Abstract
Establishment of latent infection and reactivation from latency are critical aspects of herpesvirus infection and pathogenesis. Interfering with either of these steps in the herpesvirus life cycle may offer a novel strategy for controlling herpesvirus infection and associated disease pathogenesis. Prior studies show that mice deficient in gamma interferon (IFN-γ) or the IFN-γ receptor have elevated numbers of cells reactivating from murine gammaherpesvirus 68 (γHV68) latency, produce infectious virus after the establishment of latency, and develop large-vessel vasculitis. Here, we demonstrate that IFN-γ is a powerful inhibitor of reactivation of γHV68 from latency in tissue culture. In vivo, IFN-γ controls viral gene expression during latency. Importantly, depletion of IFN-γ in latently infected mice results in an increased frequency of cells reactivating virus. This demonstrates that IFN-γ is important for immune surveillance that limits reactivation of γHV68 from latency.
Gammaherpesviruses characteristically establish latent and persistent infections in their hosts after immunologically mediated clearance of the acute infection. Chronic infection is critical to the life cycle of the virus, as latency and persistent infection serve as viral reservoirs for spread and likely contribute to disease pathogenesis. Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV) are human gammaherpesviruses that have been associated with the development of cancers, especially in immunocompromised patients. EBV is associated with undifferentiated nasopharyngeal carcinoma (23), posttransplant lymphoproliferative disease (19), and endemic Burkitt's lymphoma (40); KSHV is associated with KS lesions (5, 34), pleural effusion lymphomas (36), multicentric Castleman's disease (14), and in one report pulmonary hypertension (8). Despite the prevalence of chronic gammaherpesvirus infections and their extensive impact on the immunocompromised patient population, there is much to be learned about how these chronic infections are controlled by the host.
The species specificity of EBV and KSHV limits pathogenesis and immunity studies. Murine gammaherpesvirus 68 (γHV68) provides a tractable small animal model with which to study gammaherpesvirus infection. γHV68 has areas of colinear sequence homology with EBV, KSHV, and the primate gammaherpesvirus herpesvirus saimiri (59). Over the past several years, multiple laboratories have provided important insights into gammaherpesvirus pathogenesis and immunity using this model system. Similar to the human gammaherpesviruses, γHV68 establishes both acute and chronic infections, the latter associated with the development of disease in immunocompromised mice. Chronic infection with γHV68 is associated with atherosclerosis, tumor induction, and severe arteritis in immunocompromised mice (1, 9, 10, 47, 52, 61). Given the ability of γHV68 to establish latent infection and induce diseases in immunocompromised hosts, γHV68 is a useful model for investigating control of chronic gammaherpesvirus infection.
Gamma interferon (IFN-γ) has been implicated in the control of chronic gammaherpesvirus infection in both humans and mice. In a small study, an IFN-γ gene polymorphism associated with low production of IFN-γ positively segregated with development of posttransplant lymphoproliferative disease in renal transplant patients (56). Recently, this same gene polymorphism was associated with the development of EBV-positive lymphoproliferative disease in the human peripheral blood lymphocyte-SCID mouse model (11). Additionally, lymphocytes from patients with undifferentiated carcinoma of nasopharyngeal type were shown to have depressed IFN-γ secretion (65).
These data are consistent with work demonstrating that the murine pathogen, γHV68, is susceptible to control by IFN-γ. Interestingly, the absence of IFN-γ signaling has no effect on the acute phase of γHV68 infection (43, 61). However, mice deficient in the IFN-γ receptor develop a large-vessel vasculitis after chronic infection with γHV68 (61). IFN-γ−/− mice have elevated numbers of cells reactivating from viral latency, as well as production of infectious virus after the establishment of latency, referred to here as persistent replication (18, 53). IFN-γ is required for the antiviral activity of T cells in B-cell-deficient mice (6). Furthermore, IFN-γ is required for CD4 T-cell-mediated control of γHV68 reactivation efficiency and the number of latently infected cells, as well as CD4 helper function-independent control of viral replication (46). Together, these studies demonstrate that IFN-γ signaling is essential for control of chronic γHV68 infection, persistent viral replication, and the resulting vasculitis.
Based on these data, we hypothesized that IFN-γ is a key regulator of the transition between latent infection and the emergence of γHV68 from latently infected cells. Reactivation of a herpesvirus from latency consists of many stages (including initiation of lytic gene expression, viral protein synthesis, viral assembly, and viral release). With this in mind, we refer here to reactivation as the whole process by which a previously latent cell produces infectious virus.
In this study, we demonstrate that IFN-γ suppresses γHV68 reactivation from latency. We describe the kinetics of viral reactivation from latently infected cells and IFN-γ-mediated inhibition of this phenomenon by an ex vivo reactivation assay. Further, we demonstrate that IFN-γ controls viral gene expression in vivo during chronic infection. Finally, we show that in vivo depletion of IFN-γ after the establishment of latency leads to increased reactivation of virus from latently infected cells. Together, these data demonstrate a critical role for IFN-γ in immune surveillance of γHV68 reactivation and gene expression.
MATERIALS AND METHODS
Virus and mouse infections.
γHV68 clone WUMS (ATCC VR1465) was passaged, and titers were determined by plaque assay of NIH 3T12 cells (60). Mice were housed at the Washington University School of Medicine in accordance with all federal and university guidelines. C57BL/6J mice (B6; Jackson no. 000664) were obtained from the Jackson Laboratory (Bar Harbor, Maine). Wild-type mice, mice deficient in IFN-γ receptor chain 1 (IFN-γR−/−) (24), and mice lacking both the IFN-α receptor chain 1 and the IFN-γ receptor chain 1 (IFN-αβγR−/−) (57) on the 129 Pas background (13) were obtained from B & K Universal (Aldbrough, England). Unless otherwise stated, mice were infected intraperitoneally between 8 and 12 weeks of age with 106 PFU of γHV68 in 0.5 ml of Dulbecco's modified Eagle medium containing 10% fetal calf serum. Mice were sacrificed, and peritoneal cells from four to five mice per group were harvested and pooled as previously described (21, 62).
Reagents and antibodies.
Recombinant mouse IFN-γ was generously supplied by Robert Schreiber and commercially obtained from R & D Systems. Hybridomas producing the IFN-γ-specific monoclonal antibody, H22 (2, 45), and its isotype control antibody, PIP (32), were obtained from Robert Schreiber. These hybridomas were grown in protein-free medium (Gibco Media; Invitrogen, Carlsbad, Calif.) in Celline CL1000 flasks (Integra Biosciences, Ijamsville, Maryland). Supernatants from these flasks were sterile filtered and quantified by enzyme-linked immunosorbent assay. The concentration of antibody (approximately 1 μg/ml) needed to neutralize 10 U of IFN-γ/ml used was determined by enzyme-linked immunosorbent assay for macrophage-inducible nitric oxide synthase production (12; data not shown). We used 10 μg/ml to fully neutralize 10 U of IFN-γ/ml in the ex vivo neutralization experiments.
Determination of the frequency of reactivation and frequency of infected cells by limiting dilution.
Reactivation from latency was assayed as previously described (60) by plating limiting dilutions of cells onto permissive mouse embryonic fibroblast (MEF) monolayers and scoring for cytopathic effect (CPE) as a result of infectious virus 3 weeks later. Serial twofold dilutions of cells (24 wells/dilution) were plated onto an indicator monolayer of IFN-αβγR−/− MEFs in 96-well tissue culture plates. To distinguish between reactivation from latency and preformed infectious virus present in these cells, we plated parallel cell samples after mechanical disruption. This procedure destroys >99% of the cells but has minimal effect on preformed infectious virus, thus allowing distinction between reactivation from latency (which requires live cells) and preformed infectious virus (62). For kinetic analysis of reactivation, plates were freeze-thawed three times, and supernatants from these plates were plated onto new MEF monolayers in 96-well plates to detect infectious virus. To determine the effect of IFN-γ on detection of CPE in MEF cultures, an indicator monolayer of IFN-αβγR−/− MEFs in 96-well tissue culture plates was infected with γHV68 in the presence or absence of IFN-γ and scored for infectious virus 10 days later. The sensitivity of this assay was determined to be approximately 0.3 PFU per well. To determine the frequency of cells carrying the γHV68 genome, single-copy sensitivity nested-PCR assays for γHV68 gene 72 were performed on serial dilutions of cells by a previously published method (53, 58, 62).
RPA.
Eight- to 12-week-old mice were infected intranasally with 100 PFU γHV68. At 28 days postinfection, peritoneal cells were harvested and total RNA was prepared by using TRIzol reagent (Invitrogen, Carlsbad, Calif.) and mechanical disruption. Total RNA was prepared from spleen via mechanical disruption with silica beads in the presence of TRIzol. A total of 10 to 20 μg total RNA was hybridized overnight with the γ6 riboprobe set specific for selected γHV68 transcripts (41). RNase protection reactions were performed using the RiboQuant RNase protection assay (RPA) kit according to the manufacturer's instructions (BD Biosciences, San Jose, Calif.). The resulting protected probes were resolved on a 6% acrylamide denaturing urea gel (40-cm length), and the signal was visualized using a STORM phosphorimager and quantified with ImageQuant software (GE Healthcare, Chalfont St. Giles, United Kingdom).
Depletion of IFN-γ in vivo after the establishment of latency.
At 16 days postinfection, mice were injected with 0.5 mg anti-IFN-γ antibody (H22) or 0.5 mg isotype control antibody (PIP) every 5 days until day 28 postinfection (20, 28, 50, 51, 66). At that time, mice were sacrificed, and peritoneal cells were analyzed by limiting dilution for frequency of reactivation or by limiting dilution PCR for the frequency of genome-bearing cells.
Statistical analysis.
All data points represent the mean ± the standard error of the mean for all experiments. To quantify the frequency of cells from which the virus reactivated, data were subjected to nonlinear regression (sigmoidal dose curve with a nonvariable slope) by using GraphPad Prism (GraphPad, San Diego, Calif.). Frequencies of reactivation events were determined on the basis of the Poisson distribution by calculating the cell density at which 63.2% of the wells scored positive for reactivation. To calculate significance, frequencies of reactivation events were statistically analyzed by paired t tests over all cell dilutions. For the neutralization experiments, statistical analysis (analysis of variance) was performed by Avril Adelman at the Division of Biostatistics at Washington University School of Medicine.
RESULTS
IFN-γ decreases the frequency of peritoneal cells that reactivate from γHV68 latency.
To determine whether IFN-γ inhibits reactivation of γHV68 from latency, we performed limiting dilution assays on wild-type mouse peritoneal cells harvested 16 days postinfection. At this time, latency has been established, and preformed infectious virus is not detectable in cells from wild-type mice (4, 48, 53, 60). Therefore, the presence of cytopathic effect 3 weeks postplating represents virus that has reactivated from latency (53). We selected peritoneal cells for these assays, since studies with IFN-γ−/− mice indicated that IFN-γ plays an important role in regulating latency in peritoneal but not splenic cells (53). Peritoneal cells were harvested and plated in the presence of various concentrations of IFN-γ onto indicator IFN-αβγR−/− MEF monolayers (Fig. 1A). Consistent with published results, the frequency of reactivation of wild-type peritoneal cells was approximately 1/1,000 cells (53). The reactivation of peritoneal cells treated with 100 U and 10 U of IFN-γ/ml was reduced such that a frequency could not be calculated by using the Poisson distribution but was conservatively estimated at ≤1/40,000 cells, a >40-fold decrease compared to untreated controls. At 1 U of IFN-γ/ml, the frequency of peritoneal cells reactivating from viral latency was approximately 10-fold lower than untreated peritoneal cells (1/9,600 cells reactivated).
FIG. 1.
IFN-γ controls the frequency of peritoneal cells that reactivate from γHV68 latency. (A) Ex vivo reactivation. The statistical differences between medium and 1 U/ml, medium and 10 U/ml, and medium and 100 U/ml of IFN-γ are P = 0.002, P < 0.0001, and P < 0.0001, respectively. (B) IFN-γ does not affect the ability of γHV68 to cause CPE on the MEF monolayer. (C) IFN-γ does not activate peritoneal cells such that the ability of γHV68 to cause CPE on the MEF monolayer is altered. n, number of independent experiments.
We wished to determine whether the effect of IFN-γ on reactivation from latency was due to an effect on the latently infected cells plated or to an effect on fibroblasts of the indicator monolayer. To assure that interferon effects were not due to effects on the indicator monolayer, IFN-αβγR−/− MEFs were used in all experiments. We also determined whether the presence of IFN-γ affected the capacity of γHV68 to infect and induce cytopathic effect in these MEFs. Virus was serially diluted and plated in media containing IFN-γ (1 to 100 U/ml), and the presence of infectious virus was assessed 10 days later. IFN-γ had no significant effect on γHV68 infectivity or cytopathic effect on IFN-αβγR−/− MEFs (Fig. 1B).
We also considered the possibility that IFN-γ activates peritoneal cells ex vivo such that virus released during or after reactivation from latency is inactivated. For example, IFN-γ can activate peritoneal macrophages to kill bacteria (37). To address this possibility, peritoneal cells were harvested from uninfected B6 mice and plated onto MEF monolayers in medium with IFN-γ at various concentrations. Five days later, virus was serially diluted and added to wells, and plates were scored for the presence of infectious virus 2 weeks later. IFN-γ pretreatment of peritoneal cells ex vivo did not result in inactivated infectious virus (Fig. 1C). This assay was performed with limiting dilutions of infectious virus to optimize detection of IFN-γ-induced inactivation of even very small amounts of virus. These data are consistent with IFN-γ acting on latently infected peritoneal cells to inhibit reactivation from latency.
IFN-γ-mediated inhibition of γHV68 reactivation occurs during the first few days of explant culture.
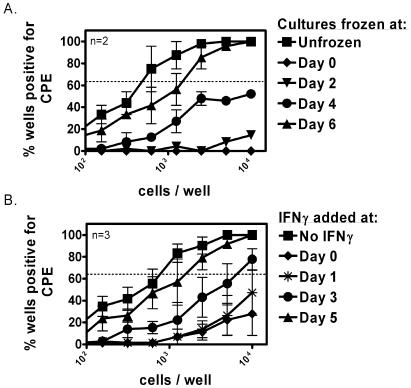
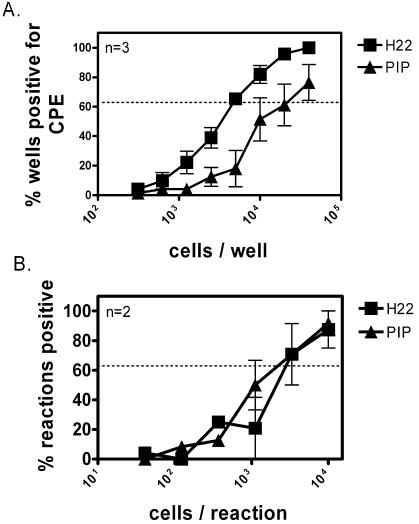
To determine the kinetics and specificity with which IFN-γ inhibits reactivation from latency, we neutralized IFN-γ at various times by addition of a neutralizing monoclonal antibody to IFN-γ (H22) to cultures. We considered this important, since purified IFN-γ might contain immunologically active substances such as lipopolysaccharide that activate macrophages carrying latent γHV68 in peritoneal cells. Peritoneal cells were harvested 16 days postinfection from wild-type mice and plated in either medium alone or 10 U of IFN-γ/ml in medium. At various days after cell plating (1 to 15 days), one set of IFN-γ-treated limiting dilution plates received H22 and another set received PIP, an isotype control antibody for H22. When H22 was added 1, 2, 3, or 5 days after cell plating, it fully neutralized the effect of IFN-γ. PIP added in parallel on any day did not significantly change the reactivation of peritoneal cells from latency compared to the IFN-γ-only treated plates (Fig. 2). IFN-γ-treated cultures receiving H22 at days 1, 2, 3, and 5 reactivated with a frequency of 1/2,320, 1/2,905, 1/2,915, and 1/6,380, respectively; the cultures receiving PIP at each of these days were indistinguishable from the IFN-γ-only treated cultures (≤1/18,000). The ability of H22 to counteract IFN-γ-mediated inhibition of viral reactivation progressively waned, as evidenced by the loss of its effect at days 8, 12, and 15; these H22-treated cultures reactivated with a frequency of 1/12,400, ≤1/20,000, and ≤1/20,000, respectively, while the parallel cultures treated with PIP reactivated at ≤1/20,000. These data demonstrate that IFN-γ has significant effects during the first few days after explantation of latently infected cells. Furthermore, they show the specificity of IFN-γ effects on reactivation from latency.
FIG. 2.
IFN-γ-mediated inhibition of γHV68 reactivation is neutralizable by anti-IFN-γ antibody early after explantation. Ex vivo reactivation is shown. The statistical difference between medium and plates with 10 U of IFN-γ/ml is P < 0.0001. PIP-treated plates were not statistically different from IFN-γ plates on any day. H22-treated plates were statistically different from IFN-γ plates at days 1, 2, 3, and 5 with the following P values: P = 0.03, P = 0.04, P = 0.05, and P = 0.05, respectively. At day 4, the difference between H22-treated plates and IFN-γ plates was not significant (P = 0.07). The capacity of neutralizing antibody to inhibit reactivation waned over time. By day 8, the ability to neutralize IFN-γ with H22 was lost. the statistical difference between day 1 H22-treated plates and day 8 H22-treated plates was significant at P = 0.01. n, number of independent experiments.
IFN-γ has significant effects on reactivation in the first 4 days after latently infected cells are explanted.
To better define when IFN-γ has effects on reactivation from latency, we sought to determine the kinetics in which IFN-γ acts during the reactivation of γHV68 ex vivo. We first assayed for the presence of infectious virus released from untreated latently infected wild-type peritoneal cells at various times after plating (Fig. 3A). As expected, no reactivation was observed on day 0 ex vivo, which is consistent with the absence of preformed infectious virus in peritoneal cells isolated from a wild-type mouse 16 days postinfection (62). Infectious virus was first detected at day 4 postplating. By day 6, a significant number of reactivation events had occurred (compare cultures frozen at day 6 to the unfrozen plates).
FIG. 3.
IFN-γ-mediated inhibition of γHV68 reactivation acts during viral reactivation ex vivo. (A) Ex vivo reactivation stopped at various days by three freeze-thaw cycles. The statistical significance of the difference between unfrozen and frozen results on day 0, day 2, and day 4 is P = 0.015. (B) Ex vivo reactivation after addition of IFN-γ at various days. The statistical significance of the difference between medium and day 0, day 1, and day 2 is P = 0.001, P = 0.02, P = 0.04, respectively. n, number of independent experiments.
To determine when IFN-γ acts on reactivating cells, IFN-γ was added to cultures on days 1 through 5 after cell plating. Significant reactivation events occurred when IFN-γ was added on day 3 or thereafter; cells reactivated with a frequency of 1/5,900 and 1/1,300 when IFN-γ was added on day 3 or day 5, respectively, compared to ≤1/20,000 cells reactivated when IFN-γ was added at day 0 or day 1 after cell plating (Fig. 3B). Thus, IFN-γ must be present during the first 3 days after latently infected cells are explanted to have a maximal effect. These data corroborate previous results that IFN-γ acts during cell reactivation and not on infectious virus (Fig. 1). By day 4, when IFN-γ can no longer be added to cultures to suppress reactivation (Fig. 3B), a significant number of reactivation events already have occurred (Fig. 3A). IFN-γ has no effect of the infectivity of preformed virus on IFN-nonresponsive indicator cells (Fig. 1B and C).
IFN-γ receptor expression on latently infected cells is required for IFN-γ-mediated inhibition of reactivation.
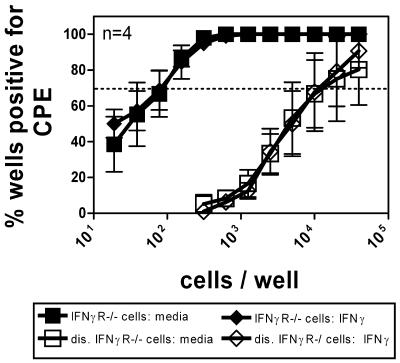
We next sought to determine whether the IFN-γ receptor on latently infected cells is required for the effects of IFN-γ on reactivation. To address this, we determined the effect of IFN-γ on reactivation from cells isolated from IFN-γR−/− mice. Peritoneal cells from IFN-γR−/− mice were harvested, and intact cells were plated in 100 U of IFN-γ/ml or in medium only. Peritoneal cells isolated from IFN-γR−/− mice contain both latently infected cells and a small but detectable amount of persistent infectious virus, demonstrating the critical role of IFN-γ in containing chronic γHV68 infection (Fig. 4). No significant difference in the frequency of cells reactivating virus in IFN-γR−/− peritoneal cells was observed between the IFN-γ-treated cultures and the untreated cultures. Also, in accordance with data presented in Fig. 1, no significant difference was observed in detection of persistent replication as measured by detection of preformed γHV68 in mechanically disrupted samples treated with IFN-γ or untreated samples. From these data, we conclude that the IFN-γ receptor expressed on latently infected cells is necessary for IFN-γ inhibition of reactivation from latency. These data, together with the experiments using neutralizing antibody to IFN-γ (Fig. 2), conclusively demonstrate that the effects of IFN-γ on reactivation from latency are not due to contaminants in our IFN-γ preparation and that signals dependent on the IFN-γ receptor directly or indirectly regulate γHV68 reactivation.
FIG. 4.
IFN-γ inhibits reactivation from latency through the IFN-γ receptor. (A) Ex vivo reactivation. At 28 days postinfection, peritoneal cells from IFN-γR−/− mice were harvested and assayed for frequency of reactivation by limiting dilution in the presence or absence of 100 U of IFN-γ/ml. Parallel samples were mechanically disrupted (dis.) to determine the presence of persistent virus at the time of plating. n, number of independent experiments.
IFN-γ does not suppress reactivation of γHV68 through a paracrine effect on infected cells.
Having shown that IFN-γR expression on cells from latently infected mice is essential, we next determined whether IFN-γ acts indirectly via induction of a paracrine factor that inhibits γHV68 reactivation from latency. To determine if a paracrine effect inhibits reactivation, we compared the effects of 100 U of IFN-γ/ml on reactivation from wild type, IFN-γR−/−, and mixtures of these two latently infected peritoneal cell types. We reasoned that if the effects of IFN-γ were mediated by a paracrine factor, independent of the IFN-γR, the IFN-γ responsive cells in mixed cultures would secrete such a factor, which would then act on the IFN-γR−/− cells to suppress reactivation from latency. No decrease in the frequency of cells reactivating γHV68 was observed, even at the highest ratio of wild-type cells to IFN-γR−/− cells (Fig. 5), ruling out a significant effect of a paracrine factor. Additionally, these data support the conclusion that the IFN-γR on latently infected cells is key to IFN-γ-mediated inhibition of viral reactivation.
FIG. 5.
IFN-γ does not suppress reactivation of γHV68 through a paracrine effect on infected cells. Ex vivo reactivation is shown. The numbers in the legend indicate the ratios in which the wild-type peritoneal cells were mixed with the IFN-γR−/− peritoneal cells. The concentration of IFN-γ used was 100 U/ml. The statistical significance of the difference between wild-type cells plated in the presence and absence of IFN-γ was P = 0.02.
IFN-γR−/− peritoneal cells have a different profile of viral gene expression than wild-type peritoneal cells.
The experiments above demonstrate that IFN-γ added to explant reactivation cultures inhibited reactivation of γHV68 from latency. If this effect was physiologically relevant, we predicted that IFN-γ would inhibit expression of viral genes in vivo. Therefore, we determined whether peritoneal cells from wild-type and IFN-γR−/− mice expressed different profiles of viral genes in vivo by RPA. This assay is sensitive enough to detect γHV68 transcription in the spleen and mesenteric lymph nodes of wild-type mice 16 days after intranasal infection (41). Mice were infected with 100 PFU γHV68 intranasally; 28 days postinfection, RNA from peritoneal cells was isolated. Note that there was persistent replication in IFN-γR−/− peritoneal cells. As predicted, wild-type and IFN-γR−/− peritoneal cells had significantly different expression profiles of select viral gene transcripts (Fig. 6). Consistent with prior experiments using reverse transcriptase PCR on latently infected in vivo samples (42), no significant transcription of viral genes was detected by this method in peritoneal cells derived from wild-type mice. However, viral transcripts for M9, M3, gB, M8, Rta, and K3 were readily detected in peritoneal cells isolated from infected IFN-γR−/− mice. These data indicate that signaling through the IFN-γ receptor plays a significant role in regulating viral gene expression during chronic infection in vivo.
FIG. 6.
Peritoneal cells from chronically infected IFN-γR−/− mice have a different profile of viral gene expression than peritoneal cells from wild-type mice. (A) RNase protection assay. RNA was isolated from peritoneal cells of infected wild-type or IFN-γR−/− mice and analyzed by RPA for expression of γHV68 genes. RNA from three mice (lanes A, B, and C) were analyzed for each group. The positive control is RNA extracted from infected OMK cells. Shown is a representative phosphorimage from three experiments. (B) Phosphorimager quantitation for viral mRNAs and host mRNA L32.
In vivo depletion of IFN-γ after the establishment of latency increases the frequency of cells that reactivate virus.
If IFN-γ plays a significant role in control of reactivation in vivo, then depletion of IFN-γ in vivo should alter the reactivation phenotype of latently infected cells derived from wild-type mice. We therefore determined if neutralization of IFN-γ in vivo increased the frequency of latently infected cells capable of reactivation ex vivo. Importantly, to assure that we distinguished effects of IFN-γ during latent infection from effects during acute infection, we depleted IFN-γ by neutralizing antibody injection after the establishment of latency at 16 days postinfection. On day 28 postinfection, cells from these mice were harvested, and the frequency of cells capable of reactivation and the frequency of infected cells were determined. Measurement of serum IFN-γ by cytometric bead array revealed complete depletion of IFN-γ in the H22-treated mice and no change in IFN-γ levels in the control PIP-treated mice (data not shown). In the IFN-γ-depleted mice, cells reactivated ex vivo with a frequency of 1/5,180 compared to 1/21,594 from the control-treated mice (Fig. 7A). This result was not due to an increase in the number of latently infected cells, since the frequency of cells carrying viral genome was not changed in mice depleted of IFN-γ (Fig. 7B). There was no preformed infectious virus in the peritoneal cells (data not shown). These data indicate that IFN-γ signaling is critical for control of the frequency of reactivation in chronic γHV68 infection. Importantly, these data demonstrate the critical role of IFN-γ after the establishment of latency and during the maintenance of latency.
FIG. 7.
In vivo depletion of IFN-γ after the establishment of latency leads to an increase in the frequency of cells reactivating from latency. (A) Ex vivo reactivation. The difference in these reactivation curves is statistically significant (P = 0.003). (B) Frequency of genome-bearing cells. n, number of independent experiments.
DISCUSSION
Effects of viral genes on infected host cells play a critical role in determining the nature of chronic herpesvirus infection. One key measure of chronic infection regulated by viral genes is the efficiency with which latently infected cells reactivate from latency ex vivo (7, 18, 25, 35, 58). However, chronic γHV68 infection is also regulated by host genes, among which IFN-γ is of particular importance. Effects of IFN-γ on infected cells are presumably extrinsic to the latently infected cells, since the major source of IFN-γ during infections is thought to be T cells and NK cells (3), while γHV68 latently infects B cells, dendritic cells, and macrophages (15, 49, 63). In this study, we show that IFN-γ controls reactivation of γHV68 from latency. This effect required expression of the IFN-γ receptor, correlated with viral gene expression changes in vivo, and depended on the continuous presence of IFN-γ after the establishment of latency in vivo. This indicates that maintenance of stable γHV68 latency requires continuous IFN-γ-mediated immune surveillance and regulation of viral gene expression.
Generality of the role of IFN-γ in controlling herpesvirus latency.
The ability of IFN-γ to control chronic herpesvirus infection and reactivation from latency is not unique to gammaherpesviruses. We showed in 1998 that IFN-γ inhibits reactivation of murine cytomegalovirus, a betaherpesvirus, from latency (39). The physiologic relevance of this observation was uncovered by studies from the Koszinowski laboratory demonstrating that IFN-γ plays a key role in controlling in vivo reactivation and dissemination of murine cytomegalovirus (31, 38). In addition, IFN-γ regulates alphaherpesvirus latency (30, 33). IFN-γ is expressed for prolonged periods in trigeminal ganglia latently infected with HSV (30). Importantly, CD8 T cells can restrict HSV-1 reactivation from latency, and this effect is partly attributable to the effects of IFN-γ (27, 29, 30). Together with the data presented here, these studies demonstrate that IFN-γ is critical for controlling chronic and latent infection of alpha, beta, and gammaherpesviruses and specifically controls reactivation of herpesvirus latency. The conservation of this effect across all three subfamilies of herpesviruses raises the possibility that components or targets of IFN-γ signaling represent a common mechanism central to the maintenance and reactivation of latent herpesvirus infection.
Physiologic relevance of the effects of IFN-γ on γHV68 reactivation from latency.
The effects of IFN-γ during chronic infection must be interpreted in light of its lack of a significant role during acute infection. IFN-γ is required for control of chronic but not acute γHV68 infection (9, 43, 53, 61). During chronic infection, the absence of either IFN-γ or the IFN-γ receptor leads to increased reactivation from viral latency, persistent viral replication, and vasculitis (9, 10, 53, 61). The ability of IFN-γ to inhibit γHV68 reactivation from latency reported here is consistent with and may provide a reasonable explanation for these phenotypes.
Depletion of IFN-γ after the establishment of latency led to an increased frequency of reactivation, demonstrating that this cytokine is being continuously produced and is required for controlling chronic infection. One possible source of IFN-γ is activated T cells present in mice that are latently infected with γHV68 (54, 55). Depletion of IFN-γ did not alter the frequency of latently infected cells, an observation consistent with studies in IFN-γ−/− mice (53), but did alter their capacity to reactivate. It is interesting to speculate that chronically produced IFN-γ has an immune surveillance role in vivo, limiting γHV68 gene expression and blocking reactivation. Consistent with this hypothesis is the production of IFN-γ by wild-type splenic and lymph node cells restimulated in vitro 70 days after infection (44).
Inhibition of persistent replication.
Persistent replication is observed in normal mice at a very low level after clearance of productive infection (16) but is present at much higher levels in mice that are unresponsive to IFN-γ (7, 9, 10, 18, 26, 53, 61). Increased persistent replication has serious consequences for the host, including severe elastic artery vasculitis (9, 10, 61) and high levels of reactivating cells (17). Might the ability of IFN-γ to inhibit reactivation from latency contribute to the presence of high levels of persistent replication in IFN-γ-unresponsive mice? While there are other possible explanations, we prefer the hypothesis that persistent replication is derived from reactivation events. Support for this concept comes from genetic studies of γHV68 mutants lacking either the v-cyclin or v-Bcl-2 proteins (18, 22, 58). While these genes are dispensable for productive replication during the first days of γHV68 infection (18, 58), they are required for both efficient reactivation from latency and high levels of persistent replication, as well as vascular disease in IFN-γ-unresponsive mice (18, 58). These data show that the processes that result in productive acute infection are fundamentally different from those giving rise to persistent infection, since they require different viral genes. Furthermore, the requirement for v-cyclin and v-Bcl-2 during both reactivation from latency and persistent replication suggests a mechanistic link between these two viral processes. This is consistent with IFN-γ controlling persistent replication indirectly by decreasing reactivation from latency, as shown in this study.
Mechanism of IFN-γ-mediated inhibition of reactivation from latency.
While the molecular mechanism underlying the ability of IFN-γ to suppress reactivation of latent virus is unknown, our experiments support the concept that a primary site of IFN-γ action is directly on latently infected cells. The presence of IFN-γ-responsive latently infected cells in explant cultures did not reduce the frequency of reactivation when mixed with IFN-γ-unresponsive latently infected cells. This result argues against the generation of an IFN-γ-inducible soluble factor or activation of a cell population (such as T cells) (27, 29) that inhibits reactivation from latency. The most reasonable interpretation of these data is that IFN-γ acts in explant cultures by direct signaling in latently infected cells. This suggests that the well-documented immunomodulatory effects of IFN-γ are not involved in inhibition of γHV68 reactivation from latency in explant cultures. We note, however, that reciprocal bone marrow transplant studies show that IFN-γ regulates pathology in the vascular system via both direct antiviral effects on somatic cells and immunoregulatory effects on hematopoietic cells (9). Thus, a role for IFN-γ immunomodulation in control of reactivation from γHV68 latency in vivo cannot be ruled out. Studies in which only infected cells are either IFN-γ responsive or IFN-γ unresponsive will be required to definitively address this question.
IFN-γ regulation of the transition from an early to a late form of latency.
IFN-γ expression is also necessary for the transition from an early to a late form of latency. γHV68 establishes an early form of latency after clearance of productive acute infection that is characterized by efficient reactivation of latently infected cells in explant cultures. Thus, 16 days after infection, nearly every latently infected cell reactivates upon explantation (53, 63). However, by 42 days after infection in normal mice, the efficiency of reactivation from latency decreases such that only approximately 10% of latently infected cells reactivate when explanted (53). The molecular and cellular bases for this transition from high efficiency to low efficiency of reactivation are undefined. However, this transition is disrupted in mice lacking IFN-γ or the IFN-γ receptor, since nearly 100% of these latently infected cells harvested 42 days after infection reactivate in explant cultures (53).
It is possible that IFN-γ is required for latently infected cells to transition to a transcriptionally quiescent state from which reactivation is inefficient. Support for IFN-γ regulation of viral transcription during chronic infection comes from our demonstration that there is significantly enhanced transcription of a number of viral genes in chronically infected mice lacking IFN-γ. Of particular interest is the upregulation of genes that are known to play a critical role in efficient reactivation of γHV68 from latency such as the Rta and v-cyclin genes (58, 64). Interestingly, the pattern of viral gene expression in the absence of IFN-γ is not what one would expect for standard lytic infection, since known lytic cycle genes such as gB and pol were not dramatically upregulated. It is tempting to speculate that IFN-γ blocks reactivation of γHV68 from latency via regulation of the transcription of viral genes. While these data are consistent with the hypothesis that IFN-γ blocks reactivation from latency via regulation of latent gene expression and thereby fosters establishment of the late form of γHV68 latency, this must be tested directly. An important experiment will be to determine the effects of IFN-γ on γHV68 gene expression in different cell types on a genome-wide basis.
In this report, we demonstrate that the host cytokine IFN-γ is a strong suppressor of reactivation from latency in the murine model of γHV68 infection. Consistent with this conclusion, human patients with a specific IFN-γ expression polymorphism are at increased risk for developing EBV-associated posttransplant lymphoproliferative disease (56), indicating that IFN-γ is likely critical for controlling latent gammaherpesvirus infections in humans. Human gammaherpesvirus leads to lifelong infections, and its latency-associated pathogenesis is particularly problematic in the immunocompromised patient. Treatments aimed at controlling reactivation from latency may be valuable. Further understanding of the mechanism by which IFN-γ controls gammaherpesvirus reactivation may therefore lead to the development of appropriate therapeutics.
Acknowledgments
H.W.V. was supported by NIH grants CA074730, HL60090, and CA096511; A.L.S. was supported by a Ruth L. Kirschstein National Research Service Award, grant 5 T32 GM07200, and American Heart Association grant 0515435Z. R.R. and M.L.L. were supported by NIH grant AG09822.
We thank members of the Virgin laboratory for helpful discussions. We thank Robert Schreiber for supplying critical reagents and Darren Kreamalmeyer for expert assistance with mouse strains.
REFERENCES
- 1.Alber, D. G., K. L. Powell, P. Vallance, D. A. Goodwin, and C. Grahame-Clarke. 2000. Herpesvirus infection accelerates atherosclerosis in the apolipoprotein E-deficient mouse. Circulation 102:779-785. [DOI] [PubMed] [Google Scholar]
- 2.Bancroft, G. J., R. D. Schreiber, G. C. Bosma, M. J. Bosma, and E. R. Unanue. 1987. A T cell-independent mechanism of macrophage activation by interferon-gamma. J. Immunol. 139:1104-1107. [PubMed] [Google Scholar]
- 3.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 4.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 6.Christensen, J. P., R. D. Cardin, K. C. Branum, and P. C. Doherty. 1999. CD4(+) T cell-mediated control of a gamma-herpesvirus in B cell-deficient mice is mediated by IFN-gamma. Proc. Natl. Acad. Sci. USA 96:5135-5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clambey, E. T., H. W. Virgin IV, and S. H. Speck. 2000. Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. J. Virol. 74:1973-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cool, C. D., P. R. Rai, M. E. Yeager, D. Hernandez-Saavedra, A. E. Serls, T. M. Bull, M. W. Geraci, K. K. Brown, J. M. Routes, R. M. Tuder, and N. F. Voelkel. 2003. Expression of human herpesvirus 8 in primary pulmonary hypertension. N. Engl. J. Med. 349:1113-1122. [DOI] [PubMed] [Google Scholar]
- 9.Dal Canto, A. J., P. E. Swanson, A. K. O'Guin, S. H. Speck, and H. W. Virgin. 2001. IFN-gamma action in the media of the great elastic arteries, a novel immunoprivileged site. J. Clin. Investig. 107:R15-R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dal Canto, A. J., H. W. Virgin IV, and S. H. Speck. 2000. Ongoing viral replication is required for gammaherpesvirus 68-induced vascular damage. J. Virol. 74:11304-11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dierksheide, J. E., R. A. Baiocchi, A. K. Ferketich, S. Roychowdhury, R. P. Pelletier, C. F. Eisenbeis, M. A. Caligiuri, and A. M. VanBuskirk. 2005. IFN-gamma gene polymorphisms associate with development of EBV+ lymphoproliferative disease in hu PBL-SCID mice. Blood 105:1558-1565. [DOI] [PubMed] [Google Scholar]
- 12.Dighe, A. S., M. A. Farrar, and R. D. Schreiber. 1993. Inhibition of cellular responsiveness to interferon-γ (IFNγ) induced by overexpression of inactive forms of the IFNγ receptor. J. Biol. Chem. 268:10645-10653. [PubMed] [Google Scholar]
- 13.Dunn, G. P., A. T. Bruce, K. C. Sheehan, V. Shankaran, R. Uppaluri, J. D. Bui, M. S. Diamond, C. M. Koebel, C. Arthur, J. M. White, and R. D. Schreiber. 2005. A critical function for type I interferons in cancer immunoediting. Nat. Immunol. 6:722-729. [DOI] [PubMed] [Google Scholar]
- 14.Dupin, N., C. Fisher, P. Kellam, S. Ariad, M. Tulliez, N. Franck, E. van Marck, D. Salmon, I. Gorin, J. P. Escande, R. A. Weiss, K. Alitalo, and C. Boshoff. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 96:4546-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1074-1081. [DOI] [PubMed] [Google Scholar]
- 16.Flano, E., I. J. Kim, J. Moore, D. L. Woodland, and M. A. Blackman. 2003. Differential gamma-herpesvirus distribution in distinct anatomical locations and cell subsets during persistent infection in mice. J. Immunol. 170:3828-3834. [DOI] [PubMed] [Google Scholar]
- 17.Gangappa, S., S. B. Kapadia, S. H. Speck, and H. W. Virgin IV. 2002. Antibody to a lytic cycle viral protein decreases gammaherpesvirus latency in B-cell-deficient mice. J. Virol. 76:11460-11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangappa, S., L. F. Van Dyk, T. J. Jewett, S. H. Speck, and H. W. Virgin. 2002. Identification of the in vivo role of a viral bcl-2. J. Exp. Med. 195:931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanto, D. W. 1995. Classification of Epstein-Barr virus-associated posttransplant lymphoproliferative diseases: implications for understanding their pathogenesis and developing rational treatment strategies. Annu. Rev. Med. 46:381-394. [DOI] [PubMed] [Google Scholar]
- 20.Harty, J. T., R. D. Schreiber, and M. J. Bevan. 1992. Cd8 T-cells can protect against an intracellular bacterium in an interferon-gamma-independent fashion. Proc. Natl. Acad. Sci. USA 89:11612-11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heise, M. T., and H. W. Virgin IV. 1995. The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J. Virol. 69:904-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoge, A. T., S. B. Hendrickson, and W. H. Burns. 2000. Murine gammaherpesvirus 68 cyclin D homologue is required for efficient reactivation from latency. J. Virol. 74:7016-7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu, J. L., and S. L. Glaser. 2000. Epstein-Barr virus-associated malignancies: epidemiologic patterns and etiologic implications. Crit. Rev. Oncol. Hematol. 34:27-53. [DOI] [PubMed] [Google Scholar]
- 24.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 25.Jacoby, M. A., H. W. Virgin IV, and S. H. Speck. 2002. Disruption of the M2 gene of murine gammaherpesvirus 68 alters splenic latency following intranasal, but not intraperitoneal, inoculation. J. Virol. 76:1790-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapadia, S. B., B. Levine, S. H. Speck, and H. W. Virgin. 2002. Critical role of complement and viral evasion of complement in acute, persistent, and latent gamma-herpesvirus infection. Immunity 17:143-155. [DOI] [PubMed] [Google Scholar]
- 27.Khanna, K. M., R. H. Bonneau, P. R. Kinchington, and R. L. Hendricks. 2003. Herpes simplex virus-specific memory CD8(+) T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18:593-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leiby, D. A., A. H. Fortier, R. M. Crawford, R. D. Schreiber, and C. A. Nacy. 1992. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect. Immun. 60:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, T., and R. L. Hendricks. 2000. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. Investig. Ophthalmol. Vis. Sci. 41:S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, T., K. M. Khanna, B. N. Carriere, and R. L. Hendricks. 2001. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 75:11178-11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucin, P., I. Pavic, B. Polic, S. Jonjic, and U. H. Koszinowski. 1992. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J. Virol. 66:1977-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandik-Nayak, L., G. Huang, K. C. Sheehan, J. Erikson, and D. D. Chaplin. 2001. Signaling through TNF receptor p55 in TNF-alpha-deficient mice alters the CXCL13/CCL19/CCL21 ratio in the spleen and induces maturation and migration of anergic B cells into the B cell follicle. J. Immunol. 167:1920-1928. [DOI] [PubMed] [Google Scholar]
- 33.Minami, M., M. Kita, X. Q. Yan, T. Yamamoto, T. Iida, K. Sekikawa, Y. Iwakura, and J. Imanishi. 2002. Role of IFN-gamma and tumor necrosis factor-alpha in herpes simplex virus type 1 infection. J. Interferon Cytokine Res. 22:671-676. [DOI] [PubMed] [Google Scholar]
- 34.Moore, P. S., S.-J. Gao, G. Dominguez, E. Cesarman, O. Lungu, D. M. Knowles, R. Garber, P. E. Pellett, D. J. McGeoch, and Y. Chang. 1996. Primary characterization of a herpesvirus agent associated with Kaposi's sarcoma. J. Virol. 70:549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moorman, N. J., H. W. Virgin IV, and S. H. Speck. 2003. Disruption of the gene encoding the γHV68 v-GPCR leads to decreased efficiency of reactivation from latency. Virology 307:179-190. [DOI] [PubMed] [Google Scholar]
- 36.Nador, R. G., E. Cesarman, A. Chadburn, D. B. Dawson, M. Q. Ansari, J. Sald, and D. M. Knowles. 1996. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood 88:645-656. [PubMed] [Google Scholar]
- 37.Peck, R. 1989. Gamma-interferon induces monocyte killing of Listeria monocytogenes by an oxygen-dependent pathway: alpha- or beta-interferons by oxygen-independent pathways. J. Leukoc. Biol. 46:434-440. [DOI] [PubMed] [Google Scholar]
- 38.Polic, B., H. Hengel, A. Krmpotic, J. Trgovcich, I. Pavic, P. Lucin, S. Jonjic, and U. H. Koszinowski. 1998. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J. Exp. Med. 188:1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Presti, R. M., J. L. Pollock, A. J. Dal Canto, A. K. O'Guin, and H. W. Virgin. 1998. Interferon-gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J. Exp. Med. 188:577-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 41.Rochford, R., M. L. Lutzke, R. S. Alfinito, A. Clavo, and R. D. Cardin. 2001. Kinetics of murine gammaherpesvirus 68 gene expression following infection of murine cells in culture and in mice. J. Virol. 75:4955-4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roy, D. J., B. C. Ebrahimi, B. M. Dutia, A. A. Nash, and J. P. Stewart. 2000. Murine gammaherpesvirus M11 gene product inhibits apoptosis and is expressed during virus persistence. Arch. Virol. 145:2411-2420. [DOI] [PubMed] [Google Scholar]
- 43.Sarawar, S. R., R. D. Cardin, J. W. Brooks, M. Mehrpooya, A. M. Hamilton-Easton, X. Y. Mo, and P. C. Doherty. 1997. Gamma interferon is not essential for recovery from acute infection with murine gammaherpesvirus 68. J. Virol. 71:3916-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarawar, S. R., R. D. Cardin, J. W. Brooks, M. Mehrpooya, R. A. Tripp, and P. C. Doherty. 1996. Cytokine production in the immune response to murine gammaherpesvirus 68. J. Virol. 70:3264-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schreiber, R. D., L. J. Hicks, A. Celada, N. A. Buchmeier, and P. W. Gray. 1985. Monoclonal antibodies to murine gamma interferon which differentially modulate macrophage activation and antiviral activity. J. Immunol. 134:1609-1618. [PubMed] [Google Scholar]
- 46.Sparks-Thissen, R. L., D. C. Braaten, K. Hildner, T. L. Murphy, K. M. Murphy, and H. W. Virgin. 2005. CD4 T cell control of acute and latent murine gammaherpesvirus infection requires IFN gamma. Virology 338:201-208. [DOI] [PubMed] [Google Scholar]
- 47.Sunil-Chandra, N. P., J. Arno, J. Fazakerley, and A. A. Nash. 1994. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am. J. Pathol. 145:818-826. [PMC free article] [PubMed] [Google Scholar]
- 48.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gammaherpesvirus 68. J. Gen. Virol. 73:2347-2356. [DOI] [PubMed] [Google Scholar]
- 49.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 73:3275-3279. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki, Y., M. A. Orellana, R. D. Schreiber, and J. S. Remington. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516-518. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki, Y., and J. Remington. 1990. The effect of anti-IFN-gamma antibody on the protective effect of Lyt2+ immune T cells against toxoplasmosis in mice. J. Immunol. 144:1954-1956. [PubMed] [Google Scholar]
- 52.Tarakanova, V. S. F. S., M. T. A. Jacoby, K. E. Weck, J. H. Hess, S. H. Speck, and H. W. Virgin IV. 2005. Association of murine γherpesvirus 68 with lymphoproliferative disorders in β2 microglobulin deficient mice. J. Virol. 79:14668-14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tibbetts, S. A., L. F. van Dyk, S. H. Speck, and H. W. Virgin IV. 2002. Immune control of the number and reactivation phenotype of cells latently infected with a gammaherpesvirus. J. Virol. 76:7125-7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tripp, R. A., A. M. Hamilton-Easton, R. D. Cardin, P. Nguyen, F. G. Behm, D. L. Woodland, P. C. Doherty, and M. A. Blackman. 1997. Pathogenesis of an infectious mononucleosis-like disease induced by a murine gamma-herpesvirus: role for a viral superantigen? J. Exp. Med. 185:1641-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Usherwood, E. J., A. J. Ross, D. J. Allen, and A. A. Nash. 1996. Murine gammaherpesvirus-induced splenomegaly: a critical role for CD4 T cells. J. Gen. Virol. 77:627-630. [DOI] [PubMed] [Google Scholar]
- 56.VanBuskirk, A. M., V. Malik, D. Xia, and R. P. Pelletier. 2001. A gene polymorphism associated with posttransplant lymphoproliferative disorder. Transplant. Proc. 33:1834. [DOI] [PubMed] [Google Scholar]
- 57.Van Den Broek, M. F., U. Muller, S. Huang, M. Aguet, and R. M. Zinkernagel. 1995. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 69:4792-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Dyk, L. F., H. W. Virgin IV, and S. H. Speck. 2000. The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency. J. Virol. 74:7451-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Virgin, H. W., IV, P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weck, K. E., M. L. Barkon, L. I. Yoo, S. H. Speck, and H. W. Virgin. 1996. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J. Virol. 70:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weck, K. E., A. J. Dal Canto, J. D. Gould, A. K. O'Guin, K. A. Roth, J. E. Saffitz, S. H. Speck, and H. W. Virgin. 1997. Murine gammaherpesvirus 68 causes severe large vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus induced vascular disease. Nat. Med. 3:1346-1353. [DOI] [PubMed] [Google Scholar]
- 62.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. B cells regulate murine gammaherpesvirus 68 latency. J. Virol. 73:4651-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu, T. T., E. J. Usherwood, J. P. Stewart, A. A. Nash, and R. Sun. 2000. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J. Virol. 74:3659-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zanussi, S., E. Vaccher, C. Caffau, C. Pratesi, C. Crepaldi, M. T. Bortolin, R. Tedeschi, D. Politi, L. Barzan, U. Tirelli, and P. de Paoli. 2003. Interferon-gamma secretion and perforin expression are impaired in CD8(+) T lymphocytes from patients with undifferentiated carcinoma of nasopharyngeal type. Cancer Immunol. Immunother. 52:28-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong, G, E. M. Peterson, C. W. Czarniecki, R. D. Schreiber, and L. M. de la Maza. 1989. Role of endogenous gamma interferon in host defense against Chlamydia trachomatis infections. Infect. Immun. 57:152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]