Abstract
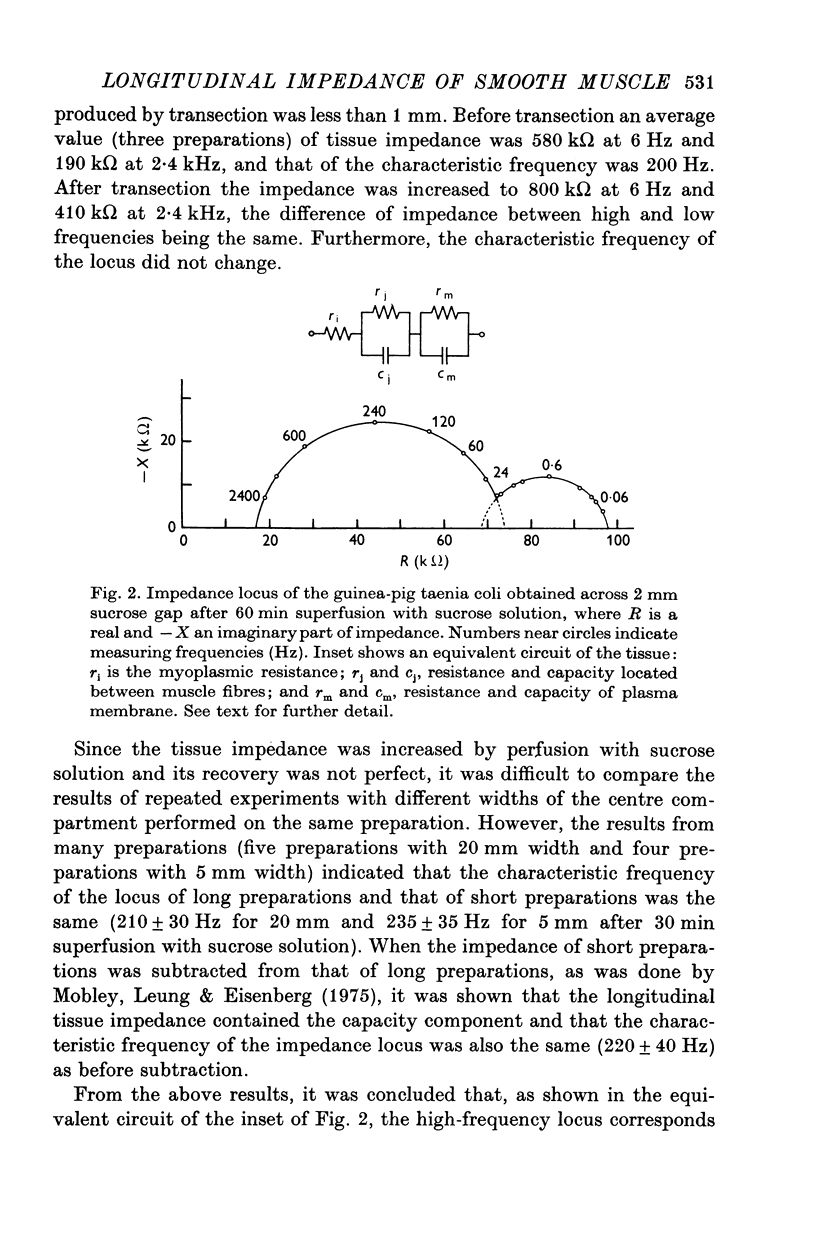
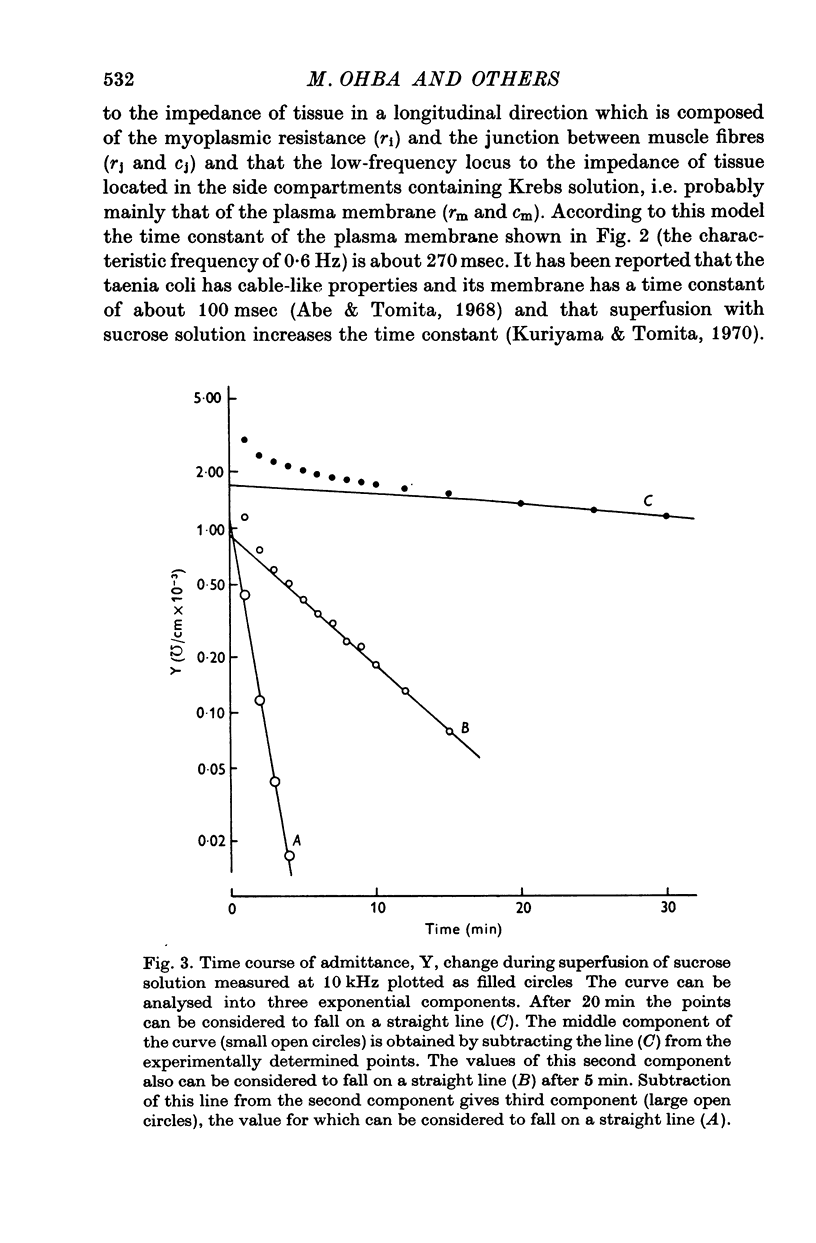
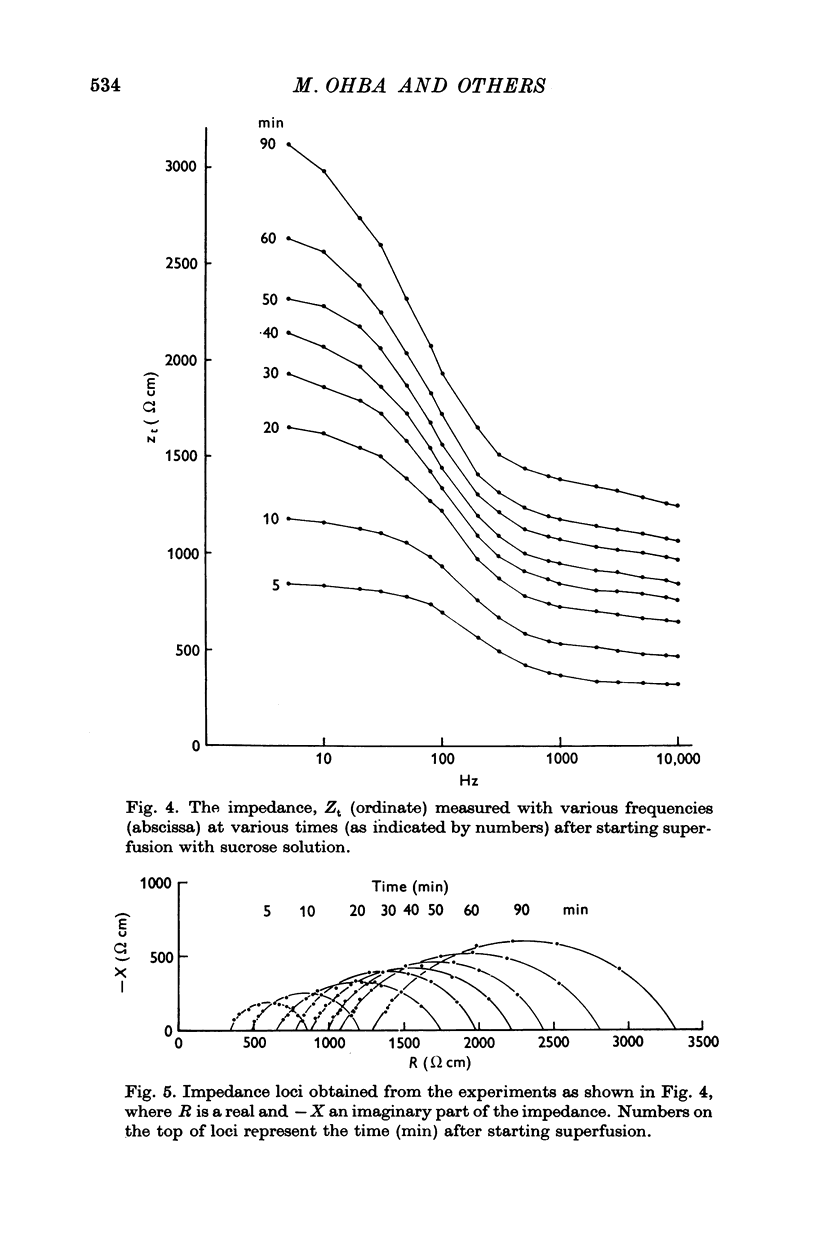
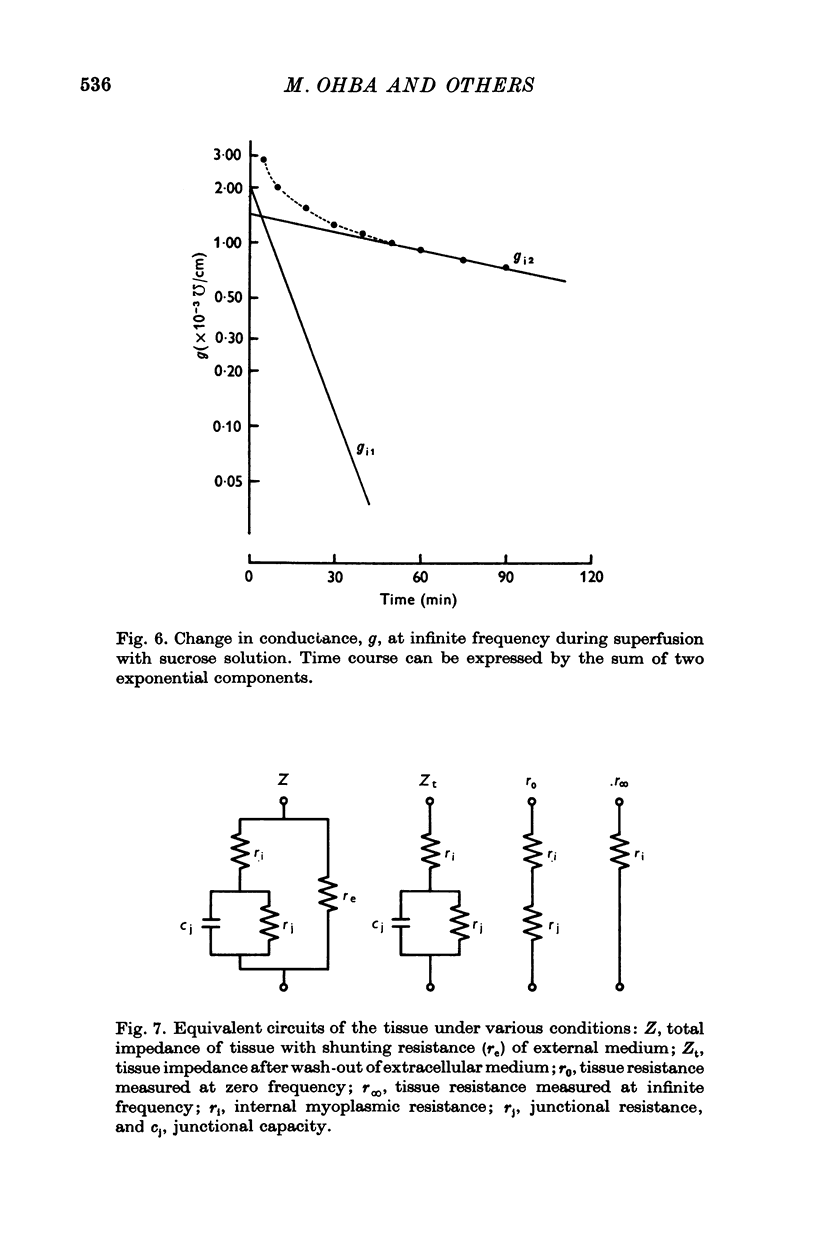
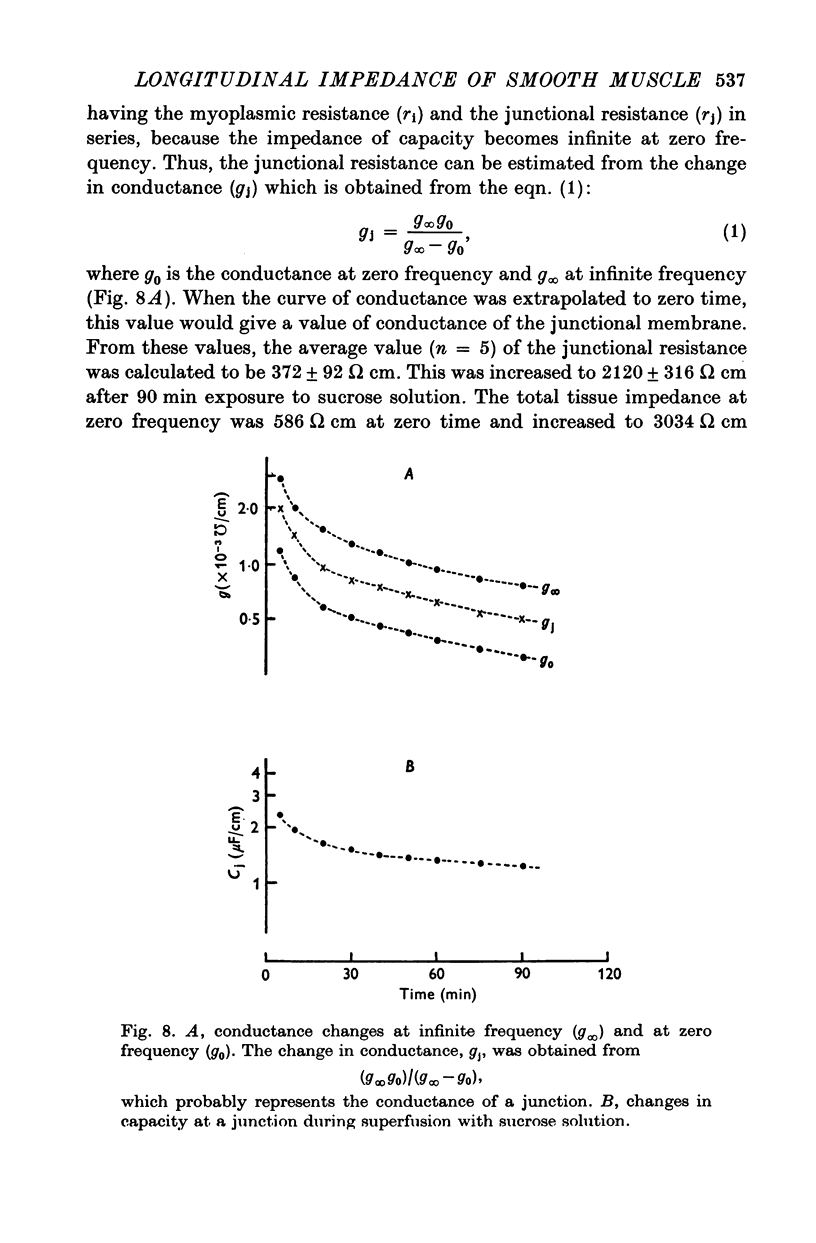
1. When the tissue impedance of the guinea-pig taenia coli was measured across a 2 mm sucrose gap in a longitudinal direction, the impedance locus could be fitted by two different circular arcs. Their characteristic frequencies were about 0-6 and 240 Hz after 60 min superfusion with sucrose solution. From the effects of changing the width of sucrose gap and of transection of tissue, and also from taking the difference between impedances measured at two distances, it was concluded that the low-frequency locus corresponds to the transverse impedance of the plasma membrane and the high-frequency locus to the longitudinal tissue impedance. 2. A change in the longitudinal tissue impedance was measured during superfusion with sucrose solution, using a frequency range between 5 Hz and 10 kHz. The admittance decreased with time of superfusion and this time course could be expressed by the sum of three exponential terms. The fastest component, having a time constant of 1-3 min at 10 kHz, was interpreted to correspond to a process of wash-out of extracellular medium. 3. Admittances at zero and infinite frequencies were obtained from the impedance locus. The decrease in these admittances with the time was analysed and the values at the start of washing were obtained by extrapolating the admittance change to zero time. 4. From these values it was estimated that the myoplasmic resistance was 214 omega cm, the junctional resistance 372 omega cm, and the junctional capacity 3-1 muF/cm at 25 degrees C. In these calculations the equivalent circuit of tissue was assumed to be expressed by two components in series: one for the myoplasmic resistance and the other for the junction which has the junctional resistance and capacity in parallel. 5. After 90 min superfusion with sucrose solution, the total tissue impedance measured at zero frequency was increased from 586 to 3034 omega cm. In the total impedance the myoplasmic resistance was increased from 214 to 914 omega cm and the junctional resistance from 372 to 2120 omega cm. Thus, the change in junctional resistance was greater than that in myoplasmic resistance during superfusion of sucrose solution.
Full text
PDF

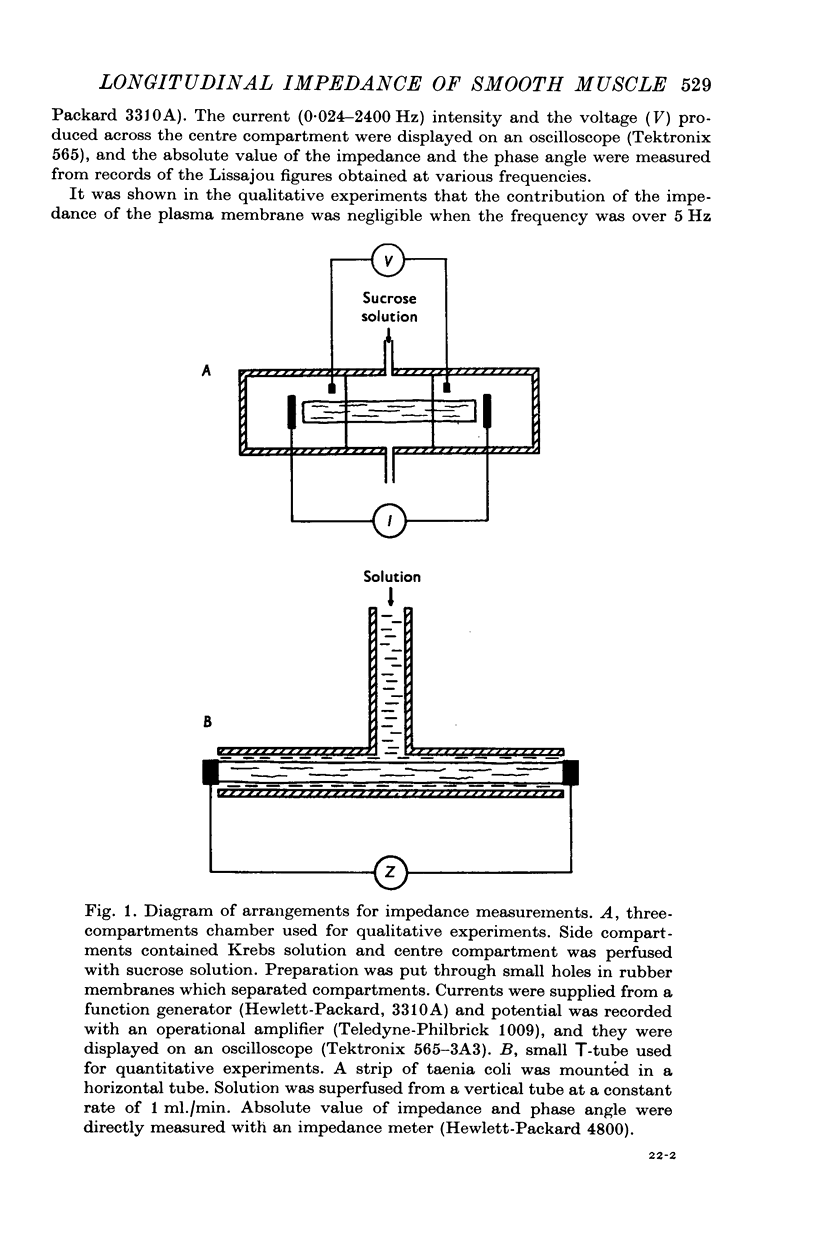






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F. Analysis of the effluxes of sodium, potassium and chloride ions from smooth muscle in normal and hypertonic solutions. J Physiol. 1971 May;214(3):393–416. doi: 10.1113/jphysiol.1971.sp009440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEWEY M. M., BARR L. A STUDY OF THE STRUCTURE AND DISTRIBUTION OF THE NEXUS. J Cell Biol. 1964 Dec;23:553–585. doi: 10.1083/jcb.23.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey M. M., Barr L. Intercellular Connection between Smooth Muscle Cells: the Nexus. Science. 1962 Aug 31;137(3531):670–672. doi: 10.1126/science.137.3531.670-a. [DOI] [PubMed] [Google Scholar]
- FATT P. AN ANALYSIS OF THE TRANSVERSE ELECTRICAL IMPEDANCE OF STRIATED MUSCLE. Proc R Soc Lond B Biol Sci. 1964 Mar 17;159:606–651. doi: 10.1098/rspb.1964.0023. [DOI] [PubMed] [Google Scholar]
- Freygang W. H., Trautwein W. The structural implications of the linear electrical properties of cardiac Purkinje strands. J Gen Physiol. 1970 Apr;55(4):524–547. doi: 10.1085/jgp.55.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kléber A. G. Effects of sucrose solution on the longitudinal tissue resistivity of trabecular muscle from mammalian heart. Pflugers Arch. 1973 Dec 18;345(3):195–205. doi: 10.1007/BF00586334. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Tomita T. The action potential in the smooth muscle of the guinea pig taenia coli and ureter studied by the double sucrose-gap method. J Gen Physiol. 1970 Feb;55(2):147–162. doi: 10.1085/jgp.55.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann F. V., Stibitz G. R., Huguenin J. Studies of impedance in cardiac tissue using sucrose gap and computer techniques. I. The influence of sucrose and oil as insulating media. Biophys J. 1973 Nov;13(11):1183–1199. doi: 10.1016/S0006-3495(73)86054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley B. A., Leung J., Eisenberg R. S. Longitudinal impedance of single frog muscle fibers. J Gen Physiol. 1975 Jan;65(1):97–113. doi: 10.1085/jgp.65.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New W., Trautwein W. Inward membrane currents in mammalian myocardium. Pflugers Arch. 1972;334(1):1–23. doi: 10.1007/BF00585997. [DOI] [PubMed] [Google Scholar]
- Tomita T. The longitudinal tissue impedance of the smooth muscle of guinea-pig taenia coli. J Physiol. 1969 Mar;201(1):145–159. doi: 10.1113/jphysiol.1969.sp008748. [DOI] [PMC free article] [PubMed] [Google Scholar]


