Abstract
To evaluate the role of fluid and Na+ balance in the development of exercise-associated hyponatremia (EAH), changes in serum Na+ concentrations ([Na+]) and in body weight were analyzed in 2,135 athletes in endurance events. Eighty-nine percent of athletes completed these events either euhydrated (39%) or with weight loss (50%) and with normal (80%) or elevated (13%) serum [Na+]. Of 231 (11%) athletes who gained weight during exercise, 70% were normonatremic or hypernatremic, 19% had a serum [Na+] between 129-135 mmol/liter, and 11% a serum [Na+] of <129 mmol/liter. Serum [Na+] after racing was a linear function with a negative slope of the body weight change during exercise. The final serum [Na+] in a subset of 18 subjects was predicted from the amount of Na+ that remained osmotically inactive at the completion of the trial. Weight gain consequent to excessive fluid consumption was the principal cause of a reduced serum [Na+] after exercise, yet most (70%) subjects who gained weight maintained or increased serum [Na+], requiring the addition of significant amounts of Na+ (>500 mmol) into an expanded volume of total body water. This Na+ likely originated from osmotically inactive, exchangeable stores. Thus, EAH occurs in athletes who (i) drink to excess during exercise, (ii) retain excess fluid because of inadequate suppression of antidiuretic hormone secretion, and (iii) osmotically inactivate circulating Na+ or fail to mobilize osmotically inactive sodium from internal stores. EAH can be prevented by insuring that athletes do not drink to excess during exercise, which has been known since 1985.
Keywords: endurance, exchangeable Na+ stores, fluid overload, overdrinking, syndrome of inappropriate ADH secretion
There have been eight reported fatalities from exercise-associated hyponatremic encephalopathy (EAHE) in marathon runners (1-6) and Army recruits (7-9) in the past 8 years. All deaths have occurred in the United States. The incidence of exercise-associated hyponatremia (EAH) (1, 2, 10-12) has also increased substantially during the same period in the United States. In contrast, EAH has become less common in South Africa (13-15), where the condition was first described (16) and in New Zealand (17), where it was once more prevalent (17-26). The reasons for this anomaly clearly invite scientific inquiry.
Some argue that EAH is caused by dehydration associated with large Na+ losses (27-33). In contrast, others propose that the most dangerous form of EAH, EAHE, occurs only in athletes who retain fluid in excess during exercise and who do not have a greater Na+ deficit after racing than do control runners without EAH (13, 26, 34) and that the condition can be reproduced in athletes who ingest fluid to excess at rest even without the development of a significant Na+ deficit (21, 35).
A theoretical diagram explaining how serum Na+ concentration [Na+] will alter with changes in Na+ and fluid balance has been published (figure 6.4 in ref. 36). In this model, weight loss during exercise causes hyponatremia as a result of dehydration and large, unreplaced Na+ losses (29). In contrast, excessive drinking leading to weight gain reduces the serum [Na+] by the dilution of the equally reduced whole-body Na+ content in an expanded volume of total body water (TBW). According to this theory, a plot of serum [Na+] and body weight change during exercise must be in the form of an inverted-U (36) (Fig. 4, which is published as supporting information on the PNAS web site).
There are no reports of EAH before 1981 (37), when athletes were advised to avoid drinking during exercise (38). Rather athletes developed elevated serum [Na+] (hypernatremia) during exercise (39-46). Since the first scientific description of EAH (16), a number of studies have reported serum [Na+] after exercise and associated body weight changes in competitors in endurance events, including 42-km marathon footraces (12, 47) and 226-km Ironman triathlons (13-15, 20, 22, 24, 26, 48). In addition are a large number of individual case studies of athletes treated for EAHE (5, 18, 23, 25, 27, 34, 49-54). Analysis of these data should be sufficient to identify the alterations in fluid and Na+ balance necessary to cause EAH.
We have combined and reanalyzed our previous data as well as the currently published case reports to answer the following three questions. First, does a plot of serum [Na+] after racing against body weight change during exercise confirm the inverted-U relationship predicted by the theory that EAH is caused by a large Na+ deficit associated with dehydration or overhydration (29, 30, 36)? Second, do athletes who develop EAHE (1, 3-9) gain or lose weight during these competitions? Third, is there any evidence that the storage of osmotically active Na+ in an osmotically inactive form contributes to the development of EAH as occurs in other forms of hyponatremia (55, 56)?
Materials and Methods
Definitions of Terms. EAH is defined as the occurrence of hyponatremia in individuals engaged in prolonged physical exercise and is defined as a serum or plasma [Na+] below the reference range for the laboratory performing the test. For most laboratories, this is a [Na+] of <35 mmol/liter (57).
Two distinct forms of EAH are recognized (57); this distinction is made on the basis of specific symptoms indicating altered function of the CNS. Athletes with any typical CNS symptoms are considered to have EAHE, whereas subjects without these specific symptoms but with a serum [Na+] of <135 mmol/liter are diagnosed as having asymptomatic hyponatremia. In view of the retrospective nature of this study, we cannot be absolutely certain that all cases of EAHE were correctly identified, but misclassification is likely only in those with either mild or absent CNS symptoms.
Body weight changes and serum [Na+] after racing from eight recent endurance events [1997 New Zealand 226-km Ironman triathlon (24); 2000 and 2001 South Africa 226-km Ironman triathlons (14, 15); 2004 New Zealand 226-km Ironman triathlon (48); 2002 Christchurch, New Zealand marathon (47); 2002 Boston marathon (12); 2003 Houston marathon (unpublished data); 2003 109-km Argus Cycle Tour, Cape Town South Africa (54)] were analyzed retrospectively for this study. Historical data were also included: four athletes each in the 1966 Witney, Oxford, United Kingdom 42-km marathon (40) and the 1970 Commonwealth Games 42-km marathon in Edinburgh, United Kingdom (41); 13 athletes competing in the 1972 Cape Town 160-km footrace (46); and 18 athletes in the 1976 42-km Jackie Gibson marathon in Johannesburg (44).
Additional data collected from all cases of EAHE occurring in marathon and Ironman triathlon events reported in the literature (49, 51-54) and in which either the body weight changes during the race or the fluid balance (difference between volumes of fluids administered or ingested and urinary losses from the time of hospital admission until the serum [Na+] normalized) changes during recovery was reported were also included. The study sample size is 2,135. Ethical approval was obtained from the Research Ethics Committee of the Faculty of Health Sciences, University of Cape Town (13-15, 54); the North Health Ethics Committee (24); the Canterbury Ethics Committee (47); the Committee on Clinical Investigation, Children's Hospital (12); and the Waikato Ethics Committee (48).
The detailed methodology is described in the published references and involved weighing and reweighing athletes before and after these races with blood drawn for the measurement of serum [Na+].
At present, the best method to determine the level of dehydration during exercise is to measure changes in TBW with deuterium oxide (58). Because this is impractical in large field studies, we used exercise-induced changes in body weight as a surrogate measure of each athlete's hydration status after racing. Because not all of the weight lost during exercise represents a fluid deficit (22, 43, 59-62), this method does not predict the exact changes in TBW. Instead on the basis of the body weight change during exercise and certain assumptions, we have classified all athletes into different subgroups based on their probable fluid status at the end of the race. In addition, we have also included a series of cut-off points for different serum [Na+] after racing, according to the probability that such low serum [Na+] levels are clinically important.
The different serum [Na+] cut-off points are the following: (i) >145 mmol/liter is referred to as hypernatremia, (ii) 135-144.9 mmol/liter is defined as normonatremia, (iii) 129-134.9 mmol/liter is defined as biochemical hyponatremia, and (iv) <128.9 mmol/liter is defined as clinically significant hyponatremia. In addition, EAHE was defined by the presence of specific CNS symptoms regardless of the actual serum [Na+]. These cut-off points are based on our joint clinical experience, which suggests that clinically significant hyponatremia begins to appear when the serum [Na+] falls below ≈129 mmol/liter (50). In chronic hyponatremia, symptoms of encephalopathy are likely to develop only at even lower serum [Na+] (55, 56).
The following cut-off points for weight changes were chosen: (i) Weight gain more than +0.0 kg is a state of overhydration, (ii) weight change between -0.01 and -3.0% body weight is considered to be euhydration, and (iii) weight change more than -3% body weight indicates increasing levels of dehydration. The calculation for euhydration is based on the evidence that not all of the weight lost during exercise is due to a fluid loss that needs to be replaced to ensure euhydration. During a 42-km marathon footrace or a 226-km Ironman triathlon, a total of ≈0.6-0.8 kg will be lost from the irreversible oxidation of fuel reserves with the production of ≈0.4 kg of metabolic water (22, 43, 59, 63). In addition, ≈1.5 kg of water stored with glycogen will be released without reducing the exchangeable TBW (43, 59). This water bound to glycogen represents a store of water that can be lost as sweat but which does not contribute to any reduction in exchangeable TBW. Thus a total weight loss of at least 2.2 kg (3.1%) could occur in a 70-kg athlete who nevertheless completed the 226-km Ironman triathlon without a change in TBW, that is, in a state of euhydration.
Detailed analysis was also performed of the fluid and electrolyte balance of 18 subjects who were studied during recovery from EAH of various severity (Tables 2 and 3, which are published as supporting information on the PNAS web site). During recovery, these athletes received either 0.9% saline i.v. (26, 34), oral food and fluids for which the Na+ content was known (23, 26), or nothing (13). The details of these calculations are described in Supporting Text, which is published as supporting information on the PNAS web site.
Statistical Analysis. Data were analyzed with statistica 5.5 (Stat-Soft, Tulsa, OK). A Pearson's product moment correlation coefficient was used to determine the relationship between serum [Na+] after racing and the percentage change in body weight relative to starting body weight before and after the endurance event. To standardize data for differences in body weight, serum [Na+] after racing was plotted against the percentage of weight change during exercise. Statistical significance was accepted as P < 0.05.
Results
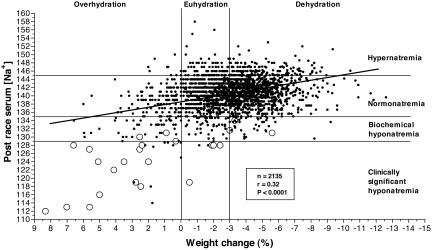
Fig. 1 shows the distribution of serum [Na+] after racing versus relative weight changes (%) for the total group of 2,135 athletes. There is a significant (P < 0.0001) linear relationship with a negative slope between these two variables so that increasing levels of weight loss are associated with higher serum [Na+]. There is no evidence for an inverted-U-shaped relationship as required by the alternate hypothesis of Armstrong (36) (Fig. 4).
Fig. 1.
The relationship between the serum [Na+] after racing and the weight change during exercise in the 2,135 athletes in this study is a linear function with a negative slope (P < 0.0001). Asymptomatic athletes are denoted by •, and athletes with symptoms considered compatible with EAHE are denoted by ○.
Table 1 provides the detailed breakdown of all 2,135 athletes classified into one of four groups on the basis of their serum [Na+] after racing and into one of three groups on the basis of their hydration state after racing. Table 1 shows that 1,712 athletes (80% of total) finished these races with serum [Na+] between 135-145 mmol/liter, defined as normonatremia. The majority (51%) of athletes with normonatremia were dehydrated (numbers read horizontally). Of all of the athletes, 269 (13%) completed these races hypernatremic with serum [Na+] of >145 mmol/liter. The majority (59%) of these athletes were dehydrated.
Table 1. Classification of 2,135 athletes on the basis of four different serum [Na+] and three different states of hydration after racing.
| Category | Hypernatremia | Normonatremia | Biochemical hyponatremia | Clinically significant hyponatremia | Total |
|---|---|---|---|---|---|
| Overhydration | |||||
| n | 11 | 151 | 44 | 25 | 231 |
| % [Na+] group | 4 | 9 | 36 | 81 | – |
| % BW group | 5 | 65 | 19 | 11 | – |
| % total | 0.5 | 7 | 2 | 1 | 11 |
| Euhydration | |||||
| n | 100 | 680 | 41 | 6 | 827 |
| % [Na+] group | 37 | 40 | 33 | 19 | – |
| % BW group | 12 | 82 | 5 | 1 | – |
| % total | 5 | 32 | 2 | 0.5 | 39 |
| Dehydration | |||||
| n | 158 | 881 | 38 | 0 | 1,077 |
| % [Na+] group | 59 | 51 | 31 | 0 | – |
| % BW group | 15 | 82 | 3 | 0 | – |
| % total | 7 | 41 | 2 | 0 | 50 |
| Total | |||||
| n | 269 | 1,712 | 123 | 31 | 2,135 |
| % total | 13 | 80 | 6 | 1 | 100 |
Values listed for “% BW group” refer to percentages of the groups of athletes categorized as overhydration, euhydration, and dehydration, which are defined as changes in fluid level of less than +0%, between –3% and +0.01%, and more than –3%, respectively. BW, body weight. Values listed for “% [Na+]” refer to percentages of the groups of athletes categorized as hypernatremia, normonatremia, biochemical hyponatremia, and clinically significant hyponatremia, which are defined as >145, 135–144.9, 129–134.9, and <128.9 mmol/liter [Na+], respectively. Values for “% total” are percentages of the total group of 2,135 athletes.
One hundred twenty-three athletes (6% of total) finished with serum [Na+] after racing of <135 mmol/liter but >128.9 mmol/liter, defined as biochemical hyponatremia. Of athletes in this group, 69% were either overhydrated (36%) or euhydrated (33%). Six of these athletes were symptomatic, three of whom were overhydrated; one was euhydrated; and two lost between -3.3 and -5.3% body weight.
Thirty-one athletes (1% of total) finished races with serum [Na+] of <128.9 mmol/liter, defined as clinically significant hyponatremia. All of these athletes were either overhydrated (81%) or euhydrated (19%); none were dehydrated. Eighteen of these athletes were overhydrated and four were euhydrated (Fig. 1). Thus, of the 24 athletes diagnosed with EAHE, the majority (75%) were overhydrated and only 5% were dehydrated. It is possible that some subjects with mild hyponatremia were incorrectly classified as EAHE.
When analyzed by fluid status after racing, 231 athletes (11% of total) finished these races overhydrated. Of this group, 70% had serum [Na+] of >135 mmol/liter and were either hypernatremic (5%) or normonatremic (65%). Of the remaining 30% of athletes who were overhydrated, 19% developed biochemical hyponatremia, but only 11% developed clinically significant hyponatremia.
Of the total number of athletes, 827 (39%) finished these races euhydrated, 82% of which also had normal serum [Na+]; 12% were hypernatremic, 5% had biochemical hyponatremia, and six athletes (1%) had serum [Na+] of <128.9 mmol/liter.
There were 1,077 athletes (50% of total) who completed these races in a dehydrated state, 82% of which had normal serum [Na+]; 15% were hypernatremic, and only 3% (38 athletes) had serum [Na+] between 129 and 134.9 mmol/liter. No dehydrated athlete had a serum [Na+] of <128.9 mmol/liter (Fig. 1).
The mean ± SD serum [Na+] was 136.1 ± 6.4 mmol/liter for athletes who gained weight during these races; 140.5 ± 3.7 mmol/liter for those who finished the race euhydrated; 141.1 ± 3.7 mmol/liter for those who were dehydrated and 140.3 ± 4.3 mmol/liter for the total group (Fig. 5, which is published as supporting information on the PNAS web site). Serum [Na+] was therefore significantly lower in the overhydrated group than in the euhydrated (P < 0.001) or dehydrated (P < 0.001) groups. Serum [Na+] was also significantly different between the euhydrated and dehydrated groups (P < 0.001). Notably, the SD was much greater in the group that gained weight during the race compared with the other groups.
Table 2 lists data from 18 athletes hospitalized for the treatment of EAHE and in whom both the Na+ deficit and whole-body fluid excess was measured during their hospital recovery (23, 26, 34). The mean Na+ deficit (ΔE mmol) was -104 ± 87 mmol (mean ± SD). Thirteen athletes had a Na+ deficit less than -133 mmol, and the largest Na+ deficit was -307 mmol. This range of Na+ deficit is not different from values measured in a control group of runners who completed ultramarathon races in South Africa with a mean Na+ deficit of 187 ± 37 (mean ± SEM) but who did not develop hyponatremia (34, 64). The average fluid excess excreted during recovery in these subjects (ΔTBW) was 2.5 ± 1.5 liters, indicating that all were overhydrated (Table 2).
Predicted serum [Na+] after racing based on fluid and electrolyte balance during the race exceeded the measured concentrations in 14 of these 18 athletes (Table 2, [Na+2p] - [Na+1p]), indicating the loss of osmotically active Na+ unaccounted for by external losses incurred during the race. This loss most likely occurred through the inactivation of osmotically active Na+. The amounts of Na+ so inactivated ranged from to -23 to -368 mmol and the mean ± SD of Na+ inactivation for all 18 athletes was -82.9 ± 154.3 mmol (Table 2). The range of osmotic activation in the four runners who showed the opposite response was from +9 to +214 mmol/liter. These amounts can be compared with the estimated average of 9 mmol of Na+ per kg of body weight that is likely to be osmotically active but exchangeable (Supporting Text).
Table 3 uses the same calculations and logic to calculate the amount of Na+ that was osmotically activated or inactivated during the period of recovery (Table 3) when the patient's symptoms had resolved (athletes 3, 7,12, and 14) or the serum [Na+] had returned to the normal range (>135 mmol/liter). Eight athletes exhibited osmotic activations of 27-357 mmol (mean, 152.9 ± 110.0 mmol) during recovery, whereas the remaining 10 athletes showed osmotic inactivation of -5 to 382 mmol of Na+ during recovery (mean, -180.0 ± -129.4 mmol). Differences in the extent of osmotic activation or inactivation between groups were significant (P < 0.0001).
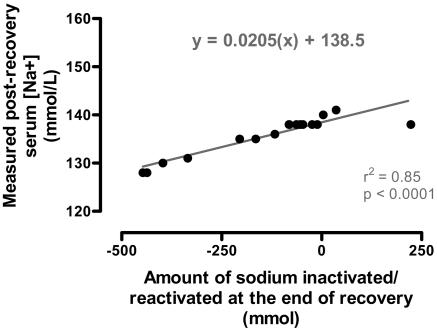
Subjects who osmotically activated sodium during recovery (Table 3) finished the trial with significantly (P < 0.005) higher serum [Na+] after racing than did those who osmotically inactivated Na+ during recovery (Table 3). Subjects who inactivated Na+ during recovery completed the trial with net Na+ deficit compared with the start of the trial because of persisting Na+ inactivation (Table 3). As a result, total Na+ balance from before the race to after recovery was significantly different between groups (P < 0.005) and was positive in the group that activated sodium during recovery. There also was a significant linear relationship between the [Na+] after recovery and the magnitude of the residual osmotic inactivation of Na+ during exercise and recovery (Fig. 2).
Fig. 2.
A significant linear (r2 = 0.85; P < 0.0001) relationship exists between the serum [Na+] and the calculated residual sodium that remained osmotically inactivated at the end of the study.
Fig. 6, which is published as supporting information on the PNAS web site, shows the risk to athletes of developing hyponatraemia of various severity with different degrees of body weight change. Thus, the probability was zero that an athlete who lost >6% body weight would develop either a serum [Na+] ≤ 135 mmol/liter or EAHE. The risk that EAH would develop first arose at a body weight loss of -6 to -2% and increased to >10% at a body weight change of -2 to 0%. Thereafter, the risk rose progressively so that those with a weight gain >4% had an ≈85% probability of developing EAH, including a 45% probability of developing EAHE.
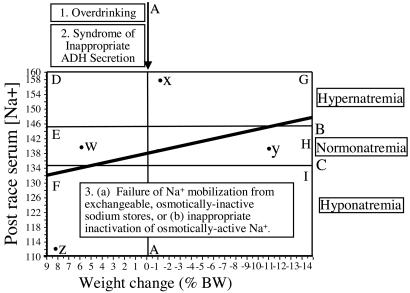
Fig. 3 summarizes the factors that explain how EAH develops. Line A divides those who gained weight (to the left of the line) from those who lost weight during exercise (to the right of the line). The area between lines B and C defines serum [Na+] in the normal range between 135 and 145 mmol/liter (normonatremia). Serum [Na+] levels above line B are elevated above the normal range (hypernatremia), whereas concentrations below line C are reduced below the normal range (hyponatremia). Also indicated in Fig. 3 are six zones, labeled D-I, that demarcate different combinations of body weight change and serum [Na+]. This relationship is also indicated for four different athletes (labeled w-z).
Fig. 3.
Three abnormalities are required for the development of EAH. The majority of subjects maintain normal serum [Na+] (normonatremia) (groups E and H between lines B and C), although they lose or gain weight during prolonged exercise. For the serum [Na+] to fall to <129 mmol/liter, (most) subjects must increase TBW by gaining weight (blocks D, E, and F to the left of Line A) as a result of overdrinking with the development of SIADH. However, the majority (70%) of athletes who gain weight during exercise nevertheless maintain normal or elevated serum [Na+]. This extra Na+ must originate from the osmotically inactive, exchangeable sodium body stores. In addition, athletes who develop severe EAH show inactivation of osmotically active Na+ during exercise (Table 2), with evidence for reactivation during recovery in some athletes (Table 3). Subjects who develop hyponatremia without an increase in TBW (Group I) may lose excessive amounts of Na+ during exercise. It is more likely that these athletes also osmotically inactivate Na+ during exercise or fail to activate osmotically inactive sodium to buffer the normal sweat and urinary Na+ losses during exercise. Points w, x, y, and z refer to individual athletes as described in the text.
Discussion
There was a linear relationship with a negative slope between the serum [Na+] after racing and the degree of weight loss in 2,135 athletes who completed 42-km marathon, 109-km cycling, or 226-km Ironman triathlon events on four continents (Fig. 1). This finding refutes the prediction that a large acute Na+ deficit associated with weight loss is an important cause of EAH (29-31, 36). Were that theory correct, this relationship would be in the form of an inverted U (Fig. 4).
Instead, the reconfirmation (14, 15, 19, 20, 24, 33) of this linear relationship with a negative slope in the largest sample yet reported proves that factors determining body weight changes during exercise are the principal determinants of the serum [Na+] after exercise. Increasing levels of weight gain during exercise increased the probability that EAH would occur (Fig. 5). Thus, athletes who gained >4% body weight during exercise had a 45% probability of developing EAHE.
The primary cause of such weight gain can only be the overconsumption of fluid during exercise (4, 12) because of an exaggerated thirst drive during exercise or because of behavioral conditioning (65). However, even in the face of significant overconsumption of fluid, weight gain should not normally occur (57). The maximum excretory capacity of the kidneys is between 750 and 1,440 ml/h (21, 35, 66). Because the rate of sweating is ≈500 ml/h, even in slow marathon runners (67), athletes should be able to safely ingest fluid at rates in excess of 1.5 liters/h without the development of EAH.
Instead, it seems likely that fluid retention occurs at substantially lower rates of fluid overconsumption (23, 26, 54, 68, 69) because of the syndrome of inappropriate ADH secretion (SIADH) (55-57, 70, 71).
Thus, Fig. 3 shows that the inadequate suppression of ADH secretion would produce the fluid retention that causes subjects who drink to excess during exercise to gain weight and, thus, to finish to the left of line A drawn at a weight change of 0%. Two lines of evidence argue that the overconsumption of fluid and SIADH cannot be the sole etiological factors for this condition.
First is the finding that only 69 (30%) of the 231 subjects who completed these races with a positive weight gain (Fig. 3, blocks D-F, and corresponding data in Fig. 1 and Table 1) developed EAH (Fig. 3, block F, and corresponding data in Fig. 1 and Table 1). Thus, another factor protected 70% of those who overdrank and gained weight during exercise from developing EAH.
Second is the finding that at any degree of weight loss or gain, the range of serum [Na+] after racing varied by as much as 34 mmol/liter (athletes with a weight loss of 0.5% in Fig. 1). For a TBW of 40 liters, this difference in serum [Na] represents a difference of 1,360 mmol (31 g of Na+ or 80 of NaCl) in their respective exchangeable, ionized Na+ stores. There is no evidence that such large differences in serum [Na+] exist before exercise in healthy athletes (14, 15, 46, 51); thus, they must develop during exercise. Nor could these differences be due to individual differences in Na+ intake (usually ≈300 mmol) or Na+ losses (usually ≈300-500 mmol) (Table 2) during exercise.
The only possibility is that some athletes can mobilize the same amount of Na+ from internal sodium sources, the so-called osmotically inactive, exchangeable sodium stores (72). Alternatively, some athletes may prevent the inappropriate osmotic inactivation of Na+ during exercise. Already in 1936, Harrison et al. (73) reported that “about one-fourth of the total sodium in the body must exist in bone and cartilage either in an insoluble compound or in a compound in which the sodium is not dissociated” (p. 528), that is, in an osmotically inactive form. Subsequently, a growing collection of papers have considered the role of the osmotically inactive but exchangeable sodium stores in health and disease (e.g., refs. 74-76). Redistribution of Na+ within these different stores during exercise could explain the logical anomalies identified in Fig. 1.
Indeed, the data presented in Tables 2 and 3 provide evidence for osmotic inactivation of Na+ during exercise in 14 of 18 subjects who developed EAH and whose fluid and Na+ balance was studied during recovery (Table 2). There was also evidence for reactivation of osmotically inactive sodium during recovery in eight of these subjects (Table 3). In general, the extent of inactivation (Table 2) and subsequent reactivation (Table 3) was greatest in those with the lowest serum [Na+] after racing so that the two athletes with the lowest serum [Na+] after racing (113 mmol/liter) (Tables 2 and 3, athletes 1 and 2) inactivated 368 and 262 mmol, respectively, during the 90-km Comrades marathon and then reactivated essentially the same amounts (357 and 228 mmol, respectively) during recovery.
Na+ inactivation continued during recovery in 10 athletes (Table 3) so that there was a significant inverse relationship between the serum [Na+] at the end of the study and the total amount of sodium that remained osmotically inactivated at that time (Fig. 2). The gradient of this relationship is defined by the size of the TBW for this group and predicts a TBW of 48.8 liters compared with the likely real TBW of 38.5 liters (Table 2). Thus, the amount of electrolyte that were osmotically modified during recovery was underestimated by ≈27%. Simultaneous osmotic inactivation of Na+ and K+ in a molar ratio of ≈2:1 would explain this finding.
We are not the first to identify the possible contribution of osmotic inactivation of Na+ in acute hyponatraemia (72). The original studies of SIADH (56, 70, 71) reported that the quantities of the Na+ lost and the water retained could not explain the extent by which serum [Na+] was reduced in severely hyponatraemic patients or the extent to which it increased during recovery (56). Rather, the conversion of osmotically active Na+ to stored osmotically inactive sodium was the probable explanation (56, 72) with reactivation of osmotically inactive exchangeable sodium during recovery (55), as we also show in our subjects (Table 3).
Thus, the 70% of athletes who overconsumed fluid in this study maintained their serum [Na+] despite an increase in TBW during exercise and sizeable Na+ losses in sweat, because (i) they osmotically activated sodium from exchangeable stores and (ii) they did not osmotically inactivate Na+ (and K+) during exercise. In contrast, athletes with the most severe EAH osmotically inactivated substantial Na+ during exercise (Table 2).
A failure to mobilize these exchangeable sodium stores to compensate for the inevitable Na+ losses would also explain why the serum [Na+] can fall in some athletes who do not gain weight during exercise (Fig. 3, block I, and relevant data in Fig. 1 and Table 1).
The magnitude of this effect is remarkable. For example, to achieve their measured serum Na+ concentrations after racing, the athletes identified as w and x in Fig. 3 must have mobilized 588 and ≈700 mmol Na+ from the osmotically inactive stores over and above the normal losses of ≈200 mmol Na+ expected in the 42-km marathon race that each ran. Similarly, the athlete with the lowest serum [Na+] after racing (Table 3, athlete 1) reactivated 357 mmol Na+ during recovery.
In contrast, athletes y and z (Fig. 3) must either have lost in urine and sweat or osmotically inactivated ≈700 and 448 mmol of Na+ to explain their respective serum Na+ concentrations after racing. Similarly, the subset of subjects who developed the most severe EAH, osmotically inactivated ≈300 mmol of Na+ during exercise (Table 2) or up to 450 mmol during recovery (Table 3).
Accordingly, these data establish that EAH occurs as a result of at least three different biological mechanisms working in tandem: overdrinking; inappropriate ADH secretion; and a failure to mobilize Na+ from the osmotically inactive, exchangeable sodium stores or, alternatively, osmotic inactivation of Na+ as reported in other conditions (72, 77, 78). Only in the presence of all three abnormalities can athletes move from the normal response (Fig. 3, Group H) to one in which fluid overload develops but the serum [Na+] is protected (Fig. 3, Group E) or even increased (Fig. 3, Group D) to the group in which EAH occurs (Fig. 3, Group F).
These findings have important clinical implications.
First, the vast majority (80%) of athletes (Table 1) homeostatically regulate their serum [Na+] between 135-145 mmol/liter despite losing or gaining TBW, even within the range of body weight changes of -12 to +6% body weight (Fig. 1). This effective homeostasis must result from the buffering effect of the exchangeable osmotically inactive body sodium stores in response to the loss of osmotically active Na+ in urine and sweat during exercise and simultaneous changes in TBW (78).
Second, subjects who maintained their serum [Na+] in the presence of fluid overload (Fig. 3, blocks D and E, and relevant data in Fig. 1 and Table 1) remained asymptomatic.
Third, it is frequently claimed that the ingestion of sports drinks during prolonged exercise will prevent EAH (29, 32, 79-81). However, persons with an increased TBW as a result of SIADH retain fluid but excrete any Na+ present in the ingested or infused fluids (56) as a result of (i) suppression of aldosterone secretion consequent to an increase in the extracellular fluid volume; (ii) an increase in the filtered load of Na+ resulting from an increase in the glomerular filtration rate; or (iii) suppression of proximal tubular reabsorption of Na+ in response to expansion of the extracellular fluid volume, the so-called “third factor” effect. This third factor is now considered to result from intrarenal hemodynamic factors, in particular, an increased renal perfusion pressure (82).
Fluid retention with Na+ excretion when hypotonic sports drinks are ingested by athletes with SIADH must produce a progressive hyponatremia as confirmed clinically (3, 12) and experimentally (67, 83) and predicted mathematically (84). Instead, athletes with SIADH and an increase in TBW can increase their serum [Na+] only if they receive markedly hypertonic Na+ solutions orally or i.v. (1, 10), in part also to normalize the TBW (as a result of the resulting osmotic diuresis). Only the mobilization of the osmotically active exchangeable sodium stores can prevent EAH in those who overdrink and those who develop SIADH during prolonged exercise.
In summary, three independent mechanisms explain why some athletes develop EAH and EAHE during and after prolonged exercise: (i) overdrinking due to biological or psychological factors; (ii) inappropriate ADH secretion, in particular, the failure to suppress ADH secretion in the face of an increase in TBW; and (iii) a failure to mobilize Na+ from the osmotically inactive sodium stores or alternatively inappropriate osmotic inactivation of circulating Na+. Because the mechanisms causing factors ii and iii are currently unknown, it follows that the prevention of EAH and EAHE requires that athletes be encouraged to avoid overdrinking during exercise, which has been known since 1985 (16) and clinically proven since 1991 (34).
Guidelines that encourage overdrinking during exercise (32, 79, 80) should be modified (85) to negate the significant threat that these guidelines continue to pose to the health of active exercisers (4, 12, 18, 86).
Supplementary Material
Acknowledgments
We thank many colleagues in Boston, Christchurch, and Cape Town for completing the original studies on which this analysis is based and Dr. Jacqueline Hilton, Mr. John M. D. Thompson, and Dr. Lucy-May Holtzhausen for special contributions. T.D.N., K.S., T.H., and J.D. were supported by Harry Crossley and Nellie Atkinson Staff Research Funds from the University of Cape Town and by the Medical Research Council of South Africa, Discovery Health, Bromor Foods, and the National Research Foundation of South Africa through the Technology and Human Resources for Industry Programme initiative.
Author contributions: T.D.N., K.S., D.S., T.H., S.R., J.D., C.A., and P.W. designed research; T.D.N., K.S., D.S., T.H., S.R., J.D., C.A., and P.W. performed research; T.D.N., C.A., and L.W. contributed new reagents/analytic tools; and T.D.N., K.S., D.S., T.H., S.R., J.D., C.A., P.W., and L.W. analyzed data and wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: TBW, total body water; EAH, exercise-associated hyponatremia; EAHE, exercise-associated hyponatremic encephalopathy; SIADH, syndrome of inappropriate ADH secretion.
References
- 1.Ayus, J. C., Varon, J. & Arieff, A. I. (2000) Ann. Intern. Med. 132, 711-714. [DOI] [PubMed] [Google Scholar]
- 2.Hew, T. D., Chorley, J. N., Cianca, J. C. & Divine, J. G. (2003) Clin. J. Sport Med. 13, 41-47. [DOI] [PubMed] [Google Scholar]
- 3.Smith, S. (August 13, 2002) The Boston Globe, p. A1.
- 4.Noakes, T. D. (2003) Br. Med. J. 327, 113-114.12869424 [Google Scholar]
- 5.Thompson, J.-A. & Wolff, A. J. (2003) Chest 124, Suppl., 313. [Google Scholar]
- 6.Zorn, E. (October 11, 1999) Chicago Tribune, p. 1.
- 7.O'Brien, K. K., Montain, S. J., Corr, W. P., Sawka, M. N., Knapik, J. J. & Craig, S. C. (2001) Mil. Med. 166, 405-410. [PubMed] [Google Scholar]
- 8.Gardner, J. W. (2002) Mil. Med. 167, 432-434. [PubMed] [Google Scholar]
- 9.Garigan, T. P. & Ristedt, D. E. (1999) Mil. Med. 164, 234-238. [PubMed] [Google Scholar]
- 10.Davis, D. P., Videen, J. S., Marino, A., Vilke, G. M., Dunford, J. V., Van Camp, S. P. & Maharam, L. G. (2001) J. Emerg. Med. 21, 47-57. [DOI] [PubMed] [Google Scholar]
- 11.Almond, C. S., Fortescue, E. B., Shin, A. Y., Mannix, R. & Greenes, D. S. (2003) Acad. Emerg. Med. 10, 534-535. [Google Scholar]
- 12.Almond, C. S., Shin, A. Y., Fortescue, E. B., Mannix, R. C., Wypij, D., Binstadt, B. A., Duncan, C. N., Olson, D. P., Salerno, A. E., Newburger, J. W., et al. (2005) N. Engl. J. Med. 352, 1550-1556. [DOI] [PubMed] [Google Scholar]
- 13.Noakes, T. D., Sharwood, K., Collins, M. & Perkins, D. R. (2004) Br. J. Sports Med. 38, E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharwood, K. A., Collins, M., Goedecke, J. H., Wilson, G. & Noakes, T. D. (2004) Br. J. Sports Med. 38, 718-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharwood, K., Collins, M., Goedecke, J., Wilson, G. & Noakes, T. (2002) Clin. J. Sport Med. 12, 391-399. [DOI] [PubMed] [Google Scholar]
- 16.Noakes, T. D., Goodwin, N., Rayner, B. L., Branken, T. & Taylor, R. K. (1985) Med. Sci. Sports Exercise 17, 370-375. [PubMed] [Google Scholar]
- 17.Speedy, D. B., Thompson, J. M., Rodgers, I., Collins, M., Sharwood, K. & Noakes, T. D. (2002) Clin. J. Sport Med. 12, 279-284. [DOI] [PubMed] [Google Scholar]
- 18.Speedy, D. B., Rogers, I. R., Noakes, T. D., Thompson, J. M., Guirey, J., Safih, S. & Boswell, D. R. (2000) Clin. J. Sport Med. 10, 52-58. [DOI] [PubMed] [Google Scholar]
- 19.Speedy, D. B., Campbell, R., Mulligan, G., Robinson, D. J., Walker, C., Gallagher, P. & Arts, J. H. (1997) Clin. J. Sport Med. 7, 100-103. [DOI] [PubMed] [Google Scholar]
- 20.Speedy, D. B., Faris, J. G., Hamlin, M., Gallagher, P. G. & Campbell, R. G. (1997) Clin. J. Sport Med. 7, 180-184. [DOI] [PubMed] [Google Scholar]
- 21.Speedy, D. B., Noakes, T. D., Boswell, T., Thompson, J. M., Rehrer, N. & Boswell, D. R. (2001) Med. Sci. Sports Exercise 33, 1434-1442. [DOI] [PubMed] [Google Scholar]
- 22.Speedy, D. B., Noakes, T. D., Kimber, N. E., Rogers, I. R., Thompson, J. M., Boswell, D. R., Ross, J. J., Campbell, R. G., Gallagher, P. G. & Kuttner, J. A. (2001) Clin. J. Sport Med. 11, 44-50. [DOI] [PubMed] [Google Scholar]
- 23.Speedy, D. B., Noakes, T. D., Rogers, I. R., Hellemans, I., Kimber, N. E., Boswell, D. R., Campbell, R. & Kuttner, J. A. (2000) Clin. J. Sport Med. 10, 136-141. [DOI] [PubMed] [Google Scholar]
- 24.Speedy, D. B., Noakes, T. D., Rogers, I. R., Thompson, J. M., Campbell, R. G., Kuttner, J. A., Boswell, D. R., Wright, S. & Hamlin, M. (1999) Med. Sci. Sports Exercise 31, 809-815. [DOI] [PubMed] [Google Scholar]
- 25.Speedy, D. B., Rogers, I. R., Safih, S. & Foley, B. (2000) J. Emerg. Med. 18, 41-44. [DOI] [PubMed] [Google Scholar]
- 26.Speedy, D. B., Rogers, I. R., Noakes, T. D., Wright, S., Thompson, J. M., Campbell, R., Hellemans, I., Kimber, N. E., Boswell, D. R., Kuttner, J. A., et al. (2000) Clin. J. Sport Med. 10, 272-278. [DOI] [PubMed] [Google Scholar]
- 27.Hiller, W. D., O'Toole, M. L., Fortess, E. E., Laird, R. H., Imbert, P. C. & Sisk, T. D. (1987) Am. J. Sports Med. 15, 164-167. [DOI] [PubMed] [Google Scholar]
- 28.Hiller, W. D. (1989) Med. Sci. Sports Exercise 21, S219-S221. [PubMed] [Google Scholar]
- 29.Murray, B. & Eichner, E. R. (2004) Curr. Sports Med. Rep. 3, 117-118. [DOI] [PubMed] [Google Scholar]
- 30.Murray, B., Stofan, J. & Eichner, E. R. (2004) Marathon & Beyond 8, 77-92. [Google Scholar]
- 31.Murray, B. (2004) Hyponatremia In Athletes, GSSI Sports Science News, www.gssiweb.com/reflib/refs/624/ssn_hyponatremia.cfm?pid=38. Newsletter accessed April 22, 2005.
- 32.Coyle, E. F. (2004) J. Sports Sci. 22, 39-55. [DOI] [PubMed] [Google Scholar]
- 33.O'Toole, M. L., Douglas, P. S., Laird, R. H. & Hiller, D. B. (1995) Clin. J. Sport Med. 5, 116-122. [DOI] [PubMed] [Google Scholar]
- 34.Irving, R. A., Noakes, T. D., Buck, R., van Zyl, S. R., Raine, E., Godlonton, J. & Norman, R. J. (1991) J. Appl. Physiol. 70, 342-348. [DOI] [PubMed] [Google Scholar]
- 35.Noakes, T. D., Wilson, G., Gray, D. A., Lambert, M. I. & Dennis, S. C. (2001) S. Afr. Med. J. 91, 852-857. [PubMed] [Google Scholar]
- 36.Armstrong, L. E. (2000) in Performing in Extreme Environments, ed. Armstrong, L. E. (Human Kinetics, Champaign, IL), pp. 103-135.
- 37.Noakes, T. D. (1981) S. Afr. Runner (September 4), pp. 8-10.
- 38.Noakes, T. D. (1993) Exercise Sport Sci. Rev. 21, 297-330. [PubMed] [Google Scholar]
- 39.Noakes, T. D. (1995) Clin. J. Sport Med. 5, 123-128. [PubMed] [Google Scholar]
- 40.Pugh, L. G., Corbett, J. L. & Johnson, R. H. (1967) J. Appl. Physiol. 23, 347-352. [DOI] [PubMed] [Google Scholar]
- 41.Muir, A. L., Percy-Robb, I. W., Davidson, I. A., Walsh, E. G. & Passmore, R. (1970) Lancet 2, 1125-1128. [DOI] [PubMed] [Google Scholar]
- 42.Rose, L. I., Carroll, D. R., Lowe, S. L., Peterson, E. W. & Cooper, K. H. (1970) J. Appl. Physiol. 29, 449-451. [DOI] [PubMed] [Google Scholar]
- 43.Shephard, R. J. & Kavanagh, T. (1978) Physician Sports Med. 6, 90-102. [DOI] [PubMed] [Google Scholar]
- 44.Cohen, I. & Zimmerman, A. L. (1978) S. Afr. Med J. 53, 449-453. [PubMed] [Google Scholar]
- 45.McKechnie, J. K., Leary, W. P. & Noakes, T. D. (1982) S. Afr. Med. J. 61, 482-484. [PubMed] [Google Scholar]
- 46.Noakes, T. D. & Carter, J. W. (1976) S. Afr. Med. J. 50, 1562-1566. [PubMed] [Google Scholar]
- 47.Reid, S. A., Speedy, D. B., Thompson, J. M., Noakes, T. D., Mulligan, G., Page, T., Campbell, R. G. & Milne, C. (2004) Clin. J. Sport Med. 14, 344-353. [DOI] [PubMed] [Google Scholar]
- 48.Wharam, P. C., Speedy, D. B., Noakes, T. D., Thompson, J. M., Reid, S. A. & Holtzhausen, L. M. (2005) Med. Sci. Sports Exercise, in press. [DOI] [PubMed]
- 49.Frizzell, R. T., Lang, G. H., Lowance, D. C. & Lathan, S. R. (1986) J. Am. Med. Assoc. 255, 772-774. [PubMed] [Google Scholar]
- 50.Noakes, T. (2002) Curr. Sports Med. Rep. 1, 197-207. [DOI] [PubMed] [Google Scholar]
- 51.Noakes, T. D., Norman, R. J., Buck, R. H., Godlonton, J., Stevenson, K. & Pittaway, D. (1990) Med. Sci. Sports Exercise 22, 165-170. [PubMed] [Google Scholar]
- 52.Clark, J. M. & Gennari, F. J. (1993) West. J. Med. 159, 188-189. [PMC free article] [PubMed] [Google Scholar]
- 53.Surgenor, S. & Uphold, R. E. (1994) Am. J. Emerg. Med. 12, 441-444. [DOI] [PubMed] [Google Scholar]
- 54.Dugas, J. P. & Noakes, T. D. (2005) Br. J. Sports Med. 39, e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nolph, K. D. & Schrier, R. W. (1970) Am. J. Med. 49, 534-545. [DOI] [PubMed] [Google Scholar]
- 56.Bartter, F. C. & Schwartz, W. B. (1967) Am. J. Med. 42, 790-806. [DOI] [PubMed] [Google Scholar]
- 57.Hew-Butler, T. D., Almond, C. S., Ayus, J. C., Dugas, J. P., Meeuwisse, W. H., Noakes, T. D., Reid, S. A., Siegel, A. J., Speedy, D. B., Stuempfle, K. J., et al. (2005) Clin. J. Sport Med. 15, 207-213. [DOI] [PubMed] [Google Scholar]
- 58.Ellis, K. J. & Wong, W. W. (1998) J. Appl. Physiol. 85, 1056-1062. [DOI] [PubMed] [Google Scholar]
- 59.Pastene, J., Germain, M., Allevard, A. M., Gharib, C. & Lacour, J. R. (1996) Eur. J Appl. Physiol. Occup. Physiol. 73, 49-55. [DOI] [PubMed] [Google Scholar]
- 60.Rogers, G., Goodman, C. & Rosen, C. (1997) Med. Sci. Sports Exercise 29, 1477-1481. [DOI] [PubMed] [Google Scholar]
- 61.Glace, B., Murphy, C. & McHugh, M. (2002) Int. J. Sport Nutr. Exercise Metab. 12, 414-427. [DOI] [PubMed] [Google Scholar]
- 62.Glace, B. W., Murphy, C. A. & McHugh, M. P. (2002) J. Am. Coll. Nutr. 21, 553-559. [DOI] [PubMed] [Google Scholar]
- 63.Noakes, T. D. (2000) Scand. J. Med. Sci. Sports 10, 123-145. [DOI] [PubMed] [Google Scholar]
- 64.Irving, R. A., Noakes, T. D., Burger, S. C., Myburgh, K. H., Querido, D. & van Zyl, S. R. (1990) Med. Sci. Sports Exercise 22, 581-587. [DOI] [PubMed] [Google Scholar]
- 65.Hew, T. D. (2003) Clin. J. Sport Med. 13, 192-193.12792218 [Google Scholar]
- 66.Armstrong, L. E., Curtis, W. C., Hubbard, R. W., Francesconi, R. P., Moore, R. & Askew, E. W. (1993) Med. Sci. Sports Exercise 25, 543-549. [PubMed] [Google Scholar]
- 67.Twerenbold, R., Knechtle, B., Kakebeeke, T. H., Eser, P., Muller, G., von Arx, P. & Knecht, H. (2003) Br. J. Sports Med. 37, 300-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stuempfle, K. J., Lehmann, D. R., Case, H. S., Bailey, S., Hughes, S. L., McKenzie, J. & Evans, D. (2002) Alaska Med. 44, 51-55. [PubMed] [Google Scholar]
- 69.Stuempfle, K. J., Lehmann, D. R., Case, H. S., Hughes, S. L. & Evans, D. (2003) Clin. J. Sport Med. 13, 171-175. [DOI] [PubMed] [Google Scholar]
- 70.Schwartz, W. B., Bennett, W., Curelop, S. & Bartter, F. C. (1957) Am. J. Med. 23, 529-542. [DOI] [PubMed] [Google Scholar]
- 71.Kaye, M. (1966) Am. J. Med. 41, 910-926. [DOI] [PubMed] [Google Scholar]
- 72.Nguyen, M. K. & Kurtz, I. (2004) Am. J. Physiol. 287, F172-F180. [DOI] [PubMed] [Google Scholar]
- 73.Harrison, H. E., Darrow, D. C. & Yannet, H. (1936) J. Biol. Chem. 113, 515-529. [Google Scholar]
- 74.Edelman, I. S., James, A. H., Baden, H. & Moore, F. D. (1954) J. Clin. Invest. 33, 122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Wardener, H. E. & Herxheimer, A. (2000) J. Am. Soc. Nephrol. 11, 980-987.10858040 [Google Scholar]
- 76.Titze, J., Shakibaei, M., Schafflhuber, M., Schulze-Tanzil, G., Porst, M., Schwind, K. H., Dietsch, P. & Hilgers, K. F. (2004) Am. J. Physiol. 287, H203-H208. [DOI] [PubMed] [Google Scholar]
- 77.Titze, J., Bauer, K., Schafflhuber, M., Dietsch, P., Lang, R., Schwind, K. H., Luft, F. C., Eckardt, K. U. & Hilgers, K. F. (2005) Am. J. Physiol. 289, F793-F802. [DOI] [PubMed] [Google Scholar]
- 78.Stern, T. N., Cole, V. V., Bass, A. C. & Overman, R. R. (1951) Am. J. Physiol. 164, 437-449. [DOI] [PubMed] [Google Scholar]
- 79.Convertino, V. A., Armstrong, L. E., Coyle, E. F., Mack, G. W., Sawka, M. N., Senay, L. C., Jr., & Sherman, W. M. (1996) Med. Sci. Sports Exercise 28 (1), i-vii. [DOI] [PubMed] [Google Scholar]
- 80.Armstrong, L. E., Epstein, Y., Greenleaf, J. E., Haymes, E. M., Hubbard, R. W., Roberts, W. O. & Thompson, P. D. (1996) Med. Sci. Sports Exercise 28 (12), i-x. [PubMed] [Google Scholar]
- 81.Montain, S. J., Sawka, M. N. & Wenger, C. B. (2001) Exercise Sport Sci. Rev. 29, 113-117. [DOI] [PubMed] [Google Scholar]
- 82.Verbalis, J. G. (2003) Best Pract. Res. Clin. Endocrinol. Metab. 17, 471-503. [DOI] [PubMed] [Google Scholar]
- 83.Noakes, T. (2004) Br. J. Sports Med. 38, 790-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weschler, L. B. (2005) Sports Med. 35, 899-922. [DOI] [PubMed] [Google Scholar]
- 85.Hsieh, M. (2004) Sports Med. 34, 231-238. [DOI] [PubMed] [Google Scholar]
- 86.Noakes, T. D. (2003) Clin. J. Sport Med. 13, 309-318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.