virus in a relapse of Hodgkin's disease. J. Pathol. 182:475-479. [DOI] [PubMed] [Google Scholar]
- 16.Dierksheide, J. E., R. A. Baiocchi, A. K. Ferketich, S. Roychowdhury, R. P. Pelletier, C. F. Eisenbeis, M. A. Caligiuri, and A. M. VanBuskirk. 2005. IFN-gamma gene polymorphisms associate with development of EBV+ lymphoproliferative disease in hu PBL-SCID mice. Blood 105:1558-1565. [DOI] [PubMed] [Google Scholar]
- 17.Dragani, T. A. 2003. 10 years of mouse cancer modifier loci: human relevance. Cancer Res. 63:3011-3018. [PubMed] [Google Scholar]
- 18.Drake, D. R., III, and A. E. Lukacher. 1998. Beta 2-microglobulin knockout mice are highly susceptible to polyoma virus tumorigenesis. Virology 252:275-284. [DOI] [PubMed] [Google Scholar]
- 19.Dunn, G. P., A. T. Bruce, K. C. F. Sheehan, V. Shankaran, R. Uppaluri, J. D. Bui, M. S. Diamond, C. M. Koebel, C. Arthur, J. M. White, and R. D. Schreiber. 2005. A critical function for type I interferons in cancer immunoediting. [Erratum.] Nat. Immunol. 8:852. [DOI] [PubMed] [Google Scholar]
- 20.Dutia, B. M., J. P. Stewart, R. A. Clayton, H. Dyson, and A. A. Nash. 1999. Kinetic and phenotypic changes in murine lymphocytes infected with murine gammaherpesvirus-68 in vitro. J. Gen. Virol. 80:2729-2736. [DOI] [PubMed] [Google Scholar]
- 21.Efstathiou, S., Y. M. Ho, S. Hall, C. J. Styles, S. D. Scott, and U. A. Gompels. 1990. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J. Gen. Virol. 71:1365-1372. [DOI] [PubMed] [Google Scholar]
- 22.Ehtisham, S., N. P. Sunil-Chandra, and A. A. Nash. 1993. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J. Virol. 67:5247-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flaks, A. 1968. The susceptibility of various strains of neonatal mice to the carcinogenic effects of 9,10-dimethyl-1,2-benzanthracene. Eur. J. Cancer 4:579-585. [DOI] [PubMed] [Google Scholar]
- 24.Flamand, L., R. A. Zeman, J. L. Bryant, Y. Lunardi-Iskander, and R. C. Gallo. 1996. Absence of human herpesvirus 8 DNA sequences in neoplastic Kaposi's sarcoma cell lines. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13:194-197. [DOI] [PubMed] [Google Scholar]
- 25.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1074-1081. [DOI] [PubMed] [Google Scholar]
- 26.Gaidano, G., A. Gloghini, V. Gattei, M. F. Rossi, A. M. Cilia, C. Godeas, M. Degan, T. Perin, V. Canzonieri, D. Aldinucci, G. Saglio, A. Carbone, and A. Pinto. 1997. Association of Kaposi's sarcoma-associated herpesvirus-positive primary effusion lymphoma with expression of the CD138/syndecan-1 antigen. Blood 90:4894-4900. [PubMed] [Google Scholar]
- 27.Gangappa, S., S. B. Kapadia, S. H. Speck, and H. W. Virgin IV. 2002. Antibody to a lytic cycle viral protein decreases gammaherpesvirus latency in B-cell-deficient mice. J. Virol. 76:11460-11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghetie, V., J. G. Hubbard, J. K. Kim, M. F. Tsen, Y. Lee, and E. S. Ward. 1996. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur. J. Immunol. 26:690-696. [DOI] [PubMed] [Google Scholar]
- 29.Ghiotto, F., F. Fais, A. Valetto, E. Albesiano, S. Hashimoto, M. Dono, H. Ikematsu, S. L. Allen, J. Kolitz, K. R. Rai, M. Nardini, A. Tramontano, M. Ferrarini, and N. Chiorazzi. 2004. Remarkably similar antigen receptors among a subset of patients with chronic lymphocytic leukemia. J. Clin. Investig. 113:1008-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hislop, A. D., N. H. Gudgeon, M. Callan, C. Fazou, H. Hasegawa, M. Salmon, and A. Rickinson. 2001. EBV-specific CD8+ T cell memory: relationships between epitope specificity, cell phenotype, and immediate effector function. J. Immunol. 167:2019-2029. [DOI] [PubMed] [Google Scholar]
- 31.Jung, J. U., J. K. Choi, A. Ensser, and B. Biesinger. 1999. Herpesvirus saimiri as a model for gammaherpesvirus oncogenesis. Semin. Cancer Biol. 9:231-239. [DOI] [PubMed] [Google Scholar]
- 32.Kim, I. J., E. Flano, D. L. Woodland, and M. A. Blackman. 2002. Antibody-mediated control of persistent γ-herpesvirus infection. J. Immunol. 168:3958-3964. [DOI] [PubMed] [Google Scholar]
- 33.Kuperwasser, C., G. D. Hurlbut, F. S. Kittrell, E. S. Dickinson, R. Laucirica, D. Medina, S. P. Naber, and D. J. Jerry. 2000. Development of spontaneous mammary tumors in BALB/c p53 heterozygous mice. A model for Li-Fraumenni syndrome. Am. J. Pathol. 157:2151-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Botet, M., M. Llano, F. Navarro, and T. Bellon. 2000. NK cell recognition of non-classical HLA class I molecules. Semin. Immunol. 12:109-119. [DOI] [PubMed] [Google Scholar]
- 35.Malek, S. N., D. I. Dordai, J. Reim, H. Dintzis, and S. Desiderio. 1998. Malignant transformation of early lymphoid progenitors in mice expressing an activated Blk tyrosine kinase. Proc. Natl. Acad. Sci. USA 95:7351-7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melendez, L. V., M. D. Daniel, R. D. Hunt, and F. G. Garcia. 1968. An apparently new herpesvirus from primary kidney cultures of the squirrel monkey (Saimiri Sciureus). Lab. Anim. Care 18:374-381. [PubMed] [Google Scholar]
- 37.Melendez, L. V., R. D. Hunt, M. D. Daniel, F. G. Garcia, and C. E. Fraser. 1969. Herpesvirus saimiri. II. Experimentally induced malignant lymphoma in primates. Lab. Anim. Care 19:378-386. [PubMed] [Google Scholar]
- 37a.Moser, J. M., J. W. Upton, R. D. Allen III, C. B. Wilson, and S. H. Speck. 2005. Role of B-cell proliferation in the establishment of gammaherpesvirus latency. J. Virol. 79:9480-9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nevins, J. R. 2001. Cell transformation by viruses, p. 245-283. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams& Wilkins, Philadelphia, Pa. [Google Scholar]
- 39.Pappova, M., M. Stancekova, I. Spissakova, V. Durmanova, and J. Mistrikova. 2004. Pathogenetical characterization of isolate MHV-60 of mouse herpesvirus strain 68. Acta Virol. 48:91-96. [PubMed] [Google Scholar]
- 40.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. InB. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology, 3rd ed., vol.2. Lippincott-Raven, Philadelphia, Pa.
- 41.Robertson, K. A., E. J. Usherwood, and A. A. Nash. 2001. Regression of a murine gammaherpesvirus 68-positive B-cell lymphoma mediated by CD4 Tlymphocytes. J. Virol. 75:3480-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos, M., M. W. Schilham, L. H. Rademakers, J. J. Marx, M. de Sousa, and H. Clevers. 1996. Defective iron homeostasis in beta 2-microglobulin knockout mice recapitulates hereditary hemochromatosis in man. J. Exp. Med. 184:1975-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlissel, M. S., L. M. Corcoran, and D. Baltimore. 1991. Virus-transformed pre-B cells show ordered activation but not inactivation of immunoglobulin gene rearrangement and transcription. J. Exp. Med. 173:711-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen, Y., H. Zhu, and T. Shenk. 1997. Human cytomegalovirus IE1 and IE2 proteins are mutagenic and mediate “hit-and-run” oncogenic transformation in cooperation with the adenovirus E1A proteins. Proc. Natl. Acad. Sci. USA 94:3341-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simas, J. P., D. Swann, R. Bowden, and S. Efstathiou. 1999. Analysis of murine gammaherpesvirus-68 transcription during lytic and latent infection. J. Gen. Virol. 80:75-82. [DOI] [PubMed] [Google Scholar]
- 46.Sparks-Thissen, R. L., D. C. Braaten, S. Kreher, S. H. Speck, and H. W. Virgin IV. 2004. An optimized CD4 T-cell response can control productive and latent gammaherpesvirus infection. J. Virol. 78:6827-6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srinivas, S. K., J. T. Sample, and J. W. Sixbey. 1998. Spontaneous loss of viral episomes accompanying Epstein-Barr virus reactivation in a Burkitt's lymphoma cell line. J. Infect. Dis. 177:1705-1709. [DOI] [PubMed] [Google Scholar]
- 47a.Steed, A., E. S. Barton, S. A. Tibbetts, D. L. Popkin, M. L. Lutzke, R. Rochford, and H. W. Virgin IV. Gamma interferon blocks gammaherpesvirus reactivation from latency. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 48.Stevenson, P. G., R. D. Cardin, J. P. Christensen, and P. C. Doherty. 1999. Immunological control of a murine gammaherpesvirus independent of CD8+ T cells. J. Gen. Virol. 80:477-483. [DOI] [PubMed] [Google Scholar]
- 49.Stevenson, P. G., and P. C. Doherty. 1999. Non-antigen-specific B-cell activation following murine gammaherpesvirus infection is CD4 independent in vitro but CD4 dependent in vivo. J. Virol. 73:1075-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stewart, J. P., E. J. Usherwood, A. Ross, H. Dyson, and T. Nash. 1998. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J. Exp. Med. 187:1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sunil-Chandra, N. P., J. Arno, J. Fazakerley, and A. A. Nash. 1994. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am. J. Pathol. 145:818-826. [PMC free article] [PubMed] [Google Scholar]
- 52.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gammaherpesvirus 68. J. Gen. Virol. 73:2347-2356. [DOI] [PubMed] [Google Scholar]
- 53.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 73:3275-3279. [DOI] [PubMed] [Google Scholar]
- 54.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1993. Interactions of murine gammaherpesvirus 68 with B and T cell lines. Virology 193:825-833. [DOI] [PubMed] [Google Scholar]
- 55.Thorley-Lawson, D. A., and A. Gross. 2004. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 350:1328-1337. [DOI] [PubMed] [Google Scholar]
- 56.Tibbetts, S. A., J. Loh, V. van Berkel, J. S. McClellan, M. A. Jacoby, S. B. Kapadia, S. H. Speck, and H. W. Virgin IV. 2003. Establishment and maintenance of gammaherpesvirus latency are independent of infective dose and route of infection. J. Virol. 77:7696-7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tibbetts, S. A., J. S. McClellan, S. Gangappa, S. H. Speck, and H. W. Virgin IV. 2003. Effective vaccination against long-term gammaherpesvirus latency. J. Virol. 77:2522-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tibbetts, S. A., L. Van Dyk, S. H. Speck, and H. W. Virgin IV. 2002. Immune control of the number and reactivation phenotype of cells latently infected with a gammaherpesvirus. J. Virol. 76:7125-7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turcanova, V., and P. Hollsberg. 2004. Sustained CD8+ T-cell immune response to a novel immunodominant HLA-B*0702-associated epitope derived from an Epstein-Barr virus helicase-primase-associated protein. J. Med. Virol. 72:635-645. [DOI] [PubMed] [Google Scholar]
- 60.Usherwood, E. J., A. J. Ross, D. J. Allen, and A. A. Nash. 1996. Murine gammaherpesvirus-induced splenomegaly: a critical role for CD4 T cells. J. Gen. Virol. 77:627-630. [DOI] [PubMed] [Google Scholar]
- 61.Usherwood, E. J., D. J. Roy, K. Ward, S. L. Surman, B. M. Dutia, M. A. Blackman, J. P. Stewart, and D. L. Woodland. 2000. Control of gammaherpesvirus latency by latent antigen-specific CD8(+) T cells. J. Exp. Med. 192:943-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.VanBuskirk, A. M., V. Malik, D. Xia, and R. P. Pelletier. 2001. A gene polymorphism associated with posttransplant lymphoproliferative disorder. Transplant. Proc. 33:1834. [DOI] [PubMed] [Google Scholar]
- 63.Virgin, H. W., IV, P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weck, K. E., M. L. Barkon, L. I. Yoo, S. H. Speck, and H. W. Virgin IV. 1996. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J. Virol. 70:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weck, K. E., A. J. Dal Canto, J. D. Gould, A. K. O'Guin, K. A. Roth, J. E. Saffitz, S. H. Speck, and H. W. Virgin. 1997. Murine gammaherpesvirus 68 causes severe large vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus induced vascular disease. Nat. Med. 3:1346-1353. [DOI] [PubMed] [Google Scholar]
- 66.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. B cells regulate murine gammaherpesvirus 68 latency. J. Virol. 73:4651-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Willer, D. O., and S. H. Speck. 2003. Long-term latent murine gammaherpesvirus 68 infection is preferentially found within the surface immunoglobulin D-negative subset of splenic B cells in vivo. J. Virol. 77:8310-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang, T. Y., S. C. Chen, M. W. Leach, D. Manfra, B. Homey, M. Wiekowski, L. Sullivan, C. H. Jenh, S. K. Narula, S. W. Chensue, and S. A. Lira. 2000. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J. Exp. Med. 191:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshida, M., S. M. Claypool, J. S. Wagner, E. Mizoguchi, A. Mizoguchi, D. C. Roopenian, W. I. Lencer, and R. S. Blumberg. 2004. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 20:769-783. [DOI] [PubMed] [Google Scholar]
- 71.Young, L. S., and A. Rickinson. 2004. Epstein-Barr virus: 40 years on. Nat. Rev. Immunol. 4:757-768. [DOI] [PubMed] [Google Scholar]
- 72.Yu, Y., R. Okayasu, M. M. Weil, A. Silver, M. McCarthy, R. Zabriskie, S. Long, R. Cox, and R. L. Ullrich. 2001. Elevated breast cancer risk in irradiated BALB/c mice associates with unique functional polymorphism of the Prkdc (DNA-dependent protein kinase catalytic subunit) gene. Cancer Res. 61:1820-1824. [PubMed] [Google Scholar]
- 73.Zijlstra, M., M. Bix, N. E. Simister, J. M. Loring, D. H. Raulet, and R. Jaenisch. 1990. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic Tcells. Nature 344:742-746. [DOI] [PubMed] [Google Scholar]