Abstract
Epstein–Barr virus infection in vitro immortalizes primary B cells. EBNA2 is an Epstein–Barr virus-encoded transcriptional transactivator that mimics the effects of activated Notch signaling and is essential for this proliferative response. An assay using Sindbis virus (SV) as a cell death inducer revealed that, like Notch, EBNA2 also has antiapoptotic activity. We show that Nur77 is a mediator of SV-induced cell death and that EBNA2 antiapoptotic activity results from interaction with Nur77. EBNA2 colocalized with Nur77 in transfected cells and coprecipitated with Nur77 in IB4 B cells. EBNA2 binds to Nur77 through sequences in the EBNA2 amino acid 123–147 conserved domain and an EBNA2 mutant unable to bind Nur77 also lost the ability to protect cells from SV-induced apoptosis. EBNA2 exerted its antideath function by retaining Nur77 in the nucleus and preventing Nur77 from targeting mitochondria in response to apoptotic stimuli. Thus, targeting of Nur77 can be added to the list of strategies used by viruses to counter apoptosis.
Epstein–Barr virus (EBV) is associated with a variety of human malignancies (1). The nuclear latency gene products EBNA1, EBNA2, EBNA3A, and EBNA3C, and the membrane protein LMP-1 are essential for in vitro immortalization of B cells (2). Other latency proteins, EBNA-LP, LMP2A, and the proteins encoded by the BamHI-A rightward transcripts (BARTs) either contribute to immortalization efficiency or are likely to be important for the establishment and maintenance of life-long in vivo latency, which is itself a factor in the development of EBV-associated disease (3–10).
EBNA2 functions as a transcriptional transactivator to regulate the pattern of EBV latency gene expression in B cells and to modify cellular gene expression with a resultant stimulation of G0 to G1 cell cycle progression (11, 12). In its transcriptional role, EBNA2 mimics the effects of activated Notch (13, 14). Notch is an evolutionarily conserved, surface receptor that mediates cell–cell signaling to influence developmental and cell fate decisions (15, 16). Ligand binding induces proteolytic cleavage events that release the intracellular domain of Notch, NotchIC, which translocates to the nucleus where it functions as a transcriptional activator (17). EBNA2 and NotchIC both target responsive promoters through the cellular DNA binding protein CBF1/RBP-Jk (18). CBF1 acts as a transcriptional repressor by bringing to the promoter a histone deacetylase-containing complex (19–23) that deacetylates histones, favors nucleosome formation, and generates a chromatin state that is unfavorable for transcription (24). Constitutive repression occurs in part because nuclear import of CBF1 depends on preassociation with members of the corepressor complex (25). EBNA2 and NotchIC activate CBF1 bound promoters by competing away the corepressor complex (26, 27). Interactions with coactivator proteins such as pCAF, CBP, and p300 (28–30), and with the SWI/SNF complex (31) facilitate the conversion to transcriptional activation. The common mechanism of transcriptional regulation by EBNA2 and NotchIC is reflected in functional assays. EBNA2 and activated Notch can partially substitute for each other in B cell proliferation and muscle differentiation assays (32–34).
A yeast two-hybrid assay revealed that NotchIC interacts with Nur77 (35). Nur77 (TR3, NGFI-B) is an orphan member of the nuclear hormone receptor superfamily. Nur77 was originally identified as an immediate-early gene that was rapidly induced in fibroblasts by serum stimulation and in PC12 cells by nerve growth factor (36). Nur77 is a transcription factor that binds to NBRE motifs as a monomer and to palindromic NurRE sites as a dimer (37). Nur77 also heterodimerizes with the retinoid-X receptor (RXR) to down-regulate RXR activity (38). Nur77 has a second function as a mediator of apoptosis. In response to an apoptotic stimulus, the normally nuclear Nur77 translocates to the cytoplasm where it targets mitochondria to induce cytochrome c release (39). NotchIC was found to inhibit Nur77-mediated apoptosis in T cells (35).
Viral infection is an effective trigger of cellular apoptosis and viruses have evolved a variety of mechanisms to counter the apoptotic response (40). EBV has been thought to counter apoptosis during latency indirectly through LMP1-mediated activation of NF-κB-regulated genes (41, 42). The Bcl-2 homologs encoded by the EBV BHRF1 and BALF1 ORFs are expressed only during the viral lytic cycle, presumably to prevent premature cell death during active EBV replication (43–45). We now show that EBNA2 mimicry of Notch activity includes the ability to protect against apoptosis.
Sindbis virus (SV) infection has been used as an apoptotic stimulus to examine the protective effects of Bcl-2 family members and their viral homologs (46). We found that Nur77 is also a mediator of SV-induced apoptosis and that EBNA2 blocks this apoptotic response through interaction with Nur77.
A tumorigenic cell phenotype is associated not only with increased proliferation but also with an increased resistance to apoptosis. Our experiments suggest that in certain disease settings, expression of EBNA2 could be providing such a cell survival advantage by blocking the effects of stimuli that elicit Nur77-mediated cell death.
Materials and Methods
Plasmids and Viruses.
The plasmids pSG5-EBNA2 (pPDL151), Flag-NotchIC (pJH279), and pEFBOS-GST-Nur77 have been described (13, 35, 47). pSG5-EBNA2 with amino acids 123–147 deleted (pPDL328) was obtained from P. Ling (Baylor College of Medicine, Waco). Bacterial GST-EBNA2 fusion proteins were made in pGEX2T (Pharmacia; ref. 47). Nur77 was cloned into the BglII site of a modified pSG5 vector to generate HA-Nur77, into a lentivirus vector (48) to generate lenti-Nur77, and into pEGFP-C1 (CLONTECH) to generate pEGFP-Nur77. SV vectors expressing EBNA2 (SV-E2), reverse EBNA2 (SV-revE2), mutant EBNA2 (SV-E2Δ123–147), Nur77 (SV-N77), and antisense Nur77 (SV-asN77) were generated by blunt end ligation into the BstEII site of the SV vector dsTE12Q (49, 50). Virus stocks were generated and titered using BHK-21 cells as described (46). SV-BCL-xL was provided by J. M. Hardwick (Johns Hopkins University).
Cell Death.
BHK-21 or NIH 3T3 cells were infected with SV at a multiplicity of infection (moi) of 5 plaque-forming units per cell. Infections were performed in triplicate. Cell viability was determined by trypan blue exclusion as described (45), or using the lactate dehydrogenase (LDH) assay kit (Promega). The intracellular cleavage of poly (ADP-ribose) polymerase (PARP) was determined at 48 hr after infection by using whole cell extracts from SV-infected cells. Western blots were probed with anti-PARP antibody (C-2–10, CLONTECH).
Immunofluorescence Assays.
NIH 3T3 cells were seeded on chamber slides (LabTek) at 0.8 × 105 cells per well and transfected with GFP-Nur77 (0.4 μg) by using Lipofectamine Plus (GIBCO/BRL). Cells were infected with SV 12 hr after transfection. After 24 hr, cells were washed and fixed in 2% paraformaldehyde in PBS for 5 min at room temperature. Fixed cells were washed and permeabilized in 0.2% Triton X-100 in PBS for 20 min on ice. For EBNA2 staining, cells were incubated with anti-EBNA2 antibody (PE2; Dako), followed by donkey anti-mouse IgG conjugated with rhodamine (Jackson ImmunoResearch). For mitochondrial staining, MitoTracker-Red (Molecular Probes; 200 nM) was added to the medium 30 min before fixation. Slides were mounted with Vectashield containing DAPI (Vector Laboratories), and images were captured using a fluorescence microscope and image pro software (Media Cybernetic, Silver Spring, MD) or a confocal microscope with compix software.
GST Affinity Assays.
Generation of transfected cell extracts, GST binding, and Western blot analyses were carried out as described (27). Nur77 was detected using anti-Nur77 monoclonal antibody (51), or anti-HA antibody (Upstate Biotechnology, Lake Placid, NY). For mammalian GST affinity assays, 293T cells (2 × 107) were transfected with 15 μg of each plasmid by using calcium phosphate precipitation. Cells were collected 2 days after transfection and homogenized in NETN buffer [20 mM Tris⋅HCl (pH 8.0)/100 mM NaCl/0.5% Nonidet P-40/1 mM EDTA] containing protease inhibitor (0.2 μg/ml aprotinin/0.2 mM PMSF). Lysates were incubated with glutathione-sepharose 4B beads for 4 hr at 4°C. After binding, the beads were pelleted and washed six times with NETN buffer, resuspended in 35 μl of SDS sample buffer, and boiled for 5 min. Samples were analyzed by SDS/PAGE and Western blotting with anti-EBNA2 antibody, and then reprobed with anti-GST antibody (B-14; Santa Cruz Biotechnology).
Immunoprecipitation.
EBV-infected IB4 and P3HR-1 B cells (4.0 × 107) or IB4 cells infected with lenti-Nur77 (2 × 107) were harvested and lysed with RIPA buffer [1.0% Nonidet P-40/150 mM NaCl/50 mM Tris⋅HCl (pH 7.4)/1 mM DTT/1 mM NaF/1 mM Na3VO4/10 μg/ml leupeptin/2 μg/ml aprotinin/1 mM PMSF] at 4°C. Cell extracts were precleared on protein G-sepharose 4 Fast Flow (Amersham Pharmacia Biosciences). Cleared extracts were incubated with anti-EBNA2 or -Nur77 antibodies and protein G sepharose beads at 4°C. Beads were washed six times with RIPA buffer, eluted in gel loading buffer, and separated by SDS/PAGE.
Results
EBNA2 Protects Against SV-Induced Apoptosis.
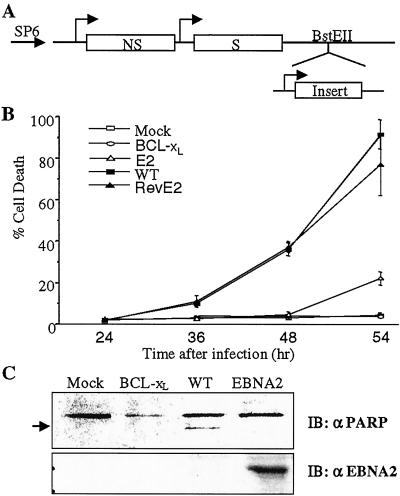
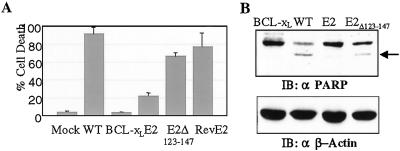
To evaluate whether EBNA2 possessed any antiapoptotic function, recombinant SV were generated that contained the EBNA2 ORF inserted in either sense or antisense orientation into a unique cloning site behind a duplicated copy of the SV subgenomic promoter (Fig. 1A). It has previously been demonstrated that SV infection induces programmed cell death and that expression of the antiapoptotic BCL-xL protein by recombinant SV is protective (50). NIH 3T3 cells were infected with SV-wt and SV-BCL-xL, as well as the sense and reverse EBNA2 SV at a moi of 5 and cell viability was followed over time by using trypan blue exclusion (Fig. 1B). The SV-wt and reverse EBNA2 SV each induced cell death in the bulk (70–90%) of the cell population by 54 hr postinfection while expression of BCL-xL was completely protective. Expression of EBNA2 from SV-E2 also resulted in complete protection at 48 hr postinfection and substantial protection at 54 hr. Apoptosis is associated with intracellular cleavage of specific protein substrates such as PARP, which is cleaved to 85- and 30-kDa fragments. Analysis of PARP in SV-infected cells showed that cleaved PARP was detected as expected in SV-wt infected cells (52), but not in SV-E2- or SV-BCL-xL-infected cells (Fig. 1C). This indicates that SV-induced apoptosis of NIH 3T3 cells was inhibited by EBNA2.
Figure 1.
Inhibition of SV-induced cell death by EBNA2. (A) Diagram of the SV vector. SP6, heterologous SP6 promoter; NS, nonstructural genes; S, structural genes. (B) NIH 3T3 cells were mock infected or infected at a moi = 5 with SV-wt, SV-BCL-xL, SV-EBNA2 (E2), or SV-revEBNA2 (revE2). Cell viability was determined by trypan blue exclusion. The data shown are the mean of three assays with the standard deviation provided. (C) NIH 3T3 cells were infected with virus at a moi = 5, and harvested at 48 hr after infection. Intact PARP and the 85-kDa cleaved product (arrowed) were detected by Western blotting. The same blot was stripped and reprobed with anti-EBNA2 antibody (Lower).
Nur77 Is a Key Mediator of SV-Induced Apoptosis.
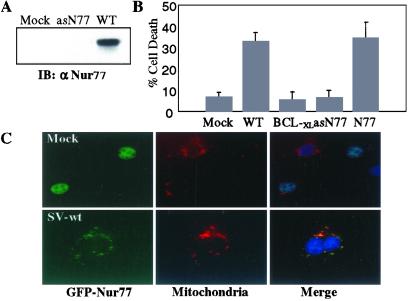
The pathways leading to SV-induced apoptosis are incompletely understood, although accumulation of ceramide and induction of caspases are known to be factors (52–54). We investigated whether Nur77 might play a role in SV-induced apoptosis. Recombinant SV carrying sense and antisense versions of the Nur77 ORF were generated and titered. NIH 3T3 cells were mock infected or infected with SV-wt or SV-asN77. Four hours postinfection, cells were harvested and Nur77 expression was analyzed by immunoprecipitation with anti-Nur77 antibody followed by Western blotting (Fig. 2A). Infection with SV-wt induced Nur77 expression and this was effectively blocked by antisense Nur77. To assess the role of Nur77 induction in SV-induced cell death, NIH 3T3 cells were mock infected or infected with SV-wt, SV-BCL-xL, SV-N77, or SV-asN77 at a moi of 5 and cell death was assessed at 48 hr postinfection by using a LDH release assay. Cell death was induced efficiently by the SV-wt and SV-N77 viruses (Fig. 2B). However, expression of antisense Nur77 was equally as effective as BCL-xL at protecting against SV-induced apoptosis.
Figure 2.
Nur77 is a mediator of SV-induced apoptosis. (A) NIH 3T3 cells (2 × 107) were infected with either SV-wt or SV-antisenseNur77 (asN77) for 4 hr. Cell lysates were immunoprecipitated with anti-Nur77 antibody and the immunoprecipitates were analyzed by immunoblotting with anti-Nur77 antibody. (B) NIH 3T3 cells were mock infected or infected at a moi = 5 with SV-wt, SV-BCL-xL, SV-Nur77 (N77), or SV-antisenseNur77 (asN77). Cell death was measured 48 hr after infection by using an LDH release assay. The data shown are the mean of three assays with the standard deviation provided. (C) NIH 3T3 cells were transfected with GFP-Nur77 and 12 hr later the cells were either mock infected (Upper) or infected with SV-wt (Lower). After 24 hr, cells were fixed, and stained with DAPI (nuclei; blue) and MitoTracker-Red (mitochondria; red). GFP-Nur77 (green) was nuclear in mock-infected cells (Upper) but colocalized with mitochondria in SV-infected cells.
Nur77 functions in the nucleus as a transcription factor, but in the presence of certain apoptotic stimuli Nur77 translocates to the cytoplasm where it targets mitochondria (39). To demonstrate that the behavior of Nur77 was consistent with a role in SV-induced apoptosis, NIH 3T3 cells were transfected with a GFP-Nur77 fusion protein and 12 hr later the cells were either mock infected or infected with SV-wt, and the intracellular localization of GFP-Nur77 was visualized. In mock-infected cells (Fig. 2C Upper), GFP-Nur77 (green) was present in the nucleus and the GFP signal was physically distinct from the cytoplasmic mitochondrial staining detected using MitoTracker-Red dye (red). After SV infection (Fig. 2C Lower), the GFP-Nur77 was detected in the cytoplasm where it colocalized with the MitoTracker-Red mitochondrial staining in merged images.
EBNA2 Interacts with Nur77.
Because Nur77 is involved in SV-induced apoptosis and EBNA2 is protective in SV infected cells, we sought to determine whether there was any interaction between EBNA2 and Nur77. Firstly, HeLa cells were cotransfected with GFP-Nur77 and EBNA2 expression vectors and the transfected cells were monitored for GFP-fluorescence and EBNA2 by using anti-EBNA2 monoclonal antibody and a rhodamine-conjugated secondary antibody. EBNA2 and Nur77 were observed to colocalize with a pattern of nuclear punctate staining in the transfected cells (Fig. 3).
Figure 3.
EBNA2 colocalizes with Nur77. IFA performed on HeLa cells cotransfected with GFP-Nur77 (green) and EBNA2 (red) and stained with anti-EBNA2 antibody. In the merged confocal images, EBNA2 and Nur77 colocalized (yellow).
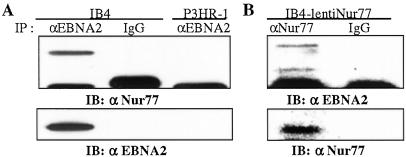
To determine whether EBNA2 and Nur77 interacted in EBV infected B cells, an immunoprecipitation was performed on extracts of IB4 cells that are latently EBV infected and express EBNA2. Extracts from P3HR-1 cells were used as a control. P3HR1 EBV carries an EBNA2 deletion. Endogenous EBNA2 was immunoprecipitated with anti-EBNA2 antibody and Western blots of the immunoprecipitated proteins were incubated with anti-Nur77 antibody. Nur77 coprecipitated with EBNA2 from IB4 cells (Fig. 4A Upper). Nur77 was not detected in the control mouse IgG precipitates, nor in the immunoprecipitate from P3HR-1 cells. The presence of EBNA2 in the IB4 immunoprecipitate was confirmed by stripping the blot and reprobing with anti-EBNA2 antibody (Fig. 4A Lower). Using lenti-Nur77-infected IB4 cells, we also demonstrated coprecipitation of EBNA2 and Nur77 in cell extracts immunoprecipitated with anti-Nur77 antibody (Fig. 4B Upper).
Figure 4.
Coprecipitation of endogenous EBNA2 and Nur77. (A) Endogenous EBNA2 protein was immunoprecipitated with anti-EBNA2 antibody (lanes 1 and 3) or with control antibody (lanes 2 and 4) from extracts of IB4 or control P3HR-1 cells. Western blots of the immunoprecipitates were probed with anti-Nur77 antibody (BD PharMingen). The blot was reprobed with anti-EBNA2 antibody to show the presence of EBNA2 (Lower). (B) Extracts from IB4 cells infected with lenti-Nur77 were subjected to immunoprecipitation with anti-Nur77 antibody and immunoblotting with anti-EBNA2 antibody to confirm coprecipitation of EBNA2 and Nur77. The blot was reprobed with anti-Nur77 antibody to show the presence of Nur77 (Lower).
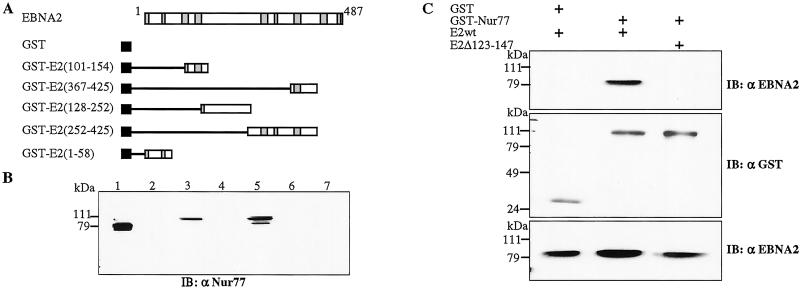
To further pursue the question of interaction between these two proteins, a GST-affinity assay was performed using GST-fusion proteins expressing different segments of EBNA2 (Fig. 5A) and extract from 293T cells transfected with HA-Nur77. Immunoblot analysis of the GST-bound proteins revealed that there was no interaction between Nur77 and GST alone (Fig. 5B, lane 2) or between Nur77 and GST fusions carrying the EBNA2 C terminus (amino acid 367–425, lane 4; amino acid 252–425, lane 6) or N terminus (amino acid 1–58, lane 7). However, Nur77 bound to GST fusion proteins carrying an internal region of EBNA2 (amino acid 101–154, lane 3; amino acid 128–252, lane 5). Interestingly, the Nur77 protein detected in lanes 3 and 5 migrated at the same position as a minor Nur77 species in the transfected cell extract and slightly more slowly than the major Nur77 band. The Nur77 species interacting with endogenous EBNA2 in IB4 cells (Fig. 4A) also migrated in the position of the modified Nur77 protein. This raises the possibility that a modified form of Nur77 might preferentially interact with EBNA2. Phosphorylation is known to modulate both the function and intracellular localization of Nur77 (38, 55).
Figure 5.
EBNA2 binds Nur77 through the EBNA2 amino acids 123–147 domain. (A) Schematic of EBNA2 and the GST-EBNA2 proteins. Regions conserved among primate EBNA2 homologs (74) are shaded. (B) GST-affinity assay in which extract from 293T cells transfected with HA-Nur77 was incubated with purified GST (lane 2) or with the GST-EBNA2 fusion proteins. Lane 3, E2 (101–154); lane 4, E2 (367–425); lane 5, E2 (128–252); lane 6, E2 (252–425); lane 7, E2 (1–58). Lane 1, transfected cell extract (10 μl). Bound protein was detected by Western blotting using anti-Nur77 antibody. (C) Mammalian GST-affinity assay showing that deletion of EBNA2 amino acids 123–147 ablates Nur77 binding. (Top) Extracts of 293T cells cotransfected with GST, GST-Nur77, EBNA2wt, or EBNA2Δ123–147 plasmids were incubated with glutathione-Sepharose beads and the bound protein was detected by immunoblotting with anti-EBNA2 antibody. (Middle) The immunoblot in A was reprobed with anti-GST antibody to demonstrate equal expression of GST and GST-Nur77. (Bottom) Immunoblot of the transfected cell extracts to show EBNA2 expression.
EBNA2 Amino Acid 123–147 Are Necessary for Nur77 Interaction and for Inhibition of Apoptosis.
The two GST-EBNA2 fusion proteins that showed interaction with Nur77 overlapped between amino acids 128 and 154, suggesting that this region was important for Nur77 binding. To directly assess the requirement for this domain, an EBNA2 mutant was tested that was deleted for amino acids 123–147. A eukaryotic GST-affinity assay was performed in 293T cells cotransfected with expression plasmids for GST-Nur77 and either EBNA2wt or EBNA2Δ123–147. Immunoblot analysis of the GST-bound protein detected EBNA2-wt bound to GST-Nur77, but no binding of EBNA2 Δ123–147 was observed (Fig. 5C Top). Equal amounts of GST protein were bound to the glutathione beads, as shown by reprobing of the immunoblot with anti-GST antibody (Fig. 5C Middle). The transfected cells expressed comparable amounts of the EBNA2wt and mutant EBNA2Δ123–147 proteins as assessed by immunoblot analysis using anti-EBNA2 monoclonal antibody (Fig. 5C Bottom).
To determine the effect of loss of Nur77 binding on the antiapoptotic function of EBNA2, a recombinant SV was generated that expressed EBNA2Δ123–147. Cell death was measured in NIH 3T3 cells infected with SV-E2, SV-revE2, SV-E2Δ123–147, or control SV recombinants (Fig. 6A). SV-E2Δ123–147 showed reduced efficacy at protecting against cell death compared with SV-E2 and was only marginally more protective than the antisense EBNA2 recombinant SV. Consistent with this observation, PARP cleavage was detected in SV-E2Δ123–147-infected cells (Fig. 6B). Thus, loss of Nur77 interaction correlated with loss of antiapoptotic function.
Figure 6.
Binding to Nur77 is essential for EBNA2 antiapoptotic activity. (A) NIH 3T3 cells were mock infected or infected with the indicated viruses and cell viability was determined 54 hr postinfection by using the LDH assay. Data shown are the mean of three assays with the standard deviation provided. The Nur77 nonbinding EBNA2Δ123–147 was nonprotective. (B) Western blot of extracts from NIH 3T3 cells infected with the indicated viruses and probed with anti-PARP antibody to detect PARP cleavage and anti-β-actin antibody to show equal loading. The 85-kDa cleaved PARP product is arrowed.
EBNA2 and NotchIC Inhibit Nur77 Nuclear Export.
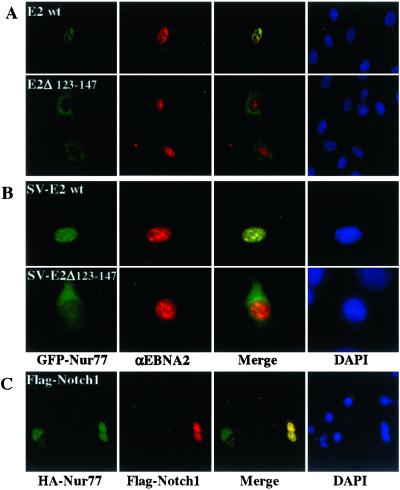
Nur77's proapoptotic activity has recently been linked to the translocation of Nur77 from the nucleus to the mitochondria (39). We examined GFP-Nur77 intracellular localization in HeLa cells that were cotransfected with either EBNA2wt or EBNA2Δ123–147 and then treated with PMA (phorbol 12-myristate 13-acetate) as an apoptotic stimulus. Cells were then stained for EBNA2 by using anti-EBNA2 antibody and Rhodamine-conjugated secondary antibody. In cells expressing EBNA2wt, cotransfected GFP-Nur77 was present in the nucleus, whereas in cells expressing the nonprotective mutant EBNA2Δ123–147 the GFP-Nur77 had relocalized to the cytoplasm (Fig. 7A). In a parallel approach, GFP-Nur77-transfected NIH 3T3 cells were infected with SV expressing either EBNA2wt or EBNA2Δ123–147. Once again the GFP-Nur77 remained nuclear in the SV-EBNA2wt-expressing cells and was relocalized to the cytoplasm in SV-EBNA2Δ123–147-expressing cells (Fig. 7B). These observations suggest that the antiapoptotic effect of EBNA2 derives from EBNA2 binding to Nur77 and preventing mitochondrial targeting by Nur77. NotchIC is known to inhibit Nur77-mediated apoptosis (35). We wondered whether NotchIC might behave similarly to EBNA2 and repeated the assay in HeLa cells, this time cotransfecting Flag-NotchIC and HA-Nur77. Treatment of the culture with PMA induced cytoplasmic export of Nur77 in the singly transfected cells, but in NotchIC-expressing cells Nur77 was retained in the nucleus (Fig. 7C).
Figure 7.
EBNA2 mediates cell survival by inhibiting Nur77 nuclear export. Immunofluorescence assays examining GFP-Nur77 localization in cells expressing EBNA2 and subjected to apoptotic stimuli. (A) HeLa cells were cotransfected with GFP-Nur77 and either E2wt or E2Δ123–147 and treated with PMA (200 ng/ml for 16 hr). (B) GFP-Nur77-transfected NIH 3T3 cells were infected with SV-E2wt (Upper) or SV-E2Δ123–147 (Lower) and fixed 24 hr after infection. (C) Flag-NotchIC- and HA-Nur77-transfected HeLa cells treated as in A. Nur77 (green) remains nuclear in E2wt- (red) and Flag-NotchIC-expressing (red) cells. Colocalized proteins appear yellow in the merged images. Nuclei were stained with DAPI (blue).
Discussion
Nur77 is a mediator of apoptosis in a variety of cell types. Much of the work on the apoptotic function of Nur77 and its family members Nurr1 and Nor1 has focused on T cell receptor (TCR)-induced apoptosis (56, 57). Nur77 synthesis is induced during this process and Nur77 is a key mediator (51, 58). Expression of antisense Nur77 or a dominant-negative Nur77 prevents apoptosis in TCR-stimulated cells, whereas constitutive expression of Nur77 in thymocytes of transgenic mice leads to extensive apoptosis (51, 59, 60). Nur77 has also been shown to be critical for surface IgM-mediated apoptosis of B cell lines (61–63). In addition, Nur77 participates in the apoptotic response induced by agents such as phorbol esters, calcium ionophores, and etoposide (39). Viral infection can now be added to this list. SV infection induced Nur77 expression and abolition of expression by antisense Nur77 was protective against SV-induced cell death.
Until recently, it had been assumed that Nur77 apoptotic function was mediated solely through its gene regulatory activity. Li et al. (39) demonstrated that appropriate apoptotic stimuli resulted in relocalization of Nur77 from the nucleus to the cytoplasm where Nur77 was found to accumulate in the cell fraction that contained mitochondria. Abrogation of mitochondrial targeting by blocking Nur77 nuclear export using leptomycin B treatment or by retargeting the protein to other cell organelles abolished its apoptotic activity. We showed that endogenous EBNA2 and Nur77 proteins from latently EBV-infected IB4 B cells coprecipitated, consistent with a role for EBNA2 in modifying Nur77 activity. EBNA2 bound Nur77 and inhibited Nur77 nuclear export induced by either SV or by treatment with PMA. EBNA2 also countered Nur77-mediated apoptosis induced by SV infection. Nur77 binding correlated with EBNA2 antiapoptotic activity in that an EBNA2 mutant unable to interact with Nur77 was also unable to prevent Nur77 nuclear export and the apoptotic cell response.
Identification of an antiapoptotic function for EBNA2 implies that EBNA2 contributes to cell survival, as well as to the proliferative response that occurs on EBV infection. Cell death can be initiated through multiple second messenger pathways and Nur77 is involved in only some of these; for example, LNCaP cells stably expressing antisense Nur77 were not protected from apoptosis induced by treatment with TNF-α (39). The anti-Nur77 protective effect of EBNA2 may complement the LMP1-induced survival signals mediated through NF-κB up-regulation of antiapoptotic genes such as A20, Bfl-1, and BCL-2 (42, 64–66) and provide initial protection before LMP1 expression during primary EBV infection of B cells. Consistent with such a role, recombinant EBV deleted for the region of EBNA2 containing the amino acids 123–147 domain transforms B cells in vitro with less than 10% of the efficiency of wild-type virus (67, 68). The majority of EBV-associated tumors do not express EBNA2, presumably because the EBNA2 and coordinately expressed EBNA3 latency proteins are highly immunogenic. However, EBNA2 is expressed in EBV-associated tumors arising in immunocompromised patients—for example, in posttransplant lymphoproliferative disease and in primary central nervous system lymphomas in AIDS patients (69–71)—and its antiapoptotic activity may be a factor in the development of these malignancies.
A dual proliferative and antiapoptotic role for EBNA2 mirrors the activities ascribed to cellular Notch (16, 72). Notch ligands are expressed on stromal cells and Notch signaling in vitro induces proliferation of CD34+CD38− pluripotent hematopoietic cells (73). Notch is believed to act at multiple stages during thymocyte development and it has been proposed that the antiapoptotic effects of Notch influence cell fate decisions by rescuing cells that would otherwise succumb to cell death by neglect or by negative selection (56). However, the way in which NotchIC blocks Nur77 apoptotic function has not been defined. We found that NotchIC also prevented cytoplasmic translocation of Nur77 in HeLa cells treated with PMA. Thus, EBNA2 mimicry of NotchIC is extended from their previously recognized shared mechanism of promoter targeting through cellular CBF1 to a similar mechanism for antiapoptotic activity. This degree of functional convergence is particularly remarkable in light of the fact that EBNA2 and Notch do not share obvious primary sequence homology.
Acknowledgments
We thank Marie Hardwick for the dsTE12Q and SV-BCL-xL plasmids and for advice on SV assays, Linzhao Cheng for the lentivirus vector pEF-GFP and helpful advice, and Paul Ling for the EBNA2Δ123–147 plasmid. We also thank Leslie Meszler of the Oncology Cell Imaging Core for assistance with confocal microscopy. This work was funded by Public Health Service Grants RO1 CA42245 (to S.D.H.) and R01 A47922 (to B.A.O.). M.W. was partially supported by Public Health Service Training Grant 5 T32 GM07445.
Abbreviations
- EBV
Epstein–Barr virus
- moi
multiplicity of infection
- PARP
poly (ADP-ribose) polymerase
- PMA
phorbol 12-myristate 13-acetate
- SV
Sindbis virus
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rickinson A B, Kieff E. In: Fields Virology. Field B N, Knipe D M, Howley P M, editors. Vol. 2. New York: Raven; 1996. pp. 2397–2446. [Google Scholar]
- 2.Kieff E. In: Fields Virology. Field B N, Knipe D M, Howley P M, editors. Vol. 2. New York: Raven; 1996. pp. 2343–2396. [Google Scholar]
- 3.Chen H, Smith P, Ambinder R F, Hayward S D. Blood. 1999;93:3026–3032. [PubMed] [Google Scholar]
- 4.Longnecker R. Adv Cancer Res. 2000;79:175–200. doi: 10.1016/s0065-230x(00)79006-3. [DOI] [PubMed] [Google Scholar]
- 5.Miyashita E M, Yang B, Lam K M, Crawford D H, Thorley-Lawson D A. Cell. 1995;80:593–601. doi: 10.1016/0092-8674(95)90513-8. [DOI] [PubMed] [Google Scholar]
- 6.Hammerschmidt W, Sugden B. Nature (London) 1989;340:393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J I, Wang F, Mannick J, Kieff E. Proc Natl Acad Sci USA. 1989;86:9558–9562. doi: 10.1073/pnas.86.23.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith P R, de Jesus O, Turner D, Hollyoake M, Karstegl C E, Griffin B E, Karran L, Wang Y, Hayward S D, Farrell P J. J Virol. 2000;74:3082–3092. doi: 10.1128/jvi.74.7.3082-3092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusano S, Raab-Traub N. J Virol. 2001;75:384–395. doi: 10.1128/JVI.75.1.384-395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Chen H, Weinmaster G, Hayward S D. J Virol. 2001;75:2946–2956. doi: 10.1128/JVI.75.6.2946-2956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinclair A J, Palmero I, Peters G, Farrell P J. EMBO J. 1994;13:3321–3328. doi: 10.1002/j.1460-2075.1994.tb06634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempkes B, Spitkovsky D, Jansen-Durr P, Ellwart J W, Kremmer E, Delecluse H-J, Rottenberger C, Bornkamm G W, Hammerschmidt W. EMBO J. 1995;14:88–96. doi: 10.1002/j.1460-2075.1995.tb06978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh J J-D, Henkel T, Salmon P, Robey E, Peterson M G, Hayward S D. Mol Cell Biol. 1996;16:952–959. doi: 10.1128/mcb.16.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strobl L J, Hofelmayr H, Marschall G, Brielmeier M, Bornkamm G W, Zimber-Strobl U. J Virol. 2000;74:1727–1735. doi: 10.1128/jvi.74.4.1727-1735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artavanis-Tsakonas S, Rand M D, Lake R J. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 16.Miele L, Osborne B. J Cell Physiol. 1999;181:393–409. doi: 10.1002/(SICI)1097-4652(199912)181:3<393::AID-JCP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Mumm J S, Kopan R. Dev Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 18.Hayward S D. EBV Rep. 1999;6:151–157. [Google Scholar]
- 19.Hsieh J J-D, Hayward S D. Science. 1995;268:560–563. doi: 10.1126/science.7725102. [DOI] [PubMed] [Google Scholar]
- 20.Ling P D, Hsieh J J-D, Ruf I K, Rawlins D R, Hayward S D. J Virol. 1994;68:5375–5383. doi: 10.1128/jvi.68.9.5375-5383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tun T, Hamaguchi Y, Matsunami N, Furukawa T, Honjo T, Kawaichi M. Nucl Acids Res. 1994;22:965–971. doi: 10.1093/nar/22.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao H-Y, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, Kintner C R, Evans R M, Kadesch T. Genes Dev. 1998;12:2269–2277. doi: 10.1101/gad.12.15.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh J J-D, Zhou S, Chen L, Young D B, Hayward S D. Proc Natl Acad Sci USA. 1999;96:23–28. doi: 10.1073/pnas.96.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Struhl K. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 25.Zhou S, Hayward S D. Mol Cell Biol. 2001;21:6222–6232. doi: 10.1128/MCB.21.18.6222-6232.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou S, Fujimuro M, Hsieh J J, Chen L, Hayward S D. J Virol. 2000;74:1939–1947. doi: 10.1128/jvi.74.4.1939-1947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou S, Fujimuro M, Hsieh J J-D, Chen L, Miyamoto A, Weinmaster G, Hayward S D. Mol Cell Biol. 2000;20:2400–2410. doi: 10.1128/mcb.20.7.2400-2410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayachandra S, Low K G, Thlick A E, Yu J, Ling P D, Chang Y, Moore P S. Proc Natl Acad Sci USA. 1999;96:11566–11571. doi: 10.1073/pnas.96.20.11566. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Wang L, Grossman S R, Kieff E. Proc Natl Acad Sci USA. 2000;97:430–435. doi: 10.1073/pnas.97.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurooka H, Honjo T. J Biol Chem. 2000;275:17211–17220. doi: 10.1074/jbc.M000909200. [DOI] [PubMed] [Google Scholar]
- 31.Wu D Y, Krumm A, Schubach W H. J Virol. 2000;74:8893–8903. doi: 10.1128/jvi.74.19.8893-8903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordadze A V, Peng R, Tan J, Liu G, Sutton R, Kempkes B, Bornkamm G W, Ling P D. J Virol. 2001;75:5899–5912. doi: 10.1128/JVI.75.13.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakai T, Taniguchi Y, Tamura K, Minoguchi S, Fukuhara T, Strobl L J, Zimber-Strobl U, Bornkamm G W, Honjo T. J Virol. 1998;72:6034–6039. doi: 10.1128/jvi.72.7.6034-6039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofelmayr H, Strobl L J, Marschall G, Bornkamm G W, Zimber-Strobl U. J Virol. 2001;75:2033–2040. doi: 10.1128/JVI.75.5.2033-2040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jehn B M, Bielke W, Pear W S, Osborne B A. J Immunol. 1999;162:635–638. [PubMed] [Google Scholar]
- 36.Winoto A, Littman D R. Cell. 2002;109,Suppl.:S57–S66. doi: 10.1016/s0092-8674(02)00710-9. [DOI] [PubMed] [Google Scholar]
- 37.Philips A, Lesage S, Gingras R, Maira M H, Gauthier Y, Hugo P, Drouin J. Mol Cell Biol. 1997;17:5946–5951. doi: 10.1128/mcb.17.10.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katagiri Y, Takeda K, Yu Z X, Ferrans V J, Ozato K, Guroff G. Nat Cell Biol. 2000;2:435–440. doi: 10.1038/35017072. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Kolluri S K, Gu J, Dawson M I, Cao X, Hobbs P D, Lin B, Chen G, Lu J, Lin F, et al. Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- 40.Hardwick J M. Semin Cell Dev Biol. 1998;9:339–349. doi: 10.1006/scdb.1998.0243. [DOI] [PubMed] [Google Scholar]
- 41.Cahir-McFarland E D, Davidson D M, Schauer S L, Duong J, Kieff E. Proc Natl Acad Sci USA. 2000;97:6055–6060. doi: 10.1073/pnas.100119497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fries K L, Miller W E, Raab-Traub N. J Virol. 1996;70:8653–8659. doi: 10.1128/jvi.70.12.8653-8659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshall W L, Yim C, Gustafson E, Graf T, Sage D R, Hanify K, Williams L, Fingeroth J, Finberg R W. J Virol. 1999;73:5181–5185. doi: 10.1128/jvi.73.6.5181-5185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khanim F, Dawson C, Meseda C A, Dawson J, Mackett M, Young L S. J Gen Virol. 1997;78:2987–2999. doi: 10.1099/0022-1317-78-11-2987. [DOI] [PubMed] [Google Scholar]
- 45.Bellows D S, Chau B N, Lee P, Lazebnik Y, Burns W H, Hardwick J M. J Virol. 2000;74:5024–5031. doi: 10.1128/jvi.74.11.5024-5031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardwick J M, Levine B. Methods Enzymol. 2000;322:492–508. doi: 10.1016/s0076-6879(00)22045-4. [DOI] [PubMed] [Google Scholar]
- 47.Ling P D, Hayward S D. J Virol. 1995;69:1944–1950. doi: 10.1128/jvi.69.3.1944-1950.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cui Y, Golob J, Kelleher E, Ye Z, Pardoll D, Cheng L. Blood. 2002;99:399–408. doi: 10.1182/blood.v99.2.399. [DOI] [PubMed] [Google Scholar]
- 49.Levine B, Huang Q, Isaacs J T, Reed J C, Griffin D E, Hardwick J M. Nature (London) 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 50.Cheng E H, Levine B, Boise L H, Thompson C B, Hardwick J M. Nature (London) 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 51.Liu Z G, Smith S W, McLaughlin K A, Schwartz L M, Osborne B A. Nature (London) 1994;367:281–284. doi: 10.1038/367281a0. [DOI] [PubMed] [Google Scholar]
- 52.Nava V E, Rosen A, Veliuona M A, Clem R J, Levine B, Hardwick J M. J Virol. 1998;72:452–459. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clem R J, Cheng E H, Karp C L, Kirsch D G, Ueno K, Takahashi A, Kastan M B, Griffin D E, Earnshaw W C, Veliuona M A, Hardwick J M. Proc Natl Acad Sci USA. 1998;95:554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jan J T, Chatterjee S, Griffin D E. J Virol. 2000;74:6425–6432. doi: 10.1128/jvi.74.14.6425-6432.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pekarsky Y, Hallas C, Palamarchuk A, Koval A, Bullrich F, Hirata Y, Bichi R, Letofsky J, Croce C M. Proc Natl Acad Sci USA. 2001;98:3690–3694. doi: 10.1073/pnas.051003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osborne B, Miele L. Immunity. 1999;11:653–663. doi: 10.1016/s1074-7613(00)80140-5. [DOI] [PubMed] [Google Scholar]
- 57.Toth R, Szegezdi E, Reichert U, Bernardon J M, Michel S, Ancian P, Kis-Toth K, Macsari Z, Fesus L, Szondy Z. Eur J Immunol. 2001;31:1382–1391. doi: 10.1002/1521-4141(200105)31:5<1382::AID-IMMU1382>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 58.Woronicz J D, Calnan B, Ngo V, Winoto A. Nature (London) 1994;367:277–281. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- 59.Weih F, Ryseck R P, Chen L, Bravo R. Proc Natl Acad Sci USA. 1996;93:5533–5538. doi: 10.1073/pnas.93.11.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calnan B J, Szychowski S, Chan F K, Cado D, Winoto A. Immunity. 1995;3:273–282. doi: 10.1016/1074-7613(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 61.Mapara M Y, Weinmann P, Bommert K, Daniel P T, Bargou R, Dorken B. Eur J Immunol. 1995;25:2506–2510. doi: 10.1002/eji.1830250915. [DOI] [PubMed] [Google Scholar]
- 62.Mittelstadt P R, DeFranco A L. J Immunol. 1993;150:4822–4832. [PubMed] [Google Scholar]
- 63.Dinkel A, Warnatz K, Ledermann B, Rolink A, Zipfel P F, Burki K, Eibel H. J Exp Med. 1998;188:2215–2224. doi: 10.1084/jem.188.12.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, Kieff E, Rickinson A. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 65.Spender L C, Cannell E J, Hollyoake M, Wensing B, Gawn J M, Brimmell M, Packham G, Farrell P J. J Virol. 1999;73:4678–4688. doi: 10.1128/jvi.73.6.4678-4688.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.D'Souza B, Rowe M, Walls D. J Virol. 2000;74:6652–6658. doi: 10.1128/jvi.74.14.6652-6658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen J I, Wang F, Kieff E. J Virol. 1991;65:2545–2554. doi: 10.1128/jvi.65.5.2545-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yalamanchili R, Harada S, Kieff E. J Virol. 1996;70:2468–2473. doi: 10.1128/jvi.70.4.2468-2473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Auperin I, Mikolt J, Oksenhendler E, Thiebaut J B, Brunet M, Dupont B, Morinet F. Neuropathol Appl Neurobiol. 1994;20:243–252. doi: 10.1111/j.1365-2990.1994.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 70.Cen H, Williams P A, McWilliams H P, Breinig M C, Ho M, McKnight J L. Blood. 1993;81:1393–1403. [PubMed] [Google Scholar]
- 71.Delecluse H J, Kremmer E, Rouault J P, Cour C, Bornkamm G, Berger F. Am J Pathol. 1995;146:1113–1120. [PMC free article] [PubMed] [Google Scholar]
- 72.Shelly L L, Fuchs C, Miele L. J Cell Biochem. 1999;73:164–175. doi: 10.1002/(sici)1097-4644(19990501)73:2<164::aid-jcb3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 73.Karanu F N, Murdoch B, Miyabayashi T, Ohno M, Koremoto M, Gallacher L, Wu D, Itoh A, Sakano S, Bhatia M. Blood. 2001;97:1960–1967. doi: 10.1182/blood.v97.7.1960. [DOI] [PubMed] [Google Scholar]
- 74.Ling P D, Ryon J J, Hayward S D. J Virol. 1993;67:2990–3003. doi: 10.1128/jvi.67.6.2990-3003.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]