Abstract
The dynamics of single-stranded DNA in an α-Hemolysin protein pore was studied at the single-molecule level. The escape time for DNA molecules initially drawn into the pore was measured in the absence of an externally applied electric field. These measurements revealed two well-separated timescales, one of which is surprisingly long (on the order of milliseconds). We characterized the long timescale as being associated with the binding and unbinding of DNA from the pore. We have also found that a transmembrane potential as small as 20 mV strongly biased the escape of DNA from the pore. These experiments have been made possible due to the development of a feedback control system, allowing the rapid modulation of the applied force on individual DNA molecules while inside the pore.
INTRODUCTION
The transport of biopolymers such as DNA, RNA, and polypeptides through protein channels is a ubiquitous process in biology. Gene expression in eukaryotic cells, for example, relies on the passage of mRNA through protein complexes connecting the cell nucleus with the cytoplasm (Alberts et al., 2002). Also, many forms of viral infection rely on the invasion of the plasma membrane followed by import of the viral genome into the cell nucleus through nuclear pore complexes (Kasamatsu and Nakanishi, 1998; Whittaker and Helenius, 1998; Salman et al., 2001). DNA is also known to be translocated through protein channels spanning the bacterial membrane during phage infection (Driselkelmann, 1994). Despite the wide interest in these problems, our understanding of the transport dynamics of biopolymers in pores is limited, and the study of biopolymer dynamics remains an important and fascinating challenge.
The α-Hemolysin (α-HL) protein complex has recently been used as an in vitro model system for the study of DNA and RNA transport through narrow pores (Kasianowicz et al., 1996; Akeson et al., 1999; Meller et al., 2000, 2001). The α-HL protein, secreted by Staphylococcus aureus as a water-soluble monomer, assembles on lipid membranes to form a transmembrane, heptameric channel (Song et al., 1996; Gouax, 1998). The channel conducts ions and can remain open and stable for hours. The channel complex consists of two main parts: a “cap” structure with an internal diameter of ∼30 Å that sits outside the membrane, and a narrow β-barrel region ∼50 Å in length with an internal diameter that varies between 15 Å and 18 Å. This size is comparable with the mean diameter of single-stranded DNA or RNA molecules (Bezrukov, 2000). In a process termed DNA translocation, single-stranded DNA (a negatively charged polymer) can be driven through the channel upon the application of an electric field. This process has been studied by measuring the blockades in the ion current that occur when individual DNA molecules pass through the pore. Recent experiments have suggested that DNA and RNA molecules interact with the α-HL channel and that the dynamics of DNA translocation are sensitive to the magnitude of the applied electric field (Meller et al., 2001; Meller and Branton, 2002). The DNA translocation problem has also been studied theoretically by modeling translocation as a diffusion process across a free energy barrier (Sung and Park, 1996; Muthukumar, 1999; Chuang et al., 2002), or by introducing a simple model potential to describe the DNA-protein interactions in the presence of an electric field (Lubensky and Nelson, 1999).
In this report we present a new experimental approach that allows us to study the dynamics of DNA in the α-HL channel. In particular, we find that this approach is sensitive to interactions that occur between the DNA and the channel proteins. To isolate the biasing effects of the electric field we employed two complementary methods. First, we measured the behavior of DNA in the presence of an electric field by characterizing each translocation event in terms of its duration. Next, we measured the behavior of the DNA at different electric field strengths by altering the transmembrane voltage during the passage of each molecule. For example, in some experiments a DNA molecule was driven into the channel and then the applied voltage was set to zero, allowing the unbiased dynamics of the DNA-channel system to govern the motion of the molecule for a fixed time. Our results suggest that polydeoxyadenine molecules (poly-dA) fall into one of two classes: those exhibiting fast escape dynamics and those that remain in the pore for extended periods of time. We measured the timescales associated with the two classes and we propose arguments as to their origin. Finally, the influence of the electric field was characterized by directly measuring the escape times of molecules in the channel at different electric field strengths.
MATERIALS AND METHODS
Apparatus
The basic apparatus and experimental method used for reconstituting the α-HL channel in a horizontally supported planar lipid bilayer has been described previously (Meller et al., 2001). The temperature of the system was maintained at 15.0 ± 0.1°C unless otherwise mentioned, using a custom enclosure design and a thermoelectric controller (Newport 3040, Irvine, CA). The buffer solution was 1 M KCl, 10 mM Tris-HCl with a pH of 8.5. The α-HL open pore conductance under these conditions was 8.2 × 10−10 siemens in the forward bias. The diameter of the lipid membrane supporting the channel was ∼20 μm, with a typical capacitance of 5 ± 1 pF. PAGE-purified polydeoxyadenine 60-mers (Synthetic Genetics, San Diego, CA) were buffered in 10 mM Tris, 1 mM EDTA, pH 8.5 solution, and used without further modification. The ion current was measured using a patch-clamp amplifier (Axopatch 200B, Axon Instruments, Union City, CA) and the signal was filtered using a 100 kHz low-pass four-pole Butterworth filter (Krohn Hite 3302, Avon, MA). We maintained the 100 kHz bandwidth in our measurements to minimize transient delays associated with low-pass filtering. The signal was digitized at 1 MHz/12 bits using a DAQ card (National Instruments PCI-MIO-16E-1, Austin, TX) installed in a Pentium III-based personal computer. All control and acquisition software was written using National Instruments' LabView.
Our apparatus incorporates a feedback loop used to control the applied transmembrane voltage. The control loop was used for all experiments with the exception of the translocation duration measurements. In the feedback-controlled configuration the analog trigger circuitry of the DAQ card, programmed to activate when the pore current dropped below a specified level, was used to detect DNA molecules entering the pore. When such an event was detected, a series of control voltages were generated by the DAQ card and routed to the patch-clamp amplifier which maintained the transmembrane voltage at a level proportional to the control voltage. The response time of the DAQ card to a change in the pore current was less than 1 μs. We measured the response time of the membrane potential to a step in the control voltage to be 4 ± 1 μs. Fig. 1 is a schematic representation of our setup, showing the control loop.
FIGURE 1.
A schematic diagram of the apparatus illustrating the transmembrane voltage control loop. The ion current passing through the α-HL channel is measured at the headstage and continuously monitored at the PC. When recording DNA translocation events, current blockades are continuously identified in software and saved to disk. In the feedback-controlled mode, the start of the current blockade activates a hardware trigger causing the DAQ card to generate transmembrane voltage control signals. In this manner the external electric field is rapidly switched while the DNA is in the channel.
Translocation measurements
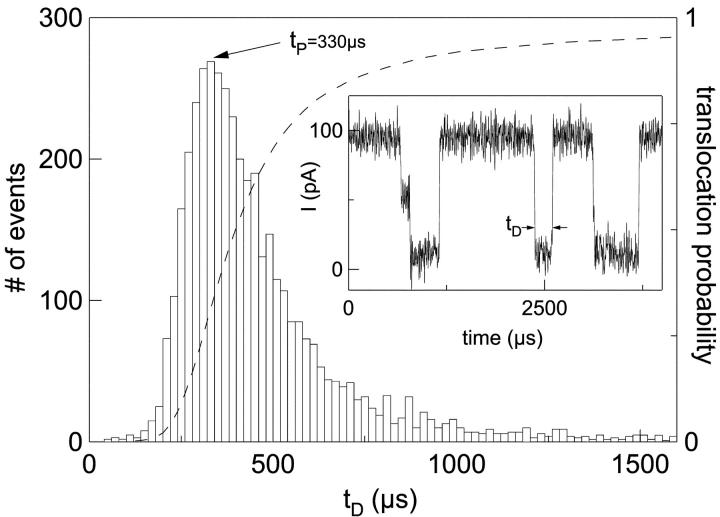
The distribution of translocation durations was measured while holding the voltage bias at a constant level of 120 mV. Blockades in the pore current were continuously identified in software and saved to disk. Low-amplitude blockades (in which the pore current was decreased by less than 80% of the open-pore value) and those with durations less than 50 μs were filtered out because they represent molecules that did not traverse from one side of the pore to the other (Kasianowicz et al., 1996). Recordings of translocation blockades were analyzed and each blockade was characterized in terms of its duration tD, as illustrated in Fig. 2 (inset). The blockade duration was defined as the time during which the ion current remained at the fully blocked state (∼10% of the open pore current level).
FIGURE 2.
Voltage-driven translocations of poly(dA)60 through an α-HL pore, at 15°C and 120 mV. The inset depicts a current trace with three blockade events corresponding to the translocation of three DNA molecules. The event duration, tD, is measured for each event and the translocation duration histogram is constructed from ∼5000 events (main figure). The most probable translocation duration (the peak of the distribution) is denoted as tp. The tail of the distribution extends to very long times (not shown). The dashed line (right axis) is the cumulative probability of translocation as a function of tD.
Feedback-controlled measurements
To study DNA dynamics in the absence of electrical bias, DNA molecules were stalled in the channel by changing the transmembrane voltage during the passage of each molecule. Overlapping current traces for two events from the feedback-controlled experiment are shown in the top panel of Fig. 3, and the corresponding applied voltage is shown in the bottom panel. Initially the transmembrane voltage was set to the driving voltage level (Vdrive) of 120 mV and the ion current through the channel was steady at 100 pA, indicating that the channel was clear. At time t = 0 the ion current dropped sharply to a level below 10 pA (the trigger threshold level), indicating the entry of a DNA molecule into the channel and triggering the generation of the control voltages. At first the applied voltage was maintained at 120 mV for a time duration denoted tdrive, allowing the DNA molecule to be drawn further into the pore. Next the applied voltage was switched to 0 mV and held at this level for a time duration referred to as the bias-free time (denoted toff), during which the DNA either escaped or remained in the channel. The third step was to apply a relatively small voltage across the membrane (40 mV unless otherwise noted) to probe the channel for the presence of DNA. The probe voltage was chosen to be small enough to not facilitate the entry of new molecules into the channel (Meller and Branton, 2002) and at the same time large enough that the resulting ion current was detectable above the noise level. When the probe voltage was applied the ion current exhibited a transient as the amplifier output was briefly overloaded before settling to a level that depended on whether or not the channel was blocked by DNA. This is illustrated by the two current traces that are plotted in the top panel of Fig. 3. In the trace plotted in black the current rose to a level corresponding to the open state of the channel (∼32 pA), indicating that the DNA escaped the channel during toff. The second trace (plotted in gray) is an example of a molecule that remained in the channel during toff. In this case the ion current first settled at a low level corresponding to the blocked state of the channel (∼7 pA) and ∼700 μs later the ion current rose abruptly to the open channel level, signaling the escape of the molecule. After a fixed time the computer reset the transmembrane voltage to 120 mV, ejecting any molecules that remained in the channel. A typical experiment consisted of ∼1000 event recordings similar to those shown in Fig. 3.
FIGURE 3.
Control of the transmembrane voltage after entry of a DNA molecule into the α-HL channel. The top panel shows two current traces corresponding to events in which the molecule left the pore at different times (low pass filtered at 70 kHz for display purposes). At time t = 0 the molecule enters the pore, triggering the generation of a series of steps in the applied transmembrane voltage as shown in the bottom panel. Initially the transmembrane voltage remains at Vdrive (120 mV) for a time tdrive, allowing the DNA to be driven further into the pore. Next, the voltage is switched off (set to 0 mV) for a time toff, during which the molecule's dynamics is not biased by the external electric field. Finally, the occupancy of the channel is probed by applying a relatively low voltage level Vprobe. The black trace corresponds to a molecule that escaped from the pore during the toff period. The current trace plotted in gray corresponds to a molecule which stayed in the pore for a time denoted tstay (see arrow). Events in which the molecule escaped the pore during tdrive were excluded from the analysis.
The current transients due to the resistance and capacitance of our system have been almost fully cancelled from the traces shown in Fig. 3. These transients were minimized using the pipette capacitance compensation circuitry in the Axopatch amplifier (see Axopatch 200B patch clamp operation manual, Axon Instruments, Foster City, CA), which injected a transient current into the headstage input to charge and discharge the system capacitance when the transmembrane voltage was changed. Minimizing the current transients had no effect on our results as was shown by experiments run with and without capacitance compensation.
Data analysis
The event recordings were analyzed to determine the fraction of DNA molecules that escaped from the channel during toff, for a given choice of tdrive and toff. Recordings in which the current returned to the open pore level during tdrive were excluded from the analysis since they represent molecules that exited the pore before the transmembrane voltage was set to zero. The averaged current level in a window 20 μs wide, measured 65 μs after the application of the probe voltage, was calculated for each recording. A histogram of these values for a typical experiment is plotted in Fig. 4. The two peaks of the histogram illustrate that the two possible states of the channel are easily distinguishable. This allows the determination of the fraction of events in which the channel is clear (no DNA present). This value, denoted Pescape (tdrive, toff), is the probability that a molecule of DNA driven into the channel for a duration tdrive has escaped the channel after a bias-free time duration toff. Note that for the purpose of analysis the 65 μs delay between the application of the probe and the actual measurement was treated as part of toff.
FIGURE 4.
A histogram of the pore current levels measured when the probe voltage is applied. The histogram was generated from ∼1000 events similar to those shown in Fig. 3. The pore current was measured 65 μs after the probe voltage was applied (averaged over 20 μs). The two peaks correspond to the two possible states of the pore: “occupied” (by a DNA molecule) or “clear.” The number of events in the upper peak divided by the total number of events is the probability that a DNA molecule will escape the pore during toff, for a given choice of tdrive and toff.
Finally, some event recordings were analyzed to determine the time for which each DNA molecule stayed in the pore after the application of Vprobe. These time durations were denoted tstay and were defined as the time for which the pore current stayed at its lower blocked state during the probing period (see Fig. 3). For these experiments the toff parameter was set to zero so that the applied voltage switched directly from Vdrive to Vprobe.
RESULTS
Translocation measurements
In the first set of measurements we recorded the translocation of roughly 5000 poly(dA)60 molecules at a stationary driving voltage of 120 mV. A histogram of the duration tD of the translocation events is displayed in Fig. 2. This distribution approaches zero for very short times and exhibits a well-defined peak at the most probable translocation duration, denoted tp. We measured tp for poly(dA)60 to be 330 ± 20 μs at 15°C. Note that the translocation duration distribution has an asymmetric shape characterized by a fast rise and a long decaying tail. Also, it was previously found that tp scales linearly with the contour length of the DNA (for DNA molecules longer than 12 nucleotides; see Meller et al., 2001). The dashed line (right axis) in Fig. 2 represents the cumulative probability of translocation for a given tD. This curve was calculated by integrating over the translocation duration histogram and normalizing to unity at infinite times.
We note that many of the translocation events observed exhibit a step-like feature at the leading edge of the event (for example, the leftmost event shown in Fig. 2, inset) during which the current level drops to approximately half of the open-pore level. The duration of the step was not included in the calculation of the translocation duration tD. We associate these steps with the time the DNA spends in the large “cap” structure of the α-HL before entering the narrow part of the channel. We have found that the duration of the steps is not correlated with the translocation duration (Bates et al., unpublished results).
DNA dynamics at zero electric field
The feedback-controlled setup allowed us to investigate the dynamics of DNA in the channel in the absence of an electric field. We measured the number of poly(dA)60 molecules that escaped the pore as a function of time under zero bias conditions. For these measurements we chose tdrive = 200 μs, which is approximately half of the most probable translocation duration (tdrive ≈ 1/2 tp). With this choice of tdrive we expected that the molecules would be threaded through the pore to approximately half their length, such that their initial condition was entropically unfavorable. The probability of escape, Pescape (tdrive, toff), was measured as explained in the Materials and Methods section for toff values ranging from 65 μs to 10065 μs. The results are plotted in Fig. 5.
FIGURE 5.
The DNA escape probability Pescape as a function of toff, with tdrive fixed at 200 μs. Each data point was evaluated from ∼1000 event recordings as illustrated in Figs. 3 and 4. Error bars were calculated by determining the fraction of events in the overlap region between the two peaks in Fig. 4. The solid line is a three-parameter fit to a sum of two exponents. The fast and slow timescales obtained from the fit are 165 ± 10 μs and 3500 ± 250 μs respectively, with a relative weight factor of 0.49 ± 0.01. The multiple data points at the same toff values represent measurements repeated using the same parameters.
For short times, Pescape displays a fast rise with a timescale of 165 ± 10 μs. At longer times we observe a transition to another regime in which Pescape is characterized by a timescale of 3500 ± 250 μs. These timescales were obtained by fitting the data in Fig. 5 to a sum of two exponents using three free parameters (solid line). The relative weight of the two exponents was found to be 0.49 ± 0.01. The appearance of two timescales is surprising because it suggests that we are measuring two populations of molecules distinguished by the rate at which they escape the pore. In the following sections we refer to these populations as the “fast” and the “slow” molecules. One mechanism that could explain the origin of the two populations would be a binding interaction between the DNA and the channel, as discussed below.
DNA escape time distributions
The sensitivity of the DNA dynamics to changes in the transmembrane electric field was studied by recording the time that DNA molecules remained in the channel in the presence of a relatively small field. Specifically, we measured the distributions of tstay (as defined above, see Fig. 3) at different electric field strengths. The initial condition of the molecules was as in the previous experiment (tdrive = 200 μs). Three distributions of tstay, measured at probe voltage levels of 20 mV, 40 mV, and 60 mV, are shown in Fig. 6.
FIGURE 6.

Distributions of the time for which DNA molecules remained in the pore (tstay) at different transmembrane voltage levels. For these experiments tdrive = 200 μs and toff = 0 μs, such that the transmembrane voltage was switched directly from Vdrive to Vprobe 200 μs after the DNA entered the pore. Distributions of tstay were measured at Vprobe levels of 20 mV, 40 mV, and 60 mV. Each of the distributions was approximated by a single exponential decay curve yielding time constants of 1010 μs, 530 μs, and 250 μs respectively. Note that the first bin in each histogram was ignored when calculating the exponential fits (see text).
For the 20 mV and 40 mV histograms we observe a large number of events in the first bin followed by a decaying number of events for longer tstay values. Although the shape of these distributions suggests two characteristic escape timescales, detailed distributions of the short events were not resolvable. Each of the longer escape time distributions were approximated by a single exponential fit, yielding decay constants of 1010 μs, 530 μs, and 250 μs corresponding to the three voltage settings. Even at the lowest probe voltage (20 mV), the timescale for DNA escape was found to be short as compared to the long escape timescale measured at zero voltage (1010 μs versus 3500 μs). Moreover, at 60 mV we observed a decrease by more than an order of magnitude in the characteristic escape time (250 μs versus 3500 μs). These observations demonstrate that the DNA dynamics in the pore are sensitive to small changes in the electric field.
DNA dynamics at varying initial conditions
In a final set of experiments we studied the effect of changing the initial condition of the molecules with respect to the channel. For these experiments toff was held constant at 365 μs and tdrive was varied between 0 μs and 600 μs. For very short tdrive values (in particular  ) we expected that the probability of escape would approach one because only a small fraction of the molecule would be initially threaded into the pore. By similar reasoning, we expected that Pescape would reach a minimum when the molecules were on average threaded halfway through the channel (tdrive ≈ 1/2 tp) and that Pescape would increase for tdrive > 1/2 tp as the molecules' average initial position approached the other side of the membrane.
) we expected that the probability of escape would approach one because only a small fraction of the molecule would be initially threaded into the pore. By similar reasoning, we expected that Pescape would reach a minimum when the molecules were on average threaded halfway through the channel (tdrive ≈ 1/2 tp) and that Pescape would increase for tdrive > 1/2 tp as the molecules' average initial position approached the other side of the membrane.
The escape probability measurements for varying tdrive are shown in Fig. 7. The fraction of molecules that were rejected from the analysis because they exited the pore before toff is plotted in Fig. 7 as a dotted line (right axis). This curve is identical to the cumulative translocation probability shown in Fig. 2. For short tdrive values our data followed the simple prediction discussed above. However, for tdrive > 200 μs our data deviates from the predicted trend. Instead of increasing, Pescape levels off and continues to decrease with a mild slope. In the discussion below we interpret this result in light of the evidence for fast and slow escape timescales presented above (see Fig. 5).
FIGURE 7.
The DNA escape probability Pescape as a function of tdrive, with toff fixed at 365 μs. Error bars were calculated by determining the fraction of events in the overlap region between the two peaks in Fig. 4. At short tdrive values the probability of escape decreased as a function of tdrive, and for longer tdrive values Pescape continued to decrease, but at a much lower rate. The observation that Pescape continued to decrease over the entire range of tdrive values was unexpected (see text). The dotted curve (right axis) is the fraction of molecules that were fully translocated during tdrive and therefore not included in our analysis (this is the same curve as plotted in Fig. 2).
DISCUSSION
The probability to escape from the channel measured at zero electric field yielded two well-separated timescales, differing by a factor of 21 (Fig. 5). This result suggests that after the DNA molecules are introduced into the channel they escape from their unfavorable initial condition by either a fast or a slow trajectory. Since there are no external forces acting on the molecules during the bias free period (toff), it is reasonable to assume that the slow molecules are hindered by some form of interaction between the DNA and the β-barrel part of the channel such as electrostatic attraction or hydrogen bonding (Bezrukov, 2000). Even if these interactions are small in magnitude (on the scale of kBT) they may significantly alter the duration of the escape trajectory if the DNA interacts with the pore at multiple sites along its length.
It was previously found that the most probable translocation duration scales linearly with the DNA contour length (for polynucleotides longer than 12 bases; see Meller et al., 2001), and therefore it is plausible to assume that tdrive determines the extent to which the molecules are on average threaded into the channel. In particular, setting tdrive ≈ 1/2 tp should result in an approximately symmetric partitioning of the polymers between the two sides of the membrane. It is therefore unlikely that asymmetric initial partitioning of the polymers was responsible for the appearance of the two timescales in our experiments. On the other hand, DNA-channel interactions have been previously proposed to affect the translocation dynamics (Meller et al., 2000; Henrickson et al., 2000; Lubensky and Nelson, 1999). Our results reinforce these observations.
In the experiments described above we also studied the influence of the electric field on the escape time of molecules in the channel. It was previously realized that the dependence of the translocation time on the electric field is not linear. In particular, a threshold voltage of 47 mV was predicted below which DNA translocation would not occur (Meller et al., 2001). Our technique enabled us to study the influence of the electric field on DNA at levels below this threshold. We found that the escape time distributions of DNA from the channel were sensitive to small changes in the transmembrane electric field (Fig. 6). For example, changing the probing voltage from 20 mV to 40 mV shortened the observed escape timescale by a factor of two.
Do DNA-protein interactions also account for the trend we observe in Pescape when we vary tdrive (Fig. 7)? In the following paragraphs we interpret the dependence of Pescape on tdrive in light of the fast (165 μs) and slow (3500 μs) escape timescales obtained in Fig. 5. Based on the fast timescale, ∼90% of the fast molecules would have escaped from the pore during the 365-μs bias-free period used in the measurements presented in Fig. 7, regardless of the value of tdrive (we approximate the fraction of molecules that escape by  ). On the other hand, the characteristic 3500 μs escape time of the slow molecules implies that ∼90% of the slow molecules remain in the channel when we probe the pore. Therefore, in this experiment the lifetime of the slow molecules at zero bias is long enough that they remain in the pore after the fast molecules have escaped. In this sense, Pescape in Fig. 7 can be thought of as the fraction of fast molecules in a population that initially includes fast and slow molecules.
). On the other hand, the characteristic 3500 μs escape time of the slow molecules implies that ∼90% of the slow molecules remain in the channel when we probe the pore. Therefore, in this experiment the lifetime of the slow molecules at zero bias is long enough that they remain in the pore after the fast molecules have escaped. In this sense, Pescape in Fig. 7 can be thought of as the fraction of fast molecules in a population that initially includes fast and slow molecules.
Following this line of reasoning, we interpret Fig. 7 in terms of a binding interaction between the DNA and the channel. For short tdrive values, only a small segment of each molecule will be threaded into the channel, restricting possible DNA-protein interactions associated with the slow molecules. As we increase tdrive longer segments of the DNA are threaded into the pore, gradually increasing the probability of binding. Therefore the relative number of slow molecules increases with tdrive and the escape probability decreases correspondingly, as suggested by our data. For tdrive > 200 μs we observe that the dependence of Pescape on tdrive levels off (with a small negative slope). This trend would be expected if the DNA-channel binding and unbinding rates at 120 mV are fast as compared to the timescale of translocation. This is supported by our data: the trend observed in Fig. 6 suggests that the DNA unbinding time at 120 mV is much smaller than 250 μs, whereas the most probable translocation time is 330 μs (Fig. 2). Thus, the ratio of bound to unbound molecules should reach a steady state for some value of tdrive < tp. Our data suggests that this state is reached ∼200 μs after the DNA enters the channel.
Based on our results, we propose a three-state picture that relates the observed timescales to the binding kinetics of the DNA. In our model (shown below) all possible configurations of the DNA in the channel are pooled into one of two states: “bound” configurations in which the DNA is bound to the channel (denoted SB), and “free” configurations in which the DNA is not bound (denoted SF). The clear pore state is represented by O:
 |
Molecules change their states from bound to free and vice versa inside the pore, with the corresponding transition rates kF and kB. The binding and unbinding rates are strongly voltage-dependent, having a much higher value at 120 mV than at zero bias. From the SF state the molecules can irreversibly escape from the pore to the free state O in a process characterized by a diffusion time, τdiff. The time for DNA to bind and unbind from the pore is denoted τreaction and defined as 1/τreaction = kB + kF. The clear separation between the slow and the fast timescales observed in Fig. 5 supports the idea that τdiff is much shorter than τreaction at zero bias, and thus τdiff can be approximated by the fast timescale (165 μs). In this picture, the fast timescale represents molecules that were initially at SF and then diffused out rapidly, without allowing for significant exchange with the bound state. At longer times the situation is different; the rate-limiting step for escaping from the pore is the reaction kinetics. With this assumption, we can approximate τreaction at zero bias by the slow component of the escape probability (3500 μs). This value may be specific to the adenine homopolymers used in this experiment. Note that the transition rates can be viewed as the probabilities of DNA binding or unbinding to the pore per unit time (Colquhoun and Hawkes, 1995), and thus have units of s−1. This definition is derived from the fact that we measure individual DNA-channel complexes, as opposed to measuring binding in bulk.
CONCLUSIONS
The α-HL model system allows us to study the dynamics of polynucleotides threaded through membrane channels. In this paper we have extended the capabilities of the system into a new regime: we have demonstrated that the transport of single DNA molecules can be controlled by varying the transmembrane potential during the passage of the molecule. This approach was used to study the escape of DNA molecules from the channel at vanishing electric field strengths and the biasing role of low electric field intensities on the DNA dynamics.
Interactions have been proposed before as an important component of the DNA translocation process (Meller et al., 2000; Henrickson et al., 2000). The data presented in Figs. 5–7 reinforce these observations. We observed two distinct timescales for DNA escape from the pore: a slow timescale which we associate with molecules that bind to the pore, and a fast timescale which we associate with molecules that escape from the pore without binding. In this manner our experiments allow us to decouple the diffusion dynamics of the DNA molecules from their binding kinetics. In the translocation experiments we believe that these timescales overlap due to the constant application of the electric field, yielding a complicated translocation duration histogram (e.g., Fig. 2). Finally, the characterization of the escape time distribution may serve as a method to evaluate the DNA-protein interactions, which were found to be very sensitive to any applied potential.
The implementation of our technique is general and it could be used to extend the scope of DNA hairpin-melting experiments (Vercoutere et al., 2001) and DNA hybridization experiments that make use of modified α-HL pores (Howorka et al., 2001). Future experiments will enable us to improve our understanding of DNA dynamics in the pore by experimenting with different DNA lengths and sequences, and to investigate alternative DNA-channel interaction mechanisms.
Acknowledgments
We thank K. Sharpe for her careful reading and comments on the manuscript. M.B. thanks Y. Nochomovitz for useful discussions. A.M. acknowledges stimulating discussions with Drs. D. Branton, S. Safran, Y. Rabin, and Y. Klafter.
We acknowledge support from members and staff at the Rowland Institute at Harvard.
References
- Akeson, M., D. Branton, J. Kasianowicz, E. Brandin, and D. Deamer. 1999. Microsecond time-scale discrimination among polycytidylic acid, polyadenylic acid, and polyuridylic acid as homopolymers or as segments within single RNA molecules. Biophys. J. 77:3227–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts, B., A. Johnson, J. Lewis, M. Raff, K. Roberts, and P. Walter. 2002. Molecular Biology of the Cell. Garland Publishing, New York.
- Bezrukov, S. M. 2000. Ion channels as molecular Coulter counters to probe metabolite transport. J. Mem. Biol. 174:1–13. [DOI] [PubMed] [Google Scholar]
- Chuang, J., Y. Kantor, and M. Kardar. 2002. Anomalous dynamics of translocation. Phys. Rev. E. 65:011802. [DOI] [PubMed] [Google Scholar]
- Colquhoun, D., and A. G. Hawkes. 1995. The principles of the stochastic interpretation of ion-channel mechanics. In Single-Channel Recording. B. Sakmann and E. Neher, editors. Plenum Press, New York.
- Driselkelmann, B. 1994. Translocation of DNA across bacterial membranes. Microbiol. Rev. 58:293–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouax, J. E. 1998. α-Hemolysin from Staphylococcus aureus: an archetype of β-barrel, channel-forming toxins. J. Struct. Biol. 121:110–122. [DOI] [PubMed] [Google Scholar]
- Henrickson, S. E., M. Misakian, B. Robertson, and J. J. Kasianowicz. 2000. Driven DNA transport into an asymmetric nanometer scale pore. Phys. Rev. Lett. 85:3057–3060. [DOI] [PubMed] [Google Scholar]
- Howorka, S., L. Movileanu, O. Braha, and H. Bayley. 2001. Kinetics of duplex formation for individual DNA strands within a single protein nanopore. Proc. Natl. Acad. Sci. USA. 98:12996–13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu, H., and A. Nakanishi. 1998. How do animal DNA viruses get to the nucleus? Annu. Rev. Microbiol. 52:627–686. [DOI] [PubMed] [Google Scholar]
- Kasianowicz, J., E. Brandin, D. Branton, and D. Deamer. 1996. Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl. Acad. Sci. USA. 93:13770–13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubensky, D. K., and D. R. Nelson. 1999. Driven polymer translocation through a narrow pore. Biophys. J. 77:1824–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller, A., and D. Branton. 2002. Single molecule measurements of DNA transport through a nanopore. Electrophoresis. 23:2583–2591. [DOI] [PubMed] [Google Scholar]
- Meller, A., L. Nivon, E. Brandin, J. Golovchenko, and D. Branton. 2000. Rapid nanopore discrimination between single polynucleotide molecules. Proc. Natl. Acad. Sci. USA. 97:1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller, A., L. Nivon, and D. Branton. 2001. Voltage-driven DNA translocations through a nanopore. Phys. Rev. Lett. 86:3435–3438. [DOI] [PubMed] [Google Scholar]
- Muthukumar, M. 1999. Polymer translocation through a hole. J. Chem. Phys. 111:10371–10374. [Google Scholar]
- Salman, H., D. Zbaida, Y. Rabin, D. Chatenay, and M. Elbaum. 2001. Kinetics and mechanism of DNA uptake into the cell nucleus. Proc. Natl. Acad. Sci. USA. 98:7247–7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, L., M. R. Hobaugh, C. Shustak, S. Cheley, H. Bayley, and J. E. Gouax. 1996. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science. 274:1859–1865. [DOI] [PubMed] [Google Scholar]
- Sung, W., and P. J. Park. 1996. Polymer translocation through a pore in a membrane. Phys. Rev. Lett. 77:783–786. [DOI] [PubMed] [Google Scholar]
- Vercoutere, W., S. Winters-Hilt, H. Olsen, D. Deamer, D. Haussler, and M. Akeson. 2001. Rapid discrimination among individual DNA hairpin molecules at single-nucleotide resolution using an ion channel. Nat. Biotech. 19:248–252. [DOI] [PubMed] [Google Scholar]
- Whittaker, G. R., and A. Helenius. 1998. Nuclear import and export of viruses and virus genomes. Virology. 246:1–23. [DOI] [PubMed] [Google Scholar]