Abstract
Earlier in vivo studies showed the involvement of IspH protein in the conversion of 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate into isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). We have demonstrated now that cell extract of an Escherichia coli strain engineered for hyperexpression of the ispH (lytB) gene catalyzes the in vitro conversion of 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate into IPP and DMAPP. The reaction requires NADH, FAD, divalent cations (preferably Co2+), and probably one or more as-yet-unidentified proteins. The low intrinsic catalytic activities of wild-type E. coli cell extract and isolated chromoplasts of red pepper (Capsicum annuum) are enhanced by the addition of purified recombinant IspH protein.
Keywords: lytB, ispH, isoprenoids, isopentenyl diphosphate, deoxyxylulose
Terpenes are one of the largest groups of natural products comprising numerous compounds with important roles in physiological and pathological processes (1). Their biosynthesis has been a central topic of metabolic studies for more than half a century. The early work with yeast and animal cells established the biosynthesis of the universal terpene precursors, isopentenyl diphosphate (IPP, 9) and dimethylallyl diphosphate (DMAPP, 10), from acetyl-CoA via mevalonate (for review see refs. 2–5). About a decade ago, a second pathway for formation of these intermediates via the carbohydrate, 1-deoxy-d-xylulose 5-phosphate (3), has been discovered in certain bacteria as well as in Ginkgo biloba (for review see refs. 6–8).
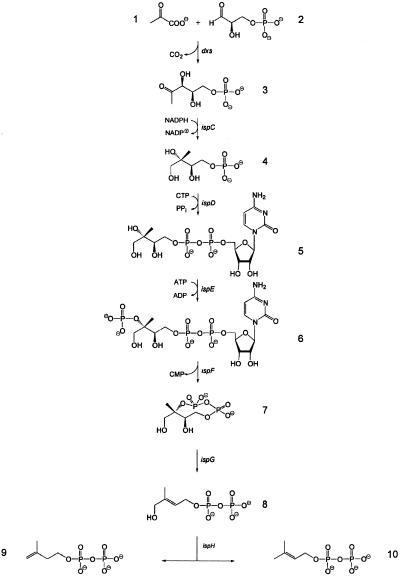
The initial step of the so-called nonmevalonate pathway is the condensation of d-glyceraldehyde 3-phosphate 2 with pyruvate 1 affording 3 and carbon dioxide (Fig. 1; refs. 9 and 10). Compound 3 is converted into the branched chain polyol 2C-methyl-d-erythritol 4-phosphate 4 by skeletal rearrangement and reduction (11). The polyol 4 is converted via 4-diphosphocytidyl-2C-methyl-d-erythritol 5 and its 2-phosphate 6 into 2C-methyl-d-erythritol 2,4-cyclodiphosphate 7 by a sequence of three reaction steps catalyzed by proteins that are specified by the ispDEF genes (12–17). In vivo experiments showed that the reductive ring opening of 7 affording 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate 8 requires a protein specified by the ispG (gcpE) gene (18, 19). The same compound was shown also to accumulate in an ispH-deficient Escherichia coli strain (20), and its formation from 14C-labeled 7 was observed in cell extracts from E. coli overexpressing the ispG (gcpE) gene (21). Subsequent in vivo studies with a recombinant E. coli strain showed that the conversion of 8 into 9 and 10 requires a protein specified by the ispH (lytB) gene (22).
Fig 1.
The nonmevalonate pathway of isoprenoid biosynthesis.
The nonmevalonate pathway is the exclusive source of terpenes in many pathogenic eubacteria and in Plasmodium spp. (for review see ref. 23). The antibiotic fosmidomycin has been shown to inhibit 2C-methyl-d-erythritol 4-phosphate synthase (24) and protect mice infected with Plasmodium vinckei (25). Hence, the enzymes of the pathway are potential targets for antiinfective drugs.
The further characterization of the enzymes of the deoxyxylulose phosphate pathway is a prerequisite for drug screening. This paper reports in vitro studies on the enzymatic conversion of 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate (8) into IPP (9) and DMAPP (10).
Experimental Procedures
Materials.
[1,2-13C2]-, [3,4,5-13C3]-, and [U-13C5]1-Deoxy-d-xylulose were prepared as described earlier (26). [2,2′-13C2]-, [1,3,4-13C3]- and [U-13C5]8 were prepared with the recombinant E. coli strain XL1-pBSxispC-G by biotransformation of [1,2-13C2]-, [3,4,5-13C3]-, and [U-13C5]1-deoxy-d-xylulose, respectively (18). The preparation of [1-3H]8 will be described elsewhere (S.H., S. Amslinger, J. Jauch, K. Kis, V. Trentinaglia, P.A., W.E., A.B., and F.R., unpublished data). Oligonucleotides were custom-synthesized by MWG Biotech (Ebersberg, Germany). IPP and DMAPP were purchased from Echelon (Salt Lake City). [4-14C]9 [57.5 μCi⋅μmol−1 (1 Ci = 37 GBq)] was purchased from NEN. Pamidronate (Aredia) was purchased from Novartis Pharma. Nylon cloth (50 μm) was obtained from Verseidag Techfab (Walbeck, Germany).
Microorganisms.
The bacterial strains and plasmids used in this study are summarized in Table 1.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant characteristic | Ref. or source |
|---|---|---|
| E. coli | ||
| XL1-Blue | RecA1, endA1, gyrA96, thi-1, hsdR17, supE44, relA1, lac, [F′, proAB, laclqZΔM15, Tn10 (tetr)] | 27 |
| XL1-pBSxispC-G | Expression strain of xylB, ispC-G | 18 |
| Plasmids | ||
| pMAL-C2 | High-copy maltose-binding protein expression vector | New England Biolabs |
| pMALlytB | Expression of ispH (lytB) from E. coli as maltose-binding fusion protein | This study |
Cloning and Expression of the ispH Gene.
The ispH gene of E. coli (formerly designated lytB, GenBank accession no. AY062212) was amplified by PCR using chromosomal E. coli DNA as template and the oligonucleotides lytBvo and lytBhi (Table 2) as primers. The amplificate was purified, treated with the restriction enzymes BamHI and PstI, and ligated into the expression vector pMAL-C2 (New England Biolabs), which had been treated with the same enzymes. The resulting plasmid pMALlytB was electrotransformed into the E. coli strain XL1-Blue (Stratagene; ref. 27) affording the recombinant strain XL1-pMALlytB.
Table 2.
Oligonucleotides used in this study
| Designation | Sequence (5′–3′) |
|---|---|
| lytBvo | TGGAGGGGATCCATGCAGATCCTGTTGGCCACC |
| lytBhi | GCATTTCTGCAGAACTTAGGC |
Purification of Recombinant IspH Protein.
The E. coli strain XL1-pMALlytB was grown in Luria Bertani broth containing 180 mg of ampicillin per liter. Cultures were incubated at 37°C with shaking. At an optical density of 0.7 (600 nm), isopropylthiogalactoside was added to a final concentration of 2 mM, and the culture was incubated for 5 h. The cells were harvested by centrifugation, washed with 0.9% (wt/vol) sodium chloride, and stored at −20°C.
Frozen cell mass (3.5 g) was thawed in 35 ml of 50 mM Tris⋅HCl, pH 8.0, containing 0.2 M sodium chloride (buffer A). The suspension was subjected to ultrasonic treatment and centrifuged. The supernatant was applied to a column of amylose resin FF (Amersham Pharmacia; column volume 22 ml) that had been equilibrated with buffer A at a flow rate of 3 ml⋅min−1. The column was washed with 200 ml of buffer A. The column was then developed with a gradient of 0–10 mM maltose in 150 ml of buffer A. The retention volume of MalE/IspH fusion protein was 40 ml. Fractions were combined and dialyzed overnight against 100 mM Tris⋅HCl, pH 8.0, containing 1 mM DTT. The solution was concentrated by ultrafiltration and stored at −80°C.
Assay of 1-Hydroxy-2-methyl-2-(E)-butenyl 4-Diphosphate Reductase Activity.
Assay mixtures contained 70 mM potassium phosphate, pH 7.0, 1 mM CoCl2, 1 mM DTT, 20 mM NaF, 1.5 mM NADH, 60 μM FAD, 7 μM (86 μCi⋅μmol−1) [1-3H]8, 0.5 mM pamidronate, and protein in a total volume of 150 μl. The assay mixtures were incubated at 37°C and placed on ice. The reaction was terminated by the addition of 10 μl of 30% (wt/vol) trichloroacetic acid followed by immediate neutralization with 20 μl of 1 M sodium hydroxide. The mixtures were centrifuged. Aliquots (85 μl) of the supernatant were analyzed by reversed-phase ion-pair HPLC using a column of Multospher 120 RP 18-AQ-3 (4.6 × 250 mm, Chromatographie Service, Langerwehe, Germany). The column was developed with a linear gradient of 11 ml of 10.5–24.5% (vol/vol) methanol and with a gradient of 8.3 ml of 24.5–52.5% (vol/vol) methanol and washed with 2.75 ml of 52.5% (vol/vol) methanol in 10 mM tetra-n-butylammonium phosphate, pH 6.0, at a flow rate of 0.55 ml⋅min−1. The effluent was monitored by online liquid scintillation analysis (Beta-RAM, Biostep, Jahnsdorf, Germany). The retention volumes of 8 and 9/10 were 30.5 and 42 ml, respectively.
Determination of Relative Amounts of DMAPP in Assay Mixtures.
The method of Satterwhite (28) was used to differentiate 9 and 10. A methanol solution of hydrogen chloride (25%, 100 μl) and 250 μl of water were added to an assay mixture of 150 μl. The mixture was incubated for 10 min at 37°C and subsequently saturated with sodium chloride. The allylic alcohol derived from 10 was extracted twice with 0.5 ml of toluene (9 resists hydrolysis and remains in the aqueous phase). The combined organic phases were dried over sodium sulfate, and radioactivity was measured by scintillation counting (bF, betascint BF 5000, Munich).
NMR Spectroscopy.
13C NMR spectra were recorded at 25°C by using an AVANCE DRX 500 spectrometer from Bruker Instruments (Karlsruhe, Germany).
Isolation of Chromoplasts from Capsicum annuum.
A procedure reported by Camara (29) was used with the following modifications. Pericarp of C. annuum (650 g) was homogenized in 750 ml of 50 mM Hepes, pH 8.0, containing 1 mM DTT, 1 mM EDTA, and 0.4 M sucrose. The suspension was filtered through four layers of nylon cloth (50 μm). The filtrate was centrifuged for 10 min at 4,500 rpm (GS3 rotor). The pellet was suspended in 250 ml of 50 mM Hepes, pH 8.0, containing 1 mM DTT, 1 mM EDTA, and 0.4 M sucrose. The mixture was centrifuged, and the pellet was resuspended in 4 ml of 50 mM Hepes, pH 8.0, containing 1 mM DTT, 1 mM EDTA, and 0.4 M sucrose. The suspension was filtered through one layer of nylon cloth. All procedures were carried out at 4°C.
Enzyme Assays Using 13C-Labeled Samples of 1-Hydroxy-2-methyl-2-(E)-butenyl 4-Diphosphate.
Reaction mixtures containing 70 mM potassium phosphate, pH 7.0, 20 mM NaF, 1 mM DTT, 0.5 mM pamidronate (Aredia), 1.5 mM NADH, 60 μM FAD, 0.5 mM CoCl2, and 3 μmol of [2,2′-13C2]8, [1,3,4-13C3]8, and [U-13C5]8, respectively, and cell extract from 0.5 g of cells of the recombinant IspH E. coli strain in a total volume of 6 ml were incubated for 60 min at 37°C. The reaction was terminated by adding DOWEX 50 (WX8, Na+ form), and the mixture was centrifugated for 10 min at 4,500 rpm and lyophilized. The residue was dissolved in 600 μl of 70% (vol/vol) d4-methanol in 2H2O and analyzed by NMR spectroscopy.
Results
The ispH gene of E. coli was fused to the 3′ end of the malE gene specifying maltose-binding protein of E. coli to facilitate affinity chromatography of the recombinant protein. The resulting plasmid, pMALlytB, was transformed into E. coli strain XL1-Blue, where it directed the expression of a protein with an apparent mass of ≈76 kDa as estimated by SDS polyacrylamide gel electrophoresis. The recombinant fusion protein accounted for ≈4% of soluble protein in crude cell extract and reacted with an antiserum against recombinant IspH protein (22) in Western blots.
Enzyme assays with cell extract of the recombinant E. coli strain were performed by using synthetic [1-3H]8 (S.H., S. Amslinger, J. Jauch, K. Kis, V. Trentinaglia, P.A., W.E., A.B., and F.R., unpublished data) as substrate. The assay mixture contained 70 mM potassium phosphate, 0.5 mM Co2+, 60 μM FAD, 1.5 mM NADH, and dialyzed cell extract of recombinant E. coli strain expressing MalE/IspH fusion protein (16 μg of recombinant protein and 400 μg of total protein). The assay mixtures were analyzed by reversed-phase ion-pair HPLC, and the effluent was monitored by online liquid scintillation counting.
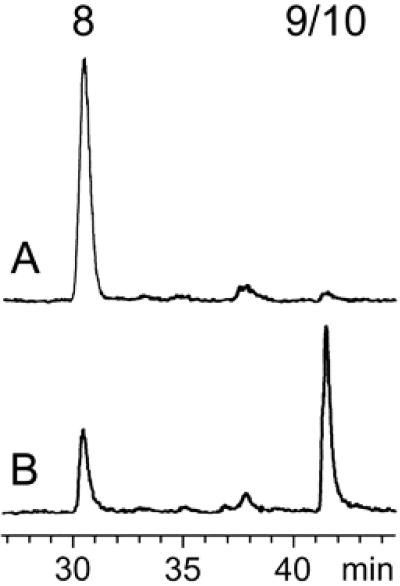
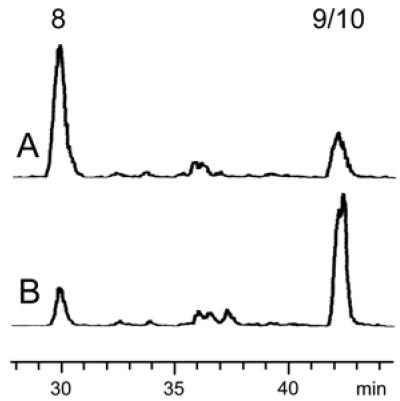
The chromatogram of a control sample retrieved before incubation of the assay mixture is shown in Fig. 2A. Fig. 2B shows that incubation at 37°C for 30 min resulted in depletion of the substrate (retention time, 30.5 min) and the formation of a radioactive peak migrating at the same velocity as an authentic sample of 14C-labeled 9 (retention time, 42 min).
Fig 2.
Assay of 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase activity in a crude extract of recombinant E. coli cells expressing MalE/IspH fusion protein by reversed-phase ion-pair HPLC. (A) Control sample before incubation. (B) Sample incubated for 30 min at 37°C. The retention times of 8 and 9/10 are 30.5 and 42 min, respectively. For details see Experimental Procedures.
The formation of product was reduced greatly when NADH or FAD were omitted from the reaction mixtures (Table 3). NADH gave higher yields of the product as compared with NADPH (data not shown). Various divalent metal ions could support the enzyme-catalyzed reaction; Co2+ had the highest efficacy (Table 4). The formation of enzyme product was suppressed completely by the addition of EDTA (20 mM) to the reaction mixture and increased by the addition of pamidronate, an inhibitor of farnesyl diphosphate synthase (ref. 30; Table 3). The pH optimum of the reaction was 7.0.
Table 3.
Cofactor dependency of recombinant 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase
| Omitted compound | Relative activity, % |
|---|---|
| None | 100 |
| Pamidronate | 42 |
| Co2+ | 20 |
| FAD | 17 |
| NADH | 12 |
Reaction conditions were as described under Experimental Procedures.
Table 4.
Activation of recombinant 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase by divalent metal ions
| Metal ion, 0.5 mM | Relative activity, % |
|---|---|
| Co2+ | 100 |
| Mn2+ | 48 |
| Fe2+ | 21 |
| Mg2+ | 17 |
| Ni2+ | 17 |
| Zn2+ | 12 |
Reaction conditions were as described under Experimental Procedures. The addition of 20 mM EDTA resulted in ≤2% relative activity.
Under the conditions of HPLC analysis used in this study, 9 and 10 migrate at the same velocity. Because our earlier in vivo studies indicated the formation of both 9 and 10 from 8 under the catalytic action of IspH protein (22), it was necessary to differentiate 9 from 10. Using the method of Satterwhite (28) based on the different susceptibility of the two compounds to acid hydrolysis (10 is hydrolyzed easily via a resonance-stabilized allyl cation), we found that 10 and 9 are formed at a ratio of ≈1:5.
To document unequivocally the structure of the major reaction product, 9, we incubated crude cell extract of the recombinant E. coli strain expressing the MalE/IspH fusion protein with various 13C-labeled isotopomers of 8 as substrate. The 13C-labeled substrate samples were obtained by in vivo biotransformation of 13C-labeled 1-deoxy-d-xylulose using a recombinant E. coli strain engineered for hyperexpression of the xylB and ispCDEFG genes (18). In that strain, the d-xylulokinase produced under the direction of the xylB gene converts exogenous deoxyxylulose into its 5-phosphate 3, which then is converted into 8 by the sequential action of the proteins specified by the recombinant ispCDEFG genes. 13C NMR spectra of extracts from recombinant cells incubated with 13C-labeled deoxyxylulose show the presence of 8 and a minor amount of its immediate biosynthetic precursor, 7.
Mixtures of cell extracts containing 13C-labeled 8 and cell extracts containing the MalE/IspH fusion protein were incubated at 37°C for 1 h. The mixtures were treated with DOWEX 50 (WX8 Na+ form) to remove Co2+ and then were analyzed by 13C NMR spectroscopy without further purification.
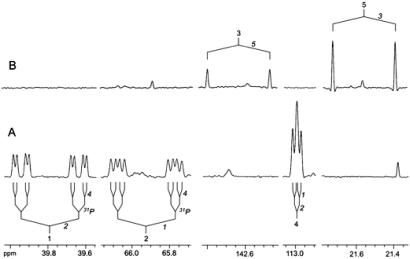
With [2,2′-13C2]8 as substrate, the 13C-labeled carbon atoms 3 and 5 of enzymatically formed 9 appeared as doublets caused by 13C13C coupling (Fig. 3B).
Fig 3.
NMR signals of [1,2,4-13C3]9 (A) and [3,5-13C2]9 (B) formed in vitro from [1,3,4-13C3] and [2,2′-13C3]8, respectively, by crude extracts of recombinant E. coli cells expressing MalE/IspH fusion protein.
With [1,3,4-13C3]8 as substrate, carbon atoms 1, 2, and 4 of enzymatically formed 9 appeared as more complex multiplets caused by 13C13C and 13C31P coupling via 1–3 bonds (Fig. 3A).
Experiments with [U-13C5]8 as substrate afforded [U-13C5]9. The observed coupling pattern reflected the coupling constants described earlier (19). For additional confirmation, internal standardization was performed by using synthetic, unlabeled 9. The signals representing the natural abundance standard appeared as singlets that were offset slightly from the centers of the multiplets of the biosynthetic material because of heavy isotope chemical shift effects (data not shown).
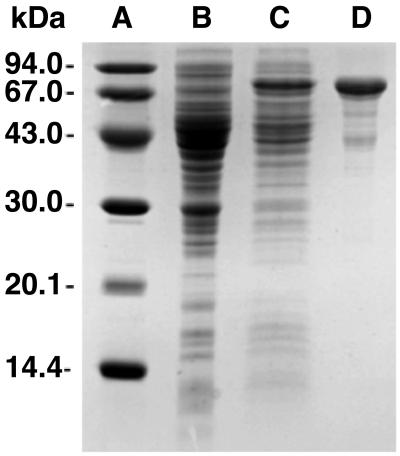
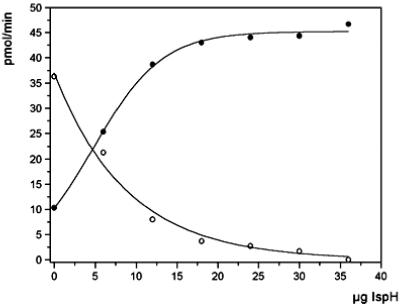
The MalE/IspH fusion protein could be purified from extract of the recombinant E. coli strain by a rapid affinity chromatography procedure using a column of amylose resin (Fig. 4). The purified protein failed to catalyze the conversion of 8 into 9 and/or 10 under a variety of experimental conditions. However, the recombinant protein enhanced the low intrinsic catalytic activity of E. coli wild-type extract up to 4.5-fold. Fig. 5 shows the depletion of [1-3H]8 and the formation of [3-3H]9/10 by mixtures of wild-type crude cell extract and purified MalE/IspH fusion protein.
Fig 4.
SDS polyacrylamide gel electrophoresis. Lane A, molecular mass markers; lane B, crude cell extract of E. coli wild type; lane C, crude cell extract of recombinant E. coli cells expressing MalE/IspH fusion protein; lane D, recombinant MalE/IspH fusion protein after affinity chromatography on amylose.
Fig 5.
Formation of 9/10 from [1-3H]8. Assay mixtures containing crude extract of wild-type E. coli and variable amounts of purified IspH fusion protein as indicated were incubated at 37°C for 30 min. The concentrations of 9/10 and 8 were monitored by reversed-phase HPLC. ○, 8; •, 9/10.
Using [1-3H]8 as substrate, we could show also that isolated chromoplasts of C. annuum catalyze the formation of 9/10 (Fig. 6A). An IPP/DMAPP ratio of ≈6:1 was estimated by the method of Satterwhite (28) as described under Experimental Procedures. The relatively low intrinsic catalytic activity of chromoplasts was increased ≈4-fold by the addition of purified, recombinant IspH protein (Fig. 6B).
Fig 6.
Formation of 9/10 from [1-3H]8. Assay mixtures containing crude C. annuum chromoplasts and/or purified recombinant MalE/IspH fusion protein were prepared as described under Experimental Procedures and incubated at 37°C for 30 min. The concentrations of 9/10 and 8 were monitored by reversed-phase HPLC. (A) C. annuum chromoplasts; (B) C. annuum chromoplasts and purified recombinant MalE/IspH fusion protein.
Discussion
In the present work we have studied the in vitro transformation of 13C- and 3H-labeled 8 into 9 and 10, respectively. The 5:1 IPP/DMAPP ratio observed in this study shows a good match with the one previously reported for the corresponding in vivo system (22). With crude cell extract the reaction required divalent cations, preferably Co2+, as well as FAD and NADH (with NADPH also displaying some activity). The in vitro formation of 9 from 8 was confirmed by NMR spectroscopy using various 13C-labeled samples of 8 as substrate; the sensitivity of the NMR experiment was insufficient to detect the formation of 10.
Purified recombinant IspH protein was unable to catalyze the in vitro transformation of 8 into 9 and 10. However, the recombinant protein increased the low, intrinsic catalytic activity of extract from E. coli wild-type cells up to 4.5-fold. The data suggest that IspH protein requires one or more as-yet-unknown proteins for activity.
Under our assay conditions, the specific activity of recombinant IspH protein (supplemented with crude cell extract) is ≈2 nmol⋅mg−1⋅min−1. The preceding enzymes in the biosynthetic pathway have much higher specific activities ranging from 10 to 500 μmol⋅mg−1⋅min−1 (23). We assume that the low apparent activity of IspH protein reflects suboptimal assay conditions. Alternatively, IspH protein may be the bottleneck of the nonmevalonate isoprenoid pathway.
Earlier we had shown that isolated plant chromoplasts (from red peppers, C. annuum, or from Narcissus pseudonarcissus) can transform 7, the biosynthetic precursor of 8, into the carotenoid precursor, phytoene (31). Not surprisingly, our studies confirm that 9 respectively 10, the precursors of phytoene, can also be generated by C. annuum chromoplasts from 8, the reaction intermediate that immediately follows 7 in the nonmevalonate pathway. The intrinsic catalytic activity of isolated chromoplasts could be enhanced ≈4-fold by the addition of recombinant IspH protein. Again, these findings support the hypothesis that IspH protein requires one or more auxiliary proteins for catalytic activity, possibly as an electron shuttle supplying the reduction equivalents.
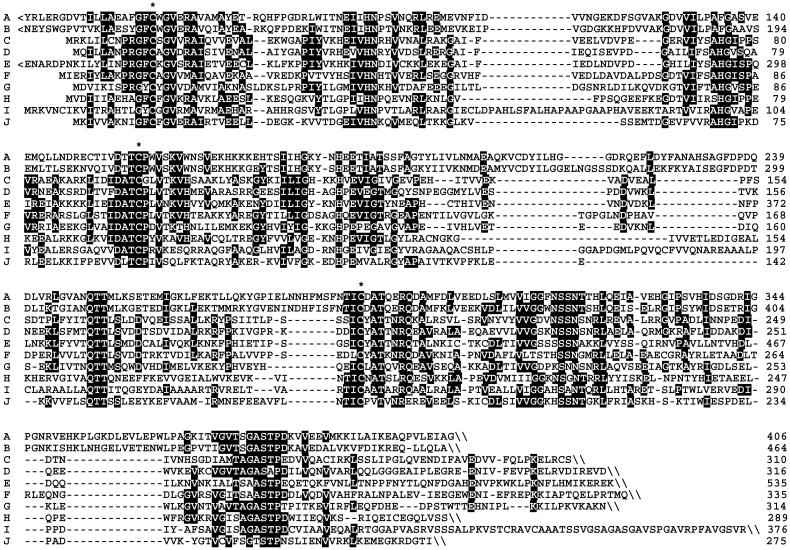
The mechanism of the enzyme-catalyzed formation of 9 and 10 from 8 remains to be established. The two-electron reduction could proceed via ionic or radical intermediates (22). Notably, the predicted amino acid sequences of IspH proteins from a wide variety of species carry three absolutely conserved cysteine residues that may be involved in iron sulfur cluster formation (Fig. 7). Two absolutely conserved histidine residues may be involved in proton-transfer reactions accompanying the reduction and elimination of water.
Fig 7.
Sequence comparison of putative IspH proteins. (A) cyanobacteria (Synechocystis sp.); (B) plants (Adonis aestivalis); (C) Chlamydia group (Chlamydophila pneumoniae); (D) proteobacteria (E. coli); (E) protozoa (Plasmodium falciparum); (F) Deinococcus group (Deinococcus radiodurans); (G) Firmicutes (Bacillus subtilis); (H) Aquificales (Aquifex aeolicus); (I) Spirochaetales (Treponema pallidum); (J) Thermotogales (Thermotoga maritima). Residues identical in more than 50% of the sequences are shown in inversed contrast. <, N-terminal extension; \, C terminus; *, conserved cysteine residues.
Acknowledgments
We thank Fritz Wendling, Katrin Gärtner, and Matthias Lee for skillful assistance and Angelika Werner for expert help with the preparation of the manuscript. We thank the Deutsche Forschungsgemeinschaft, the Fonds der Chemischen Industrie, and the Hans–Fischer Gesellschaft for support. Financial support by Novartis International AG, Basel (to D.A.) is gratefully acknowledged.
Abbreviations
IPP, isopentenyl diphosphate
DMAPP, dimethylallyl diphosphate
References
- 1.Sacchettini J. C. & Poulter, C. D. (1997) Science 277, 1788-1789. [DOI] [PubMed] [Google Scholar]
- 2.Bach T. J. (1995) Lipids 30, 191-202. [DOI] [PubMed] [Google Scholar]
- 3.Bloch K. (1992) Steroids 57, 378-382. [DOI] [PubMed] [Google Scholar]
- 4.Bochar D. A., Friesen, J. A., Stauffacher, C. V. & Rodwell, V. W. (1999) in Comprehensive Natural Product Chemistry, ed. Cane, D. (Pergamon, Oxford), Vol. 2, pp. 15–44. [Google Scholar]
- 5.Qureshi N. & Porter, J. W. (1981) in Biosynthesis of Isoprenoid Compounds, eds. Porter, J. W. & Spurgeon, S. L. (Wiley, New York), Vol. 1, pp. 47–94. [Google Scholar]
- 6.Schwarz M. & Arigoni, D. (1999) in Comprehensive Natural Product Chemistry, ed. Cane, D. (Pergamon, Oxford), Vol. 2, pp. 367–399. [Google Scholar]
- 7.Rohmer M. (1999) in Comprehensive Natural Product Chemistry, ed. Cane, D. (Pergamon, Oxford), Vol. 2, pp. 45–68. [Google Scholar]
- 8.Eisenreich W., Schwarz, M., Cartayade, A., Arigoni, D., Zenk, M. H. & Bacher, A. (1998) Chem. Biol. 5, R221-R233. [DOI] [PubMed] [Google Scholar]
- 9.Sprenger G. A., Schorke, U., Wiegert, T., Grolle, S., de Graaf, A. A., Taylor, S. V., Begley, T. P., Bringer-Meyer, S. & Sahm, H. (1997) Proc. Natl. Acad. Sci. USA 94, 12857-12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lois L. M., Campos, N., Putra, S. R., Danielsen, K., Rohmer, M. & Boronat, A. (1998) Proc. Natl. Acad. Sci. USA 95, 2105-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi S., Kuzuyama, T., Watanabe, H. & Seto, H. (1998) Proc. Natl. Acad. Sci. USA 95, 9879-9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohdich F., Wungsintaweekul, J., Fellermeier, M., Sagner, S., Herz, S., Kis, K., Eisenreich, W., Bacher, A. & Zenk, M. H. (1999) Proc. Natl. Acad. Sci. USA 96, 11758-11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuzuyama T., Takagi, M., Kaneda, K., Dairi, T. & Seto, H. (2000) Tetrahedron Lett. 41, 703-706. [Google Scholar]
- 14.Lüttgen H., Rohdich, F., Herz, S., Wungsintaweekul, J., Hecht, S., Schuhr, C. A., Fellermeier, M., Sagner, S., Zenk, M. H., Bacher, A. & Eisenreich, W. (2000) Proc. Natl. Acad. Sci. USA 97, 1062-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuzuyama T., Takagi, M., Kaneda, K., Watanabe, H., Dairi, T. & Seto, H. (2000) Tetrahedron Lett. 41, 2925-2928. [Google Scholar]
- 16.Herz S., Wungsintaweekul, J., Schuhr, C. A., Hecht, S., Lüttgen, H., Sagner, S., Fellermeier, M., Eisenreich, W., Zenk, M. H., Bacher, A. & Rohdich, F. (2000) Proc. Natl. Acad. Sci. USA 97, 2486-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takagi M., Kuzuyama, T., Kaneda, K., Watanabe, H., Dairi, T. & Seto, H. (2000) Tetrahedron Lett. 41, 3395-3398. [Google Scholar]
- 18.Hecht S., Eisenreich, W., Adam, P., Amslinger, S., Kis, K., Bacher, A., Arigoni, D. & Rohdich, F. (2001) Proc. Natl. Acad. Sci. USA 98, 14837-14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amslinger S., Kis, K., Hecht, S., Adam, P., Rohdich, F., Arigoni, D., Bacher, A. & Eisenreich, W. (2002) J. Org. Chem. 67, 4590-4594. [DOI] [PubMed] [Google Scholar]
- 20.Altincicek B., Kollas, A., Eberl, M., Wiesner, J., Sanderbrand, S., Hintz, M., Beck, F. & Jomaa, H. (2001) FEBS Lett. 499, 37-40. [DOI] [PubMed] [Google Scholar]
- 21.Wolff M., Seemann, M., Grosdemange-Billiard, C., Tritsch, D., Campos, N., Rodriguez-Concepcion, M., Boronat, A. & Rohmer, M. (2002) Tetrahedron Lett. 43, 2555-2559. [Google Scholar]
- 22.Rohdich F., Hecht, S., Gärtner, K., Adam, P., Krieger, C., Amslinger, S., Arigoni, D., Bacher, A. & Eisenreich, W. (2002) Proc. Natl. Acad. Sci. USA 99, 1158-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohdich F., Kis, K., Bacher, A. & Eisenreich, W. (2001) Curr. Opin. Chem. Biol. 5, 535-540. [DOI] [PubMed] [Google Scholar]
- 24.Kuzuyama T., Shimizu, T., Takahashi, S. & Seto, H. (1998) Tetrahedron Lett. 39, 7913-7916. [Google Scholar]
- 25.Jomaa H., Wiesner, J., Sanderbrand, S., Altinicicek, B., Weidemeyer, C., Hintz, M., Türbachova, I., Eberl, M., Zeidler, J., Lichtenthaler, H. K., Soldati, D. & Beck, E. (1999) Science 285, 1573-1576. [DOI] [PubMed] [Google Scholar]
- 26.Hecht S., Kis, K., Eisenreich, W., Amslinger, S., Wungsintaweekul, J., Herz, S., Rohdich, F. & Bacher, A. (2001) J. Org. Chem. 66, 3948-3952. [DOI] [PubMed] [Google Scholar]
- 27.Bullock W. O., Fernandez, J. M. & Short, J. M. (1978) BioTechniques 5, 376-379. [Google Scholar]
- 28.Satterwhite D. M. (1985) Methods Enzymol. 110, 92-99. [DOI] [PubMed] [Google Scholar]
- 29.Camara B. (1985) Methods Enzymol. 110, 267-273. [Google Scholar]
- 30.Dunford J. E., Thompson, K., Coxon, F. P., Luckman, S. P., Hahn, F. M., Poulter, C. D., Ebetino, F. H. & Rogers, M. J. (2001) J. Pharmacol. Exp. Ther. 296, 235-242. [PubMed] [Google Scholar]
- 31.Fellermeier M., Raschke, M., Sagner, S., Wungsintaweekul, J., Schuhr, C. A., Hecht, S., Kis, K., Radykewicz, T., Adam, P., Rohdich, F., et al. (2001) Eur. J. Biochem. 268, 6302-6310. [DOI] [PubMed] [Google Scholar]