Abstract
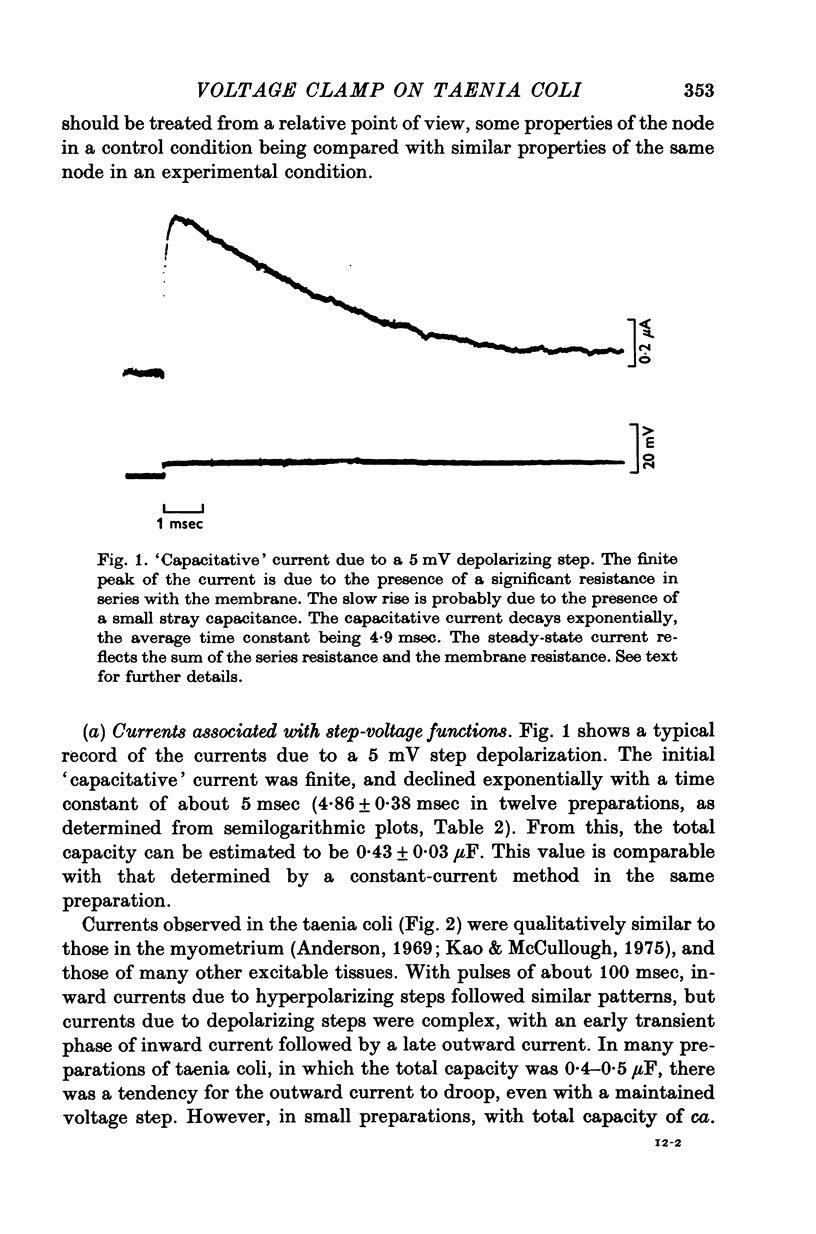
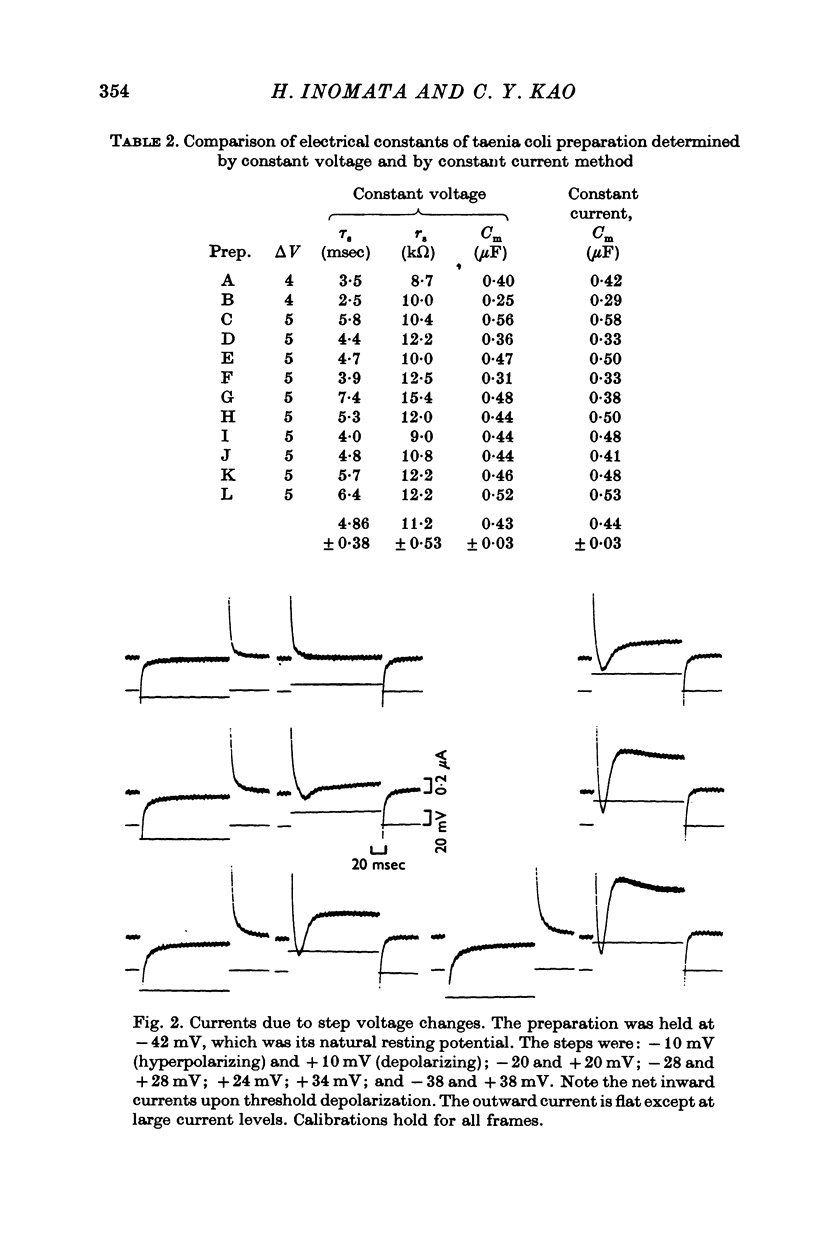
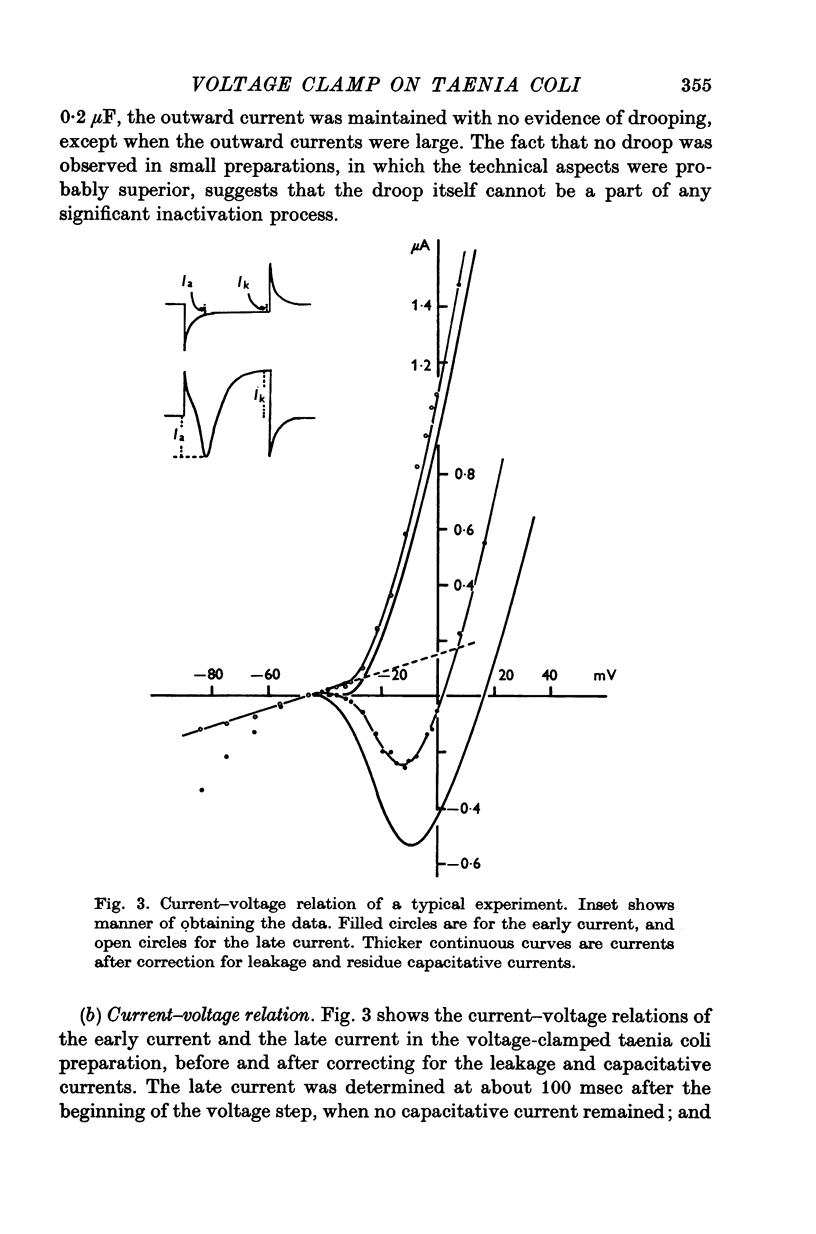
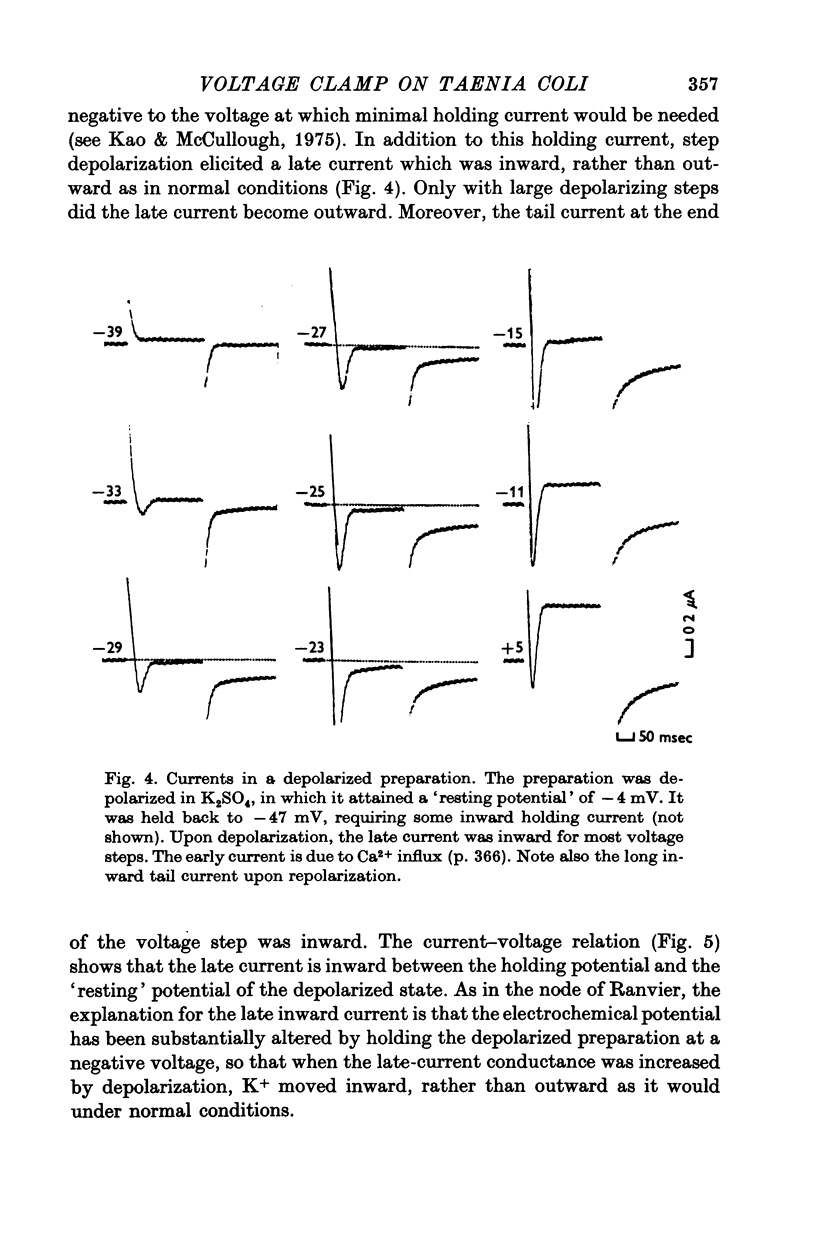
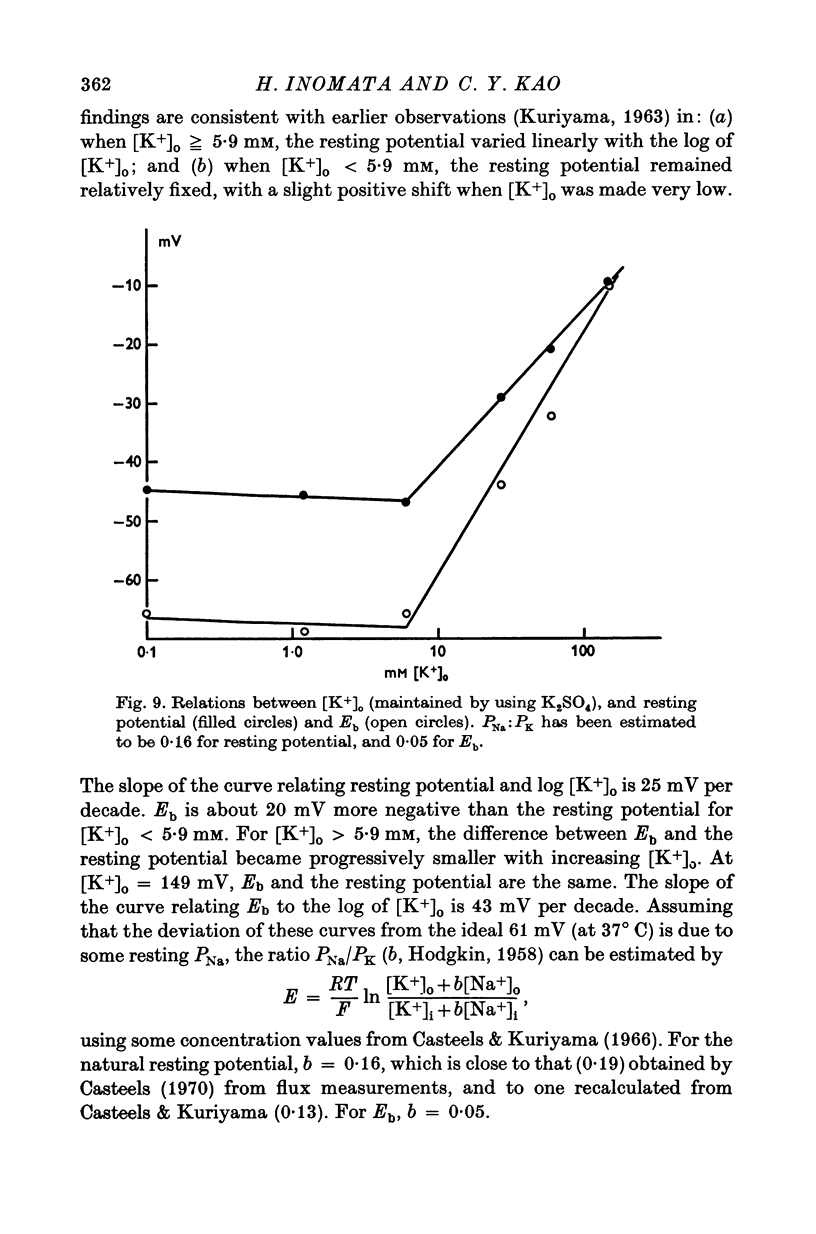
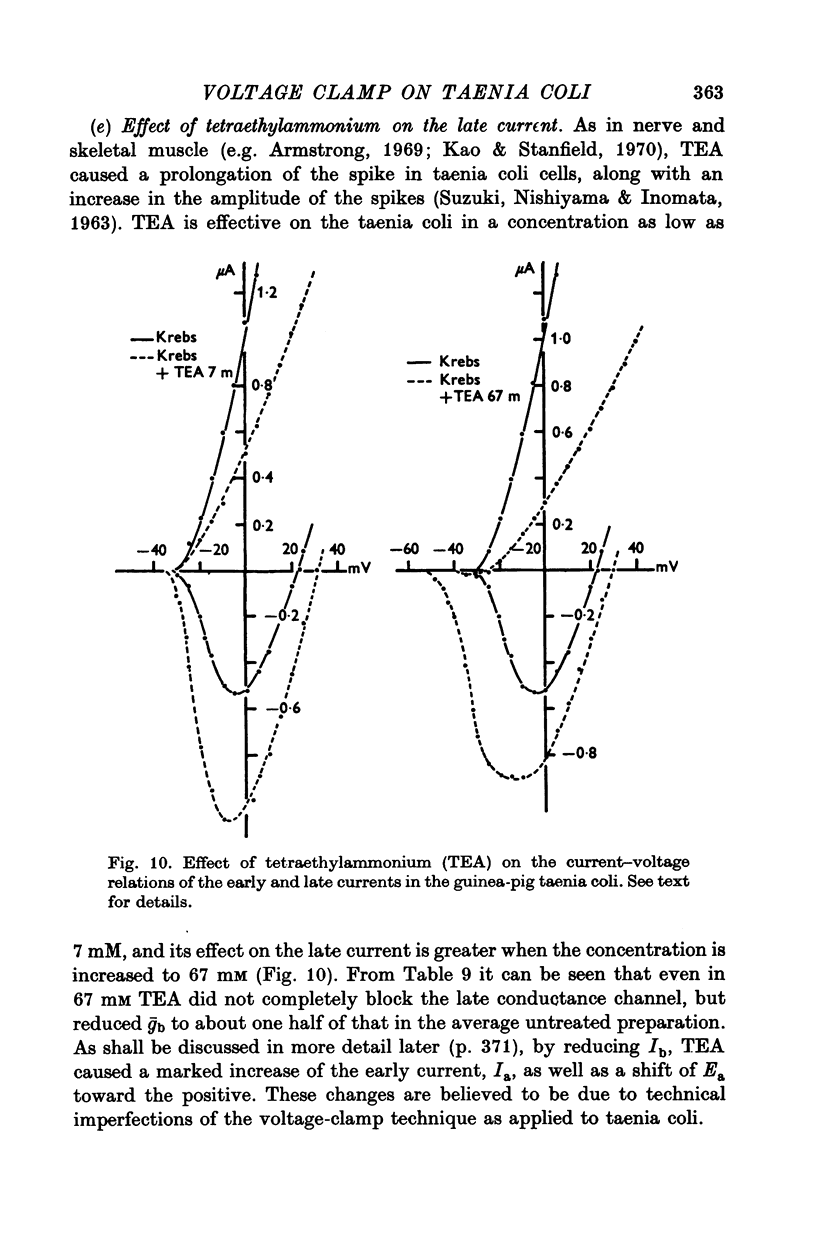
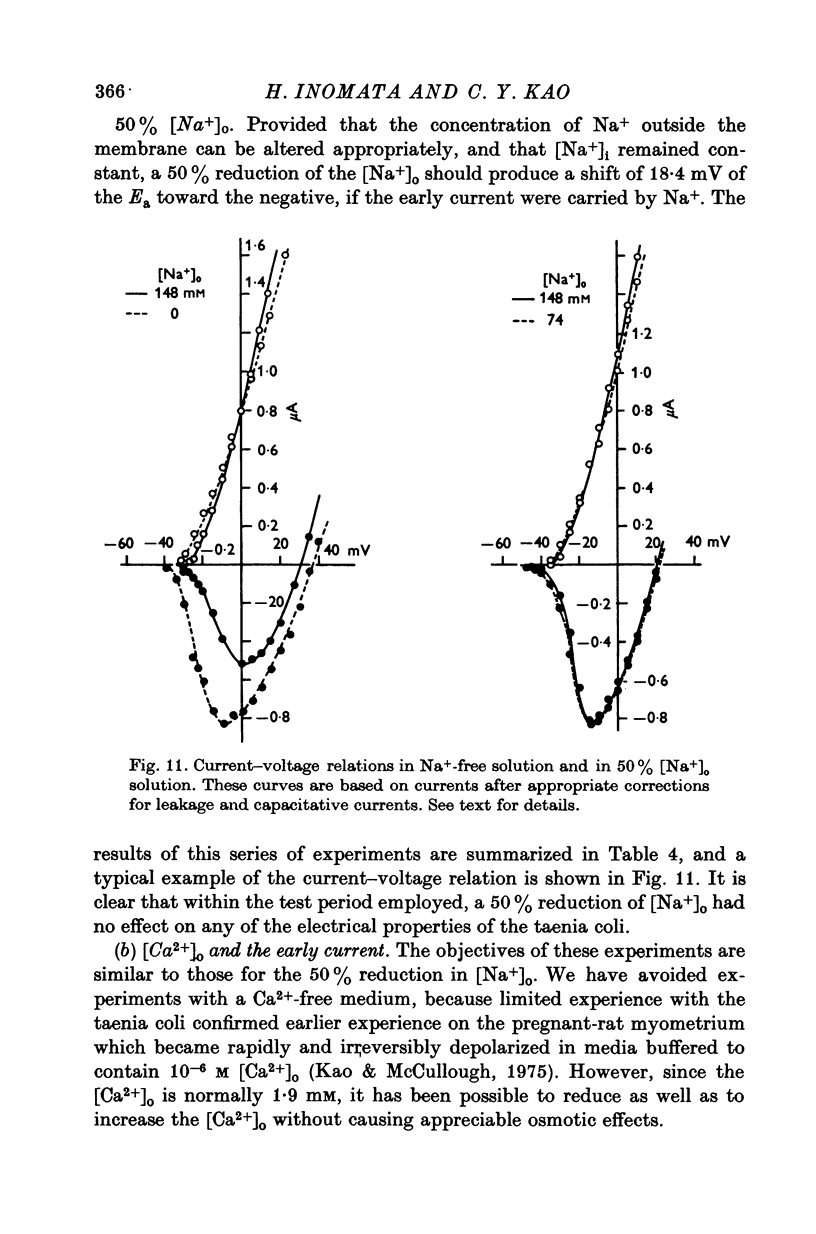
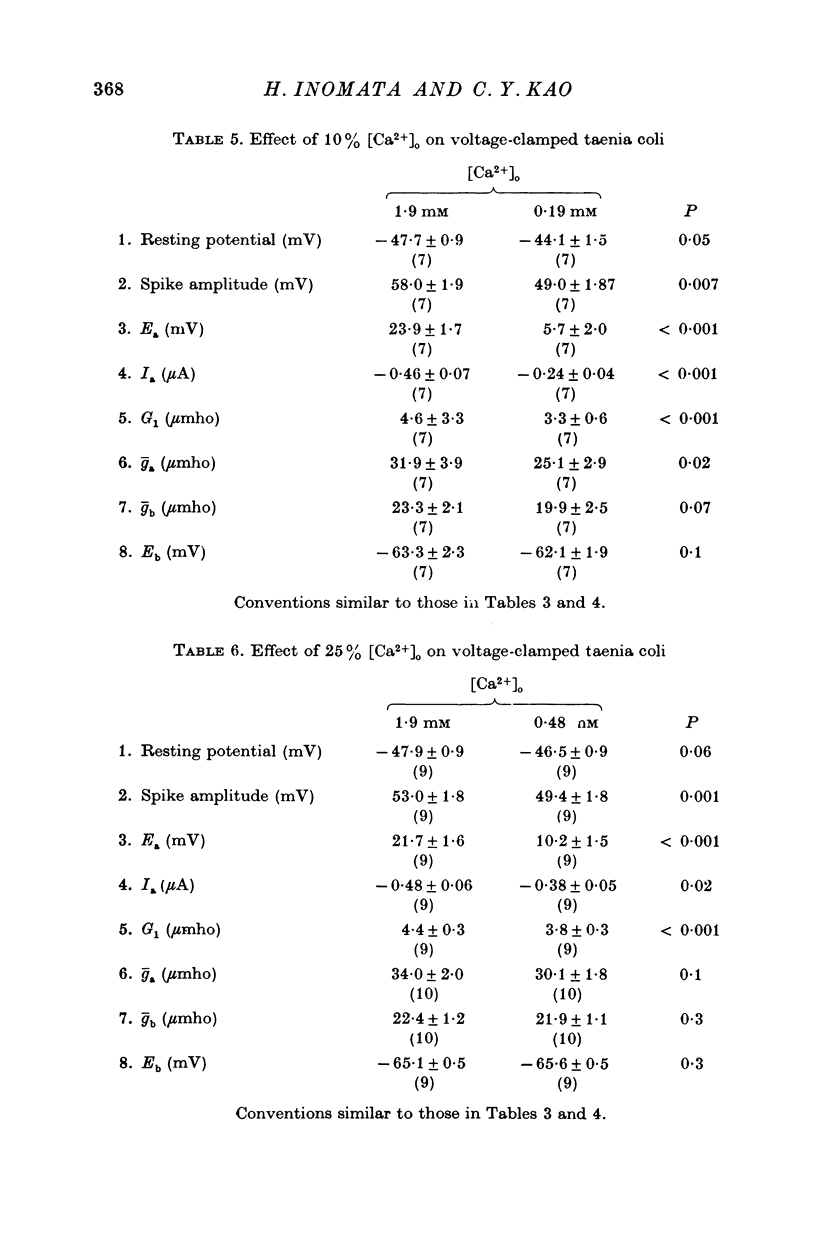
Short segments of portions of taenia coli of the guinea-pig averaging 54 mum X 219 mum X ca. 200 mum have been studied by a double sucrose-gap voltage-clamp technique. 2. The average total capacitance was 0-4 muF, corresponding to approximately 10(4) cells, if a specific membrane capacitance of 3 muF/cm2 were assumed. 3. A significant resistance, averaging 11-4omega, was in series with the membrane, and seriously limited the accuracy of the voltage control possible. 4. On depolarization, an early transient inward current was followed by a late maintained outwary current. 5. The late current was carried mainly by K+, because its direction could be reversed if the preparation were first depolarized in isotonic K2SO4 and held back to the original resting potential. 6. After appropriate corrections for residual capacitative and leakage currents, a reversal potential for the late current (Eb) was determined to be 15-20 mV more negative than the natural resting potential. It was not affected by the amplitude or the duration of the activating voltage step, but could be changed by prolonged applications of holding current. 7. At rest, the ratio of PNa:PK was 0-16:1; for Eb it was 0-05:1. 8. The reversal potential for the transient early inward current (Ea) averaged 22 mV in Krebs-bicarbonate solution, but was shifted to about 35 mV when the late current was first suppressed with tetraethylammonium ion. The shift suggested that there was some overlap of the early and late currents. 9. Reduction of [Na+]o to 50% of normal, or replacement of all Na+ with dimethyldiethanol ammonium ion and choline ion, failed to cause any significant shifts in the reversal potential of the early current or reduce the magnitude of the early current. 10. Reduction of [Ca2+]o to 0-25 or 0-1 of the normal caused shifts of the Ea toward the negative and reductions in the early current. These changes can occur without changes in the maximum chord conductance of the early current, such as might happen in ordinary Krebs-bicarbonate solution, or in preparations which had been depolarized by prior treatment with isotonic K2SO4 and then held back to the original membrane voltage. 11. Increase of [Ca2+]o to 5 times normal increased the early inward current, and the maximum chord conductances of the early and late currents, but did not shift the Ea. 12. In preparations pretreated with TEA, increasing [Ca2+]o to 5 times normal shifted Ea toward 45 mV. 13. The various observations are interpreted to mean that the early current in the taenia coli is carried principally by influx of Ca2+, and not by Na+.
Full text
PDF






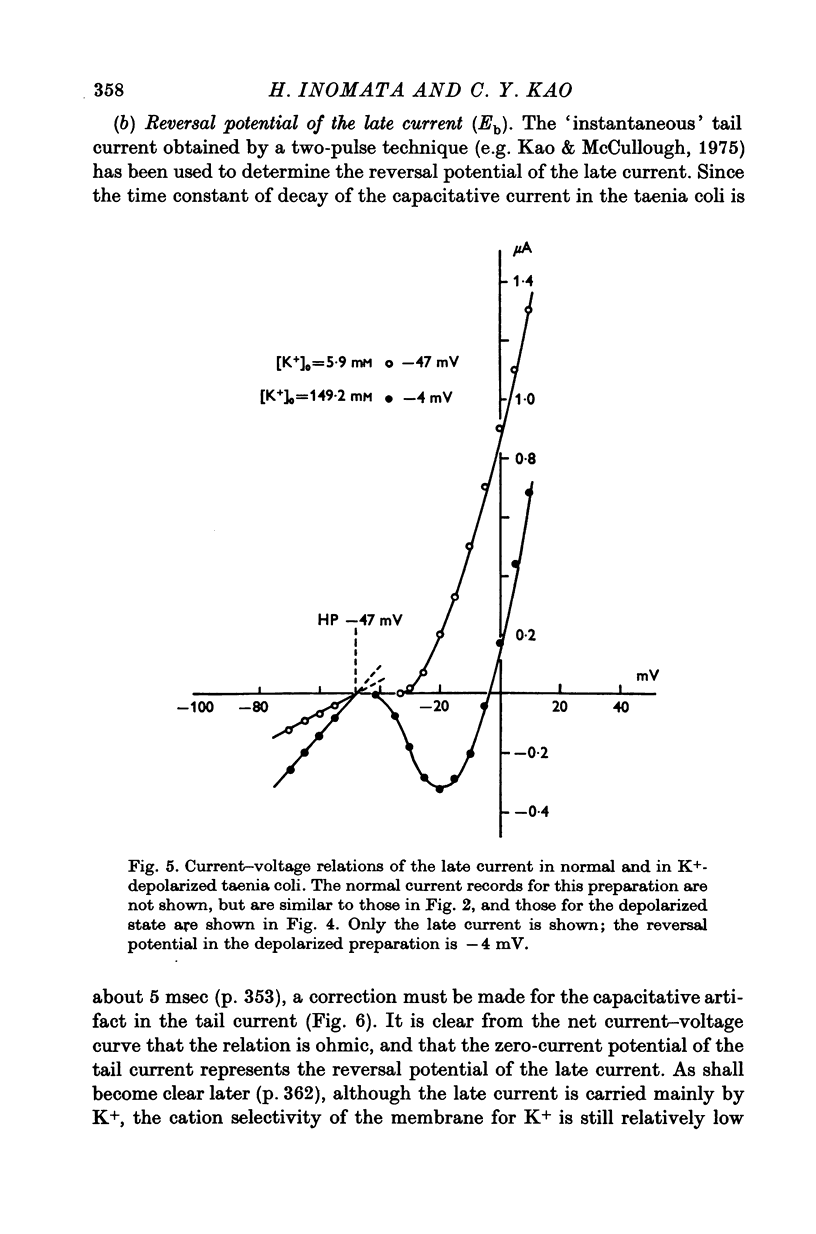
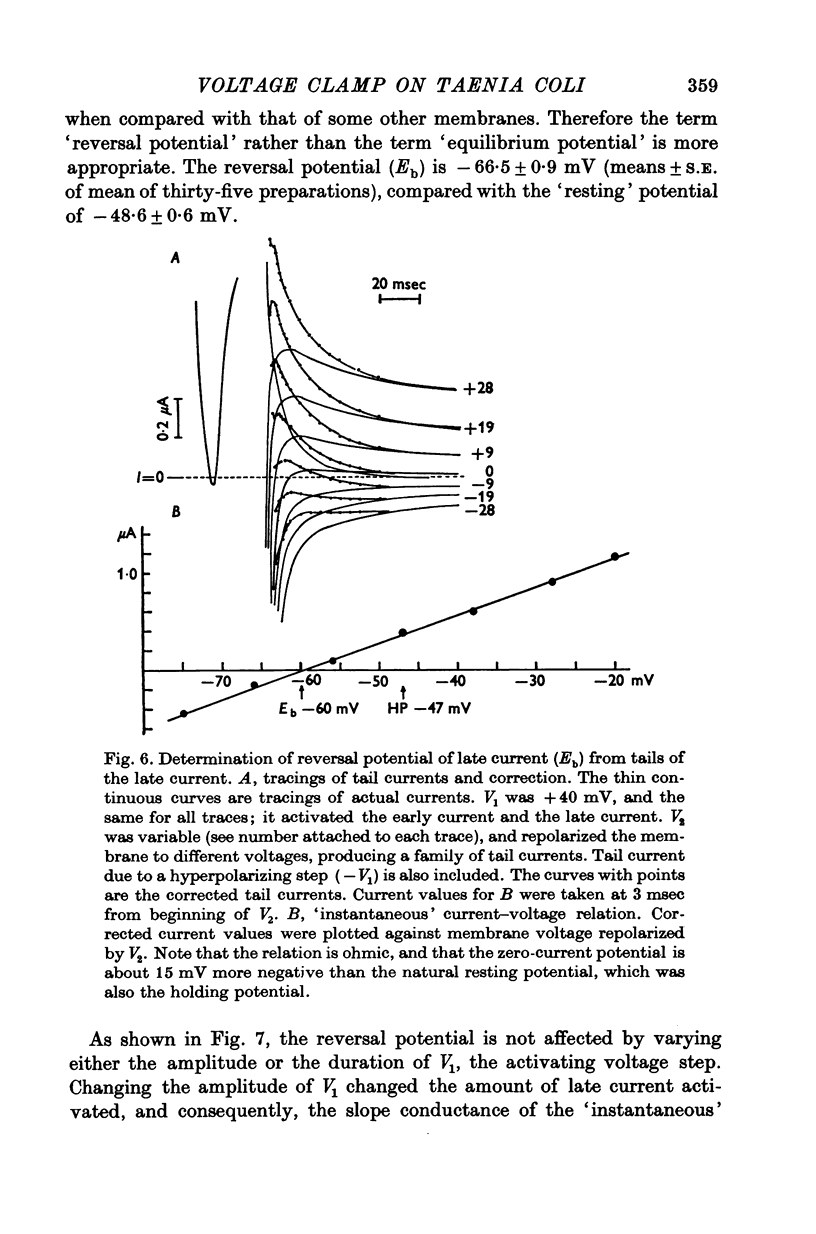
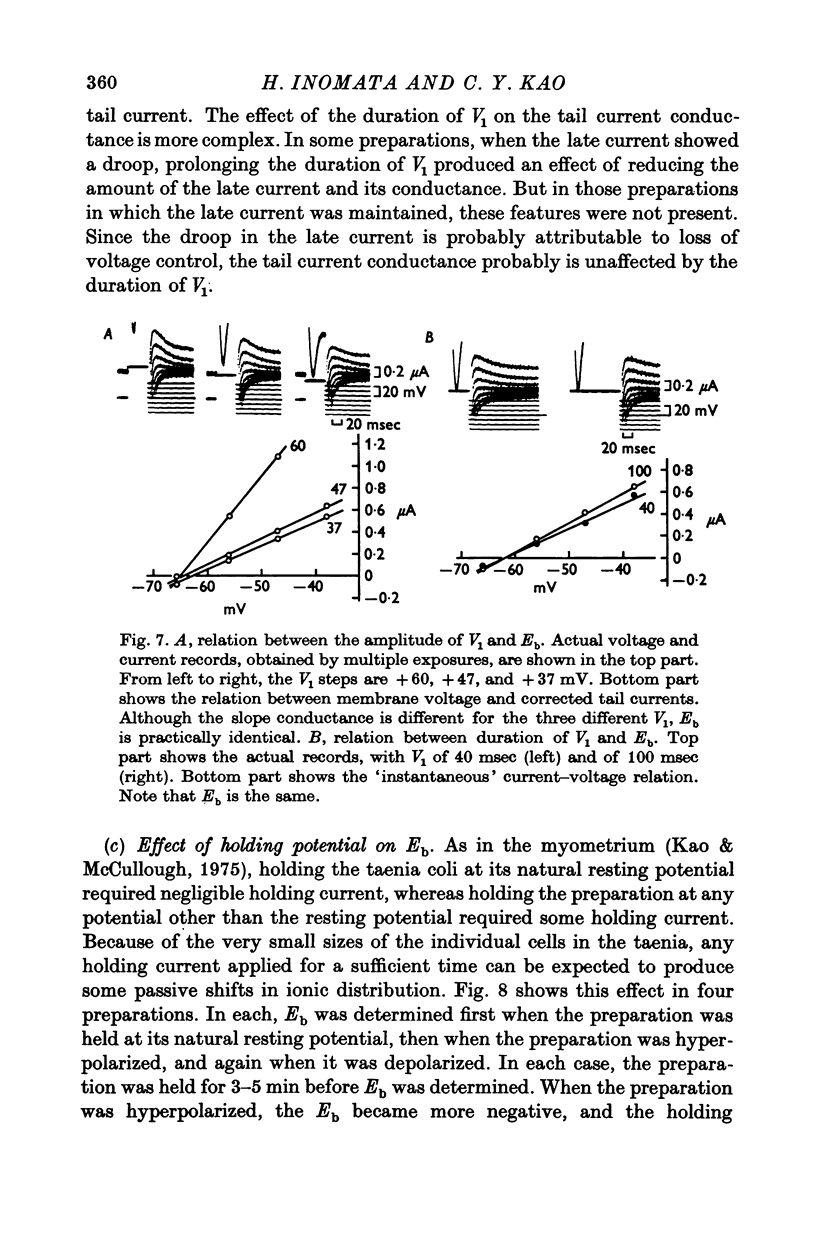
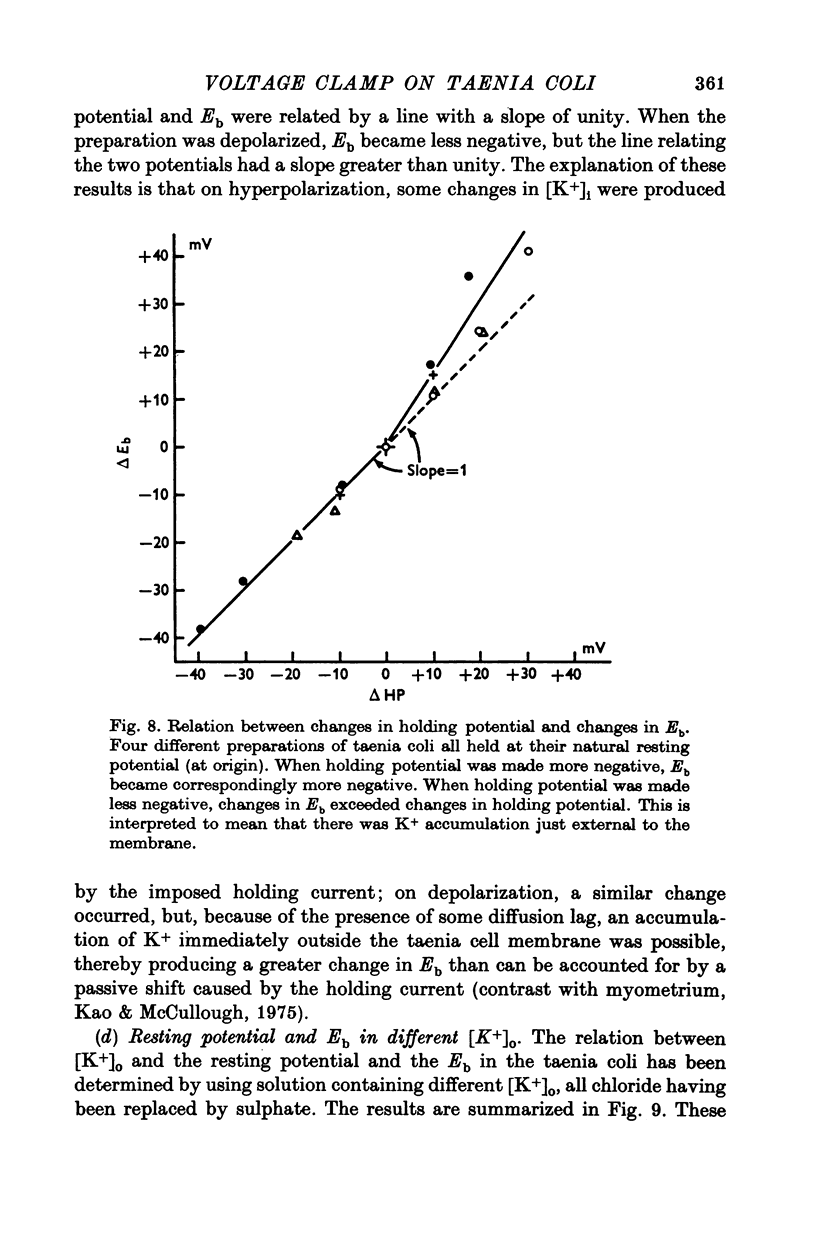









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. C., Jr Voltage-clamp studies on uterine smooth muscle. J Gen Physiol. 1969 Aug;54(2):145–165. doi: 10.1085/jgp.54.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Inactivation of the potassium conductance and related phenomena caused by quaternary ammonium ion injection in squid axons. J Gen Physiol. 1969 Nov;54(5):553–575. doi: 10.1085/jgp.54.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E., KURIYAMA H. Effects of changes in the external sodium and calcium concentrations on spontaneous electrical activity in smooth muscle of guinea-pig taenia coli. J Physiol. 1963 Apr;166:29–58. doi: 10.1113/jphysiol.1963.sp007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F. Analysis of the effluxes of sodium, potassium and chloride ions from smooth muscle in normal and hypertonic solutions. J Physiol. 1971 May;214(3):393–416. doi: 10.1113/jphysiol.1971.sp009440. [DOI] [PMC free article] [PubMed] [Google Scholar]
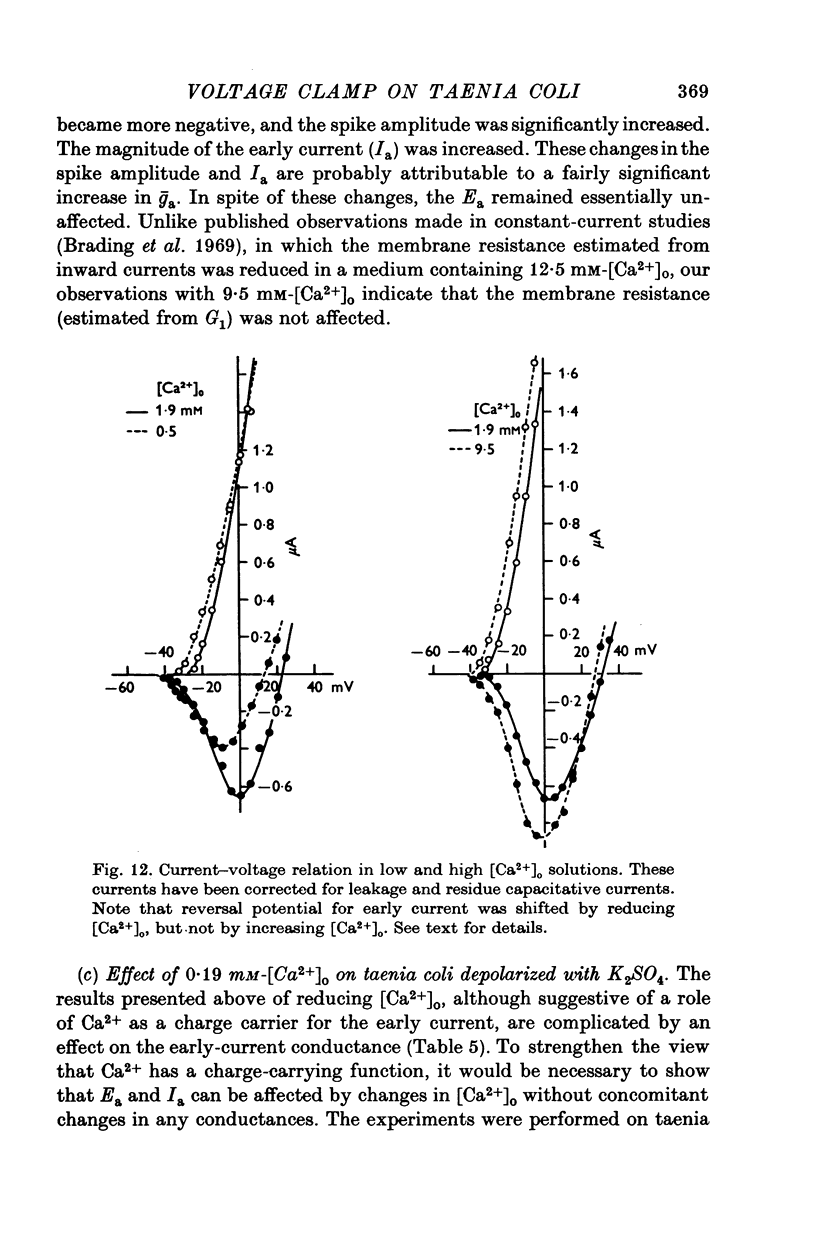
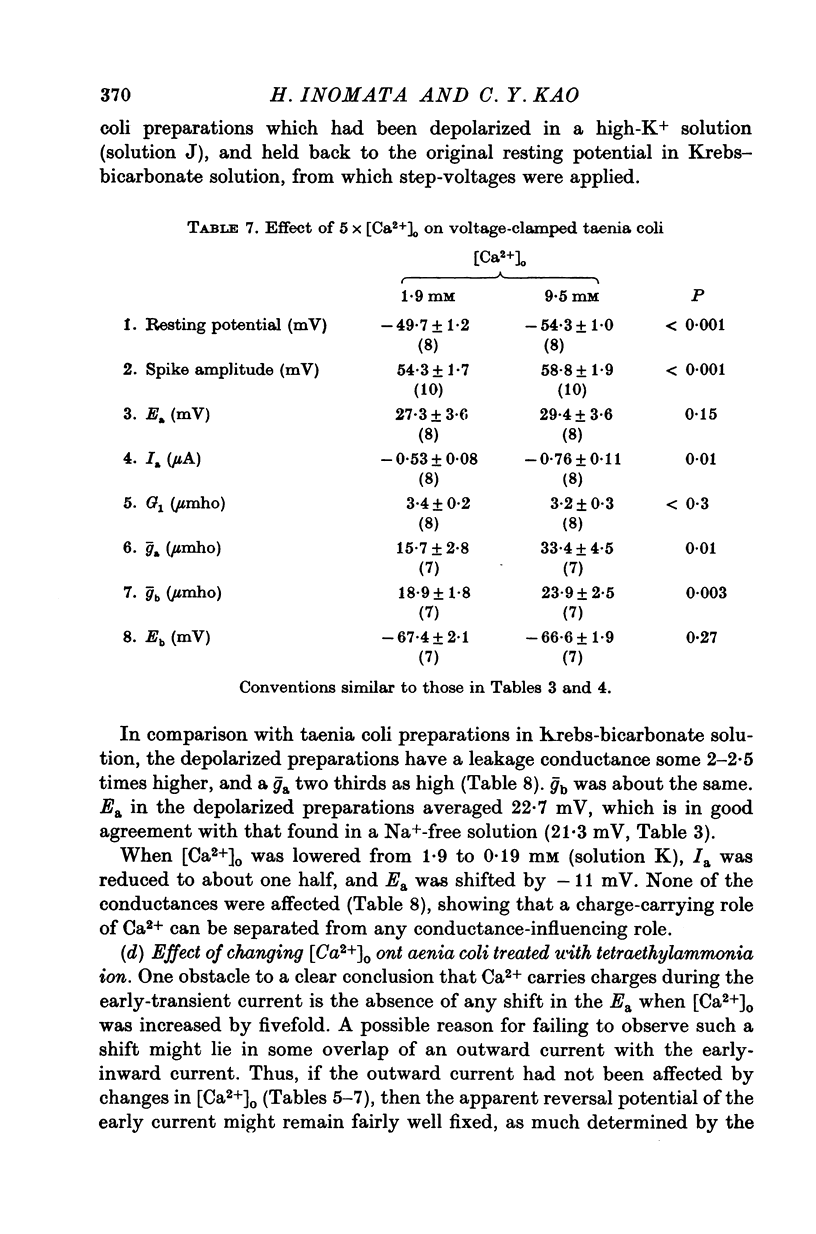
- Brading A., Bülbring E., Tomita T. The effect of sodium and calcium on the action potential of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1969 Feb;200(3):637–654. doi: 10.1113/jphysiol.1969.sp008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kuriyama H. Membrane potential and ion content in the smooth muscle of the guinea-pig's taenia coli at different external potassium concentrations. J Physiol. 1966 May;184(1):120–130. doi: 10.1113/jphysiol.1966.sp007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
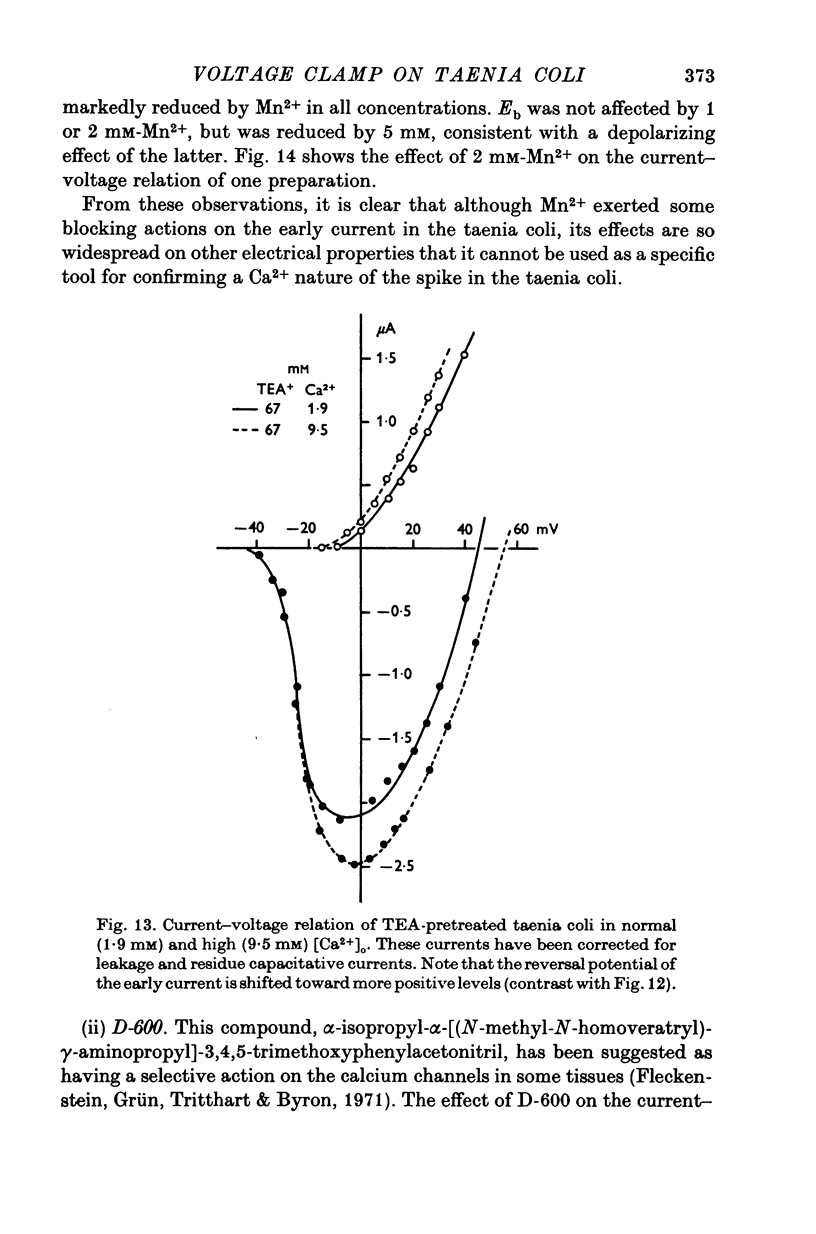
- FRANKENHAEUSER B. Delayed currents in myelinated nerve fibres of Xenopus laevis investigated with voltage clamp technique. J Physiol. 1962 Jan;160:40–45. doi: 10.1113/jphysiol.1962.sp006832. [DOI] [PMC free article] [PubMed] [Google Scholar]
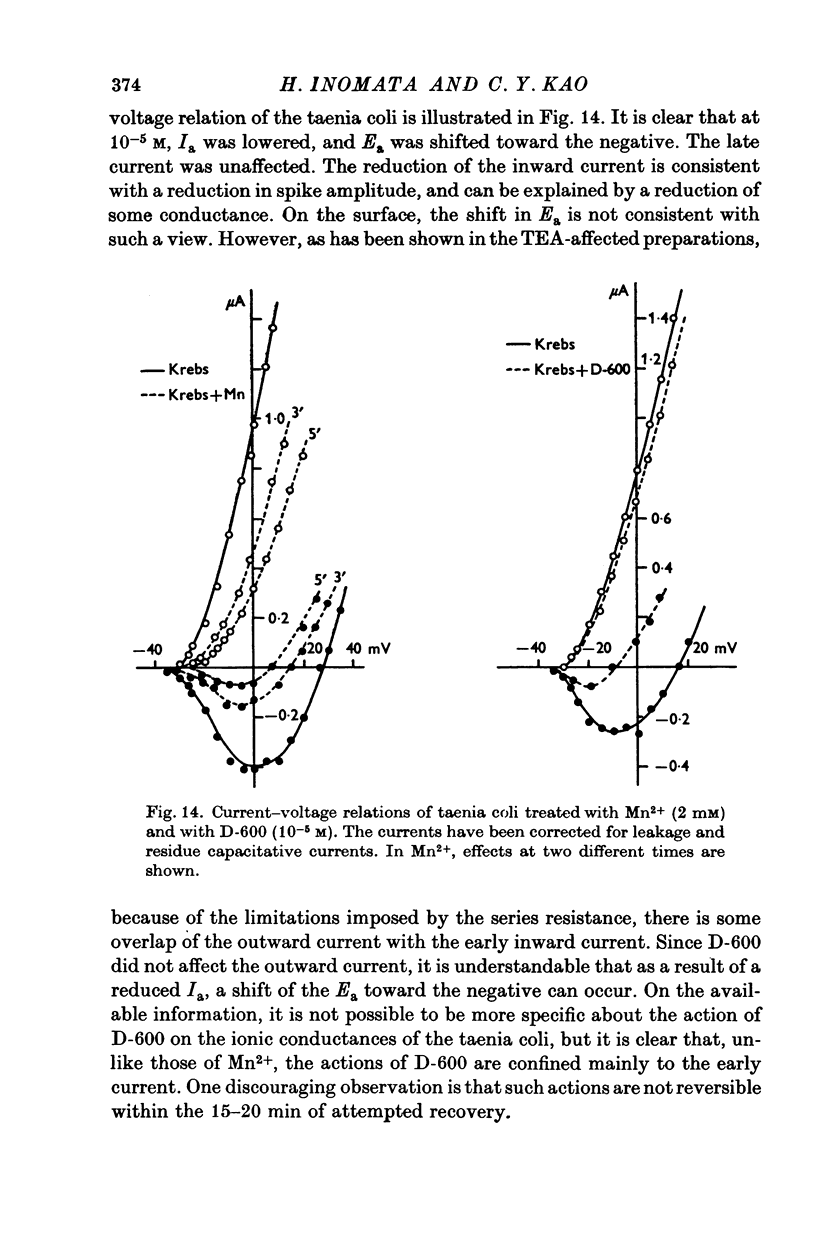
- Fleckenstein A., Grün G., Tritthart H., Byon K. Uterus-Relaxation durch hochaktive Ca plus,plus-antagonistische Hemmstoffe der elektro-mechanischen Koppelung wie Isoptin (Verapamil, Iproveratril), Substanz D 600 und Segontin (Prenylamin). Versuche am isolierten Uterus virgineller Ratten. Klin Wochenschr. 1971 Jan;49(1):32–41. doi: 10.1007/BF01494064. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L. Ionic movements and electrical activity in giant nerve fibres. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):1–37. doi: 10.1098/rspb.1958.0001. [DOI] [PubMed] [Google Scholar]
- HOLMAN M. E. The effect of changes in sodium chloride concentration on the smooth muscle of the guinea-pig's taenia coli. J Physiol. 1957 May 23;136(3):569–584. doi: 10.1113/jphysiol.1957.sp005782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966 Mar;49(4):793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H. The influence of potassium, sodium and chloride on the membrane potential of the smooth muscle of taenia coli. J Physiol. 1963 Apr;166:15–28. doi: 10.1113/jphysiol.1963.sp007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y., McCullough J. R. Ionic currents in the uterine smooth muscle. J Physiol. 1975 Mar;246(1):1–36. doi: 10.1113/jphysiol.1975.sp010878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y., Stanfield P. R. Actions of some cations on the electrical properties and mechanical threshold of frog sartorius muscle fibers. J Gen Physiol. 1970 May;55(5):620–639. doi: 10.1085/jgp.55.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Tomita T. The action potential in the smooth muscle of the guinea pig taenia coli and ureter studied by the double sucrose-gap method. J Gen Physiol. 1970 Feb;55(2):147–162. doi: 10.1085/jgp.55.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI T., NISHIYAMA A., INOMATA H. Effect of tetraethyl ammonium ion on the electrical activity of smooth muscle cell. Nature. 1963 Mar 2;197:908–909. doi: 10.1038/197908a0. [DOI] [PubMed] [Google Scholar]
- Tomita T. Membrane capacity and resistance of mammalian smooth muscle. J Theor Biol. 1966 Nov;12(2):216–227. doi: 10.1016/0022-5193(66)90114-7. [DOI] [PubMed] [Google Scholar]


