Abstract
The yeast ubiquitin-protein ligase Rsp5p regulates processes as diverse as polII transcription and endocytosis. Here, we identify Rsp5p in a screen for tRNA export (tex) mutants. The tex23-1/rsp5-3 mutant, which is complemented by RSP5, not only shows a strong nuclear accumulation of tRNAs at the restrictive temperature, but also is severely impaired in the nuclear export of mRNAs and 60S pre-ribosomal subunits. In contrast, nuclear localization sequence (NLS)-mediated nuclear protein import is unaffected in this mutant. Strikingly, the nuclear RNA export defects seen in the rsp5-3 strain are accompanied by a dramatic inhibition of both rRNA and tRNA processing, a combination of phenotypes that has not been reported for any previously characterized mutation in yeast. These data implicate ubiquitination as a mechanism coordinating the major nuclear RNA biogenesis pathways.
Introduction
Eukaryotic gene expression requires the nuclear export of several classes of RNAs, including mRNA, tRNA and rRNA, the latter in the form of ribosomal subunits. While much has been learnt about the distinct export pathways for these individual types of RNAs, little is known how these pathways are coordinated (Lei & Silver, 2002). A highly conserved export receptor, Mex67-Mtr2 in yeast and TAP/NXF-p15/NXT in metazoans, is involved in the nuclear export of mRNAs, which is connected to intranuclear export factors that couple mRNA biogenesis with nuclear export (Reed & Hurt, 2002; Stutz & Izaurralde, 2003). The export of ribosomal RNA is strictly coupled to intranuclear pre-ribosome biogenesis, which involves a complex series of steps beginning with the nucleolar transcription of rDNA, followed by pre-rRNA processing and modification, assembly of pre-ribosomal particles and final export to the cytoplasm (Johnson et al., 2002; Tschochner & Hurt, 2003). Nuclear export of the large 60S subunit requires the NES-containing adaptor Nmd3 and the Xpo1/Crm1/exportin-1 export receptor.
Although less complex than ribosome biogenesis, tRNA export also requires multiple maturation steps prior to export of the mature tRNA to the cytoplasm (Hopper, 1999; Wolin & Matera, 1999). Nuclear export of the mature tRNA is mediated by the karyopherin Xpo-t in vertebrates (Arts et al., 1998; Kutay et al., 1998) and Los1p in yeast (Hellmuth et al., 1998; Sarkar & Hopper, 1998). However, the fact that yeast Los1p is not essential and that only a subset of tRNAs exhibit nuclear accumulation in los1− mutants suggests that at least one alternative nuclear tRNA export pathway exists (Grosshans et al., 2000b).
Therefore, we set up a screen based on fluorescence in situ hybridization (FISH) to find novel tRNA export factors. In this screen for tRNA export (tex) mutants, we have identified Rsp5p, an essential ubiquitin ligase (Huibregtse et al., 1995). Previously, Rsp5p has been implicated in several processes, for example, targeting to the vacuole (Helliwell et al., 2001), remodelling of the actin cytoskeleton (Kaminska et al., 2002), polII transcription (Huibregtse et al., 1997) and activation of transcription factors (Hoppe et al., 2000). Further characterization of the rsp5-3 mutant revealed that it is strongly impaired in the nuclear export of mRNA and ribosomal 60S subunits. In a parallel work, it was recently reported that another rsp5 mutant is impaired in mRNA export (Rodriguez et al., 2003). In addition, our work showed that tRNA and rRNA export defects in the rsp5-3 mutant are preceded by a severe inhibition of pre-tRNA and pre-rRNA processing. Thus, our data show a role of the ubiquitin ligase Rsp5p in controlling the major nuclear RNA biogenesis/export pathways in yeast.
Results
To identify factors required for tRNA export, we performed a screen for temperature-sensitive (ts) mutants that accumulate tRNA inside the nucleus at the restrictive temperature. We screened a bank of randomly generated ts mutants for a tex phenotype. One mutant termed tex23-1 showed a nuclear accumulation of intron-containing and intronless tRNA after a shift to the restrictive temperature (Fig. 1A). To characterize further this putative tRNA export factor, the wild-type TEX23 gene was cloned by complementation of the tex23-1 phenotype (Fig. 1B). Unexpectedly, TEX23 is identical to RSP5, a gene encoding the essential ubiquitin ligase Rsp5p. Accordingly, the tex23-1 mutant was called rsp5-3. The rsp5-3 allele was isolated and shown to contain the mutations T104A, E673G and Q716P. Notably, the Q716P mutation lies in helix 12 of the catalytic hect domain of Rsp5p (Huang et al., 1999). To determine whether rsp5-3 cells exhibit other defects in nucleocytoplasmic transport, both nuclear export of other classes of RNA and nuclear protein import were analysed. Interestingly, rsp5-3 cells show a strong nuclear accumulation of poly(A)+ RNA (Fig. 2A). To test for nuclear export of ribosomal subunits, functional green fluorescent protein (GFP)-tagged ribosomal protein reporters Rpl25p-GFP (large subunit) and Rps2p-GFP (small subunit) were analysed by fluorescence microscopy (Fig. 2B). Nuclear export of the 60S subunit, but not of the 40S subunit, was inhibited significantly in the rsp5-3 mutant at 37 °C. In contrast, NLS-reporter constructs continued to accumulate in the nucleus of the rsp5-3 mutant (Fig. 2C). Altogether, the data suggest that Rsp5p is involved in the nuclear export of several classes of RNA.
Figure 1.
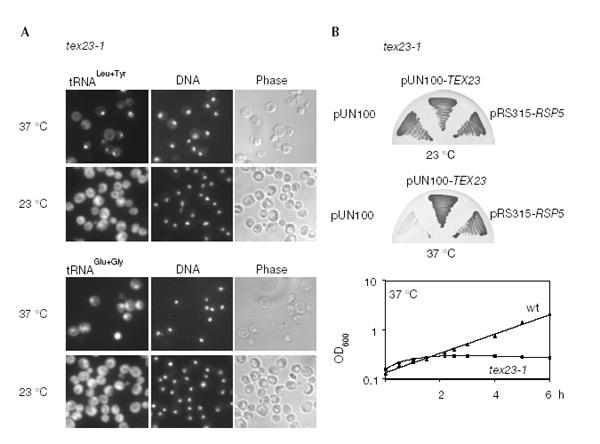
Genetic screen for tRNA export (tex) mutants identifies Rsp5p. (A) tex23-1 cells grown at 23 °C were shifted to 37 °C for 3.5 h, and localization of tRNALeu+Tyr and tRNAGlu+Gly was assessed by in situ hybridization. The DNA was stained with DAPI. (B) tex23-1 cells were transformed with pUN100, with complementing pUN100-TEX23 and with the RSP5 gene (pRS315-RSP5). Transformants were grown for 3 days at 23 or 37 °C (upper panel). Growth curve of rsp5-3 and wild-type cells in YPD medium after shift to 37 °C (lower panel).
Figure 2.
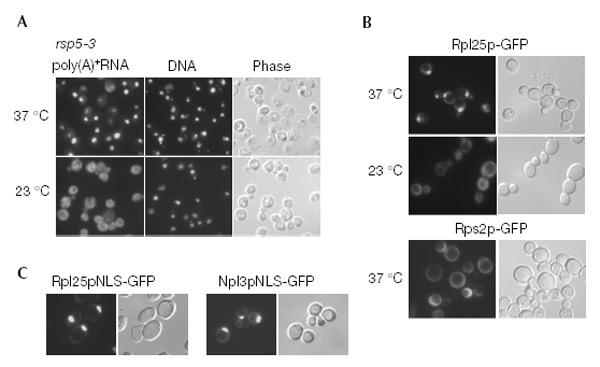
Poly(A)+ RNA and pre-ribosomal subunit export is inhibited in rsp5-3. (A) rsp5-3 cells grown at 23 °C were shifted to 37 °C for 3.5 h, and localization of poly(A)+ RNA was assessed by in situ hybridization. (B) rsp5-3 cells transformed with the respective reporter plasmids were observed in the fluorescence microscope for the localization of Rpl25p-GFP (upper panel), Rps2p-GFP (lower panel), and Rpl25pNLS-GFP and Npl3pNLS-GFP (C) after shifting for 3.5 h to 37 °C.
To determine whether nuclear accumulation of rRNA, tRNA and mRNA in rsp5-3 cells is a consequence of defects in RNA maturation, the processing of representatives of each of these classes of RNA was analysed by northern hybridization (Fig. 3) and primer extension (data not shown). These analyses revealed dramatic and unexpected defects in the maturation of both rRNAs and tRNAs. Northern analysis showed that the 35S pre-rRNA primary transcript accumulated strongly, accompanied by the appearance of the aberrant 23S RNA (Fig. 3A). In contrast, the normal products of 35S processing, the 27SA2 and 20S pre-rRNAs, were almost entirely lost within 1 h of transfer to 37 °C. These results show that the early pre-rRNA cleavages at sites A0, A1 and A2 are strongly inhibited in the rsp5-3 strain. Processing on the pathway of 5.8S and 25S synthesis is less completely inhibited, but levels of the 27SB, 7S and 6S pre-rRNAs were greatly reduced. Consistent with the pre-rRNA processing defects, the mature 18S, 25S and 5.8S rRNAs were depleted in the rsp5-3 strain progressively. However, no alteration was seen in the relative levels of the 5.8SL and 5.8SS rRNAs (Fig. 3B), and primer extension analysis (data not shown) showed no alteration in the corresponding levels of the long and short form of the 27SB pre-rRNA. The generation of the short 27SBS pre-rRNA and 5.8SS rRNA requires the cleavage of processing site A3 by the endonuclease RNase mitochondrial RNA processing (MRP), as does synthesis of the 23S RNA. We conclude that pre-rRNA cleavage by RNase MRP is not inhibited in the rsp5-3 strain. In addition, truncated fragments of the 5S rRNA appeared, presumably representing degradation intermediates. The appearance of these truncated species is not a normal consequence of the inhibition of ribosome synthesis, and may represent a defect in the RNA degradation machinery. Polysome analyses were performed on lysates from the rsp5-3 strain 5 h after transfer to 37 °C (data not shown). These showed little alteration in the ratios of free 40S and 60S to polysomes, and only a mild decrease in 80S monosomes to polysomes in the rsp5-3 strain following transfer to 37 °C. This indicates that the residual subunits that are synthesized in the mutant are fully functional.
Figure 3.
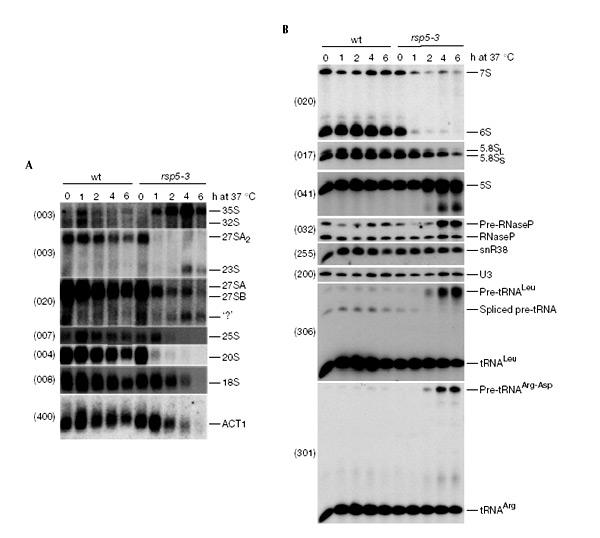
tRNA and rRNA processing is defective in the rsp5-3 strain. Northern analysis was performed on RNA extracts from the rsp5-3 and FY23 (wt) cells after shifting for 0–6 h from 23 to 37 °C. (A) High-molecular-weight RNAs separated on an agarose/formaldehyde gel. (B) Low-molecular-weight RNAs separated on a polyacrylamide/urea gel. RNA species detected are indicated on the right of the figure and oligonucleotide probes used are shown in parentheses. The identity of the aberrant processing intermediate indicated with ‘?' has not yet been established.
Pre-tRNA processing was also inhibited drastically in the rsp5-3 strain. The dimeric pre-tRNAArg–pre-tRNAAsp (Fig. 3B) strongly accumulated with depletion of the mature tRNAArg. This pre-tRNA is normally cleaved by the endonuclease RNase P. Accumulation was also seen for the full-length pre-tRNA3Leu (Fig. 3B), confirming the inhibition of processing by RNase P, and revealing an additional defect in pre-tRNA splicing. This was unexpected, since previous analyses have shown that pre-tRNA end maturation and splicing are independent events (O'Connor & Peebles, 1991). The RNA component of RNase P is processed from a 3′ extended precursor, which was also accumulated in the rsp5-3 strain at the non-permissive temperature.
In contrast to tRNA and rRNA synthesis, no clear defect was seen in the synthesis of the small nucleolar RNAs U3 or snR38 (Fig. 3B). Moreover, no defect was apparent in the splicing of the ACT1 mRNA (Fig. 3A) or of pre-U3, which contains a pre-mRNA-like intron in yeast. Thus, pre-mRNA splicing seems not to be affected by the rsp5-3 ts mutant. The level of the ACT1 mRNA was reduced in the rsp5-3 strain during incubation at 37 °C, but this decline was much slower than that seen for the pre-rRNAs, suggesting that it may be an indirect effect of the growth arrest.
The defective nuclear RNA export in rsp5-3 may be due to an inhibition of components of the respective RNA biogenesis/export machineries; however, it could also be indirect. Notably, Rsp5p is known to activate the transcription factor Spt23p via the ubiquitin/proteasome system (Hoppe et al., 2000). Spt23p controls the fatty acid desaturase Ole1p, which is thought to regulate unsaturated fatty acid levels and accordingly could affect nuclear membrane/pore biogenesis. However, nuclear membrane abnormalities were not seen in the rsp5-3 strain, and the nuclear pores appeared to be of normal size and structure in the electron microscope (Fig. 4A). Moreover, expression of a GFP-tagged nucleoporin in rsp5-3 cells did not reveal alterations in the punctate nuclear envelope staining (data not shown). However, numerous electron-dense aggregates were observed in the nucleoplasm of rsp5-3 cells (Fig. 4A). Similar electron-dense aggregates were seen in the nucleus of the mex67-5 mutant, which is impaired in nuclear mRNA export (Segref et al., 1997). We conclude that nuclear membrane/nuclear pore organization is not grossly altered in rsp5-3 cells. Intranuclear aggregates may arise from the accumulation of aberrant RNA/RNPs resulting from defective processing, assembly and/or nuclear export.
Figure 4.
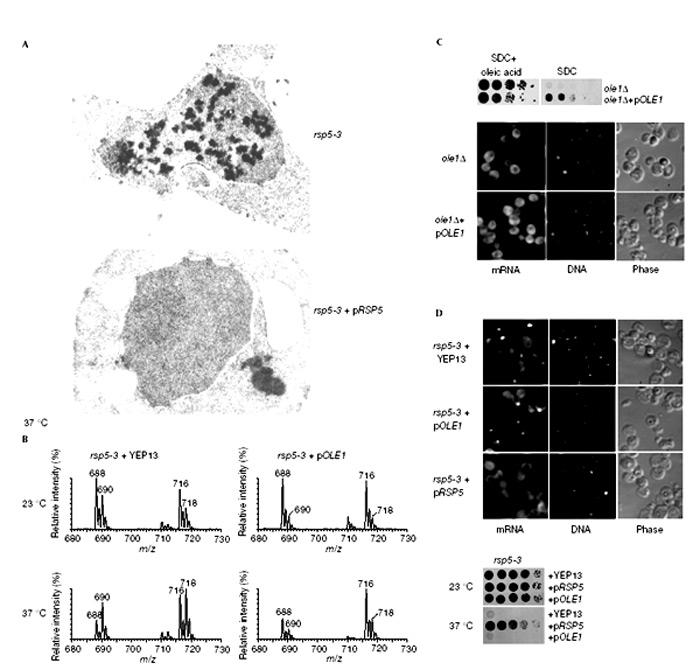
The rsp5-3 mutant is defective in nuclear structure and lipid synthesis. (A) rsp5-3 cells with and without pRSP5 grown at 23 °C and shifted to 37 °C were analysed by electron microscopy. (B) Phosphatidyl ethanolamine (PE) profiles of rsp5-3 mutant cells and rsp5-3 cells overexpressing Ole1p. (C) Growth of strains ole1Δ and ole1Δ complemented by pOLE1 in SDC medium and SDC medium containing 0.5 mM oleic acid/1% tergitol (upper panel). ole1Δ strain shifted for 18 h to SDC medium without oleic acid was analysed for nuclear export of poly(A)+ RNA (lower panel). (D) Analysis of mRNA export in the ole1Δ strain transformed with the indicated plasmids after shift for 3.5 h to 37 °C (upper panel). Cell growth was analysed after 3 days at 23 and 37 °C (lower panel).
We next sought to test whether rsp5-3 is defective in lipid synthesis as a consequence of decreased Ole1p expression. Total lipids were extracted from the rsp5-3 mutant. To analyse the unsaturated fatty acids in lipids, mass spectrometric (MS) analysis was performed and phosphatidyl ethanolamine (PE) profiles were obtained. As shown in Fig. 4B, rsp5-3 cells grown at the restrictive temperature exhibit significantly reduced levels of the di-unsaturated PE species, PE 32:2 (m/z 688) and PE 34:2 (m/z 716). The decrease of di-unsaturated PE species can be suppressed by expressing the OLE1 gene from a high-copy plasmid. Taken together, the data show that rsp5-3 is also defective in the desaturation of fatty acids, most likely due to decreased Ole1p expression.
It is possible that an altered lipid composition with decreased levels of unsaturated fatty acids is a cause for the nuclear RNA export defects in the rsp5-3 mutant. If this were true, nuclear RNA export should be defective in an ole1 mutant. No inhibition of nuclear RNA export was seen in ole1Δ cells (Fig. 4C). Moreover, overexpression of the OLE1 under a constitutive promoter (to circumvent Rsp5p/Spt23p-dependent regulation) did not abolish the mRNA (Fig. 4D) or 60S pre-ribosomal export defects in rsp5-3 (data not shown). Thus, neither the lack of unsaturated fatty acids in the nuclear membrane nor the reduced expression of Ole1p in rsp5-3 mutant cells is the cause of the observed RNA export defects.
If Rsp5p were directly involved in nuclear RNA formation, it might be expected to enter the nucleus. A functional GFP-Rsp5p was detected in both the nucleus and cytoplasm (Fig. 5A). In contrast, GFP-tagged aminoacyl-tRNA synthase is exclusively cytoplasmic and excluded from the nucleus (Fig. 5A; Galani et al., 2001). In addition, GFP-Rsp5p was concentrated at the cell periphery of bud tips and growing daughter cells (Fig. 5A).
Figure 5.
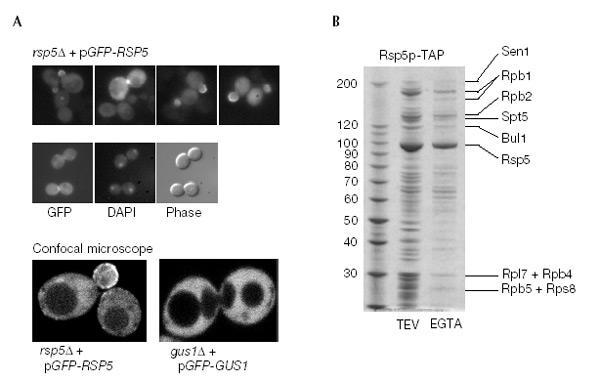
Intracellular location and purification of Rsp5p. (A) rsp5Δ cells expressing GFP-Rsp5p were analysed in the fluorescence microscope (upper panel) and also stained with DAPI (middle panel). rsp5Δ cells expressing GFP-Rsp5p were analysed in the confocal microscope (lower panel). gus1Δ cells expressing GFP-Gus1p served as control. (B) TAP-tagged Rsp5p was purified by the tandem-affinity purification method. Co-purifying proteins were separated on an SDS–4–12% polyacrylamide gradient gel and stained with Coomassie. The bands were identified by mass spectrometry and are indicated. Both the TEV protease and EGTA eluate are shown.
A functional Rsp5p-TAP fusion protein (TAP, tandem-affinity purification tag) was affinity-purified from yeast lysates. In agreement with its nuclear location and function, Rsp5p-TAP was associated with several subunits of RNA polII, the transcription factor Spt5p (Hartzog et al., 1998) and the nuclear Sen1p, which is involved in tRNA splicing and snRNA/snoRNA maturation (Ursic et al., 1997) (Fig. 5B). Rsp5p-TAP also co-precipitated Bul1p, a protein implicated in ubiquitin metabolism and associated with Rsp5p (Yashiroda et al., 1996).
Discussion
Here, we have shown that the ubiquitin ligase Rsp5p plays a role in regulating the major nuclear RNA biogenesis/export pathways in yeast. Remarkably, the rsp5-3 mutant affects concomitantly the processing and/or nuclear export of tRNA, rRNA and mRNA without clearly affecting protein import. This combination of phenotypes has not been reported previously for nucleocytoplasmic transport mutants. It is conceivable that ubiquitination by Rsp5p of a single component of the nuclear pore complex that is required by all three nuclear export systems could affect global nuclear RNA export. This would not, however, readily explain the dramatic defects seen in RNA processing. Alternatively and more likely, Rsp5p may have multiple targets, which are part of the individual RNA biogenesis/export machineries. Since specific steps in rRNA and tRNA processing are severely affected in the rsp5-3 mutant, it is likely that several substrates for Rsp5p participate in pre-rRNA and pre-tRNA processing. A high-throughput proteomic analysis recently identified many potentially ubiquitinated proteins in yeast (Peng et al., 2003), including several ribosome synthesis factors and tRNA processing enzymes. Which of these ubiquitin residues are added directly by Rsp5p remains to be determined.
Pre-tRNA splicing is inhibited by mutation of the putative RNA helicase Sen1p, which was co-precipitated with Rsp5p-TAP, making it a potential target for Rsp5p activity. The abundances of numerous other RNAs are altered in sen1-1 mutants, including rRNAs, small nuclear and small nucleolar RNAs, although the reported pre-rRNA processing defect does not resemble that of rsp5-3 strains (Ursic et al., 1997). Ubiquitinated pre-tRNA processing enzymes include the splicing endonuclease component Sen15p and the tRNA export factor Los1p/Exportin-t (Peng et al., 2003). The observed pre-tRNA end processing defect must have a different basis, since this is independent of splicing (O'Connor & Peebles, 1991). An additional potential substrate for Rsp5p is a protein component of RNase P, since both the activity and maturation of this complex were inhibited. The depletion of the mature RNase P RNA did not, however, appear to be sufficient to account for the dramatic pre-tRNA accumulation observed, indicating that maturation and function are both affected.
Several early cleavages in the pre-rRNA were almost entirely blocked in the rsp5-3 strain. This inhibition was seen within 1 h of transfer to the non-permissive temperature, and it seems probable that another direct substrate for Rsp5p can be found among the many ribosome synthesis factors that are known to be required for these cleavages (Tschochner & Hurt, 2003), several of which are reported to be ubiquitinated (Peng et al., 2003). However, pre-rRNA cleavage at site A3, which is performed by RNase MRP, was not inhibited. RNase MRP is closely related to RNase P, and the defect in RNase P activity is therefore quite specific. Notably, two proteins required for 60S subunit export, Noc2p and Noc3p, were reported to be ubiquitinated, making them potential targets for Rsp5p (Milkereit et al., 2001; Peng et al., 2003).
In analogy, we expect that specific factors involved in pre-mRNA biogenesis, which link intranuclear mRNP formation with nuclear export, could be regulated by Rsp5p. Identification of these factors should further unravel the mechanism of how the ubiquitin ligase Rsp5p can control the three major RNA export pathways.
Methods
Yeast strains and plasmids
Yeast strains are shown in Table 1. Genomic integration of the TAP tag at the 3′-end of RSP5 was performed as described previously (Grandi et al., 2002). Used plasmids were pRS316-RPL25-GFP, pRPL25-NLS-GFP (Gadal et al., 2001), pRS315-RPS2-GFP (Milkereit et al., 2002) NPL3-NLS-GFP (Senger et al., 1998) and pGFP-GUS1 (Galani et al., 2001).
Table 1.
Yeast strains used
| Name | Genotype | Origin |
|---|---|---|
| FY23 | MATa, ura3, trp1, leu2 | Derived from S288C |
| FY86 | MATα, ura3, his3, leu2 | Derived from S288C |
| rsp5-3 | MATa, ura3, trp1, leu2 | Isolated from ts collection (Amberg et al., 1992) |
| RSP5 shuffle | BY4743; MATa, his3, leu2, ura3, rsp5::kanMX4+pURA3-RSP5 | Derived from Euroscarf strain Y26124 |
| ole1Δ | BY4743; MATa, his3, leu2, ura3, lys2, ole1::kanMX4 | Derived from Euroscarf strain Y24422 |
| RSP5-TAP | MATα, ura3, leu2, trp1, his3, RSP5-CBP-TEV-protA::TRP1 | This work |
Lipid analysis
Lipids were extracted from yeast cells and analysed by MS (Brügger et al., 1997). ESI-MS/MS analysis was performed on a Micromass QII triple-stage quadrupol tandem mass spectrometer equipped with a nano-ESI source from Micromass.
Screen for tRNA export (tex) mutants
A ts mutant collection (Amberg et al., 1992) was screened for intranuclear accumulation of tRNA by FISH (intron-containing tRNALeu+Tyr and intronless tRNAGlu+Gly) at 37 °C (Grosshans et al., 2000a).
Cloning of TEX23/RSP5
The complementing gene for tex23-1 mutant cells was cloned by complementation of the ts phenotype through transformation with a yeast genomic library (Baßler et al., 2001). The rsp5-3 ts allele, which was isolated by polymerase chain reaction and sequenced, was shown to restore the viability of rsp5Δ cells at 23 °C, but caused a ts phenotype at 37 °C.
Miscellaneous
Microbiological techniques, DNA manipulation, plasmid transformation and recovery, sporulation of diploids, tetrad analysis and fluorescence microscopy were performed according to standard protocols (Baßler et al., 2001). Thinsection electron microscopy was carried out as described in Siniossoglou et al. (1998). Northern hybridization was performed as described previously (Tollervey et al., 1993).
Acknowledgments
We thank A. Hellwig (Institute for Neurobiology, Heidelberg) for performing EM analysis, H.C. Fahimi for providing access to the confocal laser scanning microscopy facility, K. Galani for confocal microscopy analysis and V. Panse for helpful discussions. The excellent technical assistance of P. Ihrig is acknowledged. We also thank S. Jentsch (MPI for Biochemistry, Martinsried, Germany) for pURA-RSP5 plasmid. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB352/B11).
References
- Amberg D.C., Goldstein A.L. & Cole C.N. ( 1992) Isolation and characterization of RAT1: an essential gene of Saccharomyces cerevisiae required for the efficient nucleocytoplasmic trafficking of mRNA. Genes Dev., 6, 1173–1189. [DOI] [PubMed] [Google Scholar]
- Arts G.-J., Fornerod M. & Mattaj I.W. ( 1998) Identification of a nuclear export receptor for tRNA. Curr. Biol., 8, 305–314. [DOI] [PubMed] [Google Scholar]
- Baßler J., Grandi P., Gadal O., Leßmann T., Tollervey D., Lechner J. & Hurt E.C. ( 2001) Identification of a 60S pre-ribosomal particle that is closely linked to nuclear export. Mol. Cell, 8, 517–529. [DOI] [PubMed] [Google Scholar]
- Brügger B., Erben G., Sandhoff R., Wieland F.T. & Lehmann W.D. ( 1997) Quantitative analysis of biological membrane lipids at the low picomole level by nano electrospray ionization tandem mass spectrometry. Proc. Natl Acad. Sci. USA, 94, 2339–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadal O., Strauß O., Kessl J., Trumpower B., Tollervey D. & Hurt E. ( 2001) Nuclear export of 60S ribosomal subunits depends on xpo1p and requires a nuclear export sequence-containing factor, Nmd3p, that associates with the large subunit protein Rpl10p. Mol. Cell. Biol., 21, 340S–341S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani K., Grasshans H., Deinert K., Hurt E.C. & Simos G. ( 2001) The intracellular location of two aminoacyl-tRNA synthetases depends on complex formation with Arc1p. EMBO J., 20, 6889–6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi P. ( 2002) 90S pre-nibosomes indude the 35S pre-rRNA, the u3 SnoRNP, and 40S subunit processing factors but predominantly lack 60S synthesis factors. Mol. Cell, 10, 10S–11S. [DOI] [PubMed] [Google Scholar]
- Grosshans H., Hurt E. & Simos G. ( 2000a) An aminoacylation-dependent nuclear tRNA export pathway in yeast. Genes Dev., 14, 830–840. [PMC free article] [PubMed] [Google Scholar]
- Grosshans H., Simos G. & Hurt E. ( 2000b) Transport of tRNA out of the nucleus—direct channeling to the ribosome? J. Struct. Biol., 129, 288–294. [DOI] [PubMed] [Google Scholar]
- Hartzog G.A., Wada T., Handa H. & Winston F. ( 1998) Evidence that Spt4, Spt5, and Spt6 control transcription elongation by RNA polymerase II in Saccharomyces cerevisiae. Genes Dev., 12, 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell S.B., Losko S. & Kaiser C.A. ( 2001) Components of a ubiquitin ligase complex specify polyubiquitination and intracellular trafficking of the general amino acid permease. J. Cell Biol., 153, 649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmuth K., Lau D.M., Bischoff F.R., Künzler M., Hurt E.C. & Simos G. ( 1998) Yeast Los1p has properties of an exportin-like nucleocytoplasmic transport factor for tRNA. Mol. Cell. Biol., 18, 6364–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe T., Matuschewski K., Rape M., Schlenker S., Ulrich H.D. & Jentsch S. ( 2000) Activation of a membrane-bound transcription factor by regulated ubiquitin/proteasome-dependent processing. Cell, 102, 577–586. [DOI] [PubMed] [Google Scholar]
- Hopper A.K. ( 1999) Nucleocytoplasmic transport: inside out regulation. Curr. Biol., 9, R803–R806. [DOI] [PubMed] [Google Scholar]
- Huang L., Kinnucan E., Wang G., Beaudenon S., Howley P.M., Huibregtse J.M. & Pavletich N.P. ( 1999) Structure of an E6AP–UbcH7 complex: insights into ubiquitination by the E2–E3 enzyme cascade. Science, 286, 1321–1326. [DOI] [PubMed] [Google Scholar]
- Huibregtse J.M., Scheffner M., Beaudenon S. & Howley P.M. ( 1995) A family of proteins structurally and functionally related to the E6-AP ubiquitin-protein ligase. Proc. Natl Acad. Sci. USA, 92, 2563–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibregtse J.M., Yang J.C. & Beaudenon S.L. ( 1997) The large subunit of RNA polymerase II is a substrate of the Rsp5 ubiquitin-protein ligase. Proc. Natl Acad. Sci. USA, 94, 3656–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.W., Lund E. & Dahlberg J. ( 2002) Nuclear export of ribosomal subunits. Trends Biochem. Sci., 27, 580–585. [DOI] [PubMed] [Google Scholar]
- Kaminska J., Gajewska B., Hopper A.K. & Zoladek T. ( 2002) Rsp5p, a new link between the actin cytoskeleton and endocytosis in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol., 22, 6946–6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U., Lipowsky G., Izaurralde E., Bischoff F.R., Schwarzmaier P., Hartmann E. & Görlich D. ( 1998) Identification of a tRNA-specific nuclear export receptor. Mol. Cell, 1, 359–369. [DOI] [PubMed] [Google Scholar]
- Lei E.P. & Silver P.A. ( 2002) Intron status and 3′-end formation control cotranscriptional export of mRNA. Genes Dev., 16, 2761–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkereit P., Gadal O., Podtelejnikov A., Trumtel S., Gas N., Petfalski E., Tollervey D., Mann M., Hurt E. & Tschochner H. ( 2001) Maturation and intranuclear transport of pre-ribosomes requires Noc proteins. Cell, 105, 499–509. [DOI] [PubMed] [Google Scholar]
- Milkereit P., Strauß D., Baßler J., Gadal O., Kühn H., Schütz S., Gas N., Lechner J., Hurt E. & Tschochner H. ( 2002) A Noc-complex specifically involved in the formation and nuclear export of ribosomal 40S subunits. J. Biol. Chem., 278, 4072–4081. [DOI] [PubMed] [Google Scholar]
- O'Connor J.P. & Peebles C.L. ( 1991) In vivo pre-tRNA processing in Saccharomyces cerevisiae. Mol. Cell. Biol., 11, 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Schwartz D., Elias J.E., Thoreen C.C., Cheng D., Marsischky G., Roelofs J., Finley D & Gygi S.P. ( 2003) A proteomics approach to understanding protein ubiquitination. Nature Biotechnol., 21, 921–926. [DOI] [PubMed] [Google Scholar]
- Reed R. & Hurt E.C. ( 2002) A conserved mRNA export machinery coupled to pre-mRNA splicing. Cell, 108, 523–531. [DOI] [PubMed] [Google Scholar]
- Rodriguez M.S., Gwizdek C., Haguenauer-Tsapis R. & Dargemont C. ( 2003) The HECT ubiquitin ligase Rsp5p is required for proper nuclear export of mRNA in Saccharomyces cerevisiae. Traffic, 4, 566–575. [DOI] [PubMed] [Google Scholar]
- Sarkar S & Hopper A.K. ( 1998) tRNA nuclear export in Saccharomyces cerevisiae: in situ hybridization analysis. Mol. Biol. Cell, 9, 3041–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segref A., Sharma K., Doye V., Hellwig A., Huber J., Lührmann R. & Hurt E.C. ( 1997) Mex67p which is an essential factor for nuclear mRNA export binds to both poly(A)+ RNA and nuclear pores. EMBO J., 16, 3256–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger B., Simos G., Bischoff F.R., Podtelejnikov A.V., Mann M. & Hurt E.C. ( 1998) Mtr10p functions as a nuclear import receptor for the mRNA binding protein Npl3p. EMBO J., 17, 2196–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniossoglou S., Santos-Rosa H., Rappsilber J., Mann M. & Hurt E. ( 1998) A novel complex of membrane proteins required for formation of a spherical nucleus. EMBO J., 17, 6449–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz F. & Izaurralde E. ( 2003) The interplay of nuclear mRNP assembly, mRNA surveillance and export. Trends Cell Biol., 13, 319–327. [DOI] [PubMed] [Google Scholar]
- Tollervey D., Lehtonen H., Jansen R.P., Kern H. & Hurt E.C. ( 1993) Temperature-sensitive mutations demonstrate roles for yeast fibrillarin in pre-rRNA processing, pre-rRNA methylation, and ribosome assembly. Cell, 72, 443–457. [DOI] [PubMed] [Google Scholar]
- Tschochner H. & Hurt E. ( 2003) Pre-ribosomes on the road from the nucleolus to the cytoplasm. Trends Cell Biol., 13, 255–263. [DOI] [PubMed] [Google Scholar]
- Ursic D., Himmel K.L., Gurley K.A., Webb F. & Culbertson M.R. ( 1997) The yeast SEN1 gene is required for the processing of diverse RNA classes. Nucleic Acids Res., 25, 4778–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin S.L. & Matera A.G. ( 1999) The trials and travels of tRNA. Genes Dev., 13, 1–10. [DOI] [PubMed] [Google Scholar]
- Yashiroda H., Oguchi T., Yasuda Y., Toh-E A. & Kikuchi Y. ( 1996) Bul1, a new protein that binds to the Rsp5 ubiquitin ligase in Saccharomyces cerevisiae. Mol. Cell. Biol., 16, 3255–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]


