Abstract
The antigenically variant major surface protein 2 (MSP2) of Anaplasma marginale is expressed from a 3.5-kb operon that contains, in a 5′-to-3′ direction, four open reading frames, opag3, opag2, opag1, and msp2. This operon structure was shown to be conserved among genotypically and phenotypically distinct A. marginale, A. ovis, and A. centrale strains. The individual OpAG amino acid sequences are highly conserved among A. marginale strains, with identities ranging from 95 to 99%. OpAG2 and OpAG3 were expressed by all examined A. marginale strains during the acute rickettsemia in the mammalian host and, like MSP2, localize to the bacterial surface. OpAG2 and OpAG3 were also expressed in an infected Ixodes scapularis tick cell line. In contrast, the same A. marginale strains expressed only OpAG2 in two different Dermacentor spp. during transmission feeding. OpAG1 expression was not detected in the infected mammalian host, the infected tick cell line, or within infected Dermacentor ticks. The differential expression of outer membrane proteins from within an operon is a novel finding in tick-transmitted bacteria, and the regulation of expression may be broadly applicable to understanding how the pathogen adapts to the mammalian host-tick vector transition.
Pathogens in the genera Anaplasma and Ehrlichia establish persistent infection in the mammalian reservoir host that provides a constant source of organisms for tick transmission. Persistent Anaplasma marginale infection within the bovine reservoir host is characterized by rickettsemic cycles, ranging from 102 to 107 bacteria/ml of blood (14). Each rickettsemic cycle is associated with the emergence of new antigenic variants of the immunodominant A. marginale major surface protein 2 (MSP2) (13). Variation is achieved by gene conversion of the single, full-length expressed copy of msp2 utilizing oligonucleotide blocks of at least nine truncated msp2 pseudogenes (6, 7). This combinatorial mechanism provides the requisite number of expressed MSP2 variants sufficient for lifelong persistence (7). Whereas msp2 pseudogenes are widely dispersed over the bacterial chromosome, the single, full-length copy of msp2 is located within an operon containing three additional open reading frames upstream of msp2 (4, 6, 24)). Analysis with the PSORT algorithm predicts that these open reading frames encode outer membrane proteins (4), as predicted and experimentally verified for MSP2 (24). The msp2 operon-associated gene 1 (opag1) directly upstream of msp2 does not resemble any sequences in the databases, whereas predicted proteins encoded by the other two open reading frames, opag2 and opag3, are similar to the outer membrane protein families OMP1 and p28 of Ehrlichia chaffeensis and E. canis (4, 17, 23).
The operon linked expression of the three putative outer membrane proteins with MSP2 would be consistent with the functional and structural relatedness of proteins expressed from bacterial operons. The operon is the only source for transcription of full-length msp2 and, correspondingly, msp2-operon transcripts and MSP2 protein are detectable in blood stages of acutely and persistently infected cattle (4). In addition, operon transcripts are also detected in infected ticks and, by using the South Idaho strain of A. marginale and the Reynolds Creek strain of Dermacentor andersoni, it has been shown that MSP2 protein is expressed from the operon (16). Thus, the operon serves as the msp2 expression site in all biologically important stages of A. marginale. However, it remains unknown whether the other open reading frames of the polycistronic operon message—opag1, opag2, and opag3—are expressed as outer membrane proteins and whether this expression is uniform in both the mammalian and tick stages of A. marginale.
If the msp2 operon-associated genes are expressed on the bacterial surface, the degree of conservation among A. marginale strains emerges as an important question. Immunization with purified outer membranes induces protection from disease upon challenge with A. marginale (9, 32). Notably, outer membrane proteins conserved among A. marginale strains are targets for CD4+ T lymphocytes derived from protected vaccinates (10). As a result, identification of highly conserved outer membrane proteins is a priority for vaccine development. In the research presented here, expression of the msp2 operon-associated genes was examined in mammalian and tick stages of A. marginale, and it was determined whether these proteins are expressed on the bacterial surface. Furthermore, the degree of conservation of the proteins encoded by the msp2 operon-associated genes was analyzed by using genotypically and phenotypically distinct A. marginale strains.
MATERIALS AND METHODS
Expression of msp2 operon-associated genes. (i) Expression of recombinant proteins.
To obtain antigen to be used as positive controls in Western blot analysis and for generation of specific antibody in mice, OpAGs were expressed as His-tagged fusion proteins in Escherichia coli by using the pET19b expression system (Novagen). Inserts containing the full-length genes of OpAG1, OpAG2, and OpAG3 of the South Idaho strain of A. marginale were generated by subcloning from full-length clones obtained in pCR4Blunt-TOPO (Invitrogen), as previously reported (4). NdeI restriction enzyme recognition sites were introduced by using insert-specific forward primers OpAG15′exp, OpAG2 5′exp, and OpAG3 5′exp (Table 1). M13Rev located downstream of the cloning site of the pCR-Blunt vector was used as reverse primer in all reactions (Inivtrogen). The PCR cycling conditions were 30 cycles of melting at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 45 s. The amplicons and the expression vector pET19b (Novagen) were double digested consecutively with NdeI and BamHI for 1.5 h each at 37°C, and the vector was subsequently treated with calf intestinal alkaline phosphatase (Roche). The vector and amplicons were gel purified by using the Bio-Rad Prep A Gene purification kit. Ligation was performed at room temperature overnight with T4 DNA ligase (Promega), followed by transformation of BL21(DE3)pLysS cells (Novagen). Correct orientation of the inserts and the reading frames was confirmed by sequencing of purified vector with the T7 promoter and terminator primers supplied by the manufacturer. A single clone of each was selected and subsequently used for protein expression. Freshly grown cultures of transformed bacteria were induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and harvested after 3 h by centrifugation. Bacterial lysates for use as positive controls in Western blots were mixed with sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) buffer and stored at −20°C. Recombinantly expressed OpAG1 was purified for the generation of specific antibodies in mice. Protein was isolated from the insoluble whole-cell extract fraction of bacterial lysates under denaturing conditions by using the His-Bind kit (Novagen). Purified protein was allowed to refold by dialysis into phosphate-buffered saline (PBS; pH 7.0) by using Slide-A-Lyzer cassettes (Pierce). Purity and specificity were determined by Western blot with the peptide antiserum to OpAG1 described below. Immunizations of mice with the purified protein were performed as described below for conjugated peptides.
TABLE 1.
Synthetic oligonucleotides used for amplification and sequencinga
| Oligonucleotide | Locationb | Sequence (5′ to 3′) |
|---|---|---|
| F0 | −115-−135 | ACG CGC TTG AAT AAA TCG TT |
| F1 | −2-27 | GTA TGT CGA TTC GCG GAA GAG CCT GTT GT |
| F2 | 401-420 | TAT GCC GTG AGC GGC AAC GC |
| F3 | 974-993 | CAA TGT TGG TAG CGC CGC AC |
| F4 | 1392-1413 | ACA CTC AGC GGG TTG TTT TTG |
| F5 | 1819-1839 | TTG CAG AGC TTT TTC CTT GAA |
| F6 | 2258-2277 | GTG TTG ATG GCT CTG GCT GC |
| F7 | 2593-2613 | TGG AGG AGC AAG GGT TGA AGT |
| R0 | 3159-3140 | GGA ACA ACC CCA ATA CCA TC |
| R1 | 3112-3095 | CAT TAC AGA AGT AGA CCC |
| R2 | 2613-2593 | ACT TCA ACC CTT GCT CCT CCA |
| R3 | 2123-2102 | TTG GAC TTG GCT GCA GTG CAG C |
| R4 | 1537-1519 | ATG TCA AGG AAG TCC ACG C |
| R5 | 1030-1010 | GCC GCT ACA TAG AGC TCA CCC |
| R6 | 521-501 | GCG CTA AGC TCG ACG GAG TTC |
| F2Ao | 456-475 | GGT GGT AGT CAT ATC GTG GG |
| F4Ao | 1508-1529 | TTA GCG TTT GTC ATG ACC TCC C |
| R4Ao | 1529-1508 | GGG AGG TCA TGA CAA ACG CTA A |
| R5Ao | 750-731 | CCT CAA CAA ACA GCG AGA GC |
| MSP2 startc | 1-20 | ATG AGT GCT GTA AGT AAT AG |
| MSP2 endc | 1230-1211 | CTA GAA GGC AAA CCT AAC AC |
| OpAG3 5′expd | 1-13 | GGA TTC CCA TAT GTC GAT TCG CG |
| OpAG2 5′expd | 893-911 | GGA TTC CCA TAT GAG TCG TAA AAG TCT G |
| OpAG1 5′expd | 1814-1830 | GGA TTC CCA TAT GGA TTG CAG AGC AT |
For the orientation of the oligonucleotides, see Fig. 1.
Numbering is based on the published sequence of the msp2 operon of the A. marginale Florida strain (4). Numbering of the primers with the designation “Ao” is based on the nucleotide sequence of the msp2 operon of A. ovis (GenBank accession no. AY132309).
Underlined sequences indicate the NdeI restriction enzyme recognition site in the primers used for subcloning of the opag genes (see Materials and Methods).
(ii) Generation of anti-msp2 OpAG1, -2, and -3 sera.
Specific antisera against the predicted proteins encoded by the msp2 operon-associated genes were generated in BALB/c mice by immunizations with synthetic peptides or recombinantly expressed OpAG1 (see above). The designations and sequences were as follows: OpAG1-1 (CPSLLRVGGEASGQQ), OpAG1-2 (CSGGGFHAAGGASATR), OpAG2 (CREFAVRENRLTAPSK), OpAG3-1 (CPTRDGFGAHYLPKYENS), and OpAG3-2 (CGTDTEEHAGGGPTLLSTTSSGVPSVDAD). An amino-terminal cysteine was included for conjugation, and 2 mg of each peptide was cross-linked to maleimide-activated keyhole limpet hemocyanin by using the Imject maleimide activated immunogen conjugation kit (Pierce) according to the manufacturer's recommendations. Each conjugated peptide or the recombinant OpAG1 was used to immunize three mice subcutaneously with 100 μg of antigen emulsified with complete Freund’s adjuvant. The mice were boosted by using four immunizations of 100 μg of antigen in incomplete Freund’s adjuvant.
(iii) Western blots.
The expression of OpAG1, OpAG2, and OpAG3 in biologically important stages of A. marginale was examined by Western blot analyses. Expression during mammalian infection was determined with blood obtained from cattle during the acute peak rickettsemia after inoculation with the Florida, South Idaho, Washington-Clarkston, Washington-Okanogan, or Virginia strains of A. marginale. The origin and characterization of the A. marginale strains have been previously reported (1, 18, 26). To examine expression of the OpAGs in the tick vector, A. marginale Virginia strain-infected adult D. andersoni and D. variabilis ticks and A. marginale South Idaho strain infected adult D. andersoni ticks were transmission fed for 3 days on uninfected, susceptible calves (3, 16). These transmission-fed ticks were dissected, and isolated salivary glands and midguts were collected in proteinase inhibition buffer (50 mM Tris [pH 8.0], 5 mM EDTA, 5 mM iodoacetamide, 0.1 mM N-α-p-tosyl-l-lysine chloromethyl ketone, and 1 mM phenylmethylsulfonyl fluoride). OpAG expression was also examined in the continuous Ixodes scapularis embryonal tick cell line IDE8 (19), which was infected with the Virginia strain of A. marginale and maintained at 34°C, as previously reported (3). Sonicated pellets of infected erythrocytes, salivary glands, and midguts from infected Dermacentor ticks and infected IDE8 cells were mixed with SDS-PAGE buffer. The number of A. marginale in each sample was normalized by using MSP5 levels, as previously described (16, 29). Electrophoresis of protein samples was carried out on precast SDS-containing 4 to 20% polyacrylamide gels (Bio-Rad) for 30 min at 200 V. After transfer to nitrocellulose, proteins were detected with the antisera described above by using the Western-Star chemiluminescence immunoblot detection system (Tropix) according to the manufacturer's instructions. Erythrocytes from an uninfected calf and salivary glands and midguts from uninfected, transmission-fed D. andersoni and D. variabilis, as well as uninfected IDE8 cells, were handled identically and served as negative antigen controls. Preimmunization mouse serum served as a negative serum control.
(iv) Agglutination assay.
Surface expression of the OpAG proteins was examined by an agglutination assay with A. marginale isolated from infected erythrocytes, as previously described (24). Briefly, a stabilate of Florida strain A. marginale maintained in liquid nitrogen was thawed and washed five times in PBS at 30,000 × g for 20 min to remove hemoglobin. The cell pellet was sonicated for 4 min at 50% power on ice, followed by two washes in PBS at 1,650 × g for 15 min to pellet the intact A. marginale. The organisms were treated with 6-carboxy fluorescein diacetate, a vital dye that labels organisms with intact outer membranes. The labeled A. marginale was incubated with individual mouse sera specific for OpAG1, OpAG2, andOpAG3 at dilutions of 1:10, 1:30, and 1:100. After incubation for 30 min at room temperature, agglutination of labeled organisms was identified by using UV microscopy as previously described (24). Preimmunization mouse serum and mouse serum against recombinant His-tagged Babesia bovis RAP1b were used as negative controls at the same dilutions. Serum from a calf infected with A. marginale served as a positive serum control.
Sequencing of Anaplasma genomic DNA and sequence analysis.
The full-length msp2 operon was sequenced using genomic DNA isolated from stabilates of the Washington-Clarkston, Washington-Okanogan, and Virginia strains of A. marginale, the Israel vaccine strain of A. centrale, and the Dubois (Idaho) strain of A. ovis (18, 20, 27). Each stabilate was thawed and washed five times in PBS at 30,000 × g. According to the manufacturer's instructions, cell pellets were collected in cell lysis buffer and digested with proteinase K overnight at 37°C, followed by incubation at 65°C for 2 h; DNA was then isolated by using the Puregene DNA isolation kit (Gentra Systems). DNA pellets were reconstituted in nuclease-free water. To amplify the full-length operon, the Elongase enzyme mix (Gibco-BRL) with buffer B (2 mM MgSO4, final concentration) and the primers F1 and R0 were used (Fig. 1 and Table 1). The PCR cycling conditions were 40 cycles of melting at 95°C for 15 s, annealing at 55°C for 15 s, and extension at 72°C for 1 min. Amplification products were detected by electrophoresis in 1% agarose gels containing ethidium bromide. To confirm sequences obtained from full-length products, shorter fragments of the operon were amplified by using PCR Master Mix (Roche) and 35 cycles of melting at 95°C for 15 s, annealing at 55°C for 15 s, and extension at 72°C for 45 s. To amplify the promoter and 5′ opag3 region, the primers F0 and R6 were used. Amplification of the 3′ terminus of msp2 utilized primers “MSP2 start” and “MSP2 end” (14). PCR products longer than 1.5 kb were gel purified and cloned using the TOPO XL PCR cloning kit (Invitrogen). Smaller fragments were gel purified with the Promega PCR purification kit and either blunt ended with Pfu polymerase (Stratagene) and cloned with the Zero-blunt TOPO PCR cloning kit for sequencing (Invitrogen) or directly cloned with the TOPO TA cloning kit for sequencing (Invitrogen). Inserts of a single clone from each reaction were sequenced at least once in both directions with the Big Dye kit and an ABI PRISM automated sequencer (PE-Applied Biosystems) using the synthetic oligonucleotides shown in Fig. 1 and Table 1. To confirm data obtained for the 5′ end of opag2 in A. ovis, amplification and cloning of the full-length operon was performed three times, followed by sequencing of the relevant region. Based on nucleotide sequence differences in the msp2 operon-associated genes of A. ovis, the primers F2, F4, R4, and R5 were not suitable for sequencing and the primers F2Ao, F4Ao, R4Ao, and R5Ao were designed to fill the sequencing gaps (Fig. 1 and Table 1). Sequences were compiled and analyzed by using the VECTOR NTI software package (InforMAX). The GenBank accession numbers for the six A. marginale strains (Florida, South Idaho, St. Maries [Idaho], Washington-Clarkston, Washington-Okanogan, and Virginia) are AY132308 and AY132310 to AY132314. The GenBank accession numbers for the A. centrale Israel vaccine strain and the A. ovis Dubois (Idaho) strain are, respectively, AY132307 and AY132309.
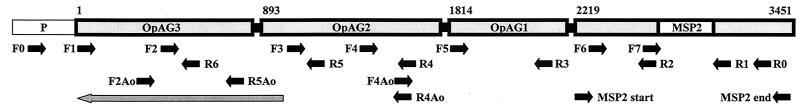
FIG. 1.
Schematic representation of the msp2 operon of A. marginale. The operon contains a 5′ promoter region (P), which is followed by three open reading frames encoding the msp2 operon-associated proteins opag3, opag2, and opag1 and ends with the full-length copy encoding MSP2 (msp2). The clear box in msp2 corresponds to the central hypervariable region of the protein (14). The numbers above the schematic designate the nucleotide position, based on the sequence of the msp2 operon in the Florida strain of A. marginale (4; GenBank accession no. AF200925), and also indicate the beginning of each open reading frame. The short black arrows under the schematic represent primers that were used for the amplification and sequencing of the operon. The sequences of the primers and their location are given in Table 1. The long gray arrow under the schematic represents the open reading frame that overlaps with and is in reverse orientation to opag3.
RESULTS
Expression of msp2 operon-associated genes in the mammalian host.
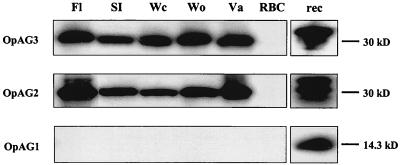
The msp2 operon of A. marginale is transcribed into a polycistronic, full-length mRNA and results in MSP2 protein expression (4, 16). Expression of the msp2 operon-associated genes was examined by Western blot analysis on blood obtained during the acute peak rickettsemia of cattle infected with genotypically and phenotypically distinct A. marginale strains. OpAG2 and OpAG3 were detected in all five examined strains by using specific antipeptide sera at dilutions of 1:500, but not in uninfected bovine erythrocytes (Fig. 2). In contrast, OpAG1 was not detected in any of the strains (Fig. 2), even when the amount of antigen was increased sixfold and the specific antipeptide serum was used at 1:25. This OpAG1-specific antiserum readily detected recombinant OpAG1 protein in bacterial lysates when used at a dilution of 1:500 (Fig. 2). OpAG1 protein was also not detected in any of the A. marginale strains in Western blots by using specific antiserum raised against recombinant expressed, purified full-length OpAG1 (data not shown).
FIG. 2.
Expression of the msp2 operon-associated proteins in erythrocytes infected with genotypically and phenotypically distinct A. marginale strains. Western blot analyses were carried out with specific mouse antisera against the OpAG given in the left margin. The blood samples were obtained during the acute peak rickettsemia of calves infected with the A. marginale strain listed above the panel: Florida (Fl), South Idaho (Si), Washington-Clarkston (Wc), Washington-Okanogan (Wo), or Virginia (Va). Uninfected erythrocytes (RBC) served as a negative control. Recombinant expressed OpAG proteins (rec) were used as positive controls. Sera were used at the following dilutions: antiserum against OpAG1 at 1:25 (infected and uninfected erythrocytes) or 1:500 (recombinant protein) and antisera against OpAG2 and OpAG3 at 1:500. The molecular size markers are indicated in the right margin in kilodaltons.
Using the PSORT prediction for protein localization sites (http://psort.nibb.ac.jp/cgi-bin), all OpAGs were predicted to localize either to the bacterial outer membrane or to the bacterial periplasmic space. Expression of the OpAGs on the bacterial surface was examined by agglutination assay using the Florida strain of A. marginale purified from infected erythrocytes (24, 25). Bacterial membranes were intact, as indicated by uptake and retention of the vital stain 6-carboxy fluorescein diacetate into A. marginale. The percentage of fluorescent bacteria agglutinated was recorded by using quadripartite scoring as follows: >75% of the bacteria agglutinated, >50% agglutinated, >25% agglutinated, and no detectable agglutination. Mouse antisera to the OpAGs were used at three dilutions, 1:10, 1:30, and 1:100. At 1:10 and 1:30, antisera to either OpAG2 or OpAG3 agglutinated A. marginale (Fig. 3). Weak agglutination was observed when antiserum to OpAG3 was diluted to 1:100, and no agglutination occurred with a 1:100 dilution of antiserum to OpAG2. No agglutination was observed with antisera to OpAG1 at any of the dilutions, a finding consistent with the lack of expression as analyzed by using Western blots. Mouse antiserum against His-tagged B. bovis RAP1 (Fig. 3) and preimmunization mouse serum (data not shown) did not agglutinate A. marginale at any dilution. The positive control antiserum from an A. marginale-infected calf, agglutinated A. marginale at all three dilutions: 1:10, 1:30, and 1:100 (Fig. 3).
FIG. 3.
Surface expression of the msp2 operon-associated proteins. Expression of the OpAGs on the bacterial surface was examined by agglutination assay using the Florida strain of A. marginale labeled with the vital dye 6-carboxy fluorescein diacetate (24). The percentage of fluorescent bacteria agglutinated was recorded by using quadripartite scoring as follows: >75% of the bacteria agglutinated, >50% agglutinated, >25% agglutinated, and no detectable agglutination (0%). Antisera to the OpAGs, positive control antiserum to A. marginale, and the negative control antiserum to B. bovis RAP1 were tested at three dilutions: 1:10, 1:30, and 1:100.
Expression of msp2 operon-associated genes in tick tissues and tick cell culture.
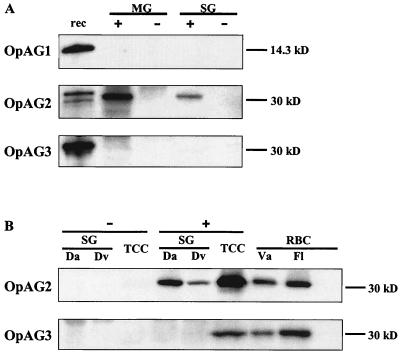
Western blot analyses were performed to examine the expression of msp2 operon-associated genes of A. marginale in Dermacentor ticks and in the I. scapularis embryonic tick cell line IDE8 (19). The salivary glands and midguts of transmission-fed adult D. andersoni ticks infected with the South Idaho strain and the salivary glands of transmission-fed adult D. andersoni and D. variabilis ticks infected with the Virginia strain were tested. OpAG2 was detected in the salivary glands of Virginia strain-infected D. variabilis, in the salivary glands of D. andersoni infected with the Virginia or South Idaho strain, and in the midguts of D. andersoni infected with the South Idaho strain with the specific antiserum at a dilution of 1:500 (Fig. 4). OpAG2 was not detected in tissues from uninfected, sham transmission-fed ticks (Fig. 4). OpAG1 and OpAG3 were not detectable in the salivary glands or midguts from infected or uninfected transmission fed Dermacentor ticks (Fig. 4), although antisera readily detected the recombinant expressed proteins OpAG1 and OpAG2 (Fig. 4A). Repeating the Western blots with sixfold-increased amounts of A. marginale-infected tick tissues and specific antisera against OpAG1 or OpAG3 at a dilution of 1:25 did not result in detectable bands (data not shown). In contrast, both OpAG2 and OpAG3 were detectable in the Virginia strain-infected IDE8 cells with specific antisera used at a dilution of 1:500. OpAG1 was not detected in infected tick cell cultures, and none of the OpAG proteins were detected in uninfected tick cell cultures.
FIG. 4.
Expression of the msp2 operon-associated proteins in Dermacentor ticks and the I. scapularis tick cell line IDE8. Western blots were performed with the specific mouse antisera against the OpAG indicated in the left margins. (A) Midguts (MG) and salivary glands (SG) of infected (+) and uninfected (−) transmission-fed D. andersoni ticks were examined. Recombinant expressed OpAG proteins (rec) were used as positive controls. (B) A. marginale Virginia strain-infected (+) and uninfected (−) salivary glands (SG) of D. andersoni (Da) and D. variabilis (Dv) ticks and the infected and uninfected permanent, embryonal I. scapularis tick cell line IDE8 (TCC) were analyzed. Erythrocytes (RBC) infected with the Florida (Fl) or Virginia (Va) strains of A. marginale were used as positive controls, whereas uninfected erythrocytes (−) served as a negative control. Antiserum against OpAG1 was used at 1:250 and antisera against OpAG2 and OpAG3 at a dilution of 1:500. The molecular size markers are given in the right margins in kilodaltons.
Conservation of OpAGs among A. marginale strains.
The DNA sequences of the full-length msp2 operon, including the 5′ promoter region previously identified (4), were obtained for the Virginia, Washington-Clarkston, and Washington-Okanogan strains of A. marginale. Predicted amino acid sequences for the individual OpAGs of these strains were aligned with those derived from the published sequences of the Florida and South Idaho strains (4). All proteins were highly conserved among these five phenotypically and genotypically distinct A. marginale strains. The amino acid sequence identities among the five strains were 95.1, 99, and 97.7% for OpAG3, OpAG2, and OpAG1, respectively.
Conservation of OpAGs among A. marginale, A. centrale, and A. ovis.
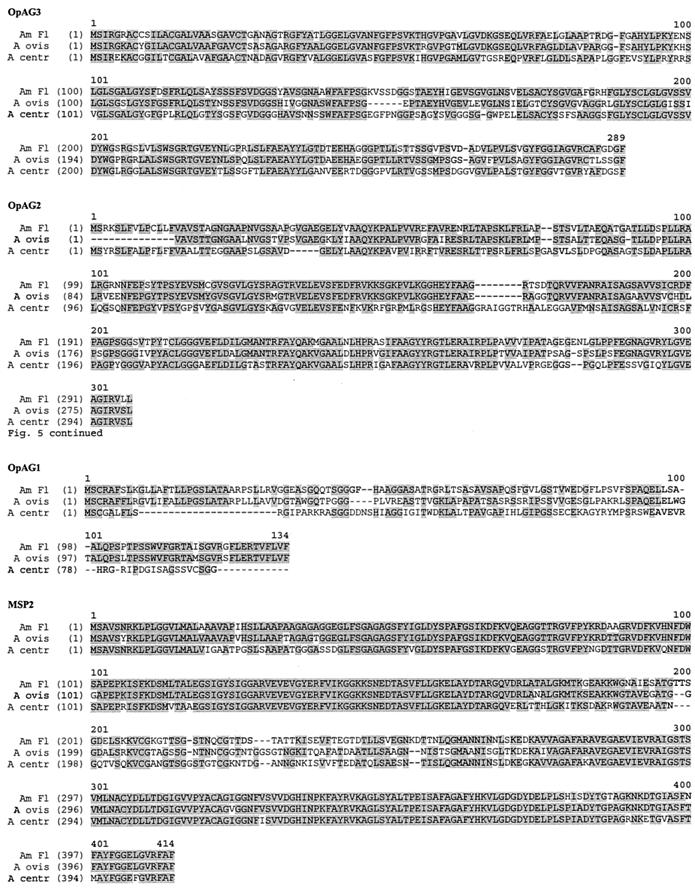
Conservation of the OpAGs was examined in A. centrale and A. ovis, which are closely related organisms that also invade and replicate in erythrocytes. Full-length msp2 operon sequences were obtained by using blood stages of the Israel vaccine strain of A. centrale and the Dubois (Idaho) strain of A. ovis. The genomic organization of the msp2 operon (shown in Fig. 1 for A. marginale), including the positioning of the genes and the intergenic spacing of the OpAGs, is conserved in A. centrale and A. ovis. The predicted amino acid sequences for the OpAGs and MSP2 of A. centrale and A. ovis were aligned with those of the Florida strain of A. marginale and are depicted in Fig. 5. The degree of conservation of the OpAGs among A. ovis, A. centrale, and A. marginale is markedly lower than that among A. marginale strains. Compared to the A. marginale Florida strain, amino acid sequence identities for OpAG2 and OpAG3 are 78.1 and 76.7%, respectively, for A. ovis and 62.2 and 62.3%, respectively, for A. centrale. The length of OpAG3 is relatively conserved between A. marginale, A. centrale, and A. ovis, as are the start and stop codons. OpAG2 of A. ovis is 16 amino acids shorter than in A. marginale and A. centrale, primarily because of an N-terminal truncation. The start ATG predicted for OpAG2 in both A. marginale and A. centrale is also present in the DNA sequence of A. ovis but is shifted out of frame due to the insertion of two thymidines directly upstream to the triplet encoding for the first depicted amino acid (valine) in A. ovis. This disruption of the 5′ terminus of the open reading frame likely results in usage of the next, yet weaker start codon (GTG) as shown in Fig. 5. The insertion of two thymidines into the A. ovis OpAG2 was present in sequences obtained in three independent amplification, cloning, and sequencing experiments. OpAG1 is the least conserved protein between A. marginale and A. ovis (58.3% identity) or between A. marginale and A. centrale (21.8%) and is 33 amino acids shorter in A. centrale than in A. marginale and A. ovis. Only a few, very short stretches of identical sequence are present in A. centrale OpAG1 compared to A. marginale and A. ovis. The A. centrale OpAG1 C terminus is truncated with utilization of a different stop codon than A. marginale and A. ovis. As previously described, the organization of MSP2 with a central hypervariable region and flanking, highly conserved regions is very similar for A. marginale, A. ovis, and A. centrale (Fig. 5) (8, 14, 31).
FIG. 5.
Conservation of the msp2 operon-associated proteins among A. marginale, A. ovis, and A. centrale. The sequences of the msp2 operon were obtained for the Dubois, Idaho, strain of A. ovis (A ovis) and the Israel vaccine strain of A. centrale (A centr). The amino acid sequences encoded by the open reading frames for each OpAG and MSP2 were aligned with those derived from the sequence of the Florida strain of A. marginale (Am Fl) (4) by using the Vector NTI software package. The numbers above the alignments indicate the amino acid positions of the consensus sequences. The numbers in the left margin correspond to the amino acid sequence positions in each individual strain. The amino acids shaded in gray are conserved.
DISCUSSION
Antigenic variation of the immunodominant outer membrane protein MSP2 allows A. marginale to evade the mammalian host immune system during persistent infection and is generated by gene conversion of the single, full-length copy of msp2 (7, 14). The msp2 gene is part of an operon that consists of a 5′ promoter region, followed by four open reading frames: opag3, opag2, and opag1, and the 3′-terminal msp2 (4). The order and the intergenic spacing of the open reading frames within the msp2 operon are conserved among genotypically and phenotypically distinct strains of A. marginale, the Dubois (Idaho) strain of A. ovis, and the Israel vaccine strain of A. centrale, a finding consistent with the functional importance of the MSP2 expression site (4, 16). Furthermore, the predicted amino acid sequences of the three OpAG are highly conserved among the Florida, South Idaho, Virginia, Washington-Clarkston, and Washington-Okanogan strains of A. marginale. The minimal polymorphism, dispersed over the entire amino acid sequences, argues against antigenic variation of these proteins and is in marked contrast to the highly polymorphic MSP2 (13, 14, 24).
A comparison of the nucleotide sequences of the msp2 operon between the Florida, South Idaho, and Oklahoma strains and at different time points during persistent infection with the South Idaho strain revealed more changes in opag3 compared to opag1 and opag2 (5). The high degree of amino acid conservation that occurs in the face of these nucleotide substitutions indicates the presence of selective pressure to retain OpAG3 structure. Although the function of the OpAG proteins is unknown, the observation that structural genes of bacteria are often organized in clusters coding for proteins whose functions are related (15) suggests that OpAG mediates interactions at the A. marginale surface. Like MSP2 (18, 24), OpAG2 and OpAG3 are expressed during acute rickettsemia of cattle infected with each of the five A. marginale strains examined and localize to the surface of A. marginale. Based on the important role MSP2 plays in the immune response to A. marginale and in the protection of cattle from disease (8, 9, 14, 32), OpAG2 and OpAG3 may also be targets for the bovine immune system. Outer membrane proteins conserved among A. marginale strains are recognized by CD4+ T lymphocytes derived from vaccinates (10), which were protected from disease upon A. marginale challenge after immunization with purified outer membranes (9, 32).
In contrast to OpAG2 and OpAG3, OpAG1 protein was not detectable by Western blot analysis in erythrocytes infected with any of the strains even after a 6-fold increase in antigen load and a 20-fold increase in antibody concentration. The lack of detectable expression with anti-peptide antibody was confirmed with antibody directed against full-length recombinant OpAg1. Both OpAG1-specific antisera readily detected recombinantly expressed protein. Whether OpAG1 protein is not expressed at all in A. marginale or is expressed at very low levels is unknown; however, it is clear that any OpAG1 expression is markedly reduced compared to OpAG2, OpAG3, and MSP2. This reduced or absent OpAG1 expression occurs despite the presence of detectable full-length operon transcript and the expression of both proteins, OpAG2 and MSP2, encoded by the genes flanking opag1. Thus, the differential expression of genes within the operon appears to be posttranscriptionally regulated. Common posttranscriptional mechanisms for differential expression of genes encoded by bacterial operons are negative regulation by a translational repressor, either in form of a protein or a small RNA, or positive regulation by a translational activator. Both systems may have a second level of regulation in the form of corepressors or inducers. Alternative regulatory mechanisms include the modification of the mRNA secondary structure, with masking of the ribosomal binding site, or cleavage of the polycistronic mRNA, with rapid degradation of certain segments, such as opag1 in the case of the msp2 operon. Although full-length msp2 operon transcripts are readily detected by reverse transcription-PCR (RT-PCR) (4), the presence of alternative transcript species has not been fully investigated. Using quantitative real-time PCR following RT-PCR, we have previously shown that opag3 cDNA is 1.5-fold less abundant than msp2 cDNA in salivary glands of infected, transmission-fed ticks (16).
The predicted amino acid sequence of OpAG1 exhibits 58.3% identity when A. ovis and A. marginale are compared and only 22% identity between A. centrale and A. marginale. This loss of identity among closely related organisms that similarly invade and replicate in mature erythrocytes raises the possibility that opag1 is a gene undergoing degeneration. The genomes of A. marginale, A. ovis, and A. centrale each reflect the process of reductive evolution (28) and progressive reduction via mutation, gene splitting, and eventual gene loss that have been been well described in the related Rickettsia genus (2, 22). This possibility is further supported by the carboxy-terminal truncation of the opag1 open reading frame in A. centrale. Thus, the lack of OpAG1 expression in A. marginale may reflect early degradation events in gene loss, but the location of the opag1 gene within the operon results in opag1 mRNA being retained.
OpAG3 is expressed during acute rickettsemia in the mammalian host but was undetectable in the salivary glands and midguts of the transmission-feeding tick vector with the same strains of A. marginale (South Idaho and Virginia). This is not simply a reflection of a specific bacterial strain and tick species combination, since the results are reproducible with the Virginia strain of A. marginale both in D. andersoni and D. variabilis ticks. Generally, the same regulatory mechanisms as described above for the differential expression within an operon also apply to the differential expression of operon-associated proteins under changing conditions. Interestingly, the msp2 operon contains an open reading frame in the opposite direction to and largely overlapping with opag3 (Fig. 1). We hypothesized that the transcript of this open reading frame could act as a small RNA repressor of translation by binding to opag3 mRNA and inducing either rapid degradation or inhibition of translation. However, no specific overlapping mRNA could be detected by RT-PCR (data not shown), and the hypothesis was therefore rejected.
Differential expression of an outer membrane protein from within an operon is a novel finding in tick-transmitted bacteria. The only other known outer membrane proteins that are expressed from an operon of a tick-transmitted bacterium are OspA and OspB of Borrelia burgdorferi (11). These proteins are organized in a bicistronic fashion, and expression in the tick vector ceases upon initiation of the feeding process (12, 30). At the same time, OspC expression is initiated and continues in the mammalian host after tick transmission (30). In contrast to the situation in the A. marginale msp2 operon, ospC is not part of the ospA/ospB operon (11). The differential expression of the Borrelia burgdorferi outer membrane proteins OspA and OspC between the tick and the mammalian host can be reproduced in infected mammalian and tick cell lines by temperature changes (21). Similarly, differential expression of OpAG3 between the tick vector and the mammalian host could reflect changes in the environmental temperature. While the core temperature of healthy calves is 38.5°C, the temperature at the body surface, where ticks attach and feed, can be more than 10°C lower. The expression of OpAG3 in the infected, permanent tick cell line IDE8, which was cultured at a temperature (34°C) closer to that in the mammalian host, suggests a possible role for temperature-dependent regulation. However, it cannot be excluded that the host cell type may play a role in the permissiveness of the IDE8 cell line for OpAG3 expression. Although the IDE8 cell line was derived from I. scapularis, which is not a vector for A. marginale, and is an embryonal derived cell line (19), the fully differentiated salivary glands and midguts examined in the present study were obtained from adult Dermacentor ticks and represent the actual site of A. marginale replication and development during tick-borne transmission. Nonetheless, the IDE8 cell line is a manipulable system that could serve as a starting point for further dissection of the regulatory mechanisms involved in the differential expression of the msp2 operon-associated genes. The identification of the differentially expressed opag genes suggests that the encoded surface proteins may have unique functions in the mammalian and tick stages of A. marginale infection. Unraveling how gene expression is regulated within an operon may be fundamentally important in understanding how the pathogen adapts to the transition between the mammalian host and the tick vector.
Acknowledgments
This work was supported by NIH grants AI44005 and AI45580 and by a postdoctoral fellowship from the Deutsche Akademie der Naturforscher Leopoldina.
The tick cell cultures and Dermacentor ticks infected with the Virginia strain were provided by Katherine Kocan and Edmour Blouin at Oklahoma State University. The technical assistance of Beverly Hunter and Carla Robertson is also greatly appreciated.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Allred, D. R., T. C. McGuire, G. H. Palmer, S. R. Leib, T. M. Harkins, T. F. McElwain, and A. F. Barbet. 1990. Molecular basis for surface antigen size polymorphisms and conservation of a neutralization-sensitive epitope in Anaplasma marginale. Proc. Natl. Acad. Sci. USA 87:3220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, S. G., A. Zomorodipour, J. O. Andersson, T. Sicheritz-Ponten, U. C. Alsmark, R. M. Podowski, A. K. Naslund, A. S. Eriksson, H. H. Winkler, and C. G. Kurland. 1998. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396:133-140. [DOI] [PubMed] [Google Scholar]
- 3.Barbet, A. F., R. Blentlinger, J. Yi, A. M. Lundgren, E. F. Blouin, and K. M. Kocan. 1999. Comparison of surface proteins of Anaplasma marginale grown in tick cell culture, tick salivary glands, and cattle. Infect. Immun. 67:102-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbet, A. F., A. Lundgren, J. Yi, F. R. Rurangirwa, and G. H. Palmer. 2000. Antigenic variation of Anaplasma marginale by expression of MSP2 mosaics. Infect. Immun. 68:6133-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbet, A. F., J. Yi, A. Lundgren, B. R. McEwen, E. F. Blouin, and K. M. Kocan. 2001. Antigenic variation of Anaplasma marginale: major surface protein 2 diversity during cyclic transmission between ticks and cattle. Infect. Immun. 69:3057-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brayton, K. A., D. P. Knowles, T. C. McGuire, and G. H. Palmer. 2001. Efficient use of a small genome to generate antigenic diversity in tick-borne ehrlichial pathogens. Proc. Natl. Acad. Sci. USA 98:4130-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brayton, K. A., G. H. Palmer, A. Lundgren, J. Yi, and A. F. Barbet. 2002. Antigenic variation of Anaplasma marginale msp2 occurs by combinatorial gene conversion. Mol. Microbiol. 43:1151-1159. [DOI] [PubMed] [Google Scholar]
- 8.Brown, W. C., T. C. McGuire, D. Zhu, H. A. Lewin, J. Sosnow, and G. H. Palmer. 2001. Highly conserved regions of the immunodominant major surface protein 2 of the genogroup II ehrlichial pathogen Anaplasma marginale are rich in naturally derived CD4+ T lymphocyte epitopes that elicit strong recall responses. J. Immunol. 166:1114-1124. [DOI] [PubMed] [Google Scholar]
- 9.Brown, W. C., V. Shkap, D. Zhu, T. C. McGuire, W. Tuo, T. F. McElwain, and G. H. Palmer. 1998. CD4+ T-lymphocyte and immunoglobulin G2 responses in calves immunized with Anaplasma marginale outer membranes and protected against homologous challenge. Infect. Immun. 66:5406-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, W. C., D. Zhu, V. Shkap, T. C. McGuire, E. F. Blouin, K. M. Kocan, and G. H. Palmer. 1998. The repertoire of Anaplasma marginale antigens recognized by CD4+ T-lymphocyte clones from protectively immunized cattle is diverse and includes major surface protein 2 (MSP-2) and MSP-3. Infect. Immun. 66:5414-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Silva, A. M., and E. Fikrig. 1997. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J. Clin. Investig. 99:377-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Silva, A. M., S. R. Telford, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French, D. M., W. C. Brown, and G. H. Palmer. 1999. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect. Immun. 67:5834-5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.French, D. M., T. F. McElwain, T. C. McGuire, and G. H. Palmer. 1998. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect. Immun. 66:1200-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewin, B. 2000. The operon, p. 275-277. In B. Lewin (ed.), Genes VII. Oxford University Press, Oxford, United Kingdom.
- 16.Löhr, C. V., F. R. Rurangirwa, T. F. McElwain, D. Stiller, and G. H. Palmer. 2002. Specific expression of Anaplasma marginale major surface protein 2 salivary gland variants occurs in the midgut and is an early event during tick transmission. Infect. Immun. 70:114-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBride, J. W., X.-J. Yu, and D. H. Walker. 2000. A conserved transcriptionally active p28 multigene locus of Ehrlichia canis. Gene 254:245-252. [DOI] [PubMed] [Google Scholar]
- 18.McGuire, T. C., G. H. Palmer, W. L. Goff, M. I. Johnson, and W. C. Davis. 1984. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect. Immun. 45:697-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munderloh, U. G., E. F. Blouin, K. M. Kocan, N. L. Ge, W. L. Edwards, and T. J. Kurtti. 1996. Establishment of the tick (Acari: Ixodidae)-borne cattle pathogen Anaplasma marginale (Rickettsiales: Anaplasmataceae) in tick cell culture. J. Med. Entomol. 33:656-664. [DOI] [PubMed] [Google Scholar]
- 20.Ndung'u, L. W., C. Aguirre, F. R. Rurangirwa, T. F. McElwain, T. C. McGuire, D. P. Knowles, and G. H. Palmer. 1995. Detection of Anaplasma ovis infection in goats by major surface protein 5 competitive inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 33:675-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obonyo, M., U. G. Munderloh, V. Fingerle, B. Wilske, and T. J. Kurtti. 1999. Borrelia burgdorferi in tick cell culture modulates expression of outer surface proteins A and C in response to temperature. J. Clin. Microbiol. 37:2137-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogata, H., S. Audic, P. Renesto-Audiffren, P. E. Fournier, V. Barbe, D. Samson, V. Roux, P. Cossart, J. Weissenbach, J. M. Claverie, and D. Raoult. 2001. Mechanisms of evolution in Rickettsia conorii and R. prowazekii. Science 293:2093-2098. [DOI] [PubMed] [Google Scholar]
- 23.Ohashi, N., N. Zhi, Y. Zhang, and Y. Rikihisa. 1998. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer, G. H., G. Eid, A. F. Barbet, T. C. McGuire, and T. F. McElwain. 1994. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect. Immun. 62:3808-3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer, G. H., and T. C. McGuire. 1984. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J. Immunol. 133:1010-1015. [PubMed] [Google Scholar]
- 26.Palmer, G. H., F. R. Rurangirwa, and T. F. McElwain. 2001. Strain composition of the ehrlichia Anaplasma marginale within persistently infected cattle, a mammalian reservoir for tick transmission. J. Clin. Microbiol. 39:631-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pipano, E., Y. Krigel, M. Frank, A. Markovics, and E. Mayer. 1986. Frozen Anaplasma centrale vaccine against anaplasmosis in cattle. Br. Vet. J. 142:553-556. [DOI] [PubMed] [Google Scholar]
- 28.Rurangirwa, F. R., K. A. Brayton, T. McGuire, D. Knowles, and G. H. Palmer. 2002. Conservation of the unique rickettsial rRNA gene arrangement in Anaplasma. Int. J. Syst. E vol. Microbiol. 52:1405-1409. [DOI] [PubMed] [Google Scholar]
- 29.Rurangirwa, F. R., D. S. Stiller, D. M. French, and G. H. Palmer. 1999. Restriction of major surface protein 2 (MSP2) variants during tick transmission of the ehrlichiae Anaplasma marginale. Proc. Natl. Acad. Sci. USA 96:3171-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shkap, V., T. Molad, K. A. Brayton, W. C. Brown, and G. H. Palmer. 2002. Expression of major surface protein 2 variants with conserved T-cell epitopes in Anaplasma centrale vaccinates. Infect. Immun. 70:642-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tebele, N., T. C. McGuire, and G. H. Palmer. 1991. Induction of protective immunity by using Anaplasma marginale initial body membranes. Infect. Immun. 59:3199-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]