Abstract
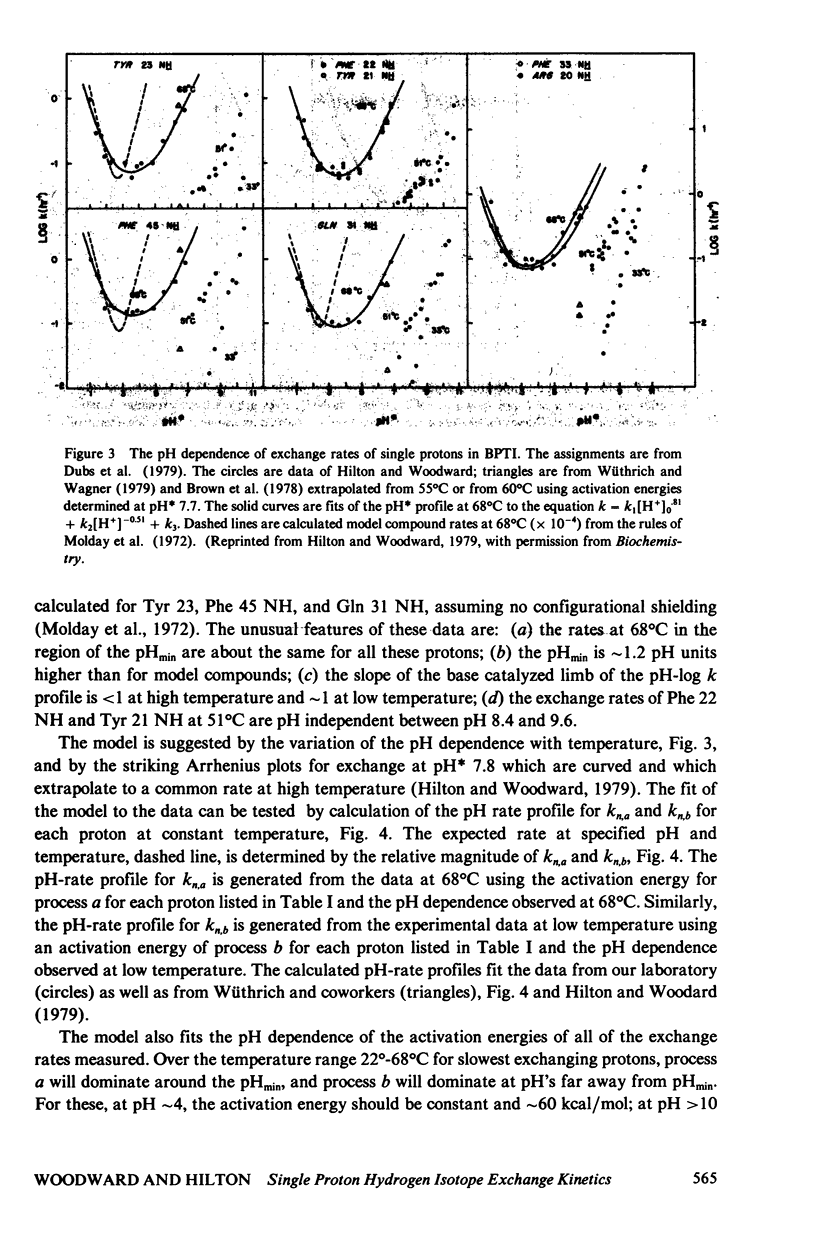
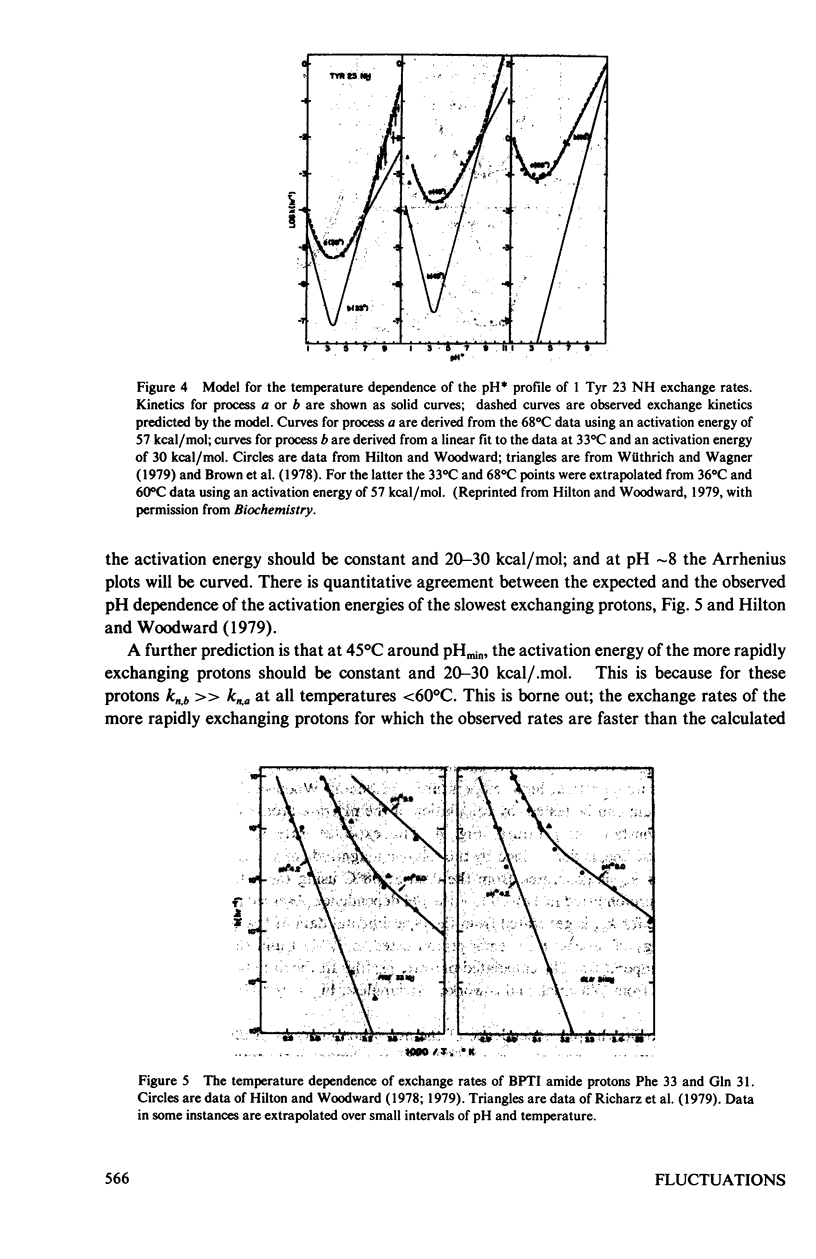
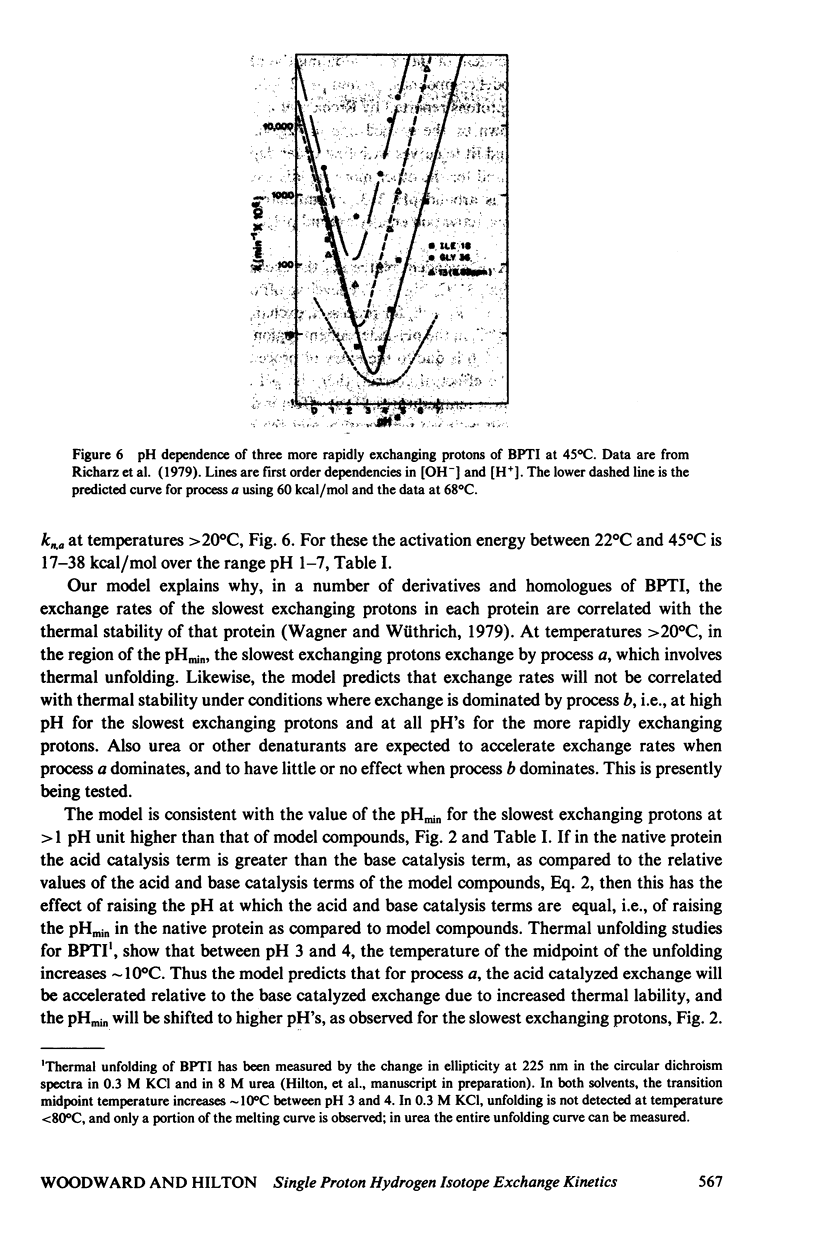
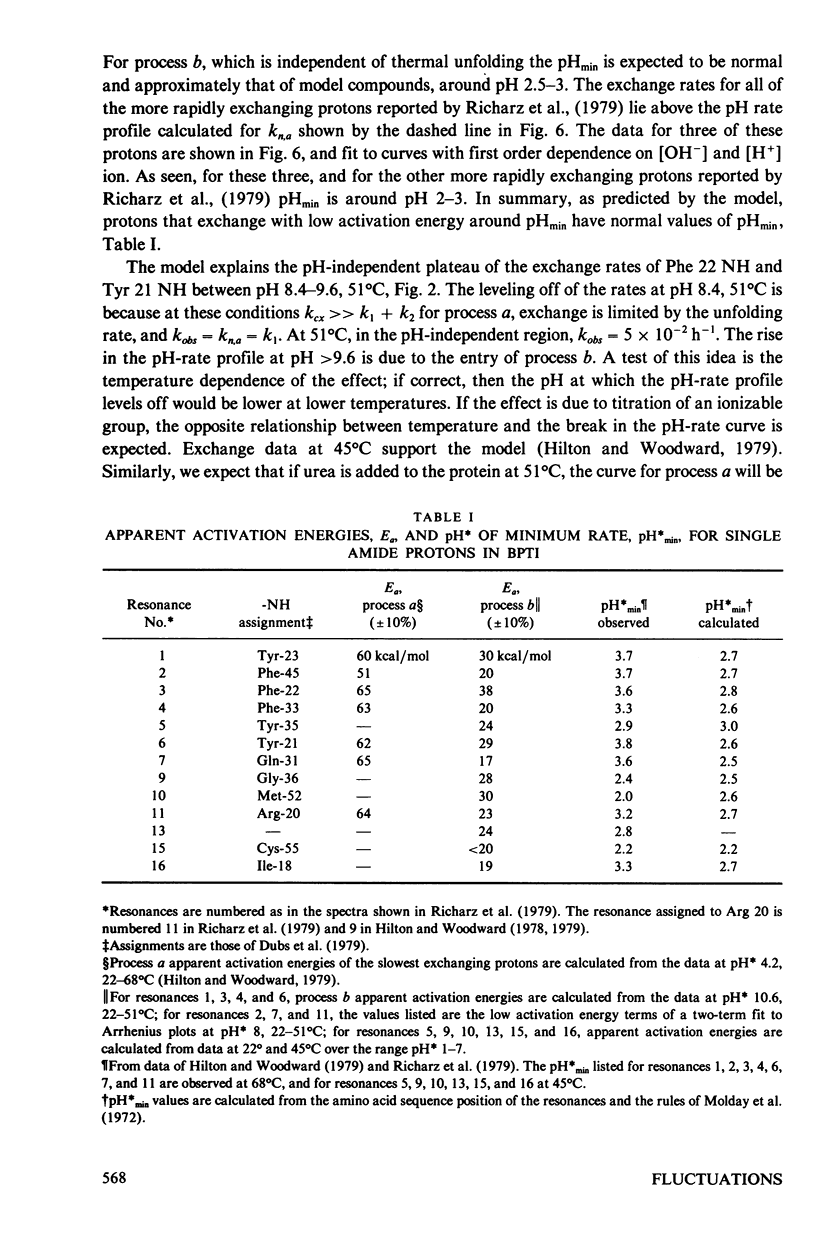
The exchange kinetics of the slowest exchanging BPTI beta-sheet protons are complex compared to model peptides; the activation energy, E alpha, and the pH dependence are temperature dependent. We have measured the exchange kinetics in the range pH 1--11, 33--71 degrees C, particularly the temperature dependence. The data are fit to a model in which exchange of each proton is determined by two discrete dynamical processes, one with E alpha approximately 65 kcal/mol and less than first order dependence on catalyst ion, and one with E alpha 20--30 kcal/mol and approaching first order in catalyst ion. The low activation energy process is the mechanism of interest in the native conformation of globular proteins and involves low energy, small amplitude fluctuations; the high activation energy process involves major unfolding. The model is simple, has a precedent in the hydrogen exchange literature, and explains quantitatively the complex feature of the exchange kinetics of single protons in BPTI, including the following. For the slowest exchanging protons, in the range 36 degrees--68 degrees C, E alpha is approximately 65 kcal/mol at pH approximately 4, 20--30 kcal/mol at pH greater than 10, and rises to approximately 65 kcal/mol with increasing temperature at pH 6--10; the Arrhenius plots converge around 70 degrees C; the pH of minimum rate, pHmin, is greater than 1 pH unit higher at 68 degrees C than for model compounds; and at high pH, the pH-rate profiles shift to steeper slope; the exchange rates around pHmin are correlated to the thermal unfolding temperature in BPTI derivatives (Wagner and Wüthrich, 1979, J. Mol. Biol. 130:31). For the more rapidly exchanging protons in BPTI the model accounts for the observation of normal pHmin and E alpha of 20--30 kcal/mol at all pH's. The important results of our analysis are (a) rates for exchange from the folded state of proteins are not correlated to thermal lability, as proposed by Wuthrich et al. (1979, J. Mol. Biol. 134:75); (b) the unfolding rate for the BPTI cooperative thermal transition is equal to the observed exchange rates of the slowest exchanging protons between pH 8.4--9.6, 51 degrees C; (c) the rates for exchange of single protons from folded BPTI are consistent with our previous hydrogen-tritium exchange results and with a penetration model of the dynamic processes limiting hydrogen exchange.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dubs A., Wagner G., Wüthrich K. Individual assignments of amide proton resonances in the proton NMR spectrum of the basic pancreatic trypsin inhibitor. Biochim Biophys Acta. 1979 Mar 27;577(1):177–194. doi: 10.1016/0005-2795(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Ellis L. M., Bloomfield V. A., Woodward C. K. Hydrogen-tritium exchange kinetics of soybean trypsin inhibitor (Kunitz). Solvent accessibility in the folded conformation. Biochemistry. 1975 Jul 29;14(15):3413–3419. doi: 10.1021/bi00686a019. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Englander J. J. Hydrogen--tritium exchange. Methods Enzymol. 1978;49:24–39. doi: 10.1016/s0076-6879(78)49005-6. [DOI] [PubMed] [Google Scholar]
- Englander S. W. Measurement of structural and free energy changes in hemoglobin by hydrogen exchange methods. Ann N Y Acad Sci. 1975 Apr 15;244:10–27. doi: 10.1111/j.1749-6632.1975.tb41518.x. [DOI] [PubMed] [Google Scholar]
- HVIDT A., LINDERSTRØM-LANG K. Exchange of hydrogen atoms in insulin with deuterium atoms in aqueous solutions. Biochim Biophys Acta. 1954 Aug;14(4):574–575. doi: 10.1016/0006-3002(54)90241-3. [DOI] [PubMed] [Google Scholar]
- Hilton B. D., Woodward C. K. Nuclear magnetic resonance measurement of hydrogen exchange kinetics of single protons in basic pancreatic trypsin inhibitor. Biochemistry. 1978 Aug 8;17(16):3325–3332. doi: 10.1021/bi00609a024. [DOI] [PubMed] [Google Scholar]
- Hilton B. D., Woodward C. K. On the mechanism of isotope exchange kinetics of single protons in bovine pancreatic trypsin inhibitor. Biochemistry. 1979 Dec 25;18(26):5834–5841. doi: 10.1021/bi00593a013. [DOI] [PubMed] [Google Scholar]
- Huber R., Kukla D., Rühlmann A., Steigemann W. Pancreatic trypsin inhibitor (Kunitz). I. Structure and function. Cold Spring Harb Symp Quant Biol. 1972;36:141–148. doi: 10.1101/sqb.1972.036.01.019. [DOI] [PubMed] [Google Scholar]
- Molday R. S., Englander S. W., Kallen R. G. Primary structure effects on peptide group hydrogen exchange. Biochemistry. 1972 Jan 18;11(2):150–158. doi: 10.1021/bi00752a003. [DOI] [PubMed] [Google Scholar]
- Nakanishi M., Tsuboi M., Ikegami A. Fluctuation of the lysozyme structure. II. Effects of temperature and binding of inhibitors. J Mol Biol. 1973 Apr 25;75(4):673–682. doi: 10.1016/0022-2836(73)90300-8. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. On the kinetics of structural transition I of some pancreatic proteins. FEBS Lett. 1969 Apr;3(1):60–64. doi: 10.1016/0014-5793(69)80097-9. [DOI] [PubMed] [Google Scholar]
- Richarz R., Sehr P., Wagner G., Wüthrich K. Kinetics of the exchange of individual amide protons in the basic pancreatic trypsin inhibitor. J Mol Biol. 1979 May 5;130(1):19–30. doi: 10.1016/0022-2836(79)90549-7. [DOI] [PubMed] [Google Scholar]
- Rosa J. J., Richards F. M. An experimental procedure for increasing the structural resolution of chemical hydrogen-exchange measurements on proteins: application to ribonuclease S peptide. J Mol Biol. 1979 Sep 25;133(3):399–416. doi: 10.1016/0022-2836(79)90400-5. [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Chicheportiche R., Lazdunski M. The conformational properties of the basic pancreatic trypsin-inhibitor. Eur J Biochem. 1971 Dec 10;23(3):401–411. doi: 10.1111/j.1432-1033.1971.tb01634.x. [DOI] [PubMed] [Google Scholar]
- Wagner G., Wüthrich K. Correlation between the amide proton exchange rates and the denaturation temperatures in globular proteins related to the basic pancreatic trypsin inhibitor. J Mol Biol. 1979 May 5;130(1):31–37. doi: 10.1016/0022-2836(79)90550-3. [DOI] [PubMed] [Google Scholar]
- Wagner G., Wüthrich K. Structural interpretation of the amide proton exchange in the basic pancreatic trypsin inhibitor and related proteins. J Mol Biol. 1979 Oct 15;134(1):75–94. doi: 10.1016/0022-2836(79)90414-5. [DOI] [PubMed] [Google Scholar]
- Wickett R. R., Ide G. J., Rosenberg A. A hydrogen-exchange study of lysozyme conformation changes induced by inhibitor binding. Biochemistry. 1974 Jul 30;13(16):3273–3277. doi: 10.1021/bi00713a015. [DOI] [PubMed] [Google Scholar]
- Woodward C. K. Dynamic solvent accessibility in the soybean trypsin inhibitor--trypsin complex. J Mol Biol. 1977 Apr 25;111(4):509–515. doi: 10.1016/s0022-2836(77)80066-1. [DOI] [PubMed] [Google Scholar]
- Woodward C. K., Ellis L. M., Rosenberg A. Solvent accessibility in folded proteins. Studies of hydrogen exchange in trypsin. J Biol Chem. 1975 Jan 25;250(2):432–439. [PubMed] [Google Scholar]
- Woodward C. K., Ellis L. M., Rosenberg A. The solvent dependence of hydrogen exchange kinetics of folded proteins. J Biol Chem. 1975 Jan 25;250(2):440–444. [PubMed] [Google Scholar]
- Woodward C. K., Hilton B. D. Hydrogen exchange kinetics and internal motions in proteins and nucleic acids. Annu Rev Biophys Bioeng. 1979;8:99–127. doi: 10.1146/annurev.bb.08.060179.000531. [DOI] [PubMed] [Google Scholar]
- Wüthrich K., Wagner G. Nuclear magnetic resonance of labile protons in the basic pancreatic trypsin inhibitor. J Mol Biol. 1979 May 5;130(1):1–18. doi: 10.1016/0022-2836(79)90548-5. [DOI] [PubMed] [Google Scholar]
- Wüthrich K., Wagner G., Richarz R., Perkins S. J. Individual assignments of the methyl resonances in the 1H nuclear magnetic resonance spectrum of the basic pancreatic trypsin inhibitor. Biochemistry. 1978 Jun 13;17(12):2253–2263. doi: 10.1021/bi00605a001. [DOI] [PubMed] [Google Scholar]


