Abstract
Pathogenicity of most Gram-negative bacterial plant pathogens depends on hrp (hypersensitive response and pathogenicity) genes, which control the ability to cause disease and to elicit specific defense responses in resistant plants. hrp genes encode a specialized type III secretion (TTS) system that mediates the vectorial delivery of bacterial effector proteins across both bacterial membranes as well as across the eukaryotic plasma membrane into the host cell cytosol. One well-studied effector protein is AvrBs3 from Xanthomonas campestris pv. vesicatoria, the causal agent of bacterial spot in pepper and tomato. AvrBs3 induces hypertrophy symptoms in susceptible plants and triggers a resistance gene-specific cell death reaction in resistant plants. Intriguingly, AvrBs3 has characteristic features of eukaryotic transcription factors, suggesting that it modulates the host’s transcriptome. Here, we discuss the TTS system of X.campestris pv. vesicatoria in the light of current knowledge on type III-dependent protein secretion in plant pathogenic bacteria.
Keywords: AvrBs3/hrp genes/pathogenicity island/PIP box/secretion
Introduction
Plants provide an attractive nutrient reservoir and ecological niche for bacterial pathogens. In most higher plants, bacterial colonization leads to a variety of severe diseases. However, disease is the exception rather than the rule since most plants possess a battery of defense mechanisms that repel invading microbes. Therefore, Gram-negative plant pathogenic bacteria have evolved sophisticated strategies to colonize their host plants. They enter the plant through natural openings such as stomata, or wounds, and multiply in the intercellular spaces of the tissue at the expense of the host.
Over the past two decades, genetic and molecular studies unraveled important mechanisms underlying bacterial pathogenicity. Essential for the molecular cross-talk between pathogens and their host plants is a specialized protein delivery system, the type III secretion (TTS) system. TTS systems are conserved in plant and animal pathogenic bacteria and mediate the vectorial delivery of bacterial effector proteins into the host cell (Hueck, 1998; Cornelis and Van Gijsegem, 2000). In plant pathogens, TTS systems are encoded by hrp (hypersensitive response and pathogenicity) genes, essential determinants of bacterial pathogenicity that control the ability to multiply in susceptible hosts and to cause disease (Alfano and Collmer, 1997). In addition, hrp genes are required to induce the hypersensitive response (HR), a rapid localized programmed death of plant cells at the infection site, in resistant host and in non-host plants (Klement, 1982). The HR is part of the plant’s innate immune response that halts bacterial ingress. Induction of the HR is due to the specific recognition of a bacterial effector protein [designated avirulence (Avr) protein] by a corresponding plant resistance (R) protein (Flor, 1971; Table I).
Table I. R gene-specified pathogen recognition according to gene-for-gene interactionsa.
| Pathogen genotype | Plant reaction |
|
|---|---|---|
| Host plant genotypeb | ||
| R1/R1 or R1/r1 | r1/r1 | |
| avr1 | HRd | Disease |
| –c | Disease | Disease |
aGene-for-gene hypothesis (Flor, 1971).
bR1, resistance locus allowing recognition of a corresponding avirulence (avr) gene (designated avr1). Most resistance (R) genes are single dominant genes. r1 refers to the absence of a functional R1 allele.
cThe avirulence gene is absent or mutated, resulting in loss of recognition by plants carrying the corresponding R gene.
dHR, hypersensitive reaction.
Among the model organisms for the molecular and genetic characterization of host–plant interactions and the functional analysis of TTS systems are Erwinia amylovora, Ralstonia solanacearum, pathovars (pv.) of Pseudo monas syringae and species (spp.) of Xanthomonas, all infecting important crop plants. The pathovar designation refers to differences in the host range of the bacteria. For some of these bacteria, the genome sequence has become available recently, initiating a new era in molecular plant pathology (Da Silva et al., 2002; Salanoubat et al., 2002; www.tigr.org). Our laboratory studies Xanthomonas campestris pv. vesicatoria, the causal agent of bacterial spot in pepper and tomato plants, which is the focus of this review.
The hrp pathogenicity island—genetic requisite for effector protein traffic
Gram-negative bacteria utilize different protein secretion systems to transport proteins across the inner and outer membrane. Among the six main groups of secretion systems, TTS systems exhibit the most complex architecture (Thanassi and Hultgren, 2000). Around 20 proteins are involved in the formation of a membrane-spanning secretion apparatus, which is associated with an extracellular filamentous structure (Hueck, 1998; see below).
Type III-mediated protein secretion into the extracellular medium was discovered initially in the animal pathogen Yersinia enterocolitica (Heesemann et al., 1984). However, the first genes encoding components of the TTS system were identified by the analysis of non-pathogenic mutants of the plant pathogens P.syringae pv. syringae and P.syringae pv. phaseolicola (Niepold et al., 1985; Lindgren et al., 1986). Except for Agrobacterium spp., hrp genes are present in all Gram-negative biotrophic plant pathogens and are generally organized in large clusters comprising >20 genes (Boucher et al., 1987; Steinberger and Beer, 1988; Barny et al., 1990; Arlat et al., 1991; Bonas et al., 1991). Based on similarities in hrp gene organization and regulation, plant pathogenic bacteria have been classified into two groups, group I (E.amylovora and P.syringae) and group II (R.solanacearum and species of Xanthomonas) (Alfano and Collmer, 1996). At least nine hrp genes (termed hrc for hrp conserved) are conserved in both groups and encode components of the TTS system, which are also present in animal pathogenic bacteria (Bogdanove et al., 1996; He, 1998; Hueck, 1998). Hrc proteins presumably constitute the core components of the secretion apparatus in the inner and outer membrane. With the exception of HrcC—the best studied Hrc protein, which belongs to the secretin family of outer membrane proteins—Hrc proteins share sequence similarities with flagellar assembly components. The flagellar assembly apparatus serves as a protein export system and probably represents an evolutionary ancestor of the TTS system (Hueck, 1998; Macnab, 1999; Aizawa, 2001; Young and Young, 2002).
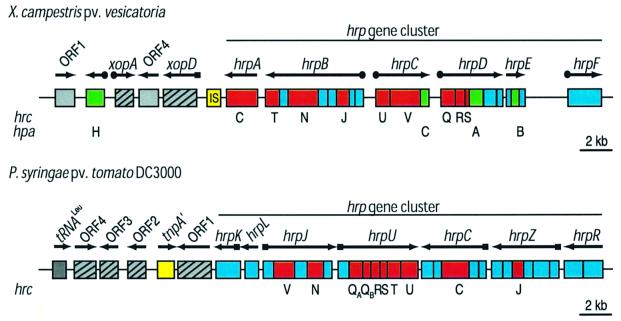
In contrast to conserved hrp genes, the precise role of non-conserved hrp genes remains to be investigated. Genetic studies of X.campestris pv. vesicatoria revealed that type III secretion requires at least six non-conserved hrp genes, some of which encode type III-secreted proteins, e.g. HrpB2 (Rossier et al., 2000; Table II). The hrp region also contains so-called hrp-associated (hpa) genes (Figure 1), which are not essential for bacterial pathogenicity but contribute to the interaction with the host plant (Huguet et al., 1998; Noël et al., 2002; O.Rossier, D.Büttner and U.Bonas, unpublished data).
Table II. Xanthomonas campestris pv. vesicatoria type III-secreted proteins.
| Proteina | Characteristics/homologyb | Expressionc | References |
|---|---|---|---|
| Components of the TTS apparatus | |||
| HrpB2‡ | Extracellular component of the TTS system | Induced; PIP box | Wengelnik and Bonas (1996); Rossier et al. (2000) |
| HrpE1‡ | Major Hrp pilus subunit | Basal expression, induction | Wengelnik and Bonas (1996); Rossier (1999); T.Ojanen-Reuhs and U.Bonas (unpublished data) |
| HrpF |
Translocon protein |
Induced; PIP box |
Wengelnik and Bonas (1996); Rossier et al. (2000); Büttner et al. (2002) |
| Xopsd | |||
| √XopA | Hpa1 (X.oryzae pv. oryzae) | Induced; PIP box | Noël et al. (2002) |
| XopB | AvrPphD (P.syringae pv. phaseolicola) | Induced | Noël (2001); Noël et al. (2001) |
| XopC | Induced | Noël (2001); Noël et al. (2001) | |
| XopD | PsvA (P.syringae pv. eriobotryae) | Induced; hrp box | Noël et al. (2002) |
| XopJ | AvrRxv/YopJ family; putative cysteine protease | Induced | Noël (2001); Noël et al. (2001) |
| √HpaA | NLS | Induced; PIP box | Wengelnik and Bonas (1996); Huguet et al. (1998) |
| AvrBs1* | AvrA (P.syringae pv. glycinea) | Constitutive | Ronald and Staskawicz, (1988); Escolar et al. (2001) |
| √AvrBs2* | Agrocinopine synthase (A.tumefaciens); phosphodiesterase (E.coli) | ND | Kearney and Staskawicz (1990); Swords et al. (1996); Mudgett et al. (2000) |
| √AvrBs3* | NLS; AAD; AvrBs3 family | Constitutivee | Van den Ackerveken et al. (1996); Rossier et al. (1999); Szurek et al. (2001); Marois et al. (2002) |
| AvrBs4* | NLS; AAD; AvrBs3 family | Constitutivee | Bonas et al. (1993); Ballvora et al. (2001) |
| AvrBsT* | AvrRxv/YopJ family; putative cysteine protease | Constitutive | Escolar et al. (2001) |
| AvrRxv | AvrRxv/YopJ family; putative cysteine protease | Constitutive; PIP box | Ciesiolka et al. (1999); Rossier et al. (1999) |
| AvrXv3* | AAD | Induced; PIP box | Astua-Monge et al. (2000a) |
| AvrXv4 | AvrRxv/YopJ family; putative cysteine protease | ND; PIP box | Astua-Monge et al. (2000b) |
a‡, essential for type III secretion in vitro; √, virulence activity demonstrated; *, indicates ability of Avr proteins to induce the HR upon transient expression in resistant plants.
bAAD, acidic activation domain; NLS, nuclear localization signal; Yop, Yersinia outer protein.
cExpression in planta or under hrp gene-inducing conditions. ND, not determined; PIP, plant-inducible promoter.
dXops, Xanthomonas outer proteins, include type III-secreted proteins with unknown destination as well as avirulence (Avr) proteins; Hpa, hrp associated.
eRecent in vitro expression experiments indicate that hrpG* leads to a 2- to 3-fold increase in expression (U.Bonas et al., unpublished data).
√, virulence activity demonstrated.
*, indicates ability of Avr proteins to induce the HR upon transient expression in resistant plants.
Fig. 1. Schematic overview of the hrp gene clusters and the left flanking regions from X.campestris pv. vesicatoria (group II) and P.syringae pv. tomato DC3000 (group I). The regions contain hrp, hrc and hpa genes (represented in blue, red and green, respectively). Arrows indicate the direction of transcription. Black dots and squares refer to the presence of PIP and hrp boxes, respectively. Hatched regions correspond to sequences with low G + C content; yellow regions refer to mobile genetic elements.
How did hrp gene clusters evolve? Genes involved in bacterial virulence often are located in regions that show characteristics of pathogenicity islands. These DNA regions usually are flanked by direct repeats, insertion sequence (IS) elements, tRNA genes and/or genes for integrases and transposases. Pathogenicity islands often differ in G + C content from the genomic DNA, indicating horizontal gene transfer (Hacker and Kaper, 2000). In X.campestris pv. vesicatoria, mobility of the hrp region has indeed been observed (Basim et al., 1999). Furthermore, sequence analyses of DNA regions flanking the hrp gene cluster revealed the presence of an IS-like element and putative effector genes with lower G + C content than the genomic DNA (Noël et al., 2002; Figure 1).
Typical features of pathogenicity islands are also present in DNA sequences flanking the hrp gene cluster of P.syringae. Here, the region adjacent to hrpK has a lower G + C content and contains sequences homologous to IS elements, transposases and tRNA genes. Interest ingly, the genes located in this region, termed exchangeable effector locus (EEL), vary in pathovars of P.syringae (Alfano et al., 2000; one example is given in Figure 1).
Entering the plant—green light for hrp gene expression
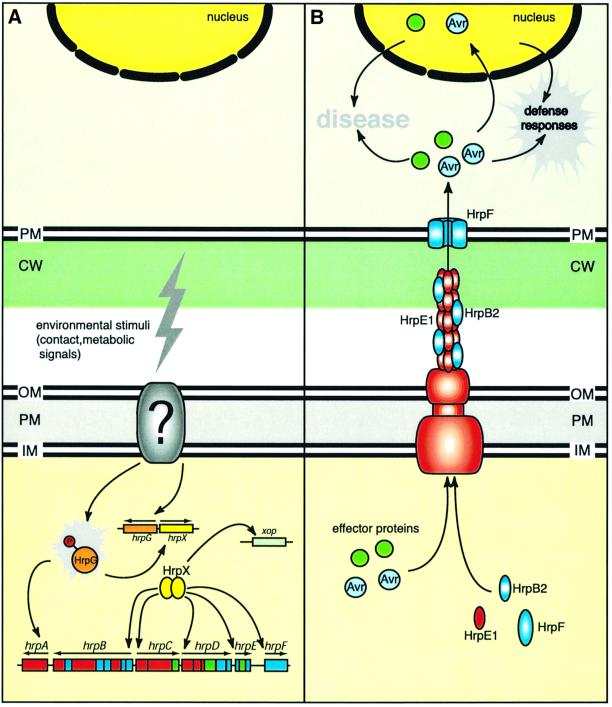
Type III secretion is a regulated process. Genes encoding components of the secretion apparatus are not constitutively expressed but activated in planta and in minimal media mimicking the environmental conditions present in the plant apoplast (Lindgren, 1997). Proteins involved in hrp gene regulation vary in the different groups of plant pathogens. In X.campestris pv. vesicatoria, hrp gene expression is controlled by HrpX, an AraC-type transcriptional activator (Wengelnik and Bonas, 1996). In minimal medium or in planta, the expression of hrpX is activated by HrpG, a transcriptional activator of the OmpR family of two-component regulators (Wengelnik et al., 1996; Figure 2A). Recent transcriptome analysis revealed that HrpG, in most cases via HrpX, controls a genome-wide regulon including hrp genes and genes encoding Xanthomonas outer proteins (Xops; Wengelnik and Bonas, 1996; Astua-Monge et al., 2000a; Noël et al., 2001, 2002).
Fig. 2. Model for hrp gene regulation and type III secretion in X.campestris pv. vesicatoria. (A) A so far uncharacterized signal transduction system in the bacterial envelope (indicated by a question mark) senses environmental stimuli and transduces the signal to HrpG. HrpG activates the expression of hrpA and, via HrpX, the expression of hrpB–hrpF as well as of a number of xop genes. (B) Expression of hrp genes is essential for the formation of the TTS apparatus, which spans both bacterial membranes and mediates secretion of Hrp and effector proteins. The TTS apparatus is associated with the Hrp pilus, which presumably spans the cell wall (200 nm thick; not drawn to scale). The major subunit of the Hrp pilus is HrpE1. Translocation of effector proteins across the plant plasma membrane requires HrpF, the putative pore-forming component of the type III translocon. Effector proteins are targeted to different locations in the plant cell and presumably modulate cellular processes leading to disease symptom formation in susceptible plants. In resistant plants, effector proteins (designated Avr proteins) can be recognized and trigger the activation of specific defense responses. CW, cell wall; IM, inner membrane; OM, outer membrane; PM, plasma membrane.
Interestingly, one of the xop genes, xopD, contains an hrp box-like motif in the promoter region (Figure 1; Table II). The hrp box is a conserved consensus sequence which was identified in promoters of hrp and effector genes in P.syringae. It presumably provides the binding site for HrpL, a member of the extracytoplasmic function family of sigma factors (Innes et al., 1993; Xiao and Hutcheson, 1994; Xiao et al., 1994; Fouts et al., 2002). In X.campestris pv. vesicatoria, however, expression of xopD is controlled by HrpG and HrpX (Noël et al., 2002). xopD encodes a putative type III effector protein with homology to the virulence factor PsvA from P.syringae pv. eriobotryae (Noël et al., 2002; Table II). The presence of an hrp box in the xopD promoter and the low G + C content of xopD support the hypothesis that genes involved in bacterial virulence might have been acquired during evolution by horizontal gene transfer.
Many hrpX-regulated genes of X.campestris pv. vesicatoria contain a PIP (plant-inducible promoter, consensus TTCGC-N15-TTCGC) box in their promoter regions. This sequence motif might be involved in the HrpX-mediated gene regulation (Fenselau and Bonas, 1995; Wengelnik and Bonas, 1996; Noël et al., 2002). However, there are also hrpX-independent promoters that contain a PIP box, e.g. avrRxv (Table II), indicating that the PIP box is not sufficient to confer inducibility by HrpX. In addition, the promoters of several xop genes that are controlled by HrpG and HrpX do not contain PIP boxes (Table II). Thus, it remains speculative whether the PIP box serves as a control element. So far, direct binding of HrpX to PIP box-containing promoter sequences could not be demonstrated (L.Escolar and U.Bonas, unpublished data).
PIP box-like motifs have also been identified in Xanthomonas axonopodis pv. citri and X.campestris pv. campestris in the promoters of hrp genes as well as genes encoding putative proteins with type II signal peptides and sequence homologies to cell wall-degrading enzymes, proteases and an iron receptor (Da Silva et al., 2002). Furthermore, PIP box-like promoter sequences have been identified in R.solanacearum, upstream of hrp transcription units, the popA gene and several avr gene homologs (Fenselau and Bonas, 1995; Wengelnik and Bonas, 1996; Salanoubat et al., 2002). In R.solanacearum, hrp genes are controlled by HrpG and HrpB, which are homologous to HrpG and HrpX, respectively, from X.campestris pv. vesicatoria (Genin et al., 1992; Brito et al., 1999). In R.solanacearum, the outer membrane protein PrhA (plant regulator of hrp genes) presumably is on top of the regulatory cascade leading to hrp gene expression. PrhA is homologous to TonB-dependent siderophore receptors and acts as a sensor for a non-diffusible molecule present in the plant cell wall (Marenda et al., 1998; Brito et al., 1999, 2002; Aldon et al., 2000). In contrast to R.solanacearum, the receptor(s) in X.campestris pv. vesicatoria that transmits external stimuli into the bacterial cell is still unknown (Figure 2).
Hrp pilus—tunnel to the host cell
TTS systems have been visualized in the animal patho gens Salmonella typhimurium, Shigella flexneri and Escherichia coli, and show striking morphological similarities to flagellar basal bodies: a membrane-embedded complex is associated with an extracellular hollow structure, the needle (Kubori et al., 1998; Tamano et al., 2000; Blocker et al., 2001; Sekiya et al., 2001).
Type III-dependent surface appendages have also been identified in plant pathogenic bacteria, i.e. P.syringae pv. tomato, E.amylovora, R.solanacearum and X.campestris pv. vesicatoria. These so-called Hrp pili have a similar diameter (6–8 nm), but are considerably longer than the needles of animal pathogens (Roine et al., 1997; Van Gijsegem et al., 2000; Hu et al., 2001; Jin et al., 2001; T.Ojanen-Reuhs and U.Bonas, unpublished data). Since Hrp pili can extend to a length of several micrometers, they have been proposed to cross the plant cell wall (Romantschuk et al., 2001; Figure 2). In R.solanacearum and P.syringae pv. tomato, the pilin, which is the major subunit of the Hrp pilus, is required for type III secretion in vitro (Van Gijsegem et al., 2000; Wei et al., 2000). Recent immunocytochemical analyses in E.amylovora and P.syringae pv. tomato elegantly demonstrated that Hrp pili serve as conduits for secreted proteins (Brown et al., 2001; Jin and He, 2001; Jin et al., 2001; Li et al., 2002). So far, there are no indications that Hrp pili also mediate bacterial contact with the host cell. In R.solanacearum, mutation of hrpY, the gene encoding the major pilus subunit, does not affect attachment of the bacteria to cultured plant cells (Van Gijsegem et al., 2000).
Getting in touch—the type III translocon
Translocation across the eukaryotic plasma membrane probably requires the presence of type III-secreted bacterial proteins that form the type III translocon, a channel-like complex in the host plasma membrane (Büttner and Bonas, 2002). Putative components of the translocon have been described mainly in animal pathogens whereas they have not been identified so far in most plant pathogenic bacteria. To our knowledge, HrpF from X.campestris pv. vesicatoria is the first known candidate for a type III translocon protein in bacterial plant pathogens. Mutant studies revealed that HrpF, which is secreted by the TTS system, is dispensable for type III secretion in vitro but essential for the interaction with the plant (Rossier et al., 2000; Büttner et al., 2002). hrpF mutants are not able to grow and cause disease in susceptible plants and to induce the HR in resistant plants. When tested in artificial lipid bilayer systems, HrpF induced pore formation, suggesting that it might be the channel-forming core component of the type III translocon (Büttner et al., 2002; Figure 2). Pore-forming activity has been demonstrated for the putative type III translocon proteins LcrV and PcrV from Yersinia pseudotuberculosis and Pseudomonas aeruginosa, respectively, which do not show any sequence similarity to HrpF (Holmström et al., 2001).
In animal pathogenic bacteria, observations of protein– protein interactions between putative translocon proteins suggest that the type III translocon is a heterogeneous protein complex. For instance, LcrV presumably interacts with YopB and YopD to build a functional translocon (Sarker et al., 1998). In X.campestris pv. vesicatoria, it remains to be investigated whether additional proteins besides HrpF are involved in the formation of the type III translocon. So far, studies to identify HrpF interaction partners failed since HrpF is a ‘sticky’ protein, making it difficult to show interaction specificity (D.Büttner and U.Bonas, unpublished data).
Carte d’accès—recognition by the TTS system
The mechanisms that control type III secretion in planta are still unknown. In Erwinia spp., P.syringae and R.solanacearum, several type III-secreted proteins could be detected in the culture supernatant after incubation of the bacteria in hrp gene-inducing medium (e.g. Gaudriault et al., 1997; Mudgett and Staskawicz, 1999; Van Gijsegem et al., 2000).
In X.campestris pv. vesicatoria, the isolation of a point mutation in hrpG (E44K, designated hrpG*), which leads to constitutive expression of hrp genes, was key for the establishment of an in vitro secretion assay (Rossier et al., 1999; Wengelnik et al., 1999). However, expression of the hrp genes is not sufficient to trigger type III secretion. The identification of secreted proteins requires the incubation of hrpG* bacteria in acidic minimal medium, which probably mimicks the plant’s apoplast. Interestingly, the X.campestris pv. vesicatoria TTS system also secretes heterologous proteins such as PopA from R.solanacearum, AvrB from P.syringae and YopE from Y.pseudo tuberculosis, indicating that the secretion signal is conserved among plant and animal pathogenic bacteria (Rossier et al., 1999).
What is the nature of the secretion signal in proteins traveling the TTS systems? It has been proposed that the signal resides in the N terminus of the secreted proteins. In Yersinia spp., the first 11–17 amino acids of Yersinia outer proteins (Yops) are sufficient to drive the type III-dependent secretion of a reporter protein (Sory et al., 1995; Schesser et al., 1996; Lloyd et al., 2001b). Similarly, in X.campestris pv. vesicatoria, the first 28 amino acids of AvrBs2 contain a functional secretion signal (Mudgett et al., 2000). Type III-secreted proteins in both plant and animal pathogens do not share any sequence conservations in their N termini. However, comparative sequence analyses of multiple type III-secreted proteins of P.syringae pathovars revealed similarities in their N-terminal amino acid composition, including a high content of serine residues (on average 16–18%; Guttman et al., 2002; Petnicki-Ocwieja et al., 2002). In X.campestris pv. vesicatoria, the serine content within the first 25 amino acids of known TTS substrates varies between 8% (HrpB2) and 32% (HrpF). This is significantly higher than the serine content in the N termini of non-secreted components of the TTS system (betweeen 0%, as in HrcN, and 12%, as in HrcT).
Since frameshift mutations in the N-terminal coding sequence did not abolish type III secretion of a reporter protein in Y.enterocolitica, the secretion signal was also predicted to reside in the 5′ region of the mRNA (Anderson and Schneewind, 1997). This hypothesis, which assumes a co-translational secretion, is, however, discussed controversially in the field. For instance, the Yersinia YopE and YopH proteins are expressed even in the absence of a functional TTS system. In addition, mutations in YopE resulting in an altered mRNA structure did not abolish its type III secretion (Lloyd et al., 2001b).
The situation is complicated further by the finding that several effector proteins from animal pathogens require specific chaperones for type III secretion and translocation (Bennett and Hughes, 2000; Lloyd et al., 2001a). Recently, TTS chaperones have also been identified in plant pathogenic bacteria. DspB from E.amylovora and ShcA from P.syringae are essential for the stability and/or secretion of the pathogenicity factor DspA and the effector protein HopPsyA, respectively (Gaudriault et al., 2002; van Dijk et al., 2002).
Quo vadis—type III-secreted proteins
Harpins
The first proteins known to be secreted by the TTS system of bacterial plant pathogens were the harpins; HrpZ from P.syringae and PopA from R.solanacearum (He et al., 1993; Arlat et al., 1994). Harpins are small, heat-stable, glycine-rich proteins that lack cysteines and elicit a necrosis-like reaction when infiltrated into non-host plants (Wei et al., 1992; He et al., 1993; Arlat et al., 1994; Alfano et al., 1996; Gaudriault et al., 1998). Interestingly, HrpZ from P.syringae was found to bind to the plant plasma membrane and to form ion-conducting pores in artificial lipid bilayers (Lee et al., 2001a,b). However, the role of harpins is not well understood. In most cases, a contribution to bacterial virulence could not be demonstrated. Only in E.amylovora, a mutation of the harpin gene hrpN results in the formation of reduced disease symptoms in susceptible plants (Wei et al., 1992; Barny, 1995).
Effector proteins
The best studied effector proteins are the products of avr genes, which were first identified genetically without knowing that they encode TTS substrates. Since the isolation of the first avr gene, avrA from P.syringae pv. glycinea (Staskawicz et al., 1984), >40 bacterial avr genes have been identified, mainly in species of Pseudomonas and Xanthomonas (Vivian and Arnold, 2000). As mentioned above, avr genes trigger an R gene-specific plant defense reaction which often culminates in the HR. The HR phenotype is easy to follow and has been instrumental in the dissection of both bacterial pathogenicity and specific defense reactions in the plant. In the absence of the corresponding R gene, no recognition occurs and the infection leads to disease. There is accumulating evidence that Avr proteins probably act as virulence factors, manipulating host cellular processes for the pathogen’s benefit and thus contributing to bacterial fitness and/or symptom formation in susceptible plants (White et al., 2000). However, it should be emphasized that mutations in putative effector genes often do not affect bacterial virulence under laboratory conditions, indicating that they play a minor role or have redundant functions.
Until recently, type III-dependent delivery of bacterial effector proteins into the host cell has not been proven. Strong indirect evidence for translocation was provided by the fact that avr genes induced an R gene-specific HR when expressed inside the plant cell (Bonas and Van den Ackerveken, 1997; Cornelis and Van Gijsegem, 2000). Furthermore, several type III-secreted proteins from plant pathogens contain typical eukaryotic features, indicating an activity inside the host cell (White et al., 2000). For instance, the putative myristoylation motifs of several Avr proteins in pathovars of P.syringae suggest a localization to the plant plasma membrane, which has indeed been shown for AvrB and AvrRpm1 (Nimchuk et al., 2000). In these proteins, the myristoylation motifs are crucial for the avirulence function. Further support for the hypothesis of type III-dependent delivery of bacterial effector proteins into the plant cell was provided by the analysis of the effector protein AvrBs2 from X.campestris pv. vesicatoria, which was fused translationally to an adenylate cyclase reporter from Bordetella pertussis (Casper-Lindley et al., 2002). Recently, the direct detection of a bacterial effector protein in the plant cell has been reported: AvrBs3 from X.campestris pv. vesicatoria could be visualized in nuclei of infected plant cells, using an AvrBs3-specific antibody (Szurek et al., 2002; see below).
Arrival—AvrBs3 localizes to the plant cell nucleus
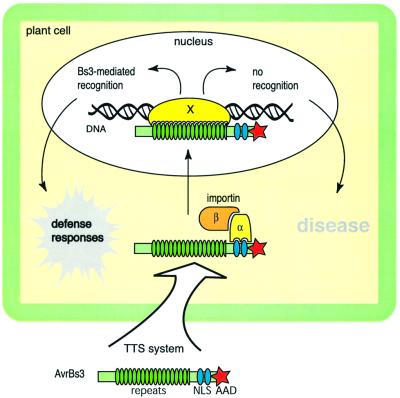
Characteristic eukaryotic protein motifs are also present in members of the AvrBs3 protein family in species of Xanthomonas (Gabriel, 1999; Lahaye and Bonas, 2001). AvrBs3-like proteins are highly homologous (90–97% amino acid sequence identity) and all contain C-terminal nuclear localization signals and an acidic activation domain, which are features of eukaryotic transcription factors (Yang and Gabriel, 1995; Van den Ackerveken et al., 1996; Zhu et al., 1998, 1999; Yang et al., 2000; Ballvora et al., 2001; Szurek et al., 2001). Differences between the family members are restricted mainly to the central protein region, which consists of 13.5–25.5 nearly perfect 34-amino-acid repeats (Lahaye and Bonas, 2001).
The AvrBs3 protein family is named after the first isolated member, AvrBs3 from X.campestris pv. vesicatoria (Bonas et al., 1989). AvrBs3 is one of the few Avr proteins for which a role in symptom formation could be demonstrated. In susceptible host plants, AvrBs3 induces hypertrophy, an enlargement of mesophyll cells (Marois et al., 2002). Since the induction of hypertrophy symptoms depends on functional nuclear localization signals and the acidic activation domain, we speculate that AvrBs3 acts as a transcription factor in the host cell nucleus. The nuclear localization signals probably provide the admission ticket for AvrBs3 to use the host’s protein traffic road into the nucleus. Indeed, yeast two-hybrid studies and in vitro pull-down assays revealed that AvrBs3 interacts with pepper importin α which, together with importin β, mediates nuclear protein import (Görlich et al., 1995; Szurek et al., 2001; Figure 3). Immunocytological analyses demonstrated that the nuclear localization signals are essential for the targeting of AvrBs3 to nuclei of infected plant cells (Szurek et al., 2002).
Fig. 3. Proposed model for the molecular mechanisms underlying virulence and avirulence activity of AvrBs3 from X.campestris pv. vesicatoria. Characteristic features of AvrBs3 are the central 17.5 nearly identical 34 amino acid repeats, two functional C-terminal nuclear localization signals (NLSs) and an acidic activation domain (AAD). Delivery of AvrBs3 into the host cell is mediated by the TTS system. In the plant cell, the NLSs bind to importin α, which together with importin β targets AvrBs3 to the plant cell nucleus. Direct or indirect (via a target protein X) interaction of AvrBs3 with the plant DNA leads to the modulation of the host’s transcriptome and presumably results in hypertrophy, a disease symptom in susceptible plants. In resistant plants, specific plant defense responses are induced upon recognition of AvrBs3 by the R protein Bs3 (Bs, bacterial spot).
The hypothesis that AvrBs3 acts as a transcription factor is supported by transcriptome analyses of infected susceptible pepper plants. cDNA-AFLP (cDNA-amplified fragment length polymorphism) studies unraveled AvrBs3-induced genes, designated upa (up-regulated by AvrBs3; Marois et al., 2002). Sequence analyses revealed that several upa genes show homologies to auxin-induced and expansin-like genes that usually play a role in cell enlargement.
Whether AvrBs3 induces gene expression with the aid of plant transcription factors or directly targets plant promoter sequences is not known (Figure 3). Support for a direct interaction of AvrBs3-like proteins with the host DNA comes from recent studies on AvrXa7, an AvrBs3 homolog from the rice pathogen Xanthomonas oryzae pv. oryzae, which directly binds to AT-rich DNA sequences (Yang et al., 2000).
Perspectives
In the past decade, tremendous progress has been made in dissecting the plethora of type III-secreted proteins in plant pathogenic bacteria. Genetic and biochemical studies have led to the identification of a variety of effector proteins that travel the TTS system, the bacterial main road into the host cell. The next major challenge is the functional analysis of effector proteins: what are their targets in the plant and how do they interfere with host cellular processes? Expression of individual effector proteins in plant cells followed by transcriptome analysis and biochemical approaches will advance our understanding of the molecular processes in infected plant cells. Inter disciplinary approaches and comparative analyses of different pathogen–host systems should not only provide a better understanding of the molecular basis of bacterial pathogenicity but also give us some clues about plant defense and last, but not least, solutions for disease management.
Acknowledgments
Acknowledgements
We are grateful to T.Lahaye, L.Escolar, R.Koebnik and G.Reuter for critical reading of the manuscript, and to all members of the lab for stimulating discussions. We apologize to all colleagues whose work could not be cited due to space limitations. Work in our laboratory was funded by grants from the Verband der Chemischen Industrie to D.B. and the Deutsche Forschungsgemeinschaft and the European Union to U.B.
References
- Aizawa S.I. (2001) Bacterial flagella and type III secretion systems. FEMS Microbiol. Lett., 202, 157–64. [DOI] [PubMed] [Google Scholar]
- Aldon D., Brito,B., Boucher,C. and Genin,S. (2000) A bacterial sensor of plant cell contact controls the transcriptional induction of Ralstonia solanacearum pathogenicity genes. EMBO J., 19, 2304–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano J.R. and Collmer,A. (1996) Bacterial pathogens in plants: life up against the wall. Plant Cell, 8, 1683–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano J.R. and Collmer,A. (1997) The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins and death. J. Bacteriol., 179, 5655–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano J.R., Bauer,D.W., Milos,T.M. and Collmer,A. (1996) Analysis of the role of the Pseudomonas syringae pv. syringae HrpZ harpin in elicitation of the hypersensitive response in tobacco using functionally non-polar hrpZ deletion mutations, truncated HrpZ fragments and hrmA mutations. Mol. Microbiol., 19, 715–728. [DOI] [PubMed] [Google Scholar]
- Alfano J.R., Charkowski,A.O., Deng,W.L., Badel,J.L., Petnicki-Ocwieja,T., van Dijk,K. and Collmer,A. (2000) The Pseudomonas syringae Hrp pathogenicity island has a tripartite mosaic structure composed of a cluster of type III secretion genes bounded by exchangeable effector and conserved effector loci that contribute to parasitic fitness and pathogenicity in plants. Proc. Natl Acad. Sci. USA, 97, 4856–4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D.M. and Schneewind,O. (1997) A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science, 278, 1140–1143. [DOI] [PubMed] [Google Scholar]
- Arlat M., Gough,C.L., Barber,C.E., Boucher,C. and Daniels,M.J. (1991) Xanthomonas campestris contains a cluster of hrp genes related to the larger hrp cluster of Pseudomonas solanacearum. Mol. Plant-Microbe Interact., 4, 593–601. [DOI] [PubMed] [Google Scholar]
- Arlat M., Van Gijsegem,F., Huet,J.C., Pernollet,J.C. and Boucher,C.A. (1994) PopA1, a protein which induces a hypersensitivity-like response on specific Petunia genotypes, is secreted via the Hrp pathway of Pseudomonas solanacearum. EMBO J., 13, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astua-Monge G., Minsavage,G.V., Stall,R.E., Davis,M.J., Bonas,U. and Jones,J.B. (2000a) Resistance of tomato and pepper to T3 strains of Xanthomonas campestris pv. vesicatoria is specified by a plant-inducible avirulence gene. Mol. Plant-Microbe Interact., 13, 911–921. [DOI] [PubMed] [Google Scholar]
- Astua-Monge G., Minsavage,G.V., Stall,R.E., Vallejos,C.E., Davis,M.J. and Jones,J.B. (2000b) Xv4-avrXv4: a new gene-for-gene interaction identified between Xanthomonas campestris pv. vesicatoria race T3 and the wild tomato relative Lycopersicon pennellii. Mol. Plant-Microbe Interact., 13, 1346–1355. [DOI] [PubMed] [Google Scholar]
- Ballvora A., Pierre,M., van den Ackerveken,G., Schornack,S., Rossier,O., Ganal,M., Lahaye,T. and Bonas,U. (2001) Genetic mapping and functional analysis of the tomato Bs4 locus governing recognition of the Xanthomonas campestris pv. vesicatoria AvrBs4 protein. Mol. Plant-Microbe Interact., 14, 629–638. [DOI] [PubMed] [Google Scholar]
- Barny M.A. (1995) Erwinia amylovora hrpN mutants, blocked in harpin synthesis, express a reduced virulence on host plants and elicit variable hypersensitive reactions on tobacco. Eur. J. Plant Pathol., 101, 333–340. [Google Scholar]
- Barny M.A., Guinebretière,M.H., Marçais,B., Coissac,E., Paulin,J.P. and Laurent,J. (1990) Cloning of a large gene cluster involved in Erwinia amylovora CFBP1430 virulence. Mol. Microbiol., 4, 777–786. [DOI] [PubMed] [Google Scholar]
- Basim H., Stall,R., Minsavage,G. and Jones,J. (1999) Chromosomal gene transfer by conjugation in the plant pathogen Xanthomonas axonopodis pv. vesicatoria. Phytopathology, 89, 1044–1049. [DOI] [PubMed] [Google Scholar]
- Bennett J.C. and Hughes,C. (2000) From flagellum assembly to virulence: the extended family of type III export chaperones. Trends Microbiol., 8, 202–204. [DOI] [PubMed] [Google Scholar]
- Blocker A., Jouihri,N., Larquet,E., Gounon,P., Ebel,F., Parsot,C., Sansonetti,P. and Allaoui,A. (2001) Structure and composition of the Shigella flexneri ‘needle complex’, a part of its type III secreton. Mol. Microbiol., 39, 652–663. [DOI] [PubMed] [Google Scholar]
- Bogdanove A. et al. (1996) Unified nomenclature for broadly conserved hrp genes of phytopathogenic bacteria. Mol. Microbiol., 20, 681–683. [DOI] [PubMed] [Google Scholar]
- Bonas U. and Van den Ackerveken,G. (1997) Recognition of bacterial avirulence proteins occurs inside the plant cell: a general phenomenon in resistance to bacterial diseases. Plant J., 12, 1–7. [DOI] [PubMed] [Google Scholar]
- Bonas U., Stall,R.E. and Staskawicz,B. (1989) Genetic and structural characterization of the avirulence gene avrBs3 from Xanthomonas campestris pv. vesicatoria. Mol. Gen. Genet., 218, 127–136. [DOI] [PubMed] [Google Scholar]
- Bonas U., Schulte,R., Fenselau,S., Minsavage,G.V., Staskawicz,B.J. and Stall,R.E. (1991) Isolation of a gene-cluster from Xanthomonas campestris pv. vesicatoria that determines pathogenicity and the hypersensitive response on pepper and tomato. Mol. Plant-Microbe Interact., 4, 81–88. [Google Scholar]
- Bonas U., Conrads-Strauch,J. and Balbo,I. (1993) Resistance in tomato to Xanthomonas campestris pv vesicatoria is determined by alleles of the pepper-specific avirulence gene avrBs3. Mol. Gen. Genet., 238, 261–269. [DOI] [PubMed] [Google Scholar]
- Boucher C.A., Van Gijsegem,F., Barberis,P.A., Arlat,M. and Zischek,C. (1987) Pseudomonas solanacearum genes controlling both pathogenicity on tomato and hypersensitivity on tobacco are clustered. J. Bacteriol., 169, 5626–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito B., Marenda,M., Barberis,P., Boucher,C. and Genin,S. (1999) prhJ and hrpG, two new components of the plant signal-dependent regulatory cascade controlled by PrhA in Ralstonia solanacearum. Mol. Microbiol., 31, 237–251. [DOI] [PubMed] [Google Scholar]
- Brito B., Aldon,D., Barberis,P., Boucher,C. and Genin,S. (2002) A signal transfer system through three compartments transduces the plant cell contact-dependent signal controlling Ralstonia solanacearum hrp genes. Mol. Plant-Microbe Interact., 15, 109–119. [DOI] [PubMed] [Google Scholar]
- Brown I.R., Mansfield,J.W., Taira,S., Roine,E. and Romantschuk,M. (2001) Immunocytochemical localization of HrpA and HrpZ supports a role for the Hrp pilus in the transfer of effector proteins from Pseudomonas syringae pv. tomato across the host plant cell wall. Mol. Plant-Microbe Interact., 14, 394–404. [DOI] [PubMed] [Google Scholar]
- Büttner D. and Bonas,U. (2002) Port of entry—the type III secretion translocon. Trends Microbiol., 10, 186–192. [DOI] [PubMed] [Google Scholar]
- Büttner D., Nennstiel,D., Klüsener,B. and Bonas,U. (2002) Functional analysis of HrpF, a putative type III translocon protein from Xanthomonas campestris pv. vesicatoria. J. Bacteriol., 184, 2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper-Lindley C., Dahlbeck,D., Clark,E.T. and Staskawicz,B. (2002) Direct biochemical evidence for type III secretion-dependent translocation of the AvrBs2 effector protein into plant cells. Proc. Natl Acad. Sci. USA, 99, 8336–8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesiolka L.D. et al. (1999) Regulation of expression of avirulence gene avrRxv and identification of a family of host interaction factors by sequence analysis of avrBsT. Mol. Plant-Microbe Interact., 12, 35–44. [DOI] [PubMed] [Google Scholar]
- Cornelis G.R. and Van Gijsegem,F. (2000) Assembly and function of type III secretory systems. Annu. Rev. Microbiol., 54, 735–774. [DOI] [PubMed] [Google Scholar]
- Da Silva A.C. et al. (2002) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature, 417, 459–463. [DOI] [PubMed] [Google Scholar]
- Escolar L., Van den Ackerveken,G., Pieplow,S., Rossier,O. and Bonas,U. (2001) Type III secretion and in planta recognition of the Xanthomonas avirulence proteins AvrBs1 and AvrBsT. Mol. Plant Pathol., 2, 287–296. [DOI] [PubMed] [Google Scholar]
- Fenselau S. and Bonas,U. (1995) Sequence and expression analysis of the hrpB pathogenicity operon of Xanthomonas campestris pv. vesicatoria which encodes eight proteins with similarity to components of the Hrp, Ysc, Spa and Fli secretion systems. Mol. Plant-Microbe Interact., 8, 845–854. [DOI] [PubMed] [Google Scholar]
- Flor H.H. (1971) Current status of the gene-for-gene concept. Annu. Rev. Phytopathol., 9, 275–296. [Google Scholar]
- Fouts D.E. et al. (2002) Genomewide identification of Pseudomonas syringae pv. tomato DC3000 promoters controlled by the HrpL alternative sigma factor. Proc. Natl Acad. Sci. USA, 99, 2275–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel D.W. (1999) The Xanthomonas avr/pth gene family. In Stacey,G. and Keen,N.T. (eds), Plant–Microbe Interactions. Vol. 4. APS Press, St Paul, MN, pp. 39–55.
- Gaudriault S., Malandrin,L., Paulin,J.P. and Barny,M.A. (1997) DspA, an essential pathogenicity factor of Erwinia amylovora showing homology with AvrE of Pseudomonas syringae, is secreted via the Hrp secretion pathway in a DspB-dependent way. Mol. Microbiol., 26, 1057–1069. [DOI] [PubMed] [Google Scholar]
- Gaudriault S., Brisset,M.N. and Barny,M.A. (1998) HrpW of Erwinia amylovora, a new Hrp-secreted protein. FEBS Lett., 428, 224–228. [DOI] [PubMed] [Google Scholar]
- Gaudriault S., Paulin,J.P. and Barny,M.A. (2002) The DspB/F protein of Erwinia amylovora is a type III secretion chaperone ensuring efficient secretion of the DspA/E essential pathogenicity factor. Mol. Plant Pathol., in press. [DOI] [PubMed] [Google Scholar]
- Genin S., Gough,C.L., Zischek,C. and Boucher,C.A. (1992) Evidence that the hrpB gene encodes a positive regulator of pathogenicity genes from Pseudomonas solanacearum. Mol. Microbiol., 6, 3065–3076. [DOI] [PubMed] [Google Scholar]
- Görlich D., Vogel,F., Mills,A.D., Hartmann,E. and Laskey,R.A. (1995) Distinct functions for the two importin subunits in nuclear protein import. Nature, 377, 246–248. [DOI] [PubMed] [Google Scholar]
- Guttman D.S., Vinatzer,B.A., Sarkar,S.F., Ranall,M.V., Kettler,G. and Greenberg,J.T. (2002) A functional screen for the type III (Hrp) secretome of the plant pathogen Pseudomonas syringae. Science, 295, 1722–1726. [DOI] [PubMed] [Google Scholar]
- Hacker J. and Kaper,J.B. (2000) Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol., 54, 641–679. [DOI] [PubMed] [Google Scholar]
- He S.Y. (1998) Type III protein secretion systems in plant and animal pathogenic bacteria. Annu. Rev. Phytopathol., 36, 363–392. [DOI] [PubMed] [Google Scholar]
- He S.Y., Huang,H.C. and Collmer,A. (1993) Pseudomonas syringae pv. syringae harpinPss: a protein that is secreted via the Hrp pathway and elicits the hypersensitive response in plants. Cell, 73, 1255–1266. [DOI] [PubMed] [Google Scholar]
- Heesemann J., Algermissen,B. and Laufs,R. (1984) Genetically manipulated virulence of Yersinia enterocolitica. Infect. Immun., 46, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmström A., Olsson,J., Cherepanov,P., Maier,E., Nordfelth,R., Pettersson,J., Benz,R., Wolf-Watz,H. and Forsberg,A. (2001) LcrV is a channel size-determining component of the Yop effector translocon of Yersinia. Mol. Microbiol., 39, 620–632. [DOI] [PubMed] [Google Scholar]
- Hu W., Yuan,J., Jin,Q.L., Hart,P. and He,S.Y. (2001) Immunogold labeling of Hrp pili of Pseudomonas syringae pv. tomato assembled in minimal medium and in planta. Mol. Plant-Microbe Interact., 14, 234–241. [DOI] [PubMed] [Google Scholar]
- Hueck C.J. (1998) Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev., 62, 379–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguet E., Hahn,K., Wengelnik,K. and Bonas,U. (1998) hpaA mutants of Xanthomonas campestris pv. vesicatoria are affected in pathogenicity but retain the ability to induce host-specific hypersensitive reaction. Mol. Microbiol., 29, 1379–1390. [DOI] [PubMed] [Google Scholar]
- Innes R.W., Bent,A.F., Kunkel,B.N., Bisgrove,S.R. and Staskawicz,B.J. (1993) Molecular analysis of avirulence gene avrRpt2 and identification of a putative regulatory sequence common to all known Pseudomonas syringae avirulence genes. J. Bacteriol., 175, 4859–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q. and He,S.Y. (2001) Role of the Hrp pilus in type III protein secretion in Pseudomonas syringae. Science, 294, 2556–2558. [DOI] [PubMed] [Google Scholar]
- Jin Q., Hu,W., Brown,I., McGhee,G., Hart,P., Jones,A.L. and He,S.Y. (2001) Visualization of secreted Hrp and Avr proteins along the Hrp pilus during type III secretion in Erwinia amylovora and Pseudomonas syringae. Mol. Microbiol., 40, 1129–1139. [DOI] [PubMed] [Google Scholar]
- Kearney B. and Staskawicz,B.J. (1990) Widespread distribution and fitness contribution of Xanthomonas campestris avirulence gene avrBs2. Nature, 346, 385–386. [DOI] [PubMed] [Google Scholar]
- Klement Z. (1982) Hypersensitivity. In Mount,M.S. and Lacy,G.H. (eds), Phytopathogenic Prokaryotes. Vol. 2. Academic Press, New York, pp. 149–177.
- Kubori T., Matsushima,Y., Nakamura,D., Uralil,J., Lara-Tejero,M., Sukhan,A., Galan,J.E. and Aizawa,S.I. (1998) Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science, 280, 602–605. [DOI] [PubMed] [Google Scholar]
- Lahaye T. and Bonas,U. (2001) Molecular secrets of bacterial type III effector proteins. Trends Plant Sci., 6, 479–485. [DOI] [PubMed] [Google Scholar]
- Lee J., Klessig,D.F. and Nürnberger,T. (2001a) A harpin binding site in tobacco plasma membranes mediates activation of the pathogenesis-related gene hin1 independent of extracellular calcium but dependent on mitogen-activated protein kinase activity. Plant Cell, 13, 1079–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. et al. (2001b) HrpZPsph from the plant pathogen Pseudomonas syringae pv. phaseolicola binds to lipid bilayers and forms an ion-conducting pore in vitro. Proc. Natl Acad. Sci. USA, 98, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.M., Brown,I., Mansfield,J., Stevens,C., Boureau,T., Romantschuk,M. and Taira,S. (2002) The Hrp pilus of Pseudomonas syringae elongates from its tip and acts as a conduit for translocation of the effector protein HrpZ. EMBO J., 21, 1909–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren P.B. (1997) The role of hrp genes during plant–bacterial interactions. Annu. Rev. Phytopathol., 35, 129–152. [DOI] [PubMed] [Google Scholar]
- Lindgren P.B., Peet,R.C. and Panopoulos,N.J. (1986) Gene-cluster of Pseudomonas syringae pv. phaseolicola controls pathogenicity of bean plants and hypersensitivity on nonhost plants. J. Bacteriol., 168, 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd S.A., Forsberg,A., Wolf-Watz,H. and Francis,M.S. (2001a) Targeting exported substrates to the Yersinia TTSS: different functions for different signals? Trends Microbiol., 9, 367–371. [DOI] [PubMed] [Google Scholar]
- Lloyd S.A., Norman,M., Rosqvist,R. and Wolf-Watz,H. (2001b) Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol. Microbiol., 39, 520–532. [DOI] [PubMed] [Google Scholar]
- Macnab R.M. (1999) The bacterial flagellum: reversible rotary propellor and type III export apparatus. J. Bacteriol., 181, 7149–7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenda M., Brito,B., Callard,D., Genin,S., Barberis,P., Boucher,C. and Arlat,M. (1998) PrhA controls a novel regulatory pathway required for the specific induction of Ralstonia solanacearum hrp genes in the presence of plant cells. Mol. Microbiol., 27, 437–453. [DOI] [PubMed] [Google Scholar]
- Marois E., Van den Ackerveken,G. and Bonas,U. (2002) The Xanthomonas type III effector protein AvrBs3 modulates plant gene expression and induces cell hypertrophy in the susceptible host. Mol. Plant-Microbe Interact., 15, 637–646. [DOI] [PubMed] [Google Scholar]
- Mudgett M.B. and Staskawicz,B.J. (1999) Characterization of the Pseudomonas syringae pv. tomato AvrRpt2 protein: demonstration of secretion and processing during bacterial pathogenesis. Mol. Microbiol., 32, 927–941. [DOI] [PubMed] [Google Scholar]
- Mudgett M.B., Chesnokova,O., Dahlbeck,D., Clark,E.T., Rossier,O., Bonas,U. and Staskawicz,B.J. (2000) Molecular signals required for type III secretion and translocation of the Xanthomonas campestris AvrBs2 protein to pepper plants. Proc. Natl Acad. Sci. USA, 97, 13324–13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niepold F., Anderson,D. and Mills,D. (1985) Cloning determinants of pathogenesis from Pseudomonas syringae pathovar syringae. Proc. Natl Acad. Sci. USA, 82, 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchuk Z., Marois,E., Kjemtrup,S., Leister,R.T., Katagiri,F. and Dangl,J.L. (2000) Eukaryotic fatty acylation drives plasma membrane targeting and enhances function of several type III effector proteins from Pseudomonas syringae. Cell, 101, 353–363. [DOI] [PubMed] [Google Scholar]
- Noël L. (2001) Utilisation de la technique de cDNA-AFLP pour l’étude d’un transcriptome procaryote: identification et caractérisation du régulon hrp chez Xanthomonas campestris pv. vesicatoria. Université de Paris-Sud, Paris, France.
- Noël L., Thieme,F., Nennstiel,D. and Bonas,U. (2001) cDNA-AFLP analysis unravels a genome-wide hrpG-regulon in the plant pathogen Xanthomonas campestris pv. vesicatoria. Mol. Microbiol., 41, 1271–1281. [DOI] [PubMed] [Google Scholar]
- Noël L., Thieme,F., Nennstiel,D. and Bonas,U. (2002) Two novel type III system-secreted proteins of Xanthomonas campestris pv. vesicatoria are encoded within the hrp pathogenicity island. J. Bacteriol., 184, 1340–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petnicki-Ocwieja T. et al. (2002) Genomewide identification of proteins secreted by the Hrp type III protein secretion system of Pseudomonas syringae pv. tomato DC3000. Proc. Natl Acad. Sci. USA, 99, 7652–7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roine E., Wei,W.S., Yuan,J., Nurmiaho-Lassila,E.L., Kalkkinen,N., Romantschuk,M. and He,S.Y. (1997) Hrp pilus: a hrp-dependent bacterial surface appendage produced by Pseudomonas syringae pv. tomato DC3000. Proc. Natl Acad. Sci. USA, 94, 3459–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romantschuk M., Roine,E. and Taira,S. (2001) Hrp pilus—reaching through the plant cell wall. Eur. J. Plant Pathol., 107, 153–160. [Google Scholar]
- Ronald P.C. and Staskawicz,B.J. (1988) The avirulence gene avrBs1 from Xanthomonas campestris pv. vesicatoria encodes a 50-kDa protein. Mol. Plant-Microbe Interact., 1, 191–198. [PubMed] [Google Scholar]
- Rossier O. (1999) Étude du système de sécretion de type III chez la bactérie phytopathogène Xanthomonas campestris pathovar vesicatoria. Université Paris, Paris, France.
- Rossier O., Wengelnik,K., Hahn,K. and Bonas,U. (1999) The Xanthomonas Hrp type III system secretes proteins from plant and mammalian pathogens. Proc. Natl Acad. Sci. USA, 96, 9368–9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier O., Van den Ackerveken,G. and Bonas,U. (2000) HrpB2 and HrpF from Xanthomonas are type III-secreted proteins and essential for pathogenicity and recognition by the host plant. Mol. Microbiol., 38, 828–838. [DOI] [PubMed] [Google Scholar]
- Salanoubat M. et al. (2002) Genome sequence of the plant pathogen Ralstonia solanacearum. Nature, 415, 497–502. [DOI] [PubMed] [Google Scholar]
- Sarker M.R., Neyt,C., Stainier,I. and Cornelis,G.R. (1998) The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J. Bacteriol., 180, 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schesser K., Frithz-Lindsten,E. and Wolf-Watz,H. (1996) Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J. Bacteriol., 178, 7227–7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya K., Ohishi,M., Ogino,T., Tamano,K., Sasakawa,C. and Abe,A. (2001) Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc. Natl Acad. Sci. USA, 98, 11638–11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sory M.P., Boland,A., Lambermont,I. and Cornelis,G.R. (1995) Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc. Natl Acad. Sci. USA, 92, 11998–12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz B.J., Dahlbeck,D. and Keen,N.T. (1984) Cloned avirulence gene of Pseudomonas syringae pv. glycinea determines race-specific incompatibility on Glycine max (L.) Merr. Proc. Natl Acad. Sci. USA, 81, 6024–6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberger E.M. and Beer,S.V. (1988) Creation and complementation of pathogenicity mutants of Erwinia amylovora. Mol. Plant-Microbe Interact., 1, 135–144. [Google Scholar]
- Swords K.M., Dahlbeck,D., Kearney,B., Roy,M. and Staskawicz,B.J. (1996) Spontaneous and induced mutations in a single open reading frame alter both virulence and avirulence in Xanthomonas campestris pv. vesicatoria avrBs2. J. Bacteriol., 178, 4661–4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurek B., Marois,E., Bonas,U. and Van den Ackerveken,G. (2001) Eukaryotic features of the Xanthomonas type III effector AvrBs3: protein domains involved in transcriptional activation and the interaction with nuclear import receptors from pepper. Plant J., 26, 523–534. [DOI] [PubMed] [Google Scholar]
- Szurek B., Rossier,O., Hause,G. and Bonas,U. (2002) Type III-dependent translocation of the Xanthomonas AvrBs3 protein into the plant cell. Mol. Microbiol., in press. [DOI] [PubMed] [Google Scholar]
- Tamano K., Aizawa,S., Katayama,E., Nonaka,T., Imajoh-Ohmi,S., Kuwae,A., Nagai,S. and Sasakawa,C. (2000) Supramolecular structure of the Shigella type III secretion machinery: the needle part is changeable in length and essential for delivery of effectors. EMBO J., 19, 3876–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanassi D.G. and Hultgren,S.J. (2000) Multiple pathways allow protein secretion across the bacterial outer membrane. Curr. Opin. Cell Biol., 12, 420–430. [DOI] [PubMed] [Google Scholar]
- Van den Ackerveken G., Marois,E. and Bonas,U. (1996) Recognition of the bacterial avirulence protein AvrBs3 occurs inside the host plant cell. Cell, 87, 1307–1316. [DOI] [PubMed] [Google Scholar]
- van Dijk K., Tam,V.C., Records,A.R., Petnicki-Ocwieja,T. and Alfano,J.R. (2002) The ShcA protein is a molecular chaperone that assists in the secretion of the HopPsyA effector from the type III (Hrp) protein secretion system of Pseudomonas syringae. Mol. Microbiol., 44, 1469–1481. [DOI] [PubMed] [Google Scholar]
- Van Gijsegem F., Vasse,J., Camus,J.C., Marenda,M. and Boucher,C. (2000) Ralstonia solanacearum produces Hrp-dependent pili that are required for PopA secretion but not for attachment of bacteria to plant cells. Mol. Microbiol., 36, 249–260. [DOI] [PubMed] [Google Scholar]
- Vivian A. and Arnold,D.L. (2000) Bacterial effector genes and their role in host pathogen interactions. J. Plant Pathol., 82, 163–178. [Google Scholar]
- Wei W., Plovanich-Jones,A., Deng,W.L., Jin,Q.L., Collmer,A., Huang,H.C. and He,S.Y. (2000) The gene coding for the Hrp pilus structural protein is required for type III secretion of Hrp and Avr proteins in Pseudomonas syringae pv. tomato. Proc. Natl Acad. Sci. USA, 97, 2247–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z.M., Laby,R.J., Zumoff,C.H., Bauer,D.W., He,S.Y., Collmer,A. and Beer,S.V. (1992) Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science, 257, 85–88. [DOI] [PubMed] [Google Scholar]
- Wengelnik K. and Bonas,U. (1996) HrpXv, an AraC-type regulator, activates expression of five of the six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria. J. Bacteriol., 178, 3462–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengelnik K., Van den Ackerveken,G. and Bonas,U. (1996) HrpG, a key hrp regulatory protein of Xanthomonas campestris pv. vesicatoria is homologous to two-component response regulators. Mol. Plant-Microbe Interact., 9, 704–712. [DOI] [PubMed] [Google Scholar]
- Wengelnik K., Rossier,O. and Bonas,U. (1999) Mutations in the regulatory gene hrpG of Xanthomonas campestris pv. vesicatoria result in constitutive expression of all hrp genes. J. Bacteriol., 181, 6828–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F.F., Yang,B. and Johnson,L.B. (2000) Prospects for understanding avirulence gene function. Curr. Opin. Plant Biol., 3, 291–298. [DOI] [PubMed] [Google Scholar]
- Xiao Y. and Hutcheson,S.W. (1994) A single promoter sequence recognized by a newly identified alternate sigma factor directs expression of pathogenicity and host range determinants in Pseudomonas syringae [published erratum appears in J. Bacteriol., 176, 6158]. J. Bacteriol., 176, 3089–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Heu,S., Yi,J., Lu,Y. and Hutcheson,S.W. (1994) Identification of a putative alternate sigma factor and characterization of a multicomponent regulatory cascade controlling the expression of Pseudomonas syringae pv. syringae Pss61 hrp and hrmA genes. J. Bacteriol., 176, 1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Zhu,W., Johnson,L.B. and White,F.F. (2000) The virulence factor AvrXa7 of Xanthomonas oryzae pv. oryzae is a type III secretion pathway-dependent nuclear-localized double-stranded DNA-binding protein. Proc. Natl Acad. Sci. USA, 97, 9807–9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. and Gabriel,D.W. (1995) Xanthomonas avirulence/patho genicity gene family encodes functional plant nuclear targeting signals. Mol. Plant-Microbe Interact., 8, 627–631. [DOI] [PubMed] [Google Scholar]
- Young B.M. and Young,G.M. (2002) YplA is exported by the Ysc, Ysa and flagellar type III secretion systems of Yersinia enterocolitica. J. Bacteriol., 184, 1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W.G., Yang,B., Chittoor,J.M., Johnson,L.B. and White,F.F. (1998) AvrXa10 contains an acidic transcriptional activation domain in the functionally conserved C terminus. Mol. Plant-Microbe Interact., 11, 824–832. [DOI] [PubMed] [Google Scholar]
- Zhu W.G., Yang,B., Wills,N., Johnson,L.B. and White,F.F. (1999) The C terminus of AvrXa10 can be replaced by the transcriptional activation domain of VP16 from the herpes simplex virus. Plant Cell, 11, 1665–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]