Abstract
Malaria sporozoites induce swift activation of antigen-specific CD8+ T cells that inhibit the intracellular development of liver-stage parasites. The length of time of functional in vivo antigen presentation, estimated by monitoring the activation of antigen-specific CD8+ T cells, is of short duration, with maximum T cell activation occurring within the first 8 h after immunization and lasting approximately 48 h. Although the magnitude of the CD8+ T cell response closely correlates with the number of parasites used for immunization, increasing the time of antigen presentation by daily immunizations does not enhance the magnitude of this response. Thus, once a primary clonal burst is established, the CD8+ T cell response becomes refractory or unresponsive to further antigenic stimulation. These findings strongly suggest that the most efficient strategy for the induction of primary CD8+ T cell responses is the delivery of a maximal amount of antigen in a single dose, thereby ensuring a clonal burst that involves the largest number of precursors to become memory cells.
The CD8+ T cells are the prime mediators of adaptive immunity against a number of intracellular parasites, bacteria, and viruses. In rodent models of malaria parasite infection, CD8+ T cells induced after immunization with sporozoites play a major role in protective immunity, inhibiting the development of the liver stages of these parasites. Adoptive transfer of CD8+ T cell clones specific for epitopes expressed in sporozoite and liver stages strongly inhibits the intracellular development of parasites (1–3). Moreover, CD8+ T cells induced by immunization with subunit vaccines expressing these plasmodial antigens can also abolish the intrahepatocytic development of these parasites and prevent the occurrence of blood infections (4–8).
Studies in humans living in malaria-endemic areas indicate that the CD8+ T cell responses to liver stages are rather restricted because they are usually found at very low levels, even in individuals living in regions with intense malaria transmission (9, 10). This phenomenon has been attributed to the existence of altered peptide ligands resulting from polymorphisms present in epitopes recognized by CD8+ T cells, which could induce antagonistic effects that may interfere with T cell priming and the survival of memory cells (11, 12). The situation found in human populations is not entirely different from that observed in experimental rodent models. Immunization of mice with Plasmodium yoelii sporozoites induces rather low CD8+ T cell responses, barely detectable by highly sensitive ex vivo assays such as the enzyme immunospot assay (ELISPOT) (7, 13, 14).
The reduced magnitude of primary CD8+ T cell responses observed in normal mice does not appear to be caused by a low immunogenicity of sporozoites. In fact, recent studies indicate that immunization with malaria sporozoites induces a strong and swift activation of naïve CD8+ T cell precursors. Using transgenic (Tg) mice bearing a T cell receptor specific for the SYVPSAEQI epitope from the P. yoelii circumsporozoite protein, we demonstrated that within 24 h of immunization, naïve CD8+ T cells already express effector functions, such as the production of IFNγ and perforin and the capacity to eliminate liver-stage parasites (15). Whereas the initiation of this T cell differentiation and proliferation appears to be driven only by antigen, IL-4-secreting CD4+ T cells are crucial to sustain the developing CD8+ T cell response (16).
To identify and characterize the underlying mechanisms influencing the magnitude and efficiency of primary CD8+ T cell responses against malaria liver stages, we evaluated the role played by antigen in the modulation and maintenance of these responses. Although it is established that sporozoite immunization induces the activation of antigen-specific CD8+ T cells, it is not known how long antigen presentation lasts and, more important, how long this antigen presentation functions to induce the activation of CD8+ T cells. Here we report studies aimed at estimating the duration of functional in vivo antigen presentation after sporozoite immunization as determined by measuring the activation of antigen-specific CD8+ T cell precursors. We also investigated the effect of prolonged antigen presentation and its effect on the magnitude of primary CD8+ T cell responses. Understanding these issues should aid in the design of vaccines not only against malaria but also for other microbial diseases.
Materials and Methods
Mice and Parasites.
Six- to eight-week-old female CB6F1 and RAG2−/− B10D2 mice were obtained from the National Cancer Institute and The Jackson Laboratory, respectively. The generation of the SYVPSAEQI-specific T cell receptor Tg mice has been described previously (15). P. yoelii 17XNL parasites were maintained as described (2). Sporozoites were collected by dissecting the salivary glands of infected Anopheles stephensi mosquitoes ≈2 weeks after an infective blood meal.
Adoptive Transfer, Immunizations, and Drug Treatment.
Spleen cells from Tg mice (CB6F1 or B10D2 background) containing 1–2 × 106 CD8+ tetramer (see below) cells were injected i.v. into syngenic mice. Reconstitution of RAG2−/− B10D2 mice was performed by i.v. transfer of ≈60 × 106 spleen cells isolated from normal syngenic mice in addition to the Tg cells. Immunizations with sporozoites were performed by i.v. injection of 3–5 × 104 radiation-attenuated (γ-source, 20 krad) sporozoites or otherwise specified in the figure legends. Infection via bites of irradiated infected mosquitoes was performed as described (17, 18). To generate activated/memory cells, mice that received Tg cells were immunized i.v. with a recombinant vaccinia virus (recVAC, 1 × 106 pfu per mouse) expressing the SYVPSAEQI epitope (4, 19). Primaquine (Sigma) was administered s.c. (60 mg/kg) as described (20).
H2Kd Tetramers, Cell Staining, and FACS Analysis.
The SYVPSAEQI-specific H2Kd tetramers were either obtained from the National Institute of Allergy and Infectious Diseases, National Institutes of Health tetramer facility or prepared as described (21, 22). Fluorescent-labeled mAb to mouse CD8α (53–6.7) were obtained from PharMingen. Staining of cells for FACS analysis was performed by using standard protocols after blocking with unconjugated streptavidin (Molecular Probes) and FC block (PharMingen). Cells were analyzed by using FACSCALIBUR and CELL QUEST software (Becton Dickinson). Between 200,000 and 500,000 live events are usually acquired.
ELISPOT Assay and Cell Culture.
The ex vivo IFNγ ELISPOT to enumerate the number of SYVPSAEQI-specific CD8+ T cells was performed as described (14). MHC-compatible A20.2J target cells were coated with the SYVPSAEQI peptide and were incubated with mouse lymphocytes for 20–24 h. Anti-mouse IFNγ (R4) and biotinylated anti-mouse IFNγ (XMG1.2) were obtained from PharMingen.
Results
Short-Term Antigen Presentation Defines the Magnitude of the CD8+ T Cell Response Against Malaria Liver Stages.
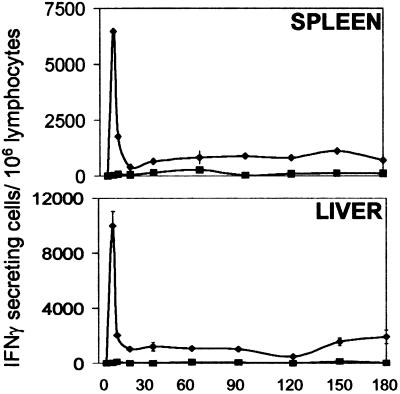
In previous studies, by using an adoptive transfer system with Tg CD8+ T cells specific for the SYVPSAEQI epitope of the P. yoelii circumsporozoite protein, we determined that the activation of CD8+ T cells and the development of their antiparasitic functions are detectable as early as 24 h after immunization. This T cell response reaches the highest magnitude on day 4, suffers a major contraction between days 6 and 7, and becomes stabilized after day 8 (15). After this initial phase, the response remains unchanged in the spleen and liver for at least 6 months (Fig. 1). These studies indicate that a single immunization drives naïve CD8+ T cells to undergo differentiation, proliferation, and later on, long-term persistence.
Figure 1.
Persistence of the CD8+ T cell response after a single immunization with sporozoites. Normal mice received transgenic (Tg) CD8+ T cells; 24 h later, they were immunized (⧫) i.v. with 5 × 104 attenuated sporozoites or were not immunized (■). The frequencies of epitope-specific CD8+ T cells in the spleen (Upper) and the liver (Lower) were determined by ELISPOT. Results represent one of two similar experiments expressed as mean values + SD.
These results raised the question regarding the length of time of in vivo antigen presentation and the influence that it may have on the generation and maintenance of CD8+ T cell memory. In studies with other microbial systems, this matter has been investigated by using activated/memory T cells obtained from immunized mice that were transferred to naïve mice to evaluate the long-term maintenance of memory CD8+ T cells in the absence of antigen. This approach, however, has been criticized as it cannot rule out the possibility that in addition to the CD8+ T cells obtained from immunized mice, antigen or antigen-bearing cells are also being transferred (23–29).
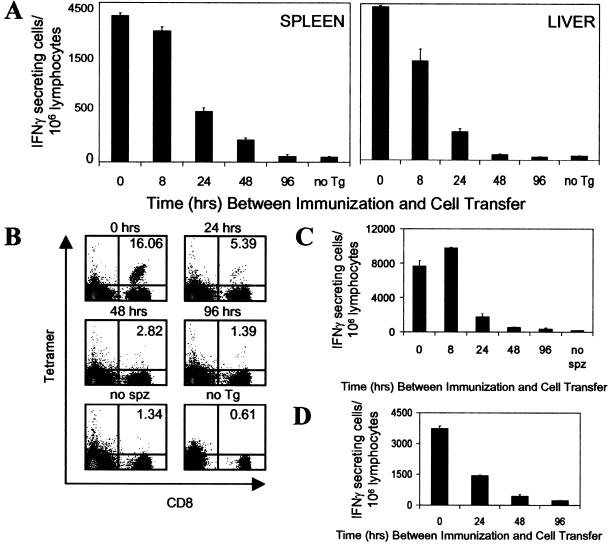
In view of this situation, and taking advantage of the specificity and sensitivity of the Tg CD8+ T cell system, we developed an approach to estimate the duration of in vivo antigen presentation. In this system, mice were injected with attenuated sporozoites, and at different time points after immunization, they received naïve Tg CD8+ T cells. Four days after the transfer of T cells into immunized mice, we evaluated the number as well as the differentiation status of the CD8+ T cells in the spleen and liver by using ELISPOT and flow cytometry with SYVPSAEQI tetramers. We found that in the spleen, maximum numbers of SYVPSAEQI-specific IFNγ-secreting CD8+ T cells were observed when the Tg CD8+ cells were transferred at the same time as sporozoite immunization (Figs. 2A Left and 3A). The number of activated cells decreased by ≈10–20% when transfer was performed 8 h later and was further reduced by ≈60 and 85% when the Tg cells were transferred 24 and 48 h after immunization, respectively. CD8+ T cells transferred 96 h after immunization or later (data not shown) showed negligible activation levels, comparable to control-immunized mice that did not receive Tg cells. Importantly, the same results were obtained when the magnitude of the CD8+ T cell response was evaluated by flow cytometry by using SYVPSAEQI-specific tetramers (Fig. 2B). We also assessed the response of T cells isolated from the liver (30), where sporozoites develop and differentiate after infection and found that the kinetics of activation of CD8+ T cells isolated from the liver is similar to that observed in the spleen (Fig. 2A Right).
Figure 2.
Short-term antigen presentation defines the magnitude of the CD8+ T cell response. (A) Normal mice were immunized i.v. with 3 × 104 attenuated sporozoites, and at different times postimmunization (8, 24, 48, 96 h), they received naïve Tg CD8+ T cells. The number and activation status of the Tg CD8+ T cells in spleens (Left) and livers (Right) for each experimental group were assessed by ELISPOT 4 days after transfer of Tg cells. As controls for full activation, mice received Tg cells at the time of immunization (0 h) and were analyzed 4 days later. Mice that were immunized but did not receive Tg cells (no Tg) served as controls for the endogenous CD8+ T cell response. (B) Spleen cells from A were stained with anti-CD8 antibodies and SYVPSAEQI tetramers. Plots were gated on lymphocytes, and the number in the upper right corner represents the frequency of CD8+ tetramer+ cells in the total CD8+ population. (C) Similar to A except that mice received day 8 activated/memory Tg CD8+ T cells. Mice that received activated/memory Tg cells but were not immunized (no spz) served as controls. The activation of the Tg CD8+ T cells in the spleen was measured by ELISPOT 4 days after transfer. (D) Similar to A except that RAG2−/− mice were used. Immunized mice received both naïve Tg CD8+ T cells and naïve spleen lymphocytes at indicated times. The activation of the Tg CD8+ T cells in the spleen was measured by ELISPOT 4 days after transfer. Results in A–D represent one of two to three similar experiments.
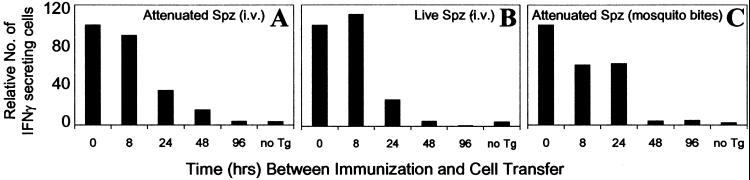
Figure 3.
Parasite viability or route of immunization does not modify the parameters of antigen presentation and T cell activation. Normal mice were immunized with 3 × 104 attenuated sporozoites (A), 3 × 104 live sporozoites (B), or four bites of irradiated infected mosquitoes (C). Naïve Tg CD8+ T cells were transferred at indicated times after immunization, and the activation of the Tg CD8+ T cells in the spleen was measured by ELISPOT 4 days after transfer. Mice that did not receive Tg CD8+ T cells (no Tg) served as control for the endogenous CD8+ T cell response. Results in A–C represent one of two similar experiments and are expressed as the relative number of CD8+ T cells compared with 0 h (100%) to facilitate comparison.
Because activated/memory CD8+ T cells may have a higher antigen-activation threshold (31–34) and therefore could display different kinetics of antigen-driven activation, we performed identical adoptive transfer experiments by using activated/memory cells. These activated/memory Tg CD8+ T cells were obtained from mice that previously received naïve Tg cells and were immunized with a recVAC (4, 19). In these experiments, we observed that the kinetics of activation and expansion of the transferred activated/memory CD8+ T cells exposed to parasite antigen were similar to those observed for naïve cells, i.e., no expansion of the CD8+ T cell response is observed when T cells are transferred 96 h after immunization. (Fig. 2C). It is noteworthy that when ELISPOT was performed 16 days after transferring either activated/memory or naïve Tg CD8+ T cells instead of on day four, which is the peak of the T cell response (15), the results were essentially the same; CD8+ T cells transferred 96 h after immunization were not activated (data not shown). These observations indicate that a low-level slowly developing T cell activation that is driven by persisting antigen does not occur.
Whereas these results suggest that functional antigen presentation occurs over a short period, they also raised the possibility that a suppressive adaptive immune response induced immediately after sporozoite immunization could be responsible for the lack of activation of CD8+ T cells transferred 96 h after immunization. This possibility was evaluated in experiments using RAG2−/− mice that lack both B and T cells (35). Along with the transfer of Tg CD8+ T cells at different hours after sporozoite immunization, we also reconstituted these mice with splenic CD4+ T cells, which are critical for the induction of the CD8+ T cell response (16). Consistent with the previous results, maximum activation of the naïve Tg CD8+ T cells was observed when they were transferred at the same time of immunization, whereas no expansion appeared to occur when the cells were transferred after 96 h (Fig. 2D).
Parasite Viability or Route of Immunization Does Not Modify the Parameters of Antigen-Driven T Cell Activation.
The preceding immunization experiments performed with radiation-attenuated sporozoites injected intravenously directly address the T cell response that occurs using the “gold standard” vaccine protocol known to induce protective immunity against malaria. However, this immunization condition may not represent the development of the immune response under normal conditions of transmission. Indeed, whereas live and attenuated sporozoites are both capable of invading hepatocytes, attenuated sporozoites undergo only limited intracellular transformations, which could limit the pool of antigen available for presentation to CD8+ T cells. Therefore, we tested the immunogenicity of live sporozoites to determine whether parasite viability affects the antigen-driven T cell activation. For this purpose, Tg cells were transferred into mice at different time points after immunization with live sporozoites, and their activation and expansion were assessed by ELISPOT 4 days later, at a time when mice already display incipient parasitemia. We found that mice immunized with live parasites display a pattern of antigen presentation and T cell activation identical to that observed in experiments using attenuated sporozoites (Fig. 3 A and B).
As the natural route of sporozoite infection is through mosquito bites that inject parasites into the host skin, we also performed experiments to evaluate the kinetics of antigen-driven activation of CD8+ T cells when sporozoites are injected by mosquito bites. Mice were exposed to the bites of four P. yoelii-infected irradiated mosquitoes and received Tg CD8+ T cells at different time points after immunization. On the basis of the data available from the literature and our recent work, we estimate that a single mosquito bite injects ≈32–95 sporozoites (17, 18). Four days after T cell transfer, the activation status of the Tg cells was assessed by ELISPOT. Maximum numbers of activated cells were observed when the Tg cells were transferred immediately before the exposure of mice to mosquito bites (Fig. 3C). Again, transfer of Tg cells 96 h later resulted in negligible levels of activation comparable to control mice that did not receive Tg cells but were exposed to mosquito bites. The results obtained in the preceding experiments strongly indicate that nearly all functional antigen presentation to CD8+ T cells was of short duration and occurred within the first hours after immunization.
Treatment with the Antimalaria Drug Primaquine and Development of the CD8+ T Cell Response.
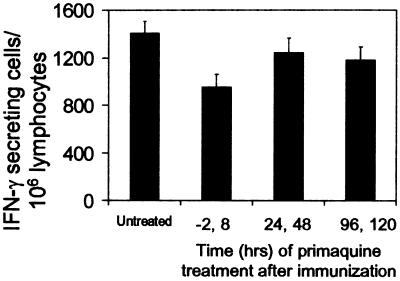
Previous studies have reported that treatment of immunized mice with the antimalaria drug primaquine reduced the immunity induced after immunization with sporozoites, and it was suggested that this drug could somehow interfere with antigen presentation to CD8+ T cells (20). Therefore, we performed experiments to determine whether the induction of CD8+ T cells is affected in mice previously receiving Tg cells and treated with primaquine at different times before and after immunization (−2, 8, 24, 48, 96 h). The activation status of the Tg cells was assessed by ELISPOT 16 days after immunization, and the results showed that CD8+ T cell responses were similar (Fig. 4). However, a small decrease in the number of CD8+ T cells could be observed in the group of mice treated with primaquine at −2 and 8 h. It is unclear whether this marginal reduction is related to the antiparasite effect of this drug or to the toxic effect on T cells described in previous studies (36). Nevertheless, our results clearly indicate that primaquine does not have a major effect on the induction and development of the CD8+ T cell response.
Figure 4.
Primaquine treatment and development of the CD8+ T cell response. Normal mice received Tg CD8+ T cells and were immunized i.v. with 3 × 104 attenuated sporozoites. At indicated times before and after immunization, mice were s.c. treated with 60 mg/kg primaquine. The frequencies of epitope-specific CD8+ T cells in the spleens were measured by ELISPOT 16 days after immunization. Results represent one of two similar experiments expressed as mean values + SD. P value(untreated vs. treated, −2,8) <0.05 (t test).
The Magnitude of the CD8+ T Cell Response Is Determined by the Size of the Priming Dose and Not by the Length of Time of Antigen Presentation.
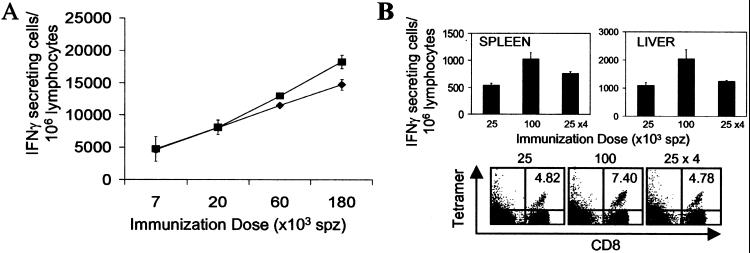
Initially, we determined that large increases in the magnitude of this CD8+ T cell response could be easily induced by increasing the amount of antigen used for immunization, i.e., the number of injected parasites (Fig. 5A). Our findings indicating a short-term in vivo antigen presentation raised the intriguing possibility that an enhanced CD8+ T cell response could be obtained not only by increasing the antigen dose but also by artificially prolonging the length of the time of antigen presentation, by performing repeated daily immunizations. Therefore, we compared the CD8+ T cell response of mice that received Tg cells and were immunized with a single dose of 10 × 104 parasites, four doses of 2.5 × 104 parasites administered in four consecutive days (total = 10 × 104), or a single dose 2.5 × 104 parasites. The activation and expansion of the CD8+ T cells were determined both by ELISPOT and FACS analysis 14 days after the first immunization. As expected, we observed a dose-dependent difference in mice immunized with 2.5 × 104 and 10 × 104 sporozoites (Fig. 5B Upper). Remarkably, however, we found that mice receiving four daily doses of 2.5 × 104 sporozoites develop, in both the spleen and liver, a CD8+ T cell response comparable in magnitude to that observed in mice that received a single dose of only 2.5 × 104 sporozoites. Similar results were obtained when the response was evaluated by FACS using SYVPSAEQI-specific tetramers (Fig. 5B Lower). The inability to significantly increase the number of activated cells after repeated antigen exposure suggests that a state of refractoriness limits the magnitude of the immune response after the single clonal burst induced by the primary immunization.
Figure 5.
Dependence of the CD8+ T cell response on priming dose and not on the length of time of antigen presentation. (A) Normal mice received and were immunized i.v. with indicated amounts of attenuated sporozoites. The activation and expansion of the Tg CD8+ T cells in spleens (⧫) and livers (■) were measured by ELISPOT 4 days after immunization. (B) Normal mice received Tg CD8+ T cells and were immunized with the indicated doses of attenuated sporozoites. The activation of the Tg CD8+ T cells in the spleens and the livers were measured by ELISPOT 14 days after the primary immunization (Upper). Spleen cells were stained with anti-CD8 antibodies and SYVPSAEQI tetramers, and plots were gated on lymphocytes; the number in the upper right corner represents the frequency of CD8+ tetramer+ cells in the total CD8+ population (Lower). Results in A and B represent one of two to three similar experiments (n = 3 mice/group). P values(100K vs. 4 × 25K for both spleen and liver) <0.05; P values(25K vs. 4 × 25K for both spleen and liver) = not significant.
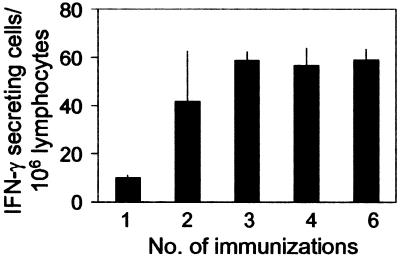
To determine whether the failure of the CD8+ T cell response to expand after repeated sporozoite immunization could also occur under conditions resembling those found in malaria endemic areas, we performed experiments in which we immunized mice by repeated exposure to two bites of irradiated infected mosquitoes. For this purpose, normal mice were immunized every 48 h such that different groups of mice received a minimum of one immunization to a maximum of six. The magnitude of the CD8+ T cell response was determined by ELISPOT 16 days after the first immunization. A clear CD8+ T cell response was observed in mice after receiving two immunizations or four mosquito bites (Fig. 6). Remarkably, however, we found no significant differences among mice receiving two, three, four, or six immunizations. Again, these results clearly indicate that once the primary T cell response becomes established at a certain level, its magnitude could not be increased despite additional exposure to antigen.
Figure 6.
A state of refractoriness limits the magnitude of the CD8+ T cell response despite repeated exposure to bites of infected mosquitoes. Normal mice were immunized every 48 h with two bites of irradiated infected mosquitoes. The frequencies of epitope-specific CD8+ T cells in the spleens were measured by ELISPOT 16 days after the first immunization. Results represent one of three similar experiments expressed as mean + SD.
Discussion
The existence of persisting antigen and the possible role it may have in the induction and maintenance of memory CD8+ T cell responses remains a controversial matter for most infectious systems. The complexity of this issue is well illustrated by the apparent contradictory results obtained in viral model systems, in which the maintenance of the CD8+ T cell response has been described as either dependent on (23, 24, 29) or independent of (25–28, 37) persisting antigen.
In this study, using Tg CD8+ T cells specific for a defined malaria antigen, we demonstrate that the length of time of in vivo antigen presentation, as measured by the activation of these cells, is relatively short but still generates a long-lasting antigen-specific CD8+ T cell response against malaria liver stages. A key finding of our study is that the antigen-driven activation of CD8+ T cell precursors occurs within the first 48 h. Maximum activation of CD8+ T cells is observed within the first 8 h after immunization, and no antigen-driven activation is observed 96 h after immunization. Although it is not possible to demonstrate the absence of antigen molecules 96 h after immunization, it is nevertheless apparent that if they exist, they have no effect on naïve or activated/memory CD8+ T cells. Our results indicate that this short-term functional in vivo antigen presentation is sufficient to initiate the differentiation and proliferation processes in T cells, which lasts for several days. This is consistent with the notion that CD8+ T cells undergo a predefined developmental program of differentiation and clonal burst immediately after antigenic stimulation (38–40).
The present study also revealed an intriguing phenomenon. Although it is clear that the magnitude of the primary CD8+ T cell response closely correlates with the number of parasites used for immunization, increasing the time of antigen presentation by daily immunizations does not enhance the magnitude of this response. These results suggest that a state of refractoriness affects the CD8+ T cell response immediately after priming, which becomes unresponsive to further repeated immunizations.
The inability of CD8+ T cells to expand further despite an increased antigen exposure during the development of the primary response is somehow reminiscent of what is observed in endemic areas where very low frequencies of T cells against liver- stage antigens are observed despite continued sporozoite inoculations through the bites of malaria-infected mosquitoes (9, 10). Indeed, in mice, we also demonstrated that repeated bites of infected mosquitoes failed to expand the number of activated cells once a primary CD8+ T cell response was established. These results are of interest as this mode of immunization not only mimics the manner by which immunity to sporozoites is acquired in endemic areas, but it is also known to induce sterile immunity against Plasmodium falciparum in human volunteers (41–44).
The low frequencies of liver stage-specific CD8+ T cells found in endemic areas have been attributed to the existence of altered peptide ligands brought about by polymorphisms in T cell epitopes of parasite antigens (11, 12). It has been suggested that T cell responses to liver stages can be negatively affected by antagonistic peptides that could interfere with T cell priming and the survival of memory T cells. This is definitely not the case for our findings, because we are dealing with a parasite strain with no polymorphic CD8+ T cell epitope. It is also possible that immune effector mechanisms, such as antibodies, can enhance the elimination of the parasites from the circulation and thus interfere with antigen processing and presentation. However, it is unlikely that such a mechanism could explain the refractoriness we observed. Under our immunization conditions, i.e., within the first 48 h, the protective immune response elicited after sporozoite immunization is mostly mediated by T cells. In fact, depletion of CD8+ T cells by antibody treatment results in a significant abrogation of the inhibition of liver stages (ref. 45 and data not shown).
It is intriguing that the inability of the CD8+ T cells to undergo further expansion contrasts with their ability to inhibit parasite development, which is detectable as early as 24 h after immunization and persists for several months (15). Therefore, despite the relative ease in inducing primary T cell responses even with minimal parasite exposure, there seems to be strong regulatory mechanisms that inhibit the further expansion of these T cells in response to additional antigen exposure. The underlying mechanisms involved in this phenomenon of refractoriness have yet to be fully elucidated.
The results presented in this study may have practical implications for the development of vaccines aimed at inducing high numbers of long-lived memory T cells. They suggest that a short-term antigen presentation rather than an extended antigen presentation is more effective at generating maximal CD8+ T cell responses. Thus, the most efficient strategy for the induction of CD8+ T cell responses would rely on the delivery of a maximal amount of antigen in a single dose. This would ensure the induction of a single clonal burst involving the largest number of naïve CD8+ T cell precursors, which, following activation, differentiate and become long-lived memory cells.
Acknowledgments
We thank Drs. Victor and Ruth Nussenzweig for review of the manuscript and helpful suggestions. We also thank G. Milon, D. Eichinger, and J. J. Lafaille for critical discussions. Tetramers were provided in part by the Tetramer Core Facility (National Institute of Allergy and Infectious Diseases, National Institutes of Health). This work was supported by National Institutes of Health Grant AI44375. J.H. was a Student Fellow of the American Liver Foundation and L.H.C. of CNPq-Brazil.
Abbreviations
- Tg
transgenic
- ELISPOT
enzyme-linked immunospot assay
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Romero P, Maryanski J L, Corradin G, Nussenzweig R S, Nussenzweig V, Zavala F. Nature (London) 1989;341:323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigues M M, Cordey A S, Arreaza G, Corradin G, Romero P, Maryanski J L, Nussenzweig R S, Zavala F. Int Immunol. 1991;3:579–585. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- 3.Weiss W R, Berzofsky J A, Houghten R A, Sedegah M, Hollindale M, Hoffman S L. J Immunol. 1992;149:2103–2109. [PubMed] [Google Scholar]
- 4.Li S, Rodrigues M, Rodriguez D, Rodriguez J R, Esteban M, Palese P, Nussenzweig R S, Zavala F. Proc Natl Acad Sci USA. 1993;90:5214–5218. doi: 10.1073/pnas.90.11.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sedegah M, Hedstrom R, Hobart P, Hoffman S L. Proc Natl Acad Sci USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renggli J, Valmori D, Romero J F, Eberl G, Romero P, Betschart B, Corradin G. Immunol Lett. 1995;46:199–205. doi: 10.1016/0165-2478(95)00043-5. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues E G, Zavala F, Eichinger D, Wilson J M, Tsuji M. J Immunol. 1997;158:1268–1274. [PubMed] [Google Scholar]
- 8.Schneider J, Gilbert S C, Blanchard T J, Hanke T, Robson K J, Hannan C M, Becker M, Sinden R, Smith G L, Hill A V. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 9.Lalvani A, Hurt N, Aidoo M, Kibatala P, Tanner M, Hill A V. Eur J Immunol. 1996;26:773–779. doi: 10.1002/eji.1830260408. [DOI] [PubMed] [Google Scholar]
- 10.Plebanski M, Aidoo M, Whittle H C, Hill A V. J Immunol. 1997;158:2849–2855. [PubMed] [Google Scholar]
- 11.Gilbert S C, Plebanski M, Gupta S, Morris J, Cox M, Aidoo M, Kwiatkowski D, Greenwood B M, Whittle H C, Hill A V. Science. 1998;279:1173–1177. doi: 10.1126/science.279.5354.1173. [DOI] [PubMed] [Google Scholar]
- 12.Plebanski M, Lee E A, Hannan C M, Flanagan K L, Gilbert S C, Gravenor M B, Hill A V. Nat Med. 1999;5:565–571. doi: 10.1038/8444. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira-Ferreira J, Miyahira Y, Layton G T, Savage N, Esteban M, Rodriguez D, Rodriguez J R, Nussenzweig R S, Zavala F. Vaccine. 2000;18:1863–1869. doi: 10.1016/s0264-410x(99)00344-8. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho L H, Hafalla J C, Zavala F. J Immunol Methods. 2001;252:207–218. doi: 10.1016/s0022-1759(01)00331-3. [DOI] [PubMed] [Google Scholar]
- 15.Sano G, Hafalla J C, Morrot A, Abe R, Lafaille J J, Zavala F. J Exp Med. 2001;194:173–180. doi: 10.1084/jem.194.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvalho L H, Sano Gi G, Hafalla J C, Morrot A, de Lafaille M A, Zavala F. Nat Med. 2002;8:166–170. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 17.Vanderberg J P. Exp Parasitol. 1977;42:169–181. doi: 10.1016/0014-4894(77)90075-3. [DOI] [PubMed] [Google Scholar]
- 18.Bruna-Romero O, Hafalla J C, Gonzalez-Aseguinolaza G, Sano G, Tsuji M, Zavala F. Int J Parasitol. 2001;31:1499–1502. doi: 10.1016/s0020-7519(01)00265-x. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues M, Li S, Murata K, Rodriguez D, Rodriguez J R, Bacik I, Bennink J R, Yewdell J W, Garcia-Sastre A, Nussenzweig R S, et al. J Immunol. 1994;153:4636–4648. [PubMed] [Google Scholar]
- 20.Scheller L F, Azad A F. Proc Natl Acad Sci USA. 1995;92:4066–4068. doi: 10.1073/pnas.92.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman J D, Moss P A, Goulder P J, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 22.Busch D H, Pilip I M, Vijh S, Pamer E G. Immunity. 1998;8:353–362. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- 23.Gray D, Matzinger P. J Exp Med. 1991;174:969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oehen S, Waldner H, Kundig T M, Hengartner H, Zinkernagel R M. J Exp Med. 1992;176:1273–1281. doi: 10.1084/jem.176.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullbacher A. J Exp Med. 1994;179:317–321. doi: 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau L L, Jamieson B D, Somasundaram T, Ahmed R. Nature (London) 1994;369:648–652. [Google Scholar]
- 27.Hou S, Hyland L, Ryan K W, Portner A, Doherty P C. Nature (London) 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 28.Bruno L, Kirberg J, von Boehmer H. Immunity. 1995;2:37–43. doi: 10.1016/1074-7613(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 29.Kundig T M, Bachmann M F, Oehen S, Hoffmann U W, Simard J J, Kalberer C P, Pircher H, Ohashi P S, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goossens P L, Jouin H, Marchal G, Milon G. J Immunol Methods. 1990;132:137–144. doi: 10.1016/0022-1759(90)90407-m. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed R, Gray D. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 32.Tanchot C, Lemonnier F A, Perarnau B, Freitas A A, Rocha B. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 33.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V, McMichael A J. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Nat Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 35.Shinkai Y, Rathbun G, Lam K P, Oltz E M, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall A M. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 36.Thong Y H, Ferrante A, Rowan-Kelly B. Trans R Soc Trop Med Hyg. 1978;72:537–539. doi: 10.1016/0035-9203(78)90181-5. [DOI] [PubMed] [Google Scholar]
- 37.Murali-Krishna K, Lau L L, Sambhara S, Lemonnier F, Altman J, Ahmed R. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 38.Mercado R, Vijh S, Allen S E, Kerksiek K, Pilip I M, Pamer E G. J Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 39.Kaech S M, Ahmed R. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Stipdonk M J, Lemmens E E, Schoenberger S P. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 41.Clyde D F, McCarthy V C, Miller R M, Hornick R B. Am J Med Sci. 1973;266:398–403. doi: 10.1097/00000441-197312000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Rieckmann K H, Carson P E, Beaudoin R L, Cassells J S, Sell K W. Trans R Soc Trop Med Hyg. 1974;68:258–259. doi: 10.1016/0035-9203(74)90129-1. [DOI] [PubMed] [Google Scholar]
- 43.Herrington D, Davis J, Nardin E, Beier M, Cortese J, Eddy H, Losonsky G, Hollingdale M, Sztein M, Levine M, et al. Am J Trop Med Hyg. 1991;45:539–547. doi: 10.4269/ajtmh.1991.45.539. [DOI] [PubMed] [Google Scholar]
- 44.Edelman R, Hoffman S L, Davis J R, Beier M, Sztein M B, Losonsky G, Herrington D A, Eddy H A, Hollingdale M R, Gordon D M, Clyde D F. J Infect Dis. 1993;168:1066–1070. doi: 10.1093/infdis/168.4.1066. [DOI] [PubMed] [Google Scholar]
- 45.Rodrigues M, Nussenzweig R S, Zavala F. Immunology. 1993;80:1–5. [PMC free article] [PubMed] [Google Scholar]